Movement Impairments May Not Preclude Visuomotor Adaptation After Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Approval and Consent to Participate and Publish
2.3. Robotic Apparatus
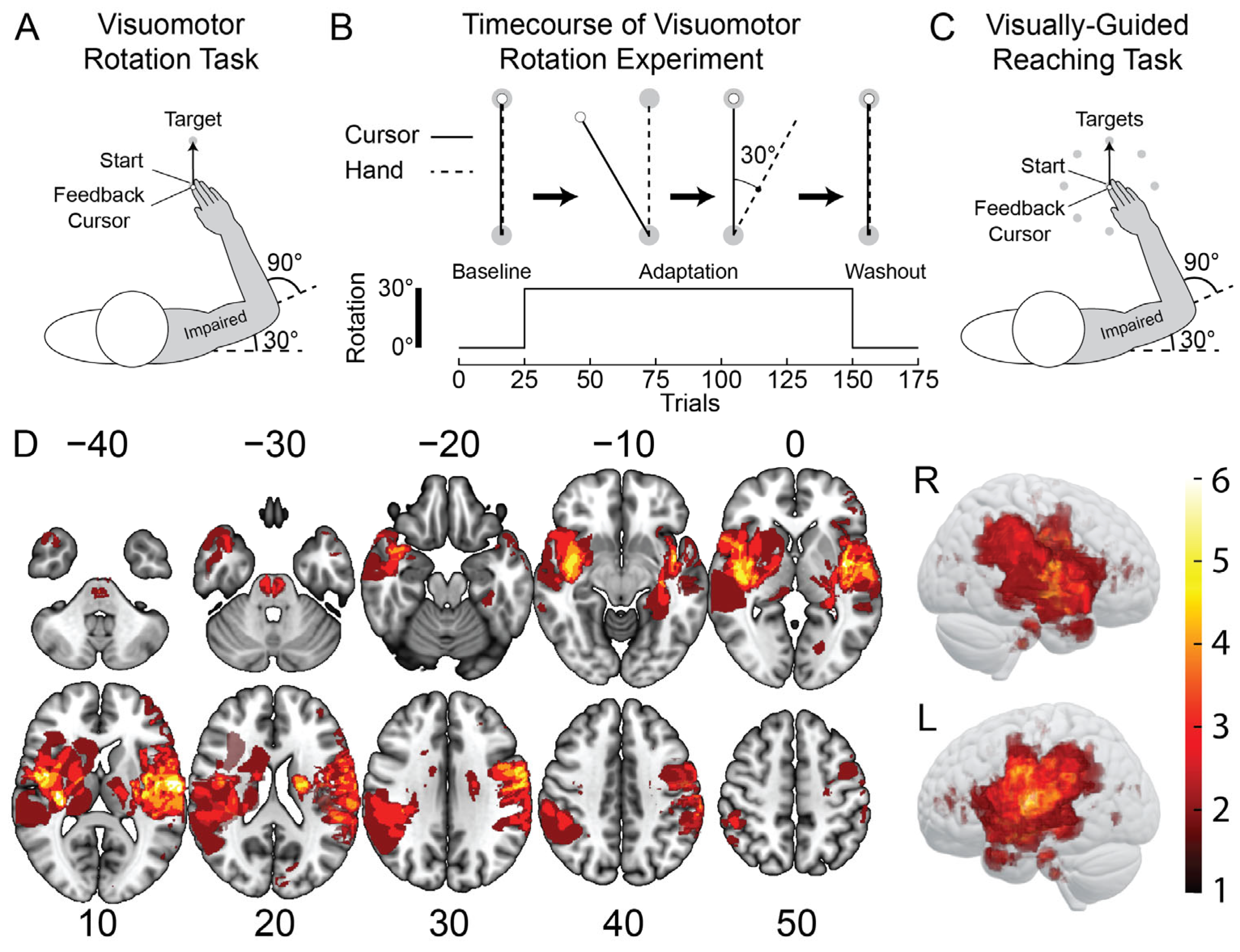
2.4. Visuomotor Rotation (VMR) Task
2.5. Visually-Guided Reaching (VGR) Task
2.6. Imaging and Lesion Delineation
2.7. Clinical Assessments
2.8. Statistical Analysis
3. Results
3.1. Representative Participants
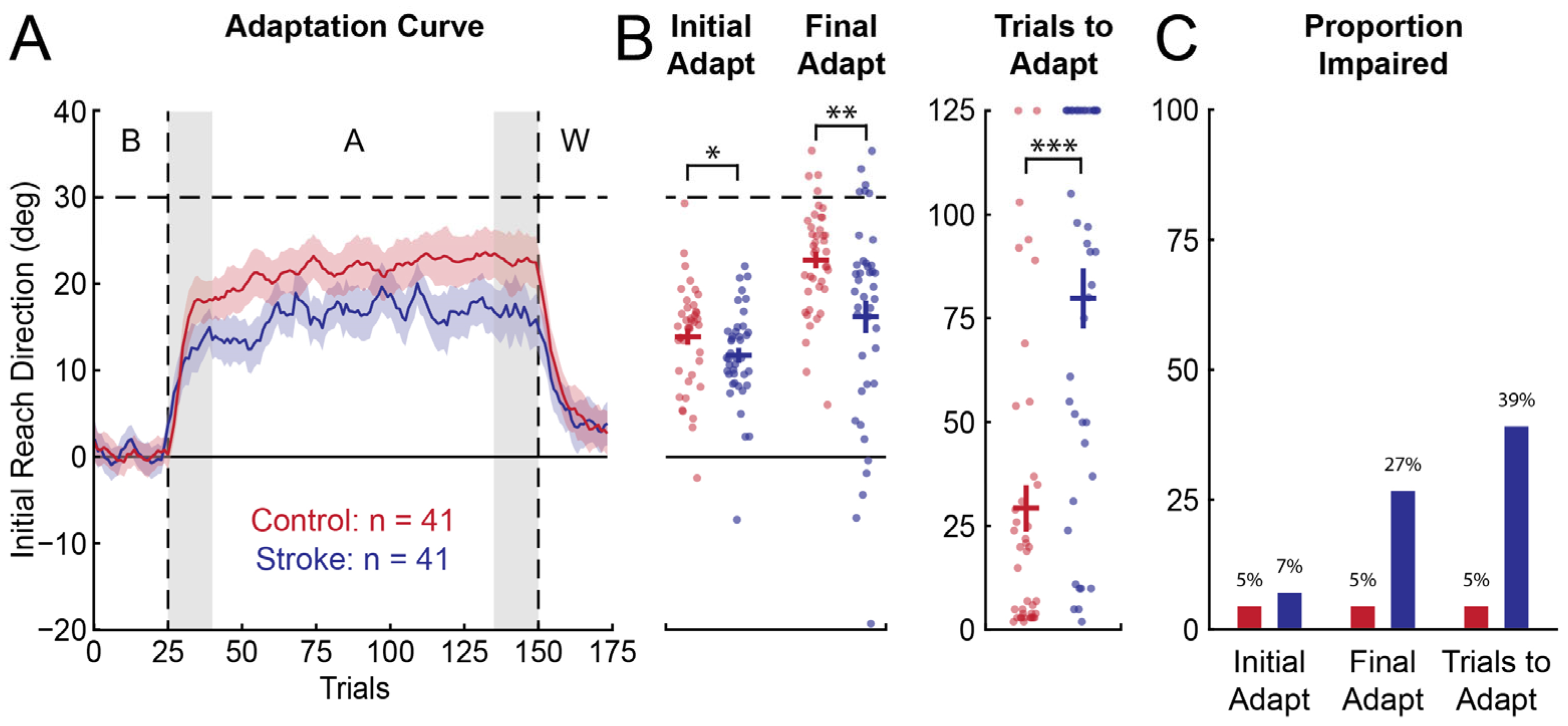
3.2. Group-Level Performance in Visuomotor Adaptation and Visually-Guided Reaching Tasks
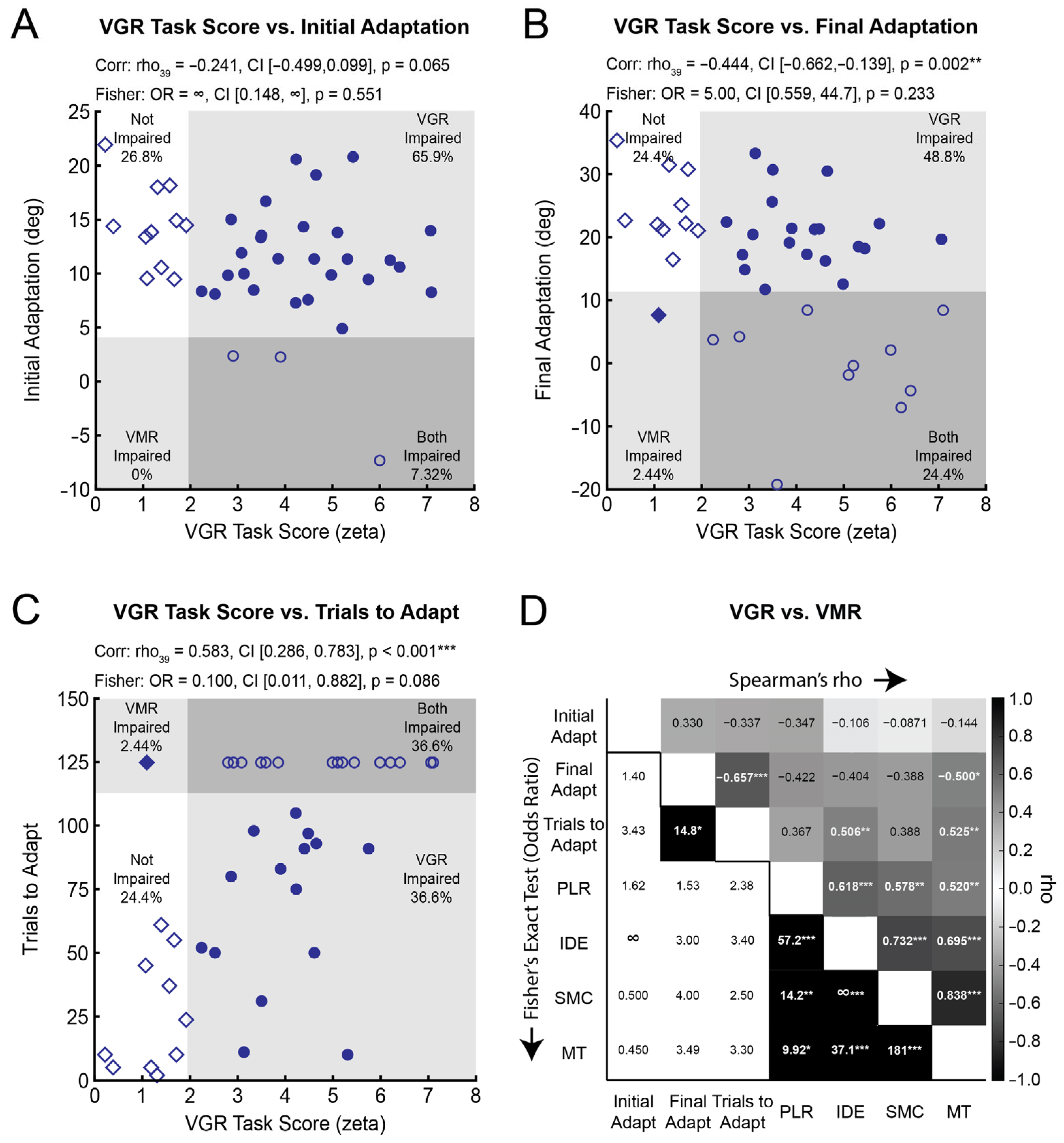
3.3. Visuomotor Adaptation Correlates with Some Measures of Visually-Guided Reaching
3.4. The Independence of Impairments in Visuomotor Adaptation and Reaching
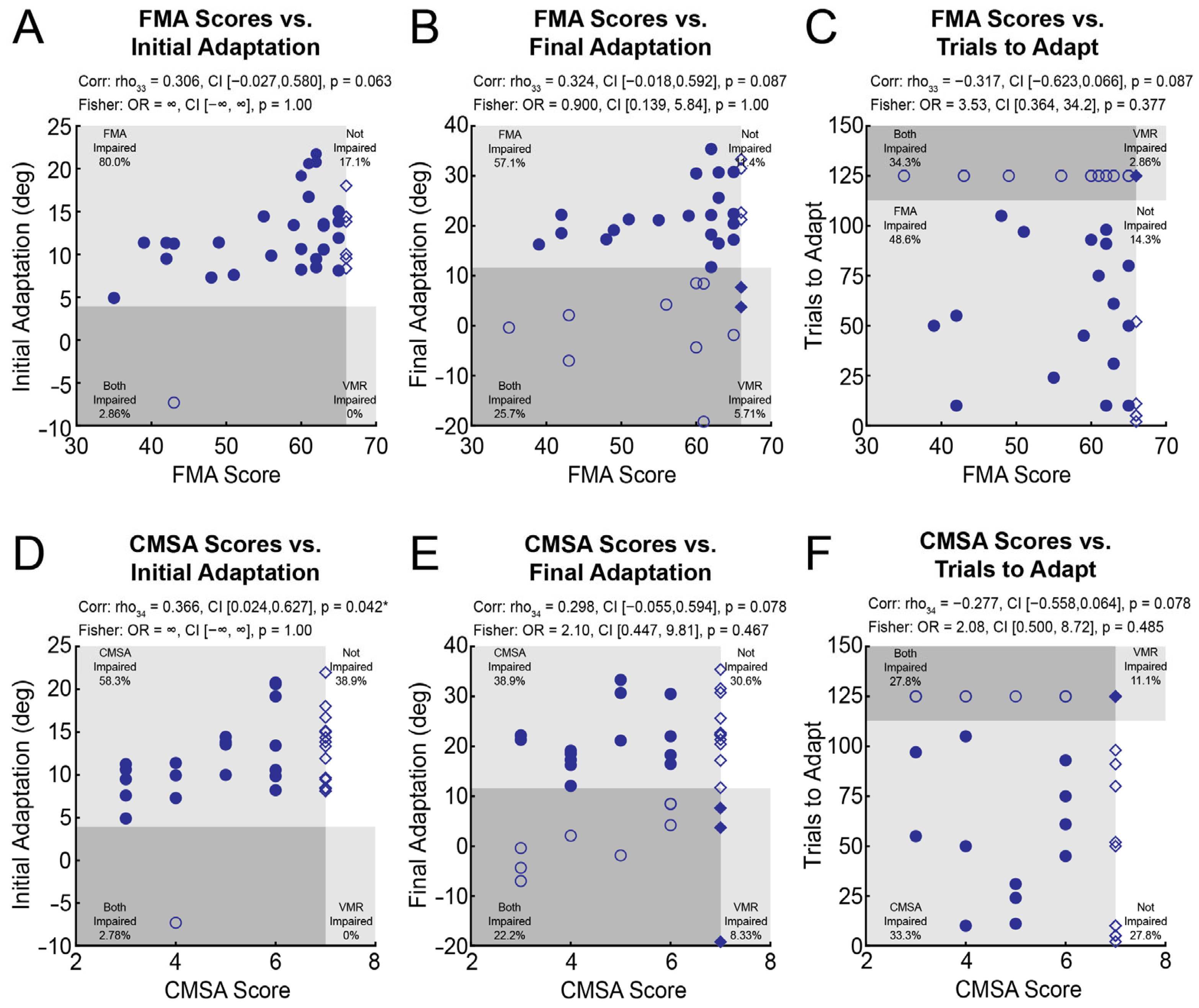
3.5. The Relationship Between Visuomotor Adaptation, FMA Scores, and CMSA Scores
4. Discussion
4.1. Visually-Guided Reaching, FMA Scores, and CMSA Scores Account for Relatively Little Variance in Visuomotor Adaptation
4.2. Reaching Impairments Do Not Preclude Normal Visuomotor Adaptation
4.3. Performance Versus Impairment
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Apparent Diffusion Coefficient |
