Resilience of Neural Networks Underlying the Stroop Effect in the Aftermath of Severe COVID-19: fMRI Pilot Study
Abstract
1. Introduction
- In healthy subjects, the neural activity elicited by Incongruent, Congruent or Neutral conditions is likely to differ between the first and the second parts of a prolonged Stroop task, particularly in the key regions of the cascade-of-control network.
- In post-COVID-19 patients, the neural activity elicited in the key regions of the cascade-of-control network by the Incongruent, Congruent and Neutral conditions is likely to differ from that in healthy subjects. Furthermore, the pattern of activity elicited by the Stroop effect may differ between individual patients.
2. Materials and Methods
2.1. Participants
2.2. Stroop Task
2.3. fMRI Data Acquisition and AnalysisStroop Task
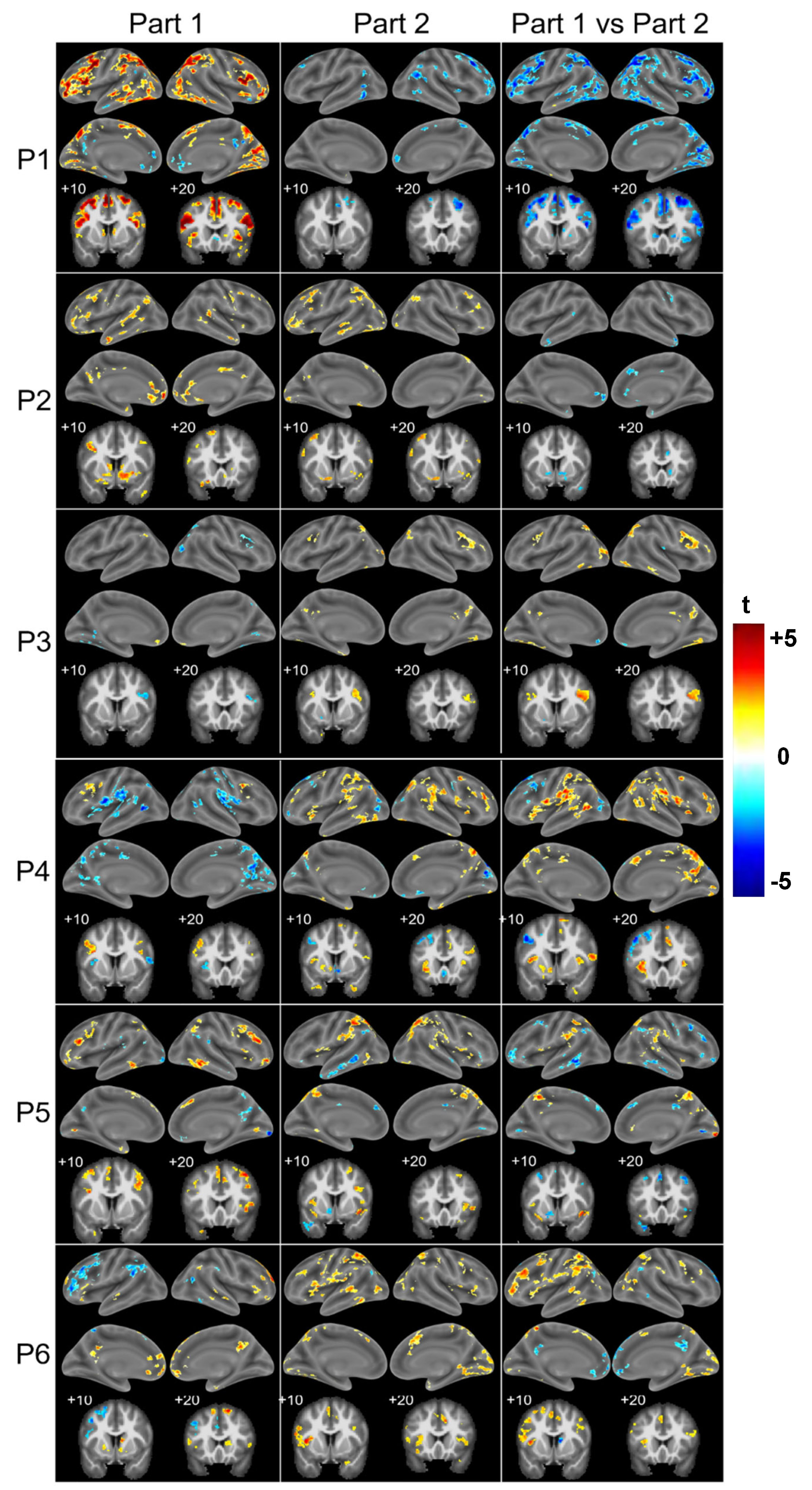
3. Results
3.1. Control Population
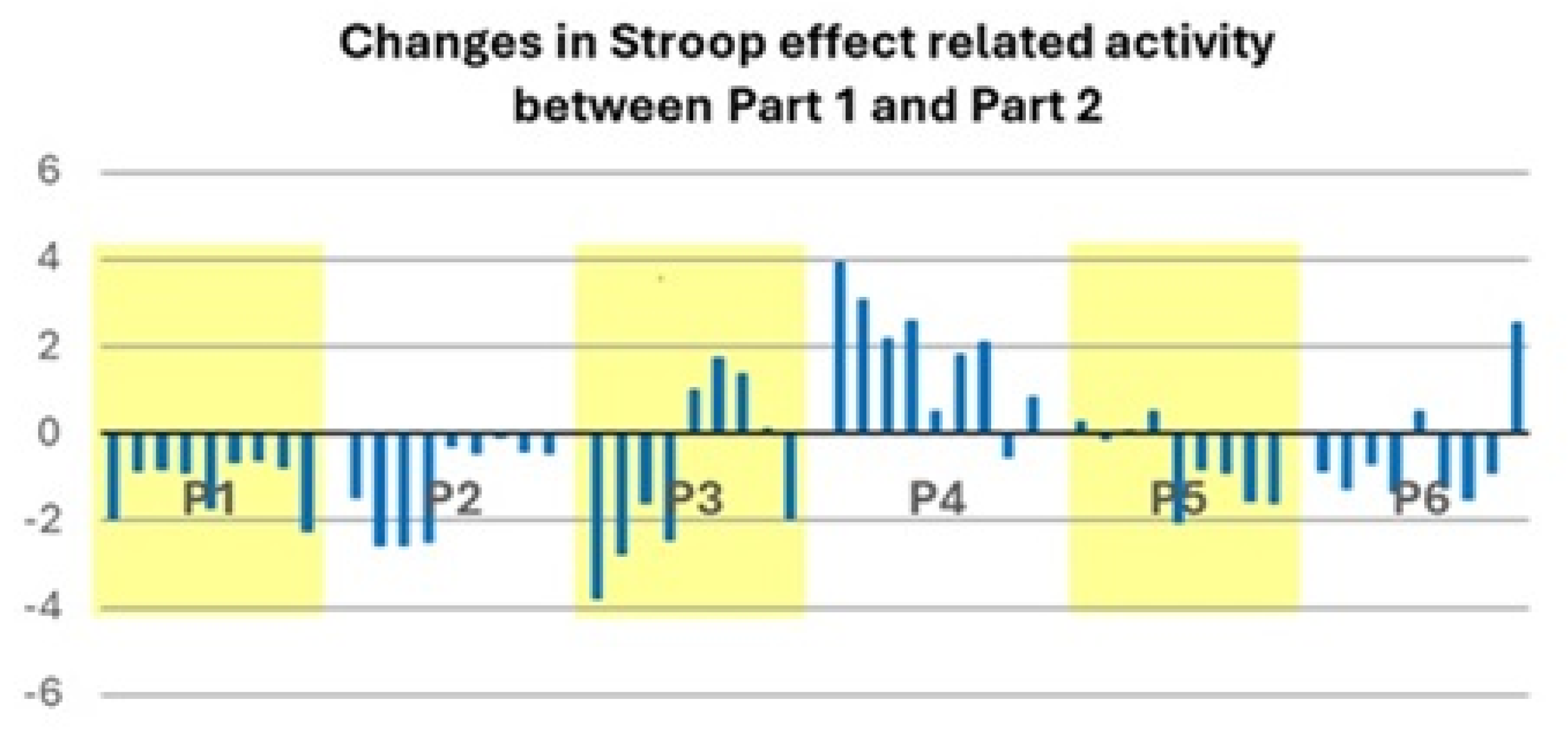
3.2. Patient Population
4. Discussion
4.1. Adaptation During a Prolonged Task in Healthy Subjects
4.1.1. Salience Network
4.1.2. Top-Down Control Network
4.1.3. Effect of a Prolonged Stroop Task
4.2. Two Profiles of Network Resilience After Severe COVID-19
4.3. Cognitive Performance and Functional Correlates in the Aftermath of COVID-19
4.4. Variety of Resting-State Functional Connectivity Profiles in COVID-19
4.5. Comparison with Activation Patterns in Chronic Fatigue Syndrome
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bamps, L.; Armenti, J.-P.; Bojan, M.; Grandbastien, B.; von Garnier, C.; Du Pasquier, R.; Desgranges, F.; Papadimitriou-Olivgeris, M.; Alberio, L.; Preisig, M.; et al. Long-Term Consequences of COVID-19: A 1-Year Analysis. J. Clin. Med. 2023, 12, 2673. [Google Scholar] [CrossRef] [PubMed]
- Beaud, V.; Crottaz-Herbette, S.; Dunet, V.; Knebel, J.-F.; Bart, P.-A.; Clarke, S. Outcome of Severe COVID-19: Spotlight on Fatigue, Fatigability, Multidomain Complaints and Pattern of Cognitive Deficits in a Case Series without Prior Brain Dysfunction and without COVID-19-Related Stroke and/or Cardiac Arrest. J. Med. Case Rep. 2024, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and Cognitive Impairment in Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Daste, C.; Ficarra, S.; Dumitrache, A.; Cariou, A.; Lefèbvre, A.; Pène, F.; Roche, N.; Roren, A.; Thery, C.; Vidal, J.; et al. Post-Intensive Care Syndrome in Patients Surviving COVID-19. Ann. Phys. Rehabil. Med. 2021, 64, 101549. [Google Scholar] [CrossRef]
- Fietsam, A.C.; Bryant, A.D.; Rudroff, T. Fatigue and Perceived Fatigability, Not Objective Fatigability, Are Prevalent in People with Post-COVID-19. Exp. Brain Res. 2023, 241, 211–219. [Google Scholar] [CrossRef]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-Discharge Persistent Symptoms and Health-Related Quality of Life after Hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive Deficits in People Who Have Recovered from COVID-19. EClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef]
- Hartung, T.J.; Neumann, C.; Bahmer, T.; Chaplinskaya-Sobol, I.; Endres, M.; Geritz, J.; Haeusler, K.G.; Heuschmann, P.U.; Hildesheim, H.; Hinz, A.; et al. Fatigue and Cognitive Impairment after COVID-19: A Prospective Multicentre Study. EClinicalMedicine 2022, 53, 101651. [Google Scholar] [CrossRef]
- Negrini, F.; Ferrario, I.; Mazziotti, D.; Berchicci, M.; Bonazzi, M.; de Sire, A.; Negrini, S.; Zapparoli, L. Neuropsychological Features of Severe Hospitalized Coronavirus Disease 2019 Patients at Clinical Stability and Clues for Postacute Rehabilitation. Arch. Phys. Med. Rehabil. 2021, 102, 155–158. [Google Scholar] [CrossRef]
