Non-Target Suppression Supports the Formation of Representational Prioritization Under High Working Memory Load
Abstract
1. Introduction
2. Experiment 1
2.1. Materials and Methods
2.1.1. Participants
2.1.2. Materials and Apparatus
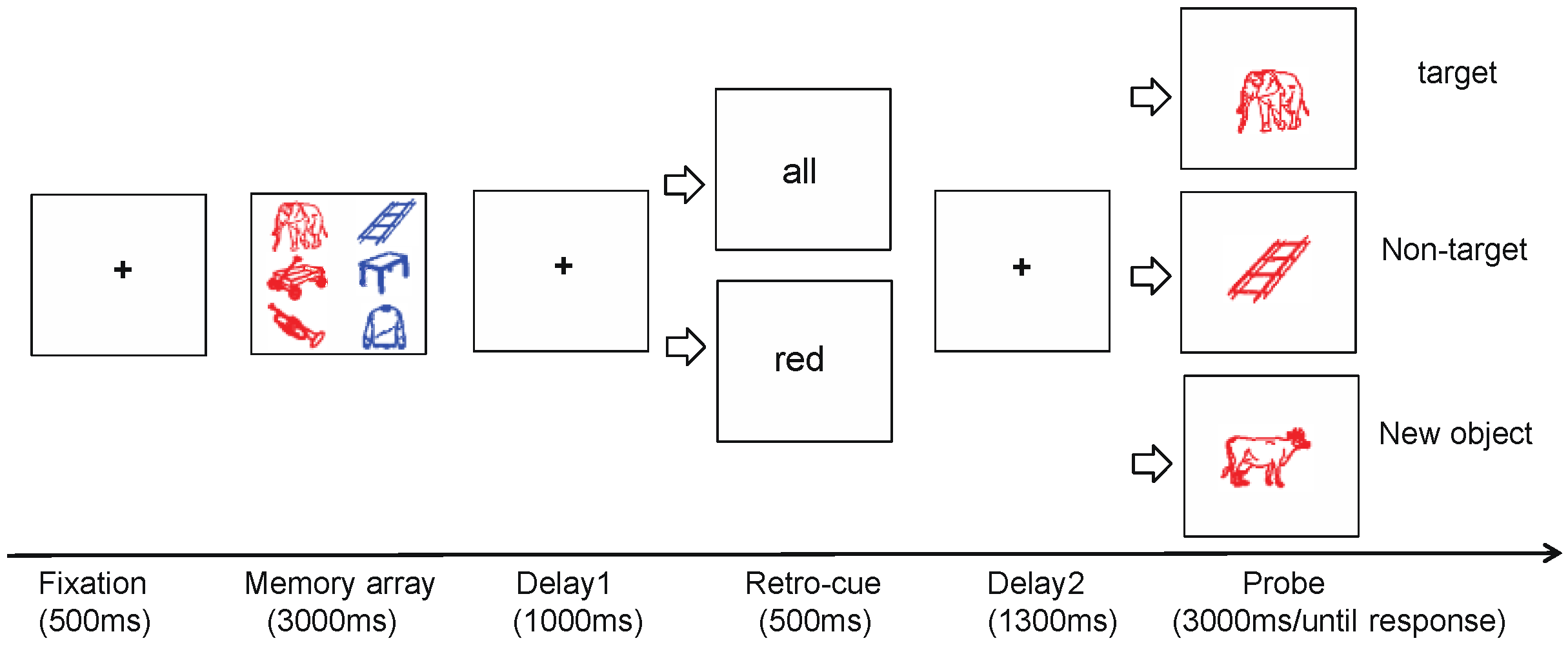
2.1.3. Experimental Design and Procedure
2.1.4. Data Analysis
2.2. Results
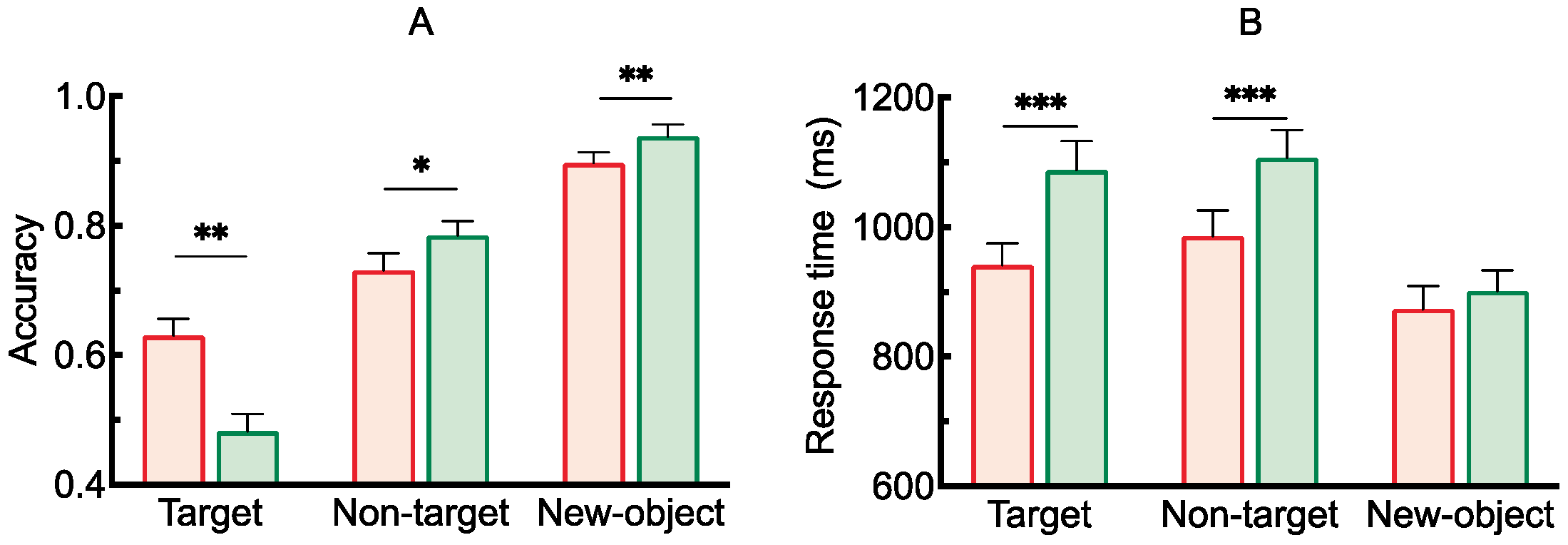
2.2.1. Accuracy and Response Time
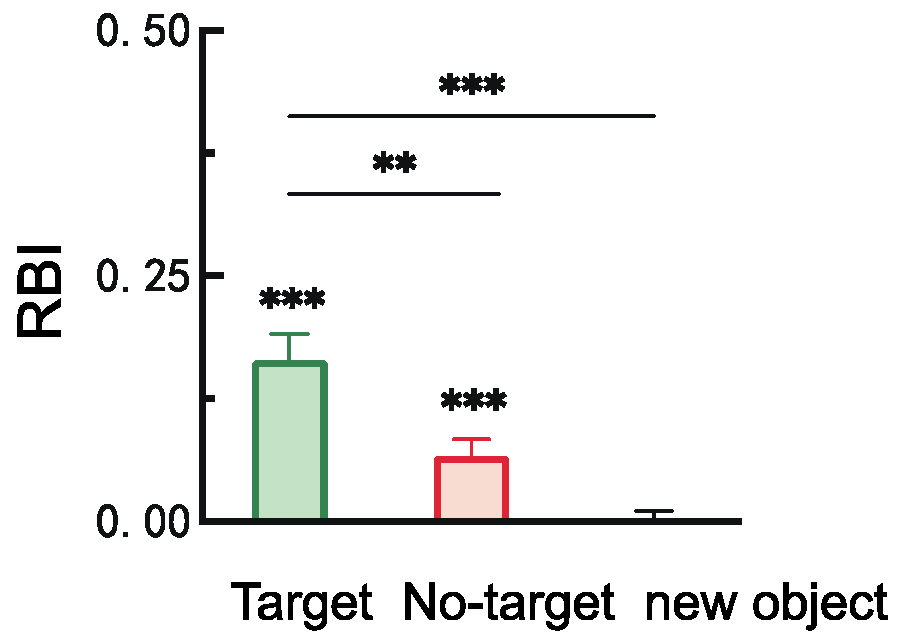
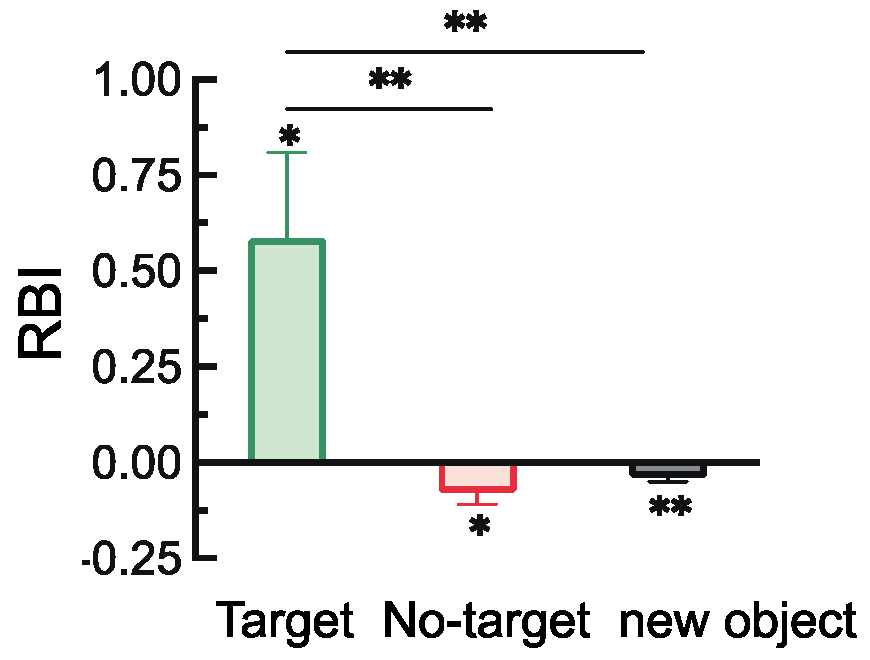
2.2.2. Retro-Cue Benefit Index (RBI)
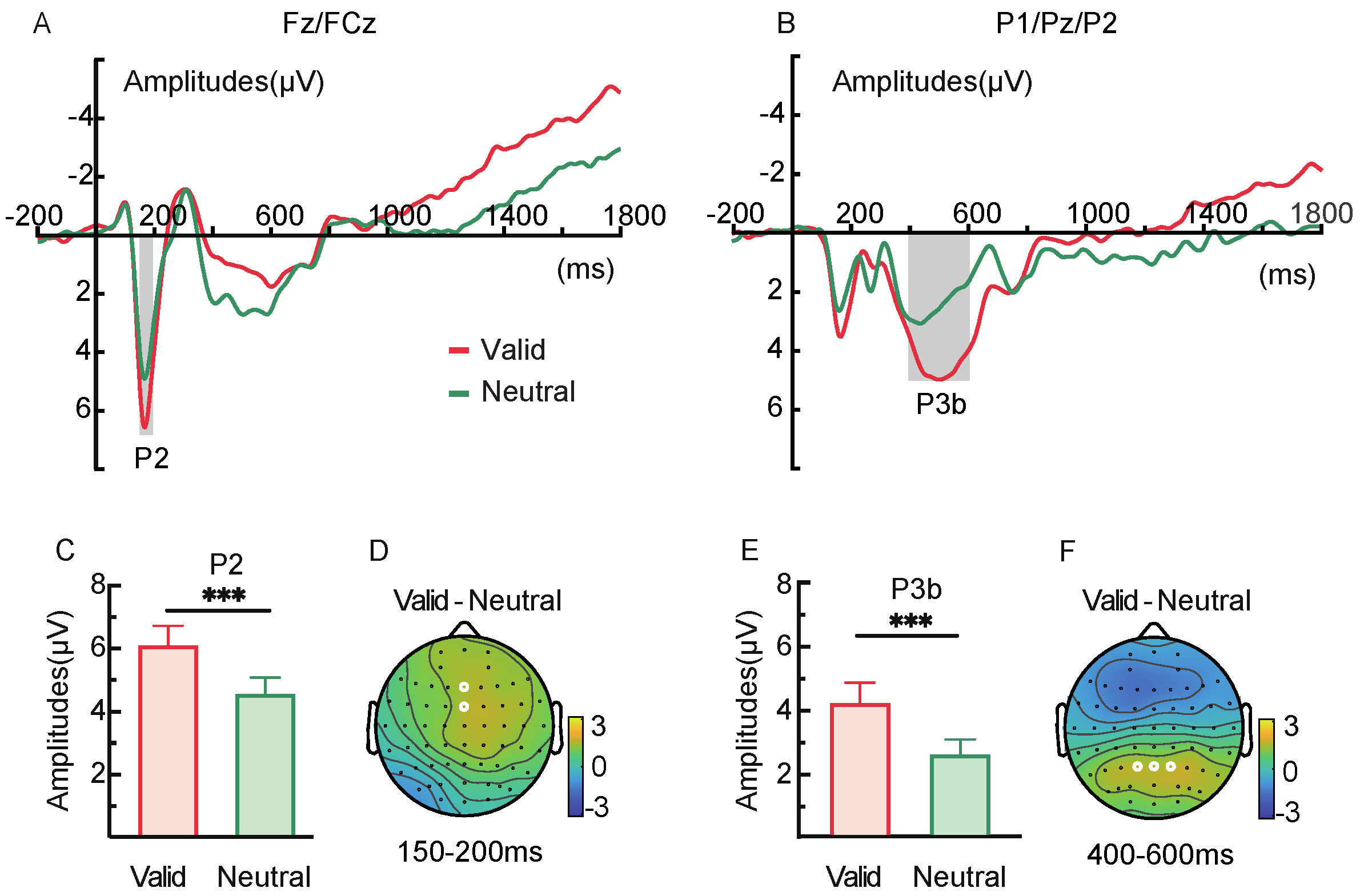
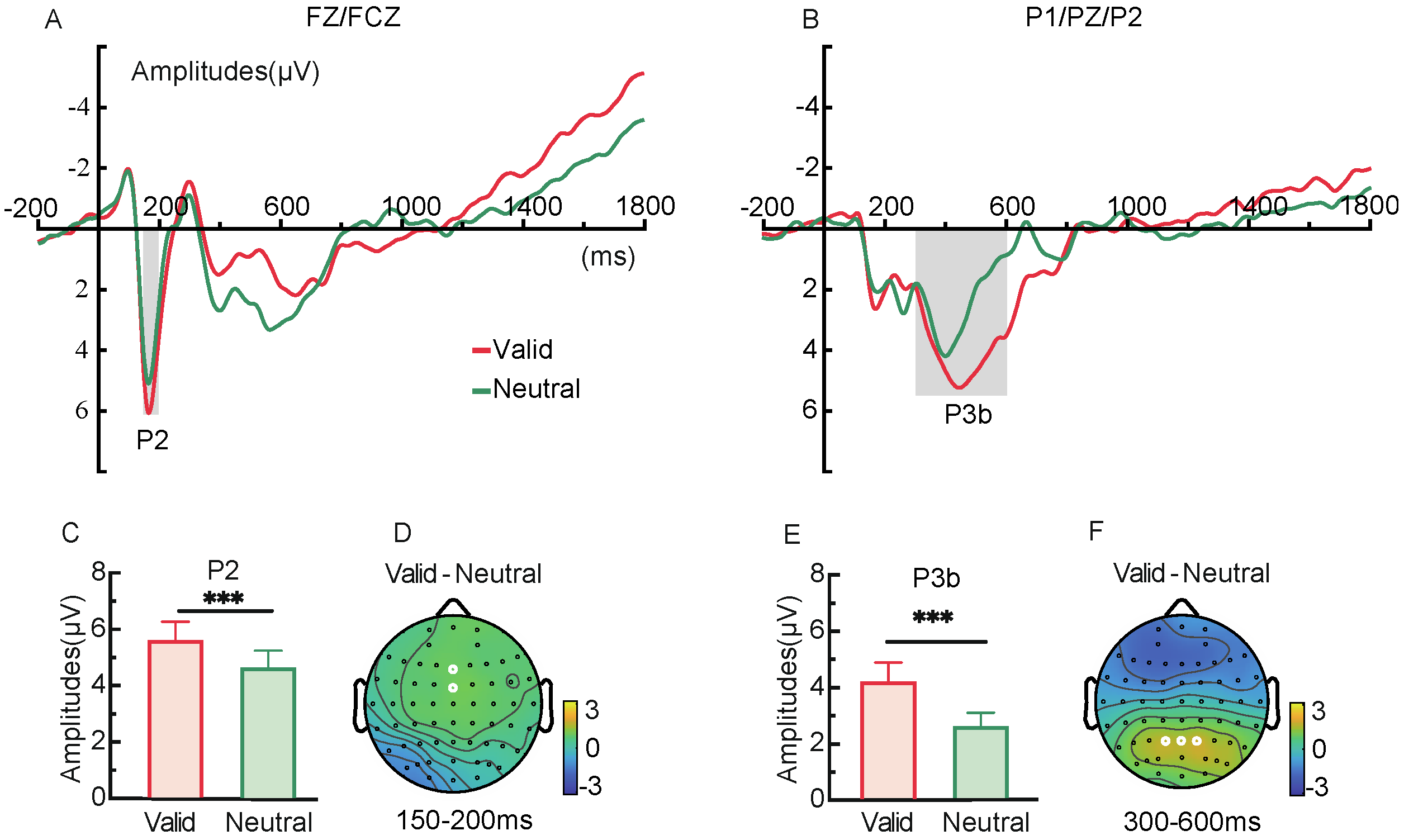
2.2.3. ERP Components
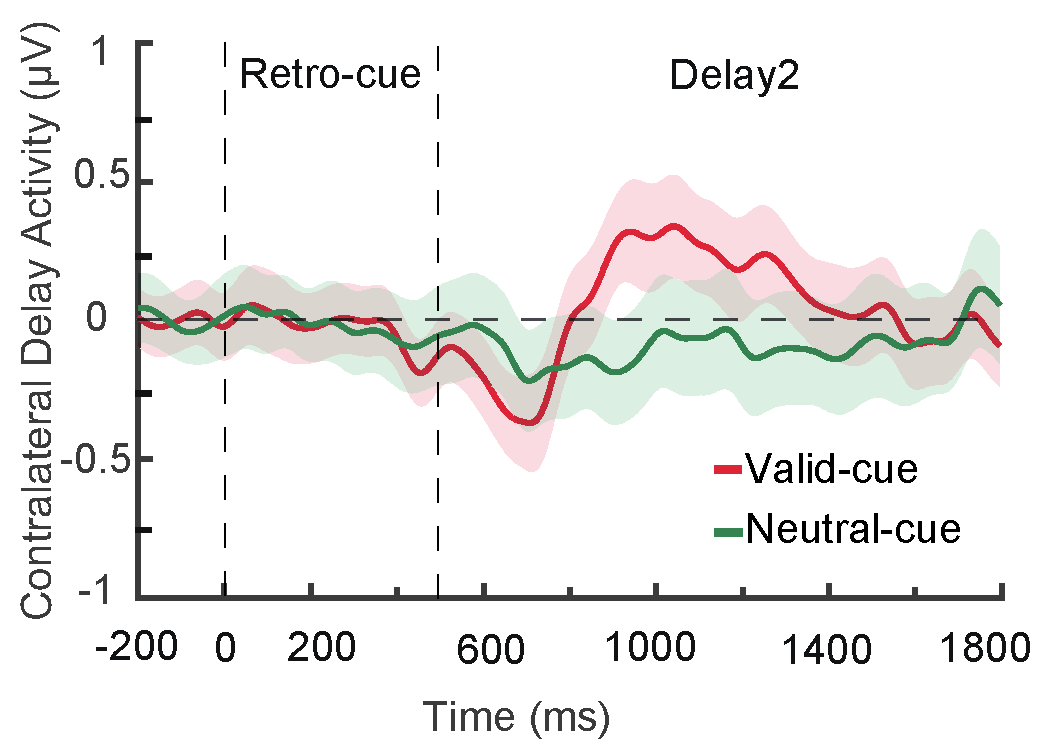
2.2.4. CDA Component
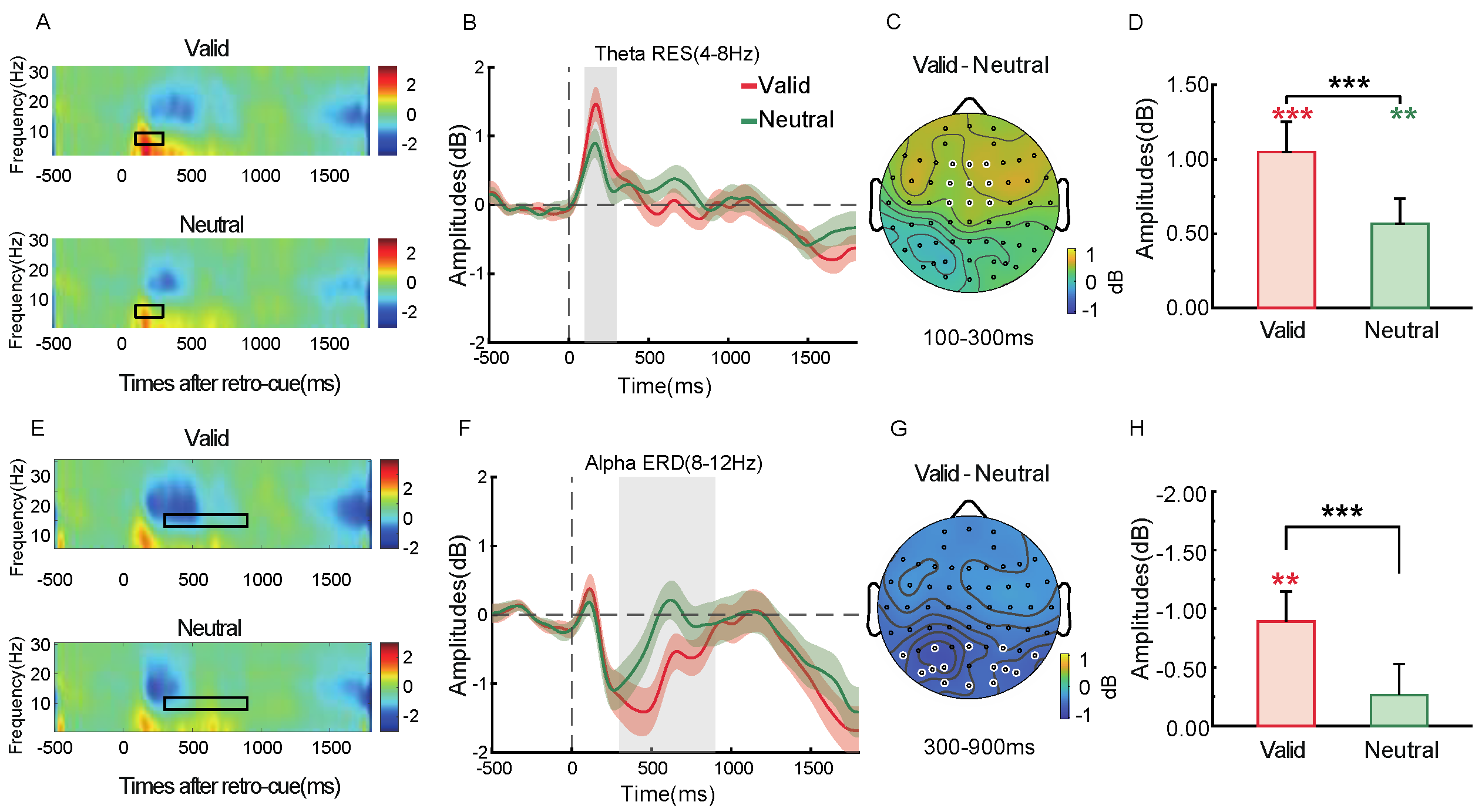
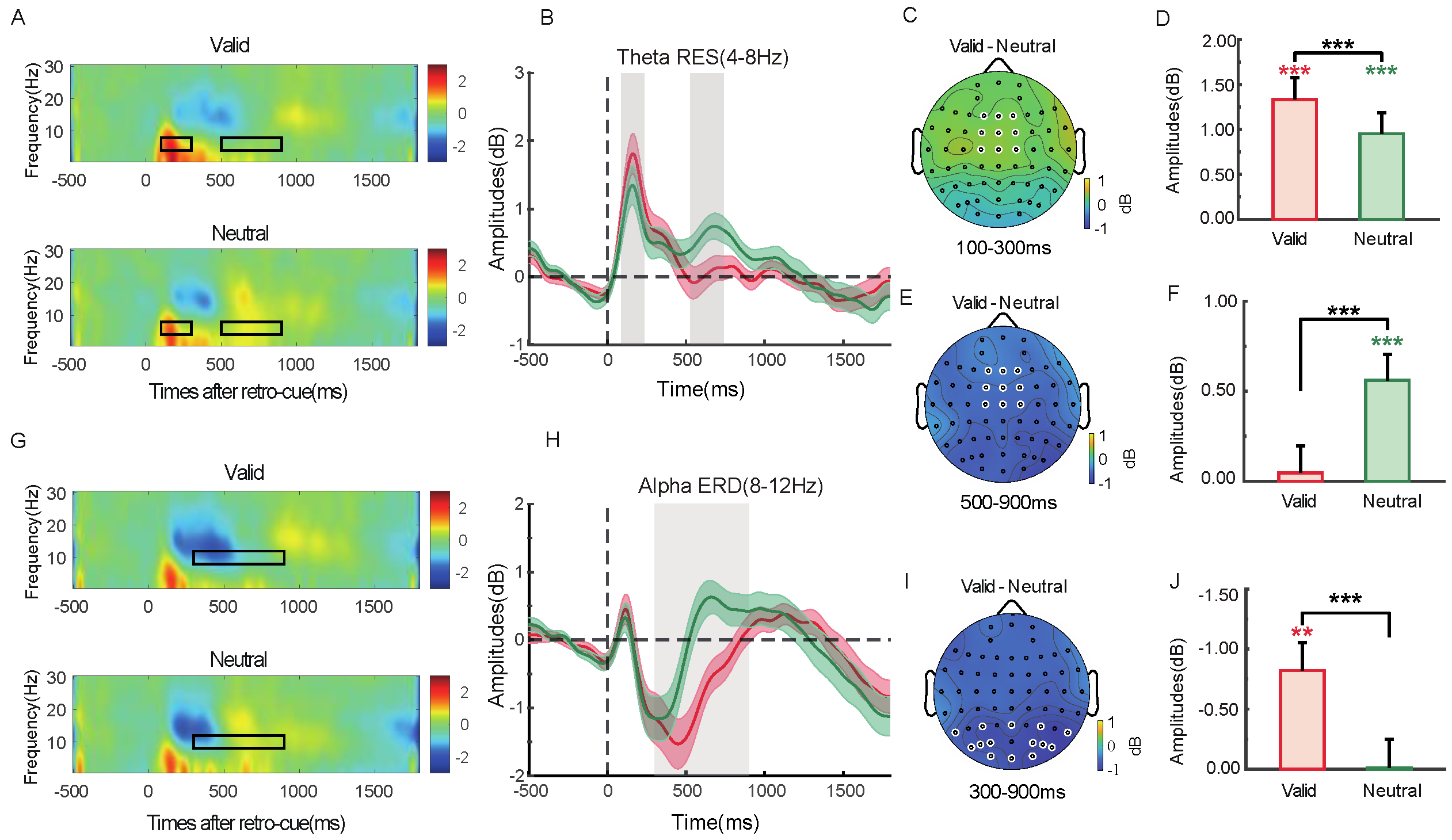
2.2.5. Time–Frequency Analysis
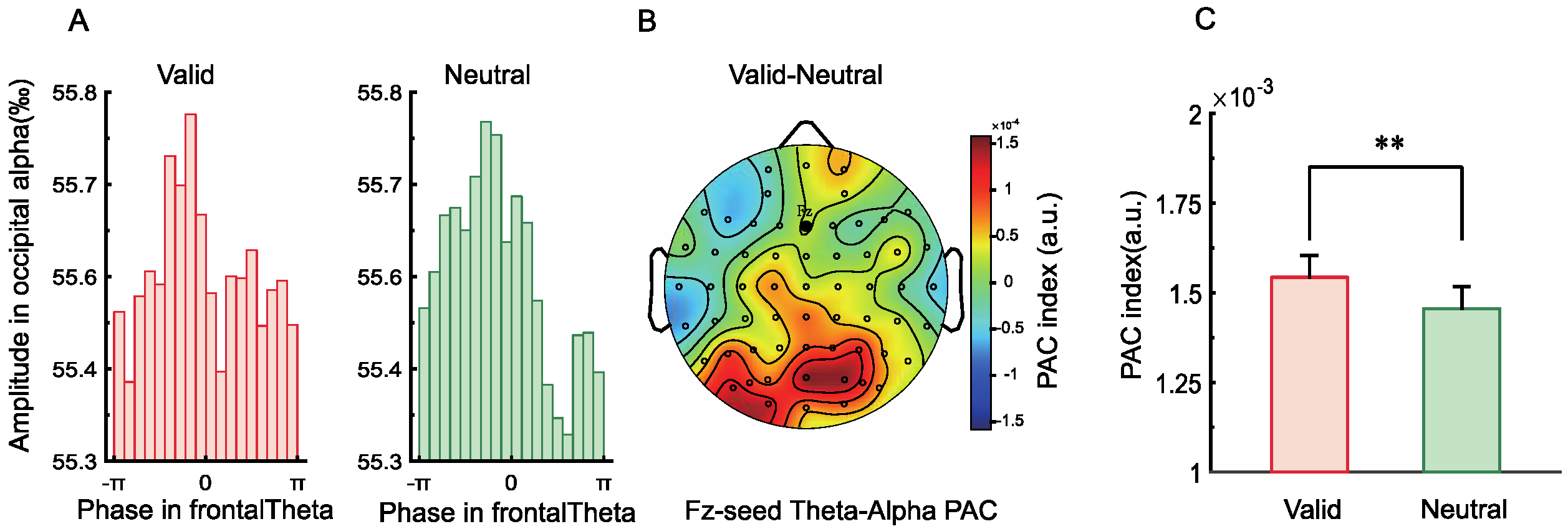
2.2.6. Phase–Amplitude Coupling (PAC)
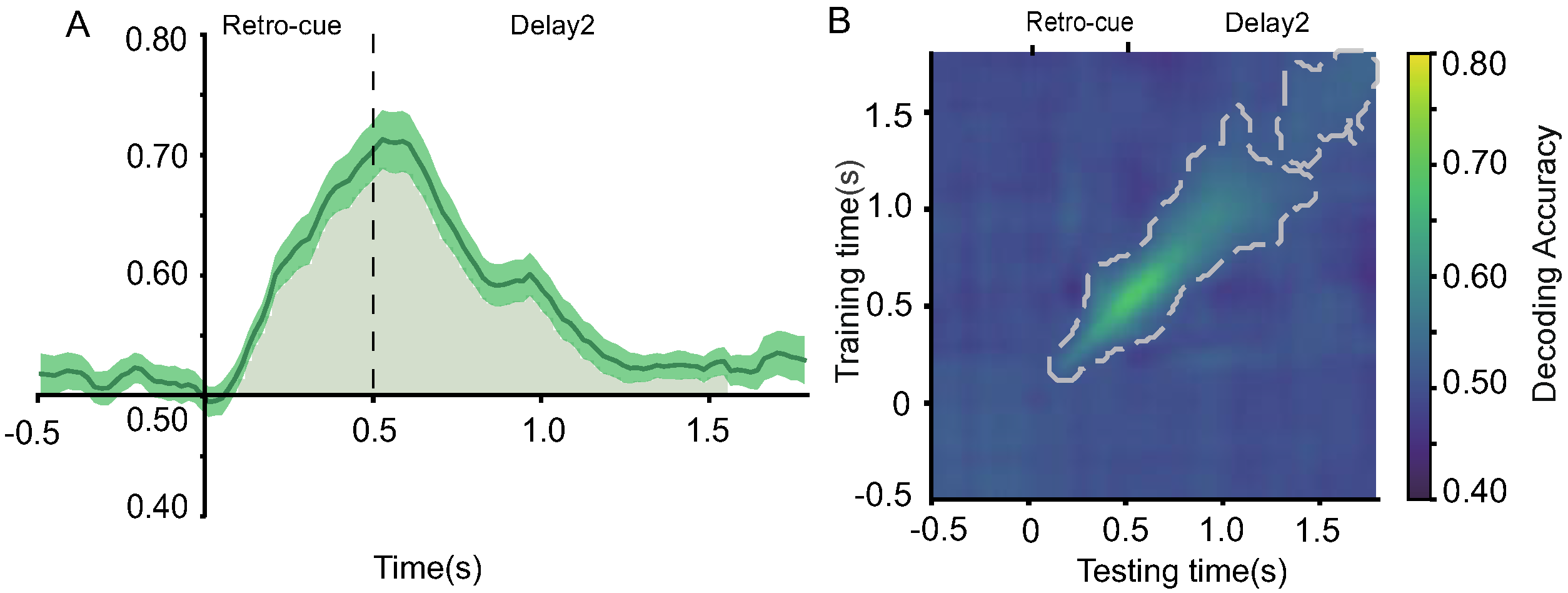
2.2.7. Decoding of Retro-Cues
2.3. Discussion
3. Experiment 2
3.1. Method
3.1.1. Participants
3.1.2. Procedure
3.2. Results
3.2.1. Accuracy and Response Time
3.2.2. Retro-Cue Benefit Index (RBI)
3.2.3. ERP Components
3.2.4. CDA Component
3.2.5. Time–Frequency Analysis
3.2.6. Phase–Amplitude Coupling (PAC)
3.2.7. Decoding of Retro-Cues
3.3. Discussion
4. General Discussion
4.1. The Generality of the Target Enhancement Mechanism
4.2. Resource Conditions for the Occurrence of Non-Target Suppression Mechanisms
4.3. Modulatory Effects of Visual Working Memory Load on Neural Oscillations in Representational Prioritization
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 2012, 63, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav. Brain Sci. 2001, 24, 87–114. [Google Scholar] [CrossRef]