| CMSA | Chedoke–McMaster Stroke Assessment—Arm Impairment Inventory |
| CT | Computed Tomography |
| DWI | Diffusion Weighted Imaging |
| FIM | Functional Independence Measure |
| FLAIR | T2 Fluid-Attenuated Inversion Recovery |
| FMA | Fugl–Meyer Assessment of Motor Recovery—Upper Extremity Motor Assessment |
| GRE | Gradient Echo |
| IDE | Initial Direction Error |
| MAS | Modified Ashworth Scale |
| MNI | Montreal Neurological Institute |
| MRC | Medical Research Council Strength Score Composite |
| MRI | Magnetic Resonance Imaging |
| MT | Movement Time |
| PLR | Path Length Ratio |
| RSS | Root Sum Square |
| SMC | Speed Maxima Count |
| SRRR | Stroke Recovery and Rehabilitation Roundtable |
| SWI | Susceptibility Weighted Imaging |
| TLT | Thumb Localization Test |
| VGR | Visually-Guided Reaching |
| VMR | Visuomotor Rotation |
References
- Reinkensmeyer, D.J.; Burdet, E.; Casadio, M.; Krakauer, J.W.; Kwakkel, G.; Lang, C.E.; Swinnen, S.P.; Ward, N.S.; Schweighofer, N. Computational Neurorehabilitation: Modeling Plasticity and Learning to Predict Recovery. J. Neuroeng. Rehabil. 