- Peskar, M.; Šimunič, B.; Šlosar, L.; Pišot, S.; Teraž, K.; Gasparini, M.; Pišot, R.; Marusic, U. Effects of COVID-19 on Cognition and Mood after Hospitalization and at 2-Month Follow-Up. Front. Psychol. 2023, 14, 1141809. [Google Scholar] [CrossRef]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and Long-Term Neurological and Neuropsychiatric Manifestations of Post-COVID-19 Syndrome: A Meta-Analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and Neuropsychiatric Presentations Associated with Severe Coronavirus Infections: A Systematic Review and Meta-Analysis with Comparison to the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Schulze, H.; Charles James, J.; Trampe, N.; Richter, D.; Pakeerathan, T.; Siems, N.; Ayzenberg, I.; Gold, R.; Faissner, S. Cross-Sectional Analysis of Clinical Aspects in Patients with Long-COVID and Post-COVID Syndrome. Front. Neurol. 2022, 13, 979152. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Lant, S.; Cividini, S.; Cattrall, J.W.S.; Goodwin, L.C.; Benjamin, L.; Michael, B.D.; Khawaja, A.; de Matos, A.M.B.; Alkeridy, W.; et al. Prognostic Indicators and Outcomes of Hospitalised COVID-19 Patients with Neurological Disease: An Individual Patient Data Meta-Analysis. PLoS ONE 2022, 17, e0263595. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-Month Neurological and Psychiatric Outcomes in 236 379 Survivors of COVID-19: A Retrospective Cohort Study Using Electronic Health Records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and Psychiatric Risk Trajectories after SARS-CoV-2 Infection: An Analysis of 2-Year Retrospective Cohort Studies Including 1 284 437 Patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent Fatigue Following SARS-CoV-2 Infection Is Common and Independent of Severity of Initial Infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and Neuropsychiatric Complications of COVID-19 in 153 Patients: A UK-Wide Surveillance Study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Writing Committee for the COMEBAC Study Group; Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.-L.; Meyrignac, O.; et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar] [CrossRef]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-Term Neurologic Outcomes of COVID-19. Nat. Med. 2022, 28, 2406–2415. [Google Scholar] [CrossRef]
- Fleischer, M.; Szepanowski, F.; Tovar, M.; Herchert, K.; Dinse, H.; Schweda, A.; Mausberg, A.K.; Holle-Lee, D.; Köhrmann, M.; Stögbauer, J.; et al. Post-COVID-19 Syndrome Is Rarely Associated with Damage of the Nervous System: Findings from a Prospective Observational Cohort Study in 171 Patients. Neurol. Ther. 2022, 11, 1637–1657. [Google Scholar] [CrossRef] [PubMed]
- Barnden, L.; Thapaliya, K.; Eaton-Fitch, N.; Barth, M.; Marshall-Gradisnik, S. Altered Brain Connectivity in Long Covid during Cognitive Exertion: A Pilot Study. Front. Neurosci. 2023, 17, 1182607. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Ryan, M.C.; Liang, H.; Zhang, X.; Cunningham, E.; Wang, J.; Wilson, E.; Herskovits, E.H.; Kottilil, S.; Ernst, T.M. Changes in Brain Activation Patterns During Working Memory Tasks in People with Post-COVID Condition and Persistent Neuropsychiatric Symptoms. Neurology 2023, 100, e2409–e2423. [Google Scholar] [CrossRef]
- Niemczak, C.E.; Ford, J.C.; Roth, R.M.; Leigh, S.M.; Parsonnet, J.; Martin, C.; Soule, S.O.; Haron, T.M.; Buckey, J.C.; Wylie, G.R. Neuroimaging Markers of Cognitive Fatigue in Individuals with Post-Acute Sequelae of SARS-CoV-2 Infection. Brain Cogn. 2025, 183, 106254. [Google Scholar] [CrossRef]
- Thomasson, M.; Voruz, P.; Cionca, A.; Jacot de Alcântara, I.; Nuber-Champier, A.; Allali, G.; Benzakour, L.; Lalive, P.H.; Lövblad, K.-O.; Braillard, O.; et al. Markers of Limbic System Damage Following SARS-CoV-2 Infection. Brain Commun. 2023, 5, fcad177. [Google Scholar] [CrossRef]
- Troll, M.; Li, M.; Chand, T.; Machnik, M.; Rocktäschel, T.; Toepffer, A.; Ballez, J.; Finke, K.; Güllmar, D.; Reichenbach, J.R.; et al. Altered Corticostriatal Connectivity in Long-COVID Patients Is Associated with Cognitive Impairment. Psychol. Med. 2025, 55, e49. [Google Scholar] [CrossRef]
- Voruz, P.; Cionca, A.; Jacot de Alcântara, I.; Nuber-Champier, A.; Allali, G.; Benzakour, L.; Lalive, P.H.; Lövblad, K.O.; Braillard, O.; Nehme, M.; et al. Brain Functional Connectivity Alterations Associated with Neuropsychological Performance 6-9 Months Following SARS-CoV-2 Infection. Hum. Brain Mapp. 2023, 44, 1629–1646. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Banich, M.T. The Stroop Effect Occurs at Multiple Points Along a Cascade of Control: Evidence From Cognitive Neuroscience Approaches. Front. Psychol. 2019, 10, 2164. [Google Scholar] [CrossRef]