- Orquin, J.L.; Perkovic, S.; Grunert, K.G. Visual Biases in Decision Making. Appl. Econ. Perspect. Policy 2018, 40, 523–537. [Google Scholar] [CrossRef]
- Van Eyghen, H. AI Algorithms as (Un)Virtuous Knowers. Discov. Artif. Intell. 2025, 5, 2. [Google Scholar] [CrossRef]
- Gazzaley, A.; Nobre, A.C. Top-down modulation: Bridging selective attention and working memory. Trends Cogn. Sci. 2012, 16, 129–135. [Google Scholar] [CrossRef]
- Souza, A.S.; Oberauer, K. In search of the focus of attention in working memory: 13 years of the retro-cue effect. Atten. Percept. Psychophys. 2016, 78, 1839–1860. [Google Scholar] [CrossRef]
- Günseli, E.; Fahrenfort, J.J.; van Moorselaar, D.; Daoultzis, K.C.; Meeter, M.; Olivers, C.N.L. EEG dynamics reveal a dissociation between storage and selective attention within working memory. Sci. Rep. 2019, 9, 13499. [Google Scholar] [CrossRef] [PubMed]
- van Moorselaar, D.; Olivers, C.N.; Theeuwes, J.; Lamme, V.A.; Sligte, I.G. Forgotten but not gone: Retro-cue costs and benefits in a double-cueing paradigm suggest multiple states in visual short-term memory. J. Exp. Psychol. Learn. Mem. Cogn. 2015, 41, 1755–1763. [Google Scholar] [CrossRef]
- Souza, A.S.; Rerko, L.; Oberauer, K. Refreshing memory traces: Thinking of an item improves retrieval from visual working memory. Ann. N. Y. Acad. Sci. 2015, 1339, 20–31. [Google Scholar] [CrossRef]
- Rerko, L.; Oberauer, K. Focused, unfocused, and defocused information in working memory. J. Exp. Psychol. Learn. Mem. Cogn. 2013, 39, 1075–1096. [Google Scholar] [CrossRef]