2016, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, J.W. Motor Learning: Its Relevance to Stroke Recovery and Neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Leech, K.A.; Roemmich, R.T.; Gordon, J.; Reisman, D.S.; Cherry-Allen, K.M. Updates in Motor Learning: Implications for Physical Therapist Practice and Education. Phys. Ther. 2021, 102, pzab250. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Hadjiosif, A.M.; Xu, J.; Wong, A.L.; Haith, A.M. Motor Learning. Compr. Physiol. 2019, 9, 613–663. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.D.; Reinkensmeyer, D.J. Hemiparetic Stroke Impairs Anticipatory Control of Arm Movement. Exp. Brain Res. 2003, 149, 131–140. [Google Scholar] [CrossRef]
- Scheidt, R.A.; Stoeckmann, T. Reach Adaptation and Final Position Control Amid Environmental Uncertainty After Stroke. J. Neurophysiol. 2007, 97, 2824–2836. [Google Scholar] [CrossRef]
- Wadden, K.P.; De Asis, K.; Mang, C.S.; Neva, J.L.; Peters, S.; Lakhani, B.; Boyd, L.A. Predicting Motor Sequence Learning in Individuals with Chronic Stroke. Neurorehabil. Neural Repair. 2017, 31, 95–104. [Google Scholar] [CrossRef]
- Boyd, L.A.; Winstein, C.J. Implicit Motor-Sequence Learning in Humans Following Unilateral Stroke: The Impact of Practice and Explicit Knowledge. Neurosci. Lett. 2001, 298, 65–69. [Google Scholar] [CrossRef]