- Derrfuss, J.; Brass, M.; Neumann, J.; von Cramon, D.Y. Involvement of the Inferior Frontal Junction in Cognitive Control: Meta-analyses of Switching and Stroop Studies. Hum. Brain Mapp. 2005, 25, 22–34. [Google Scholar] [CrossRef]
- Langenecker, S.A.; Nielson, K.A.; Rao, S.M. fMRI of Healthy Older Adults during Stroop Interference. Neuroimage 2004, 21, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Edin, F.; Klingberg, T.; Johansson, P.; McNab, F.; Tegnér, J.; Compte, A. Mechanism for Top-down Control of Working Memory Capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 6802–6807. [Google Scholar] [CrossRef] [PubMed]
- Penner, I.K.; Raselli, C.; Stöcklin, M.; Opwis, K.; Kappos, L.; Calabrese, P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): Validation of a New Instrument to Assess Multiple Sclerosis-Related Fatigue. Mult. Scler. 2009, 15, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Mairesse, O.; Damen, V.; Newell, J.; Kornreich, C.; Verbanck, P.; Neu, D. The Brugmann Fatigue Scale: An Analogue to the Epworth Sleepiness Scale to Measure Behavioral Rest Propensity. Behav. Sleep. Med. 2019, 17, 437–458. [Google Scholar] [CrossRef]
- Lepine, J.P.; Godchau, M.; Brun, P. Anxiety and Depression in Inpatients. Lancet 1985, 2, 1425–1426. [Google Scholar] [CrossRef]
- Barek, L.M.; Mercy, G.; Gautier, C.; Noquet, M.; Legros-Lafarge, E.; Fiegl, L.; Fiard, D.; Allain, P. The Use of the French Dimensional Apathy Scale (f-DAS) to Assess Apathy in Schizophrenia: Properties and Profiles. J. Affect. Disord. 2021, 294, 181–188. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- von Steinbüchel, N.; Wilson, L.; Gibbons, H.; Hawthorne, G.; Höfer, S.; Schmidt, S.; Bullinger, M.; Maas, A.; Neugebauer, E.; Powell, J.; et al. Quality of Life after Brain Injury (QOLIBRI): Scale Validity and Correlates of Quality of Life. J. Neurotrauma 2010, 27, 1157–1165. [Google Scholar] [CrossRef]
- Banich, M.T.; Milham, M.P.; Atchley, R.; Cohen, N.J.; Webb, A.; Wszalek, T.; Kramer, A.F.; Liang, Z.P.; Wright, A.; Shenker, J.; et al. fMri Studies of Stroop Tasks Reveal Unique Roles of Anterior and Posterior Brain Systems in Attentional Selection. J. Cogn. Neurosci. 2000, 12, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Crottaz-Herbette, S.; Fornari, E.; Notter, M.P.; Bindschaedler, C.; Manzoni, L.; Clarke, S. Reshaping the Brain after Stroke: The Effect of Prismatic Adaptation in Patients with Right Brain Damage. Neuropsychologia 2017, 104, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Spierer, L.; Tardif, E.; Sperdin, H.; Murray, M.M.; Clarke, S. Learning-Induced Plasticity in Auditory Spatial Representations Revealed by Electrical Neuroimaging. J. Neurosci. 2007, 27, 5474–5483. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Peters, S.K.; Dunlop, K.; Downar, J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci. 2016, 10, 104. [Google Scholar]
- Dosenbach, N.U.F.; Fair, D.A.; Cohen, A.L.; Schlaggar, B.L.; Petersen, S.E. A Dual-Networks Architecture of Top-down Control. Trends Cogn. Sci. 2008, 12, 99–105. [Google Scholar] [CrossRef]
- Menon, V. Large-Scale Brain Networks and Psychopathology: A Unifying Triple Network Model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef]
- Trambaiolli, L.R.; Peng, X.; Lehman, J.F.; Linn, G.; Russ, B.E.; Schroeder, C.E.; Liu, H.; Haber, S.N. Anatomical and Functional Connectivity Support the Existence of a Salience Network Node within the Caudal Ventrolateral Prefrontal Cortex. Elife 2022, 11, e76334. [Google Scholar] [CrossRef]
- Baliki, M.N.; Mansour, A.; Baria, A.T.; Huang, L.; Berger, S.E.; Fields, H.L.; Apkarian, A.V. Parceling Human Accumbens into Putative Core and Shell Dissociates Encoding of Values for Reward and Pain. J. Neurosci. 2013, 33, 16383–16393. [Google Scholar] [CrossRef]