- Gilchrist, A.L.; Duarte, A.; Verhaeghen, P. Retrospective cues based on object features improve visual working memory performance in older adults. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2016, 23, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Oberauer, K. Removing irrelevant information from working memory: A cognitive aging study with the modified Sternberg task. J. Exp. Psychol. Learn. Mem. Cogn. 2001, 27, 948–957. [Google Scholar] [CrossRef]
- Wöstmann, M.; Alavash, M.; Obleser, J. Alpha Oscillations in the Human Brain Implement Distractor Suppression Independent of Target Selection. J. Neurosci. 2019, 39, 9797–9805. [Google Scholar] [CrossRef]
- Zhao, C.; Li, D.; Kong, Y.; Liu, H.; Hu, Y.; Niu, H.; Jensen, O.; Li, X.; Liu, H.; Song, Y. Transcranial photobiomodulation enhances visual working memory capacity in humans. Sci. Adv. 2022, 8, eabq3211. [Google Scholar] [CrossRef]
- Makovski, T.; Pertzov, Y. Attention and memory protection: Interactions between retrospective attention cueing and interference. Q. J. Exp. Psychol. 2015, 68, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Shimi, A.; Scerif, G. The influence of attentional biases on multiple working memory precision parameters for children and adults. Dev. Sci. 2022, 25, e13213. [Google Scholar] [CrossRef]
- Riddle, J.; Scimeca, J.M.; Cellier, D.; Dhanani, S.; D’Esposito, M. Causal evidence for a role of theta and alpha oscillations in the control of working memory. Curr. Biol. 2020, 30, 1748–1754.e4. [Google Scholar] [CrossRef]
- de Vries, I.E.J.; Slagter, H.A.; Olivers, C.N.L. Oscillatory control over representational states in working memory. Trends Cogn. Sci. 2020, 24, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Gunseli, E.; van Moorselaar, D.; Meeter, M.; Olivers, C.N. The reliability of retro-cues determines the fate of noncued visual working memory representations. Psychon. Bull. Rev. 2015, 22, 1334–1341. [Google Scholar] [CrossRef]