- Schweighofer, N.; Wang, C.; Mottet, D.; Laffont, I.; Bakthi, K.; Reinkensmeyer, D.J.; Rémy-Néris, O. Dissociating Motor Learning from Recovery in Exoskeleton Training Post-Stroke. J. Neuroeng. Rehabil. 2018, 15, 89. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Rajan, V.A.; Bastian, A.J.; Krakauer, J.W.; Celnik, P.A. Motor Learning in Stroke: Trained Patients Are Not Equal to Untrained Patients With Less Impairment. Neurorehabil. Neural Repair. 2017, 31, 178–189. [Google Scholar] [CrossRef]
- Schaefer, S.Y.; Haaland, K.Y.; Sainburg, R.L. Dissociation of Initial Trajectory and Final Position Errors during Visuomotor Adaptation Following Unilateral Stroke. Brain Res. 2009, 1298, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Mutha, P.K.; Sainburg, R.L.; Haaland, K.Y. Left Parietal Regions Are Critical for Adaptive Visuomotor Control. J. Neurosci. 2011, 31, 6972–6981. [Google Scholar] [CrossRef] [PubMed]
- Mutha, P.K.; Stapp, L.H.; Sainburg, R.L.; Haaland, K.Y. Motor Adaptation Deficits in Ideomotor Apraxia. J. Int. Neuropsychol. Soc. 2017, 23, 139–149. [Google Scholar] [CrossRef]