- Milham, M.P.; Banich, M.T.; Barad, V. Competition for Priority in Processing Increases Prefrontal Cortex’s Involvement in Top-down Control: An Event-Related fMRI Study of the Stroop Task. Brain Res. Cogn. Brain Res. 2003, 17, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Milham, M.P.; Banich, M.T. Anterior Cingulate Cortex: An fMRI Analysis of Conflict Specificity and Functional Differentiation. Hum. Brain Mapp. 2005, 25, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Banich, M.T.; Jacobson, B.L.; Tanabe, J.L. Functional Dissociation of Attentional Selection within PFC: Response and Non-Response Related Aspects of Attentional Selection as Ascertained by fMRI. Cereb. Cortex 2006, 16, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Milham, M.P.; Banich, M.T.; Webb, A.; Barad, V.; Cohen, N.J.; Wszalek, T.; Kramer, A.F. The Relative Involvement of Anterior Cingulate and Prefrontal Cortex in Attentional Control Depends on Nature of Conflict. Brain Res. Cogn. Brain Res. 2001, 12, 467–473. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Miezin, F.M.; Cohen, A.L.; Wenger, K.K.; Dosenbach, R.A.T.; Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E.; et al. Distinct Brain Networks for Adaptive and Stable Task Control in Humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11073–11078. [Google Scholar] [CrossRef]
- Pattyn, N.; Neyt, X.; Henderickx, D.; Soetens, E. Psychophysiological Investigation of Vigilance Decrement: Boredom or Cognitive Fatigue? Physiol. Behav. 2008, 93, 369–378. [Google Scholar] [CrossRef]
- Richter, A.; Reinhard, F.; Kraemer, B.; Gruber, O. A High-Resolution fMRI Approach to Characterize Functionally Distinct Neural Pathways within Dopaminergic Midbrain and Nucleus Accumbens during Reward and Salience Processing. Eur. Neuropsychopharmacol. 2020, 36, 137–150. [Google Scholar] [CrossRef]
- Han, M.; Li, Q.; Yang, T.; Li, J. Amide Proton Transfer Imaging in Rats after Heatstroke. Neuroreport 2024, 35, 37–41. [Google Scholar] [CrossRef]
- Jin, P.; Cui, F.; Xu, M.; Ren, Y.; Zhang, L. Altered Brain Function and Structure Pre- and Post- COVID-19 Infection: A Longitudinal Study. Neurol. Sci. 2024, 45, 1–9. [Google Scholar] [CrossRef]
- Rusche, T.; Kaufmann, J.; Voges, J. Nucleus Accumbens Projections: Validity and Reliability of Fiber Reconstructions Based on High-Resolution Diffusion-Weighted MRI. Hum. Brain Mapp. 2021, 42, 5888–5910. [Google Scholar] [CrossRef]
- Almutairi, B.; Langley, C.; Crawley, E.; Thai, N.J. Using Structural and Functional MRI as a Neuroimaging Technique to Investigate Chronic Fatigue Syndrome/Myalgic Encephalopathy: A Systematic Review. BMJ Open 2020, 10, e031672. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.Y.; Barnden, L.R.; Kwiatek, R.A.; Bhuta, S.; Hermens, D.F.; Lagopoulos, J. Neuroimaging Characteristics of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Systematic Review. J. Transl. Med. 2020, 18, 335. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, J.N.; Thapaliya, K.; Inderyas, M.; Shan, Z.Y.; Barnden, L.R. Stroop Task and Practice Effects Demonstrate Cognitive Dysfunction in Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Sci. Rep. 2024, 14, 26796. [Google Scholar] [CrossRef]
- Nasir, S.M.; Yahya, N.; Yap, K.H.; Manan, H.A. Executive Function Deficit in Patients with Long COVID Syndrome: A Systematic Review. Heliyon 2025, 11, e41987. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Saadat, M.; Hessam, M.; Ravanbakhsh, M.; Monjezi, S. Post-COVID-19 Physical and Cognitive Impairments and Associations with Quality of Life: A Cross-Sectional Study. Front. Sports Act. Living 2024, 6, 1246585. [Google Scholar] [CrossRef]
- Gopinath, G.; Suryavanshi, C.A.; Pallavi, L.C. Long-Term Cognitive and Autonomic Effects of COVID-19 in Young Adults: A Cross-Sectional Study at 28 Months. Ann. Med. 2025, 57, 2453082. [Google Scholar] [CrossRef]
- Rubin, M.; Travis, E. COVID-19 Related Loss Is Reliably Associated with Attentional Capture and Facilitation by COVID Related Stimuli: Evidence from the Emotional Stroop Dilution Task. Cogn. Ther. Res. 2023, 435–443. [Google Scholar] [CrossRef]