- Poch, C.; Capilla, A.; Hinojosa, J.A.; Campo, P. Selection within working memory based on a color retro-cue modulates alpha oscillations. Neuropsychologia 2017, 106, 133–137. [Google Scholar] [CrossRef]
- Li, D.; Hu, Y.; Qi, M.; Zhao, C.; Jensen, O.; Huang, J.; Song, Y. Prioritizing flexible working memory representations through retrospective attentional strengthening. Neuroimage 2023, 269, 119902. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Fiebelkorn, I.C.; Kastner, S. A rhythmic theory of attention. Trends Cogn. Sci. 2019, 23, 87–101. [Google Scholar] [CrossRef]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Ghiani, A.; Maniglia, M.; Battaglini, L.; Melcher, D.; Ronconi, L. Binding mechanisms in visual perception and their link with neural oscillations: A review of evidence from tACS. Front. Psychol. 2021, 12, 643677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Cai, P.; Luo, H.; Fang, F. The causal role of α-oscillations in feature binding. Proc. Natl. Acad. Sci. USA 2019, 116, 17023–17028. [Google Scholar] [CrossRef] [PubMed]
- Daume, J.; Gruber, T.; Engel, A.K.; Friese, U. Phase-amplitude coupling and long-range phase synchronization reveal frontotemporal interactions during visual working memory. J. Neurosci. 2017, 37, 313–322. [Google Scholar] [CrossRef]
- Gregoriou, G.G.; Gotts, S.J.; Zhou, H.; Desimone, R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 2009, 324, 1207–1210. [Google Scholar] [CrossRef]
- Madore, K.P.; Wagner, A.D. Readiness to remember: Predicting variability in episodic memory. Trends Cogn. Sci. 2022, 26, 707–723. [Google Scholar] [CrossRef]