- Scardua-Silva, L.; Amorim da Costa, B.; Karmann Aventurato, Í.; Batista Joao, R.; Machado de Campos, B.; Rabelo de Brito, M.; Bechelli, J.F.; Santos Silva, L.C.; Ferreira Dos Santos, A.; Koutsodontis Machado Alvim, M.; et al. Microstructural Brain Abnormalities, Fatigue, and Cognitive Dysfunction after Mild COVID-19. Sci. Rep. 2024, 14, 1758. [Google Scholar] [CrossRef]
- Kesler, S.R.; Franco-Rocha, O.Y.; De La Torre Schutz, A.; Lewis, K.A.; Aziz, R.M.; Henneghan, A.M.; Melamed, E.; Brode, W.M. Altered Functional Brain Connectivity, Efficiency, and Information Flow Associated with Brain Fog after Mild to Moderate COVID-19 Infection. Sci. Rep. 2024, 14, 22094. [Google Scholar] [CrossRef]
- Churchill, N.W.; Roudaia, E.; Chen, J.J.; Gilboa, A.; Sekuler, A.; Ji, X.; Gao, F.; Lin, Z.; Jegatheesan, A.; Masellis, M.; et al. Effects of Post-Acute COVID-19 Syndrome on the Functional Brain Networks of Non-Hospitalized Individuals. Front. Neurol. 2023, 14, 1136408. [Google Scholar] [CrossRef]
- Sarvandani, M.N.; Sheikhi Koohsar, J.; Rafaiee, R.; Saeedi, M.; Tamijani, S.M.S.; Ghazvini, H.; Sheibani, H. COVID-19 and the Brain: A Psychological and Resting-State Functional Magnetic Resonance Imagin (fMRI) Study of the Whole-Brain Functional Connectivity. Basic Clin. Neurosci. 2023, 14, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, G.; Zhang, X.; Zhang, M.; Lu, J.; Li, H. Altered Intrinsic Brain Activity and Functional Connectivity in COVID-19 Hospitalized Patients at 6-Month Follow-Up. BMC Infect. Dis. 2023, 23, 521. [Google Scholar] [CrossRef] [PubMed]
- Paolini, M.; Palladini, M.; Mazza, M.G.; Colombo, F.; Vai, B.; Rovere-Querini, P.; Falini, A.; Poletti, S.; Benedetti, F. Brain Correlates of Subjective Cognitive Complaints in COVID-19 Survivors: A Multimodal Magnetic Resonance Imaging Study. Eur. Neuropsychopharmacol. 2023, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Snider, S.B.; Barra, M.E.; Sanders, W.R.; Rapalino, O.; Schaefer, P.; Foulkes, A.S.; Bodien, Y.G.; Edlow, B.L. Disorders of Consciousness Associated With COVID-19: A Prospective Multimodal Study of Recovery and Brain Connectivity. Neurology 2022, 98, e315–e325. [Google Scholar] [CrossRef]
- Díez-Cirarda, M.; Yus, M.; Gómez-Ruiz, N.; Polidura, C.; Gil-Martínez, L.; Delgado-Alonso, C.; Jorquera, M.; Gómez-Pinedo, U.; Matias-Guiu, J.; Arrazola, J.; et al. Multimodal Neuroimaging in Post-COVID Syndrome and Correlation with Cognition. Brain 2023, 146, 2142–2152. [Google Scholar] [CrossRef]
- Dacosta-Aguayo, R.; Torán-Monserrat, P.; Carmona-Cervelló, M.; León-Gómez, B.B.; Mataró, M.; Puig, J.; Monté-Rubio, G.; López-Lifante, V.M.; Maria Manresa-Domínguez, J.; Zamora-Putin, V.; et al. Multimodal Neuroimaging in Long-COVID and Its Correlates with Cognition 1.8 Years after SARS-CoV-2 Infection: A Cross-Sectional Study of the Aliança ProHEpiC-19 Cognitiu. Front. Neurol. 2024, 15, 1426881. [Google Scholar] [CrossRef]
- Shan, Z.Y.; Finegan, K.; Bhuta, S.; Ireland, T.; Staines, D.R.; Marshall-Gradisnik, S.M.; Barnden, L.R. Brain Function Characteristics of Chronic Fatigue Syndrome: A Task fMRI Study. NeuroImage Clin. 2018, 19, 279–286. [Google Scholar] [CrossRef]
- Lange, G.; Steffener, J.; Cook, D.B.; Bly, B.M.; Christodoulou, C.; Liu, W.-C.; Deluca, J.; Natelson, B.H. Objective Evidence of Cognitive Complaints in Chronic Fatigue Syndrome: A BOLD fMRI Study of Verbal Working Memory. Neuroimage 2005, 26, 513–524. [Google Scholar] [CrossRef]
- Cook, D.B.; O’Connor, P.J.; Lange, G.; Steffener, J. Functional Neuroimaging Correlates of Mental Fatigue Induced by Cognition among Chronic Fatigue Syndrome Patients and Controls. Neuroimage 2007, 36, 108–122. [Google Scholar] [CrossRef]
- de Lange, F.P. Neural Correlates of the Chronic Fatigue Syndrome--an fMRI Study. Brain 2004, 127, 1948–1957. [Google Scholar] [CrossRef]
- Tanaka, M.; Sadato, N.; Okada, T.; Mizuno, K.; Sasabe, T.; Tanabe, H.C.; Saito, D.N.; Onoe, H.; Kuratsune, H.; Watanabe, Y. Reduced Responsiveness Is an Essential Feature of Chronic Fatigue Syndrome: A fMRI Study. BMC Neurol. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Jones, J.F.; Drake, D.F.; Tian, H.; Unger, E.R.; Pagnoni, G. Decreased Basal Ganglia Activation in Subjects with Chronic Fatigue Syndrome: Association with Symptoms of Fatigue. PLoS ONE 2014, 9, e98156. [Google Scholar] [CrossRef] [PubMed]
- Wortinger, L.A.; Endestad, T.; Melinder, A.M.D.; Øie, M.G.; Sulheim, D.; Fagermoen, E.; Wyller, V.B. Emotional Conflict Processing in Adolescent Chronic Fatigue Syndrome: A Pilot Study Using Functional Magnetic Resonance Imaging. J. Clin. Exp. Neuropsychol. 2017, 39, 355–368. [Google Scholar] [CrossRef]
- Man, M.A.; Rosca, D.; Bratosin, F.; Fira-Mladinescu, O.; Ilie, A.C.; Burtic, S.-R.; Fildan, A.P.; Fizedean, C.M.; Jianu, A.M.; Negrean, R.A.; et al. Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccines 2024, 12, 189. [Google Scholar] [CrossRef]
- Johnson, S.; Mielke, N.; Mathew, T.; Maine, G.N.; Chen, N.-W.; Bahl, A. Predictors of Hospitalization and Severe Disease Due to Breakthrough SARS-CoV-2 Infection in Fully Vaccinated Individuals. J. Am. Coll. Emerg. Physicians Open 2022, 3, e12793. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Clarke, S.; Bindschaedler, C.; Crottaz-Herbette, S. Impact of Cognitive Neuroscience on Stroke Rehabilitation. Stroke 2015, 46, 1408–1413. [Google Scholar] [CrossRef]
- Amunts, K.; DeFelipe, J.; Pennartz, C.; Destexhe, A.; Migliore, M.; Ryvlin, P.; Furber, S.; Knoll, A.; Bitsch, L.; Bjaalie, J.G.; et al. Linking Brain Structure, Activity, and Cognitive Function through Computation. eNeuro 2022, 9, ENEURO.0316-21.2022. [Google Scholar] [CrossRef]
- Ahmad, M. Classification of Tumors in Human Brain MRI Using Wavelet and Support Vector Machine. IOSR J. Comput. Eng. 2012, 8, 25–31. [Google Scholar] [CrossRef]
- Shafi, I.; Din, S.; Farooq, S.; de la Díez, I.T.; Breñosa, J.; Espinosa, J.C.M.; Ashraf, I. Design and Development of Patient Health Tracking, Monitoring and Big Data Storage Using Internet of Things and Real Time Cloud Computing. PLoS ONE 2024, 19, e0298582. [Google Scholar] [CrossRef]
- Bispo, D.; Brandão, P.; Pereira, D.; Maluf, F.; Dias, B.; Paranhos, H.; Glehn, F.; Oliveira, A.; Regattieri, N.; Yasuda, C.; et al. Brain Microstructural Changes and Fatigue after COVID-19. Front. Neurol. 2022, 13, 1029302. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Cuffaro, L.; Capozza, A.; Rosci, C.; Maiorana, N.; Groppo, E.; Reitano, M.R.; Poletti, B.; Ticozzi, N.; Tagliabue, L.; et al. Brain Positron Emission Tomography (PET) and Cognitive Abnormalities One Year after COVID-19. J. Neurol. 2023, 270, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; COVID-19 BioB Outpatient Clinic Study group; Rovere-Querini, P.; Benedetti, F. Persistent Psychopathology and Neurocognitive Impairment in COVID-19 Survivors: Effect of Inflammatory Biomarkers at Three-Month Follow-Up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Granberg, T.; Andersen, P.; Jokhadar, E.; Kåhlin, J.; Granström, A.; Hallinder, H.; Schening, A.; Thunborg, C.; Walles, H.; et al. The Karolinska NeuroCOVID Study Protocol: Neurocognitive Impairment, Biomarkers and Advanced Imaging in Critical Care Survivors. Acta Anaesthesiol. Scand. 2022, 66, 759–766. [Google Scholar] [CrossRef]
- Hartwigsen, G.; Saur, D. Neuroimaging of Stroke Recovery from Aphasia-Insights into Plasticity of the Human Language Network. Neuroimage 2019, 190, 14–31. [Google Scholar] [CrossRef]
- Lorca-Puls, D.L.; Gajardo-Vidal, A.; PLORAS Team; Oberhuber, M.; Prejawa, S.; Hope, T.M.H.; Leff, A.P.; Green, D.W.; Price, C.J. Brain Regions That Support Accurate Speech Production after Damage to Broca’s Area. Brain Commun. 2021, 3, fcab230. [Google Scholar] [CrossRef]
- Crottaz-Herbette, S.; Fornari, E.; Clarke, S. Prismatic Adaptation Changes Visuospatial Representation in the Inferior Parietal Lobule. J. Neurosci. 2014, 34, 11803–11811. [Google Scholar] [CrossRef]
- Clarke, S.; Crottaz-Herbette, S. Modulation of Visual Attention by Prismatic Adaptation. Neuropsychologia 2016, 92, 31–41. [Google Scholar] [CrossRef]