- Bouchacourt, F.; Buschman, T.J. A flexible model of working memory. Neuron 2019, 103, 147–160. [Google Scholar] [CrossRef]
- Buschman, T.J. Balancing flexibility and interference in working memory. Annu. Rev. Vis. Sci. 2021, 7, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Hu, Z.; Ristaniemi, T.; Gendron, M.; Liu, Q. Retro-dimension-cue benefit in visual working memory. Sci. Rep. 2016, 6, 35573. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, L.; Sun, H.J.; Parviainen, T.; Zhou, Z.; Cheng, Y.; Liu, Q.; Ye, C. Sustained attention required for effective dimension-based retro-cue benefit in visual working memory. J. Vis. 2023, 23, 13. [Google Scholar] [CrossRef]
- Fu, X.; Ye, C.; Hu, Z.; Li, Z.; Liang, T.; Liu, Q. The impact of retro-cue validity on working memory representation: Evidence from electroencephalograms. Biol. Psychol. 2022, 170, 108320. [Google Scholar] [CrossRef]
- Barth, A.; Schneider, D. Manipulating the focus of attention in working memory: Evidence for a protection of multiple items against perceptual interference. Psychophysiology 2018, 55, e13062. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Chen, Y.; Yang, P.; Fu, S. Different prioritization states of working memory representations affect visual searches: Evidence from an event-related potential study. Int. J. Psychophysiol. 2023, 193, 112246. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Snodgrass, J.G.; Vanderwart, M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. Hum. Learn. Mem. 1980, 6, 174–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, M. Relation between P300 and event-related theta-band synchronization: A single-trial analysis. Clin. Neurophysiol. 2011, 122, 916–924. [Google Scholar] [CrossRef]
- Tort, A.B.; Komorowski, R.; Eichenbaum, H.; Kopell, N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 2010, 104, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ku, Y. Bridging the gap between EEG and DCNNs reveals a fatigue mechanism of facial repetition suppression. iScience 2023, 26, 108501. [Google Scholar] [CrossRef]
- Poth, C.H. Prioritization in Visual Working Memory Enhances Memory Retention and Speeds Up Processing in a Comparison Task. Cogn. Process. 2020, 21, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.; Andersen, T.S. The Effect of Exposure Duration on Visual Character Identification in Single, Whole, and Partial Report. J. Exp. Psychol. Hum. Percept. Perform. 2012, 38, 498–514. [Google Scholar] [CrossRef]
- Zhigalov, A.; Jensen, O. Alpha oscillations do not implement gain control in early visual cortex but rather gating in parieto-occipital regions. Hum. Brain Mapp. 2020, 41, 5176–5186. [Google Scholar] [CrossRef]
- Hajonides, J.E.; van Ede, F.; Stokes, M.G.; Nobre, A.C. Comparing the prioritization of items and feature-dimensions in visual working memory. J. Vis. 2020, 20, 25. [Google Scholar] [CrossRef]
- Schlichting, M.L.; Preston, A.R. Memory integration: Neural mechanisms and implications for behavior. Curr. Opin. Behav. Sci. 2015, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Vogel, E.K. Visual working memory capacity: From psychophysics and neurobiology to individual differences. Trends Cogn. Sci. 2013, 17, 391–400. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef]