- Tissieres, I.; Fornari, E.; Clarke, S.; Crottaz-Herbette, S. Supramodal Effect of Rightward Prismatic Adaptation on Spatial Representations within the Ventral Attentional System. Brain Struct. Funct. 2018, 223, 1459–1471. [Google Scholar] [CrossRef]
- Tissieres, I.; Elamly, M.; Clarke, S.; Crottaz-Herbette, S. For Better or Worse: The Effect of Prismatic Adaptation on Auditory Neglect. Neural Plast. 2017, 2017, 8721240. [Google Scholar] [CrossRef]
- Saj, A.; Cojan, Y.; Assal, F.; Vuilleumier, P. Prism Adaptation Effect on Neural Activity and Spatial Neglect Depend on Brain Lesion Site. Cortex 2019, 119, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Goedert, K.M.; Chen, P.; Foundas, A.L.; Barrett, A.M. Frontal Lesions Predict Response to Prism Adaptation Treatment in Spatial Neglect: A Randomised Controlled Study. Neuropsychol. Rehabil. 2020, 30, 32–53. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Herrera, M.; Eger, S.; Keller, I.; Hermsdörfer, J.; Saevarsson, S. Neuroanatomical and Behavioural Factors Associated with the Effectiveness of Two Weekly Sessions of Prism Adaptation in the Treatment of Unilateral Neglect. Neuropsychol. Rehabil. 2020, 30, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Banich, M.T.; Milham, M.P.; Atchley, R.A.; Cohen, N.J.; Webb, A.; Wszalek, T.; Kramer, A.F.; Liang, Z.; Barad, V.; Gullett, D.; et al. Prefrontal Regions Play a Predominant Role in Imposing an Attentional “Set”: Evidence from fMRI. Brain Res. Cogn. Brain Res. 2000, 10, 1–9. [Google Scholar] [CrossRef]
- Zysset, S.; Müller, K.; Lohmann, G.; von Cramon, D.Y. Color-Word Matching Stroop Task: Separating Interference and Response Conflict. NeuroImage 2001, 13, 29–36. [Google Scholar] [CrossRef]
- Milham, M.P.; Erickson, K.I.; Banich, M.T.; Kramer, A.F.; Webb, A.; Wszalek, T.; Cohen, N.J. Attentional Control in the Aging Brain: Insights from an fMRI Study of the Stroop Task. Brain Cogn. 2002, 49, 277–296. [Google Scholar] [CrossRef]
- Mead, L.A.; Mayer, A.R.; Bobholz, J.A.; Woodley, S.J.; Cunningham, J.M.; Hammeke, T.A.; Rao, S.M. Neural Basis of the Stroop Interference Task: Response Competition or Selective Attention? J. Int. Neuropsychol. Soc. 2002, 8, 735–742. [Google Scholar] [CrossRef]
- Norris, D.G.; Zysset, S.; Mildner, T.; Wiggins, C.J. An Investigation of the Value of Spin-Echo-Based fMRI Using a Stroop Color–Word Matching Task and EPI at 3 T. NeuroImage 2002, 15, 719–726. [Google Scholar] [CrossRef]
- Potenza, M.N.; Leung, H.-C.; Blumberg, H.P.; Peterson, B.S.; Fulbright, R.K.; Lacadie, C.M.; Skudlarski, P.; Gore, J.C. An FMRI Stroop Task Study of Ventromedial Prefrontal Cortical Function in Pathological Gamblers. Am. J. Psychiatry 2003, 160, 1990–1994. [Google Scholar] [CrossRef]
- Zysset, S.; Schroeter, M.L.; Neumann, J.; von Cramon, D.Y. Stroop Interference, Hemodynamic Response and Aging: An Event-Related fMRI Study. Neurobiol. Aging 2007, 28, 937–946. [Google Scholar] [CrossRef]
- Leung, H.C.; Skudlarski, P.; Gatenby, J.C.; Peterson, B.S.; Gore, J.C. An Event-Related Functional MRI Study of the Stroop Color Word Interference Task. Cereb. Cortex 2000, 10, 552–560. [Google Scholar] [CrossRef]
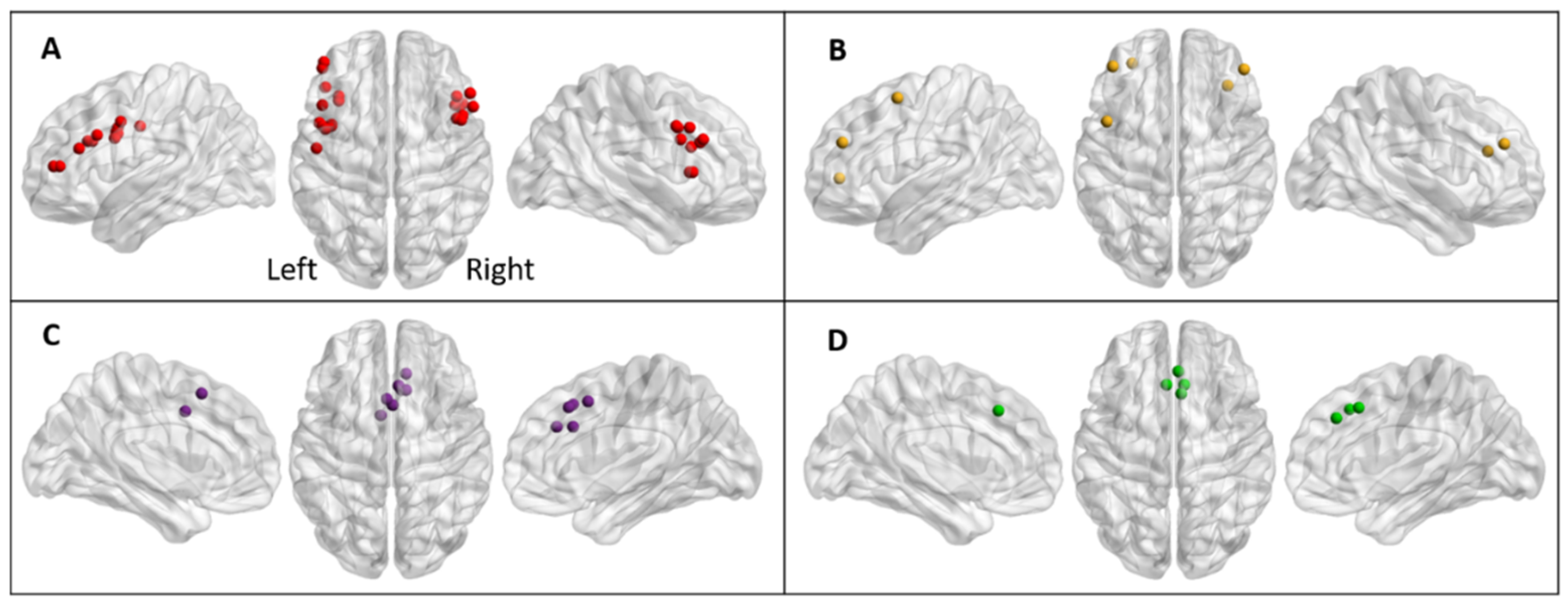
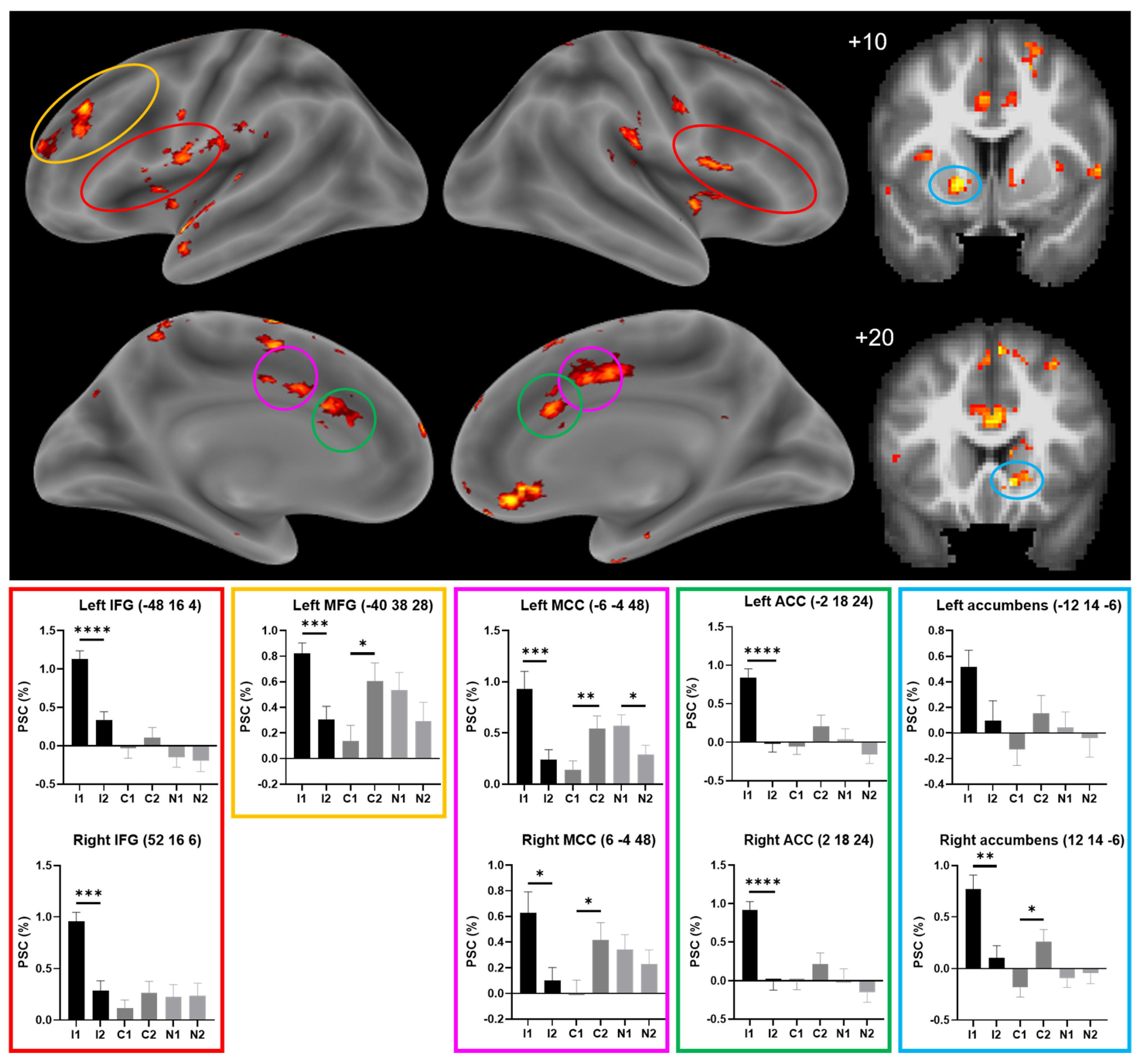
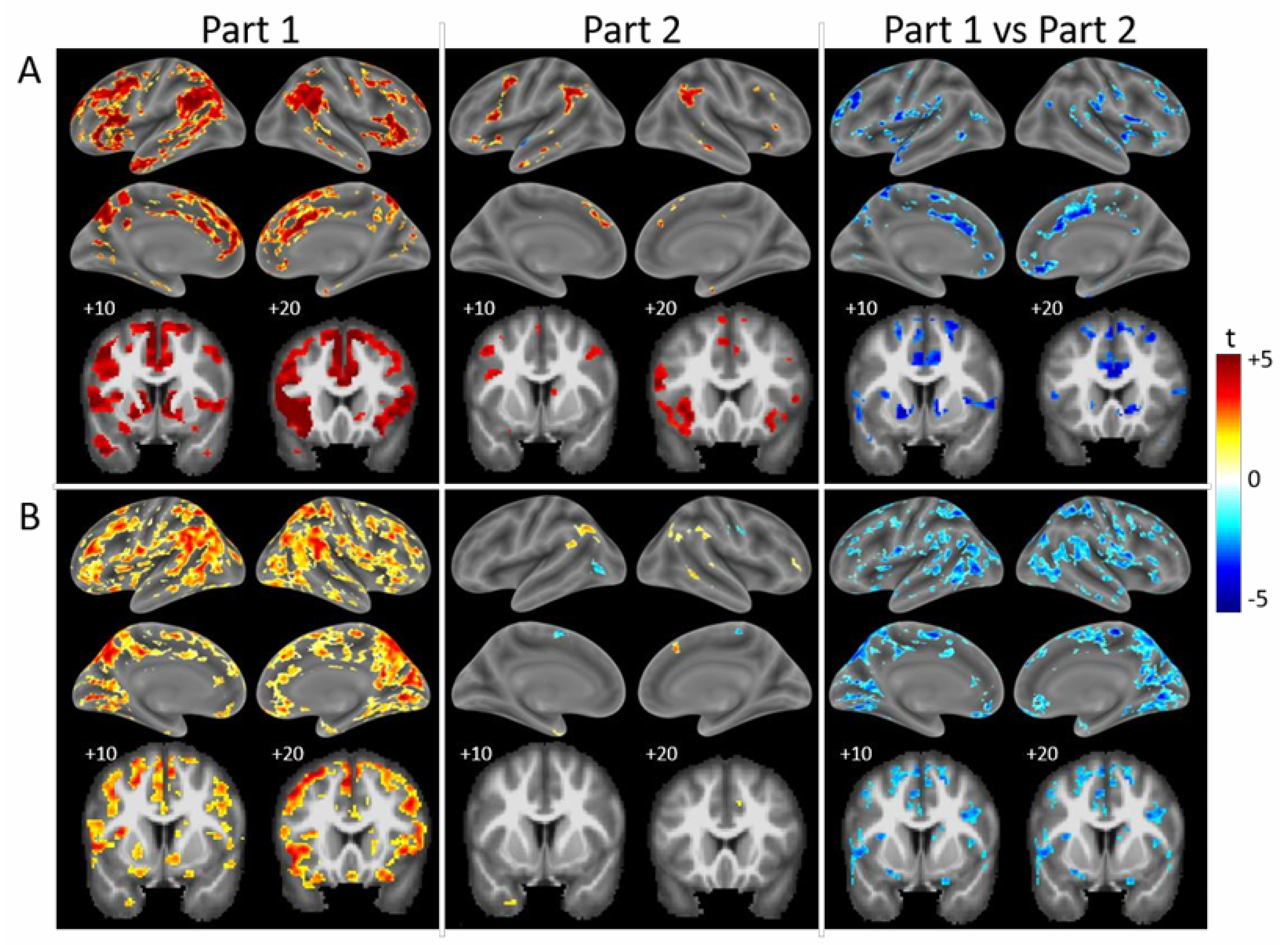
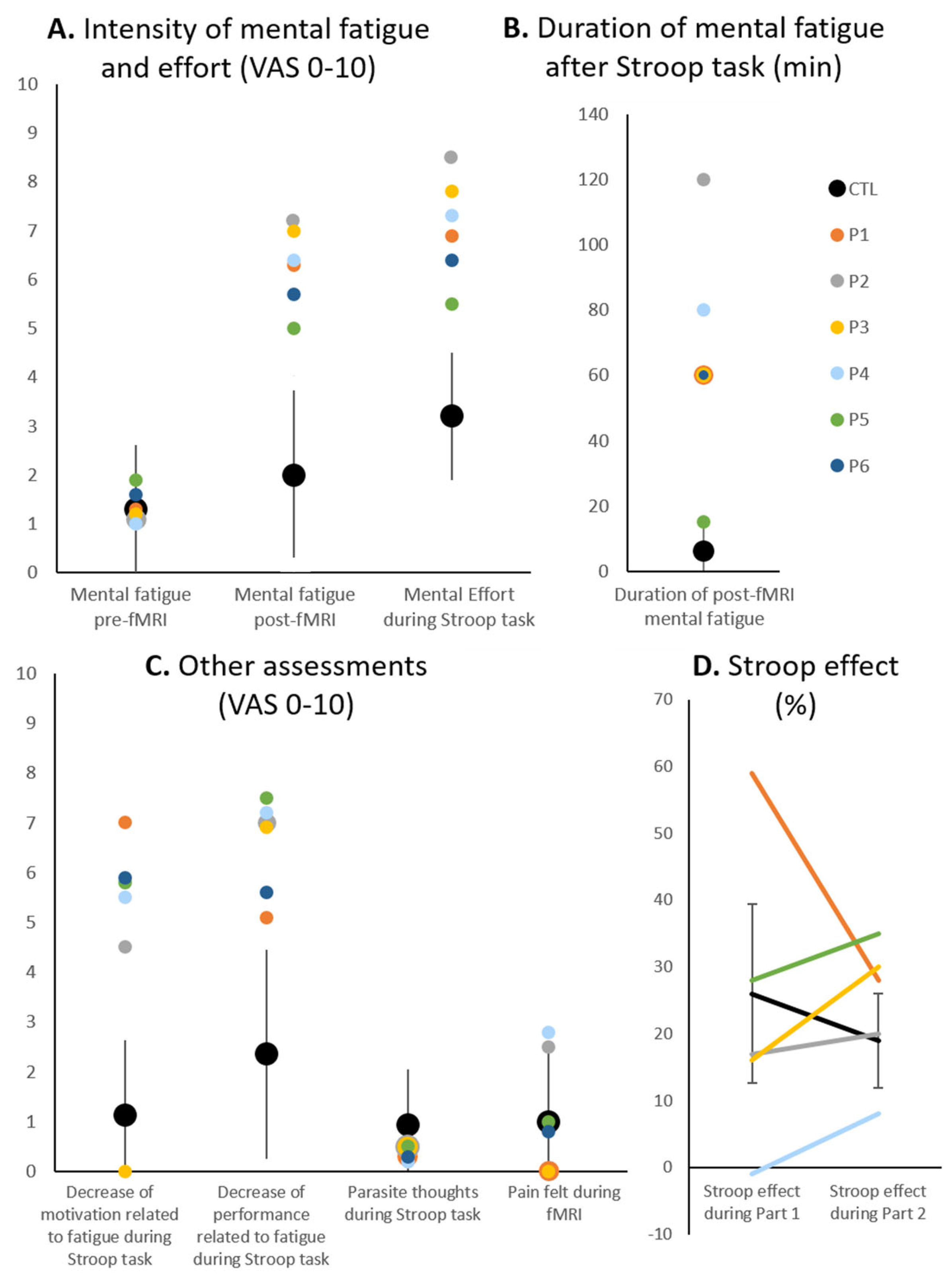
| P1 | P2 | P3 | P4 | P5 | P6 | Controls Mean ± SD | |
|---|---|---|---|---|---|---|---|
| Duration (days) of mechanical ventilation/ICU stay/acute hospitalisation/post-acute inpatient rehabilitation | 22/25/36/17 | 18/21/40/53 | 17/21/29/14 | 11/12/24/0 | 50/67/82/37 | 17/21/29/31 | -/-/-/- |
| Standardised scales and questionnaires | |||||||
| Fatigue Scale for Motor and Cognitive Functions [33]: Motor 10–50/Cognitive 10–50 | 41/40 | 30/35 | 28/34 | 26/36 | 23/27 | 26/28 | 14.8 ± 3.6/16.0 ± 3.9 |
| Brugmann Fatigue Scale [34]: Physical 0–12/Mental 0–12 | 6 /8 | 7 /8 | 4/6 | 5/7 | 4/4 | 4/4 | 1.0 ± 0.0/0.7 ± 0.5 |
| Hospital Anxiety and Depression Scale [35]: Anxiety 0–21/Depression 0–21 | 6/11 | 11/10 | 5/4 | 3/1 | 0/2 | 8 /0 | 5.1 ± 2.4/2.1 ± 1.8 |