- Peylo, C.; Hilla, Y.; Sauseng, P. Cause or consequence? Alpha oscillations in visuospatial attention. Trends Neurosci. 2021, 44, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Fahrenfort, J.J.; van Driel, J.; van Gaal, S.; Olivers, C.N.L. From ERPs to MVPA using the Amsterdam Decoding and Modeling Toolbox (ADAM). Front. Neurosci. 2018, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.C.; Nobre, A.C. Orienting attention to locations in internal representations. J. Cogn. Neurosci. 2003, 15, 1176–1194. [Google Scholar] [CrossRef]
- Lepsien, J.; Nobre, A.C. Cognitive control of attention in the human brain: Insights from orienting attention to mental representations. Brain Res. 2006, 1105, 20–31. [Google Scholar] [CrossRef]
- Fu, Y.; Guan, C.; Tam, J.; O’Donnell, R.E.; Shen, M.; Wyble, B.; Chen, H. Attention with or without working memory: Mnemonic reselection of attended information. Trends Cogn. Sci. 2023, 27, 1111–1122. [Google Scholar] [CrossRef]
- Sahan, M.I.; Verguts, T.; Boehler, C.N.; Pourtois, G.; Fias, W. Paying attention to working memory: Similarities in the spatial distribution of attention in mental and physical space. Psychon. Bull. Rev. 2016, 23, 1190–1197. [Google Scholar] [CrossRef]
- Zhang, W.; Luck, S.J. Discrete fixed-resolution representations in visual working memory. Nature 2008, 453, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Bays, P.M.; Husain, M. Dynamic shifts of limited working memory resources in human vision. Science 2008, 321, 851–854. [Google Scholar] [CrossRef]
- Wei, H.; Zhou, R. High working memory load impairs selective attention: EEG signatures. Psychophysiology 2020, 57, e13643. [Google Scholar] [CrossRef]
- Lavie, N. Distracted and confused?: Selective attention under load. Trends Cogn. Sci. 2005, 9, 75–82. [Google Scholar] [CrossRef]
- de Fockert, J.W. Beyond perceptual load and dilution: A review of the role of working memory in selective attention. Front. Psychol. 2013, 4, 287. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.E.; Chekroud, S.R.; Stokes, M.G.; Nobre, A.C. Benefits of flexible prioritization in working memory can arise without costs. J. Exp. Psychol. Hum. Percept. Perform. 2018, 44, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.J.; Awh, E. The role of alpha oscillations in spatial attention: Limited evidence for a suppression account. Curr. Opin. Psychol. 2019, 29, 34–40. [Google Scholar] [CrossRef]
- Fell, J.; Axmacher, N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 2011, 12, 105–118. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, G.; Hu, X.; Zhang, P.; Ding, J. Non-Target Suppression Supports the Formation of Representational Prioritization Under High Working Memory Load. Brain Sci. 2025, 15, 633. https://doi.org/10.3390/brainsci15060633
Zhang Y, Li G, Hu X, Zhang P, Ding J. Non-Target Suppression Supports the Formation of Representational Prioritization Under High Working Memory Load. Brain Sciences. 2025; 15(6):633. https://doi.org/10.3390/brainsci15060633
Chicago/Turabian StyleZhang, Yaya, Gongao Li, Xuezhu Hu, Peng Zhang, and Jinhong Ding. 2025. "Non-Target Suppression Supports the Formation of Representational Prioritization Under High Working Memory Load" Brain Sciences 15, no. 6: 633. https://doi.org/10.3390/brainsci15060633
APA StyleZhang, Y., Li, G., Hu, X., Zhang, P., & Ding, J. (2025). Non-Target Suppression Supports the Formation of Representational Prioritization Under High Working Memory Load. Brain Sciences, 15(6), 633. https://doi.org/10.3390/brainsci15060633