| French Dimensional Apathy Scale [36]: Executive 0–24/Emotion 0–24/Initiative 0–24 | 14/7/8 | 15/8/9 | 10/9/7 | 15/8/7 | 14/8/8 | 13/7/7 | 3.8 ± 1.6/3.3 ± 1.4/3.0 ± 1.4 |
| Perceived Stress Scale [37]: 0–40 | 20 | 10 | 8 | 10 | 10 | 10 | 10.6 ± 5.7 |
| Epworth Sleepiness Score [38]: 0–24 | 10 | 10 | 2 | 2 | 7 | 10 | 5.7 ± 4.1 |
| Insomnia Severity Index [39]: 0–28 | 4 | 7 | 5 | 1 | 3 | 8 | 6.5 ± 4.6 |
| Adapted Quality of Life after Brain Injury Questionnaire [40]: 0–100 | 65 | 62 | 95 | 84 | 77 | 78 | 83.6 ± 8.0 |
| Stroop task-associated fatigue (visual analogue scales: 1–10 or in minutes) | |||||||
| Mental fatigue pre-fMRI | 1.3 | 1.1 | 1.2 | 1 | 1.9 | 1.6 | 1.3 ± 1.3 |
| Mental fatigue post-fMRI | 6.3 | 7.2 | 7 | 6.4 | 5 | 5.7 | 2 ± 2 |
| Mental effort during Stroop task | 6.9 | 8.5 | 7.8 | 7.3 | 5.5 | 6.4 | 3.2 ± 1.3 |
| Motivation decrease related to Stroop-induced fatigue | 7 | 4.5 | 0 | 5.5 | 5.8 | 5.9 | 1.1 ± 1.5 |
| Performance decrease related to Stroop-induced fatigue | 5.1 | 7 | 6.9 | 7.2 | 7.5 | 5.6 | 2.4 ± 2.1 |
| Occurrence of parasite thoughts during Stroop task | 0.3 | 0.5 | 0.5 | 0.2 | 0.5 | 0.3 | 0.9 ± 1.1 |
| Pain felt during fMRI scanning | 0 | 2.5 | 0 | 2.8 | 1 | 0.8 | 1 ± 1.4 |
| Duration of Stroop-generated fatigue (minutes) | 60 | 120 | 60 | 80 | 15 | 60 | 6 ± 7.9 |
| Accuracy (%) | Response Times (ms) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incongruent | Congruent | Neutral | Incongruent | Congruent | Neutral | |||||||
| Part 1 | Part 2 | Part 1 | Part 2 | Part 1 | Part 2 | Part 1 | Part 2 | Part 1 | Part 2 | Part 1 | Part 2 | |
| Control population | ||||||||||||
| 81 ± 14 | 94 ± 5 | 99 ± 2 | 100 ± 1 | 99 ± 3 | 100 ± 1 | 1636 ± 342 | 1466 ± 268 | 1282 ± 273 | 1232 ± 263 | 1184 ± 211 | 1107 ± 206 | |
| p < 0.001 | p = 0.185 | p = 0.6162 | p < 0.001 | p = 0.010 | p < 0.001 | |||||||
| Patients | ||||||||||||
| P1 | 75 | 95 | 100 | 100 | 100 | 100 | 2471 | 1918 | 1429 | 1466 | 1395 | 1406 |
| P2 | 90 | 100 | 100 | 100 | 100 | 100 | 1327 | 1306 | 1136 | 1081 | 940 | 922 |
| P3 | 90 | 100 | 100 | 100 | 100 | 95 | 1850 | 1844 | 1593 | 1387 | 1328 | 1356 |
| P4 | 85 | 100 | 100 | 100 | 95 | 100 | 1606 | 1612 | 1621 | 1491 | 1355 | 1327 |
| P5 | 90 | 100 | 100 | 100 | 100 | 100 | 1728 | 1599 | 1322 | 1145 | 1254 | 1168 |
| Areas | Number of Voxels | Peak Intensity | Peak MNI Coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Three-way ANOVA (group × condition × part) | |||||
| R lingual gyrus | 33 | 9.20 | 12 | −42 | −2 |
| R cerebellum (IV–V) | 33 | 8.51 | 20 | −50 | −14 |
| R superior temporal gyrus | 31 | 8.44 | 50 | −38 | 10 |
| R putamen | 51 | 7.80 | 28 | 6 | −4 |
| R middle temporal gyrus | 19 | 7.76 | 46 | −54 | 8 |
| Two-way ANOVA (group × condition) | |||||
| L cerebellum (Crus 1) | 30 | 9.74 | −38 | −82 | −18 |
| R cerebellum (VII) | 26 | 7.14 | 40 | −60 | −42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaud, V.; Farron, N.; Fornari, E.; Dunet, V.; Crottaz-Herbette, S.; Clarke, S. Resilience of Neural Networks Underlying the Stroop Effect in the Aftermath of Severe COVID-19: fMRI Pilot Study. Brain Sci. 2025, 15, 635. https://doi.org/10.3390/brainsci15060635
Beaud V, Farron N, Fornari E, Dunet V, Crottaz-Herbette S, Clarke S. Resilience of Neural Networks Underlying the Stroop Effect in the Aftermath of Severe COVID-19: fMRI Pilot Study. Brain Sciences. 2025; 15(6):635. https://doi.org/10.3390/brainsci15060635
Chicago/Turabian StyleBeaud, Valérie, Nicolas Farron, Eleonora Fornari, Vincent Dunet, Sonia Crottaz-Herbette, and Stephanie Clarke. 2025. "Resilience of Neural Networks Underlying the Stroop Effect in the Aftermath of Severe COVID-19: fMRI Pilot Study" Brain Sciences 15, no. 6: 635. https://doi.org/10.3390/brainsci15060635
APA StyleBeaud, V., Farron, N., Fornari, E., Dunet, V., Crottaz-Herbette, S., & Clarke, S. (2025). Resilience of Neural Networks Underlying the Stroop Effect in the Aftermath of Severe COVID-19: fMRI Pilot Study. Brain Sciences, 15(6), 635. https://doi.org/10.3390/brainsci15060635