- Quattrocchi, G.; Greenwood, R.; Rothwell, J.C.; Galea, J.M.; Bestmann, S. Reward and Punishment Enhance Motor Adaptation in Stroke. J. Neurol. Neurosurg. Psychiatry 2017, 88, 730–736. [Google Scholar] [CrossRef]
- Varghese, R.; Gordon, J.; Sainburg, R.L.; Winstein, C.J.; Schweighofer, N. Adaptive Control Is Reversed between Hands after Left Hemisphere Stroke and Lost Following Right Hemisphere Stroke. Proc. Natl. Acad. Sci. USA 2023, 120, e2212726120. [Google Scholar] [CrossRef]
- Moore, R.T.; Piitz, M.A.; Singh, N.; Dukelow, S.P.; Cluff, T. The Independence of Impairments in Proprioception and Visuomotor Adaptation after Stroke. J. Neuroeng. Rehabil. 2024, 21, 81. [Google Scholar] [CrossRef]
- Moore, R.T.; Piitz, M.A.; Singh, N.; Dukelow, S.P.; Cluff, T. Assessing Impairments in Visuomotor Adaptation after Stroke. Neurorehabil. Neural Repair. 2022, 36, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Robertson, E.M.; Krakauer, J.W.; Rothwell, J.; Marshall, L.; Gerloff, C.; Wassermann, E.M.; Pascual-Leone, A.; Hummel, F.; Celnik, P.A.; et al. Consensus: Can Transcranial Direct Current Stimulation and Transcranial Magnetic Stimulation Enhance Motor Learning and Memory Formation? Brain Stimul. 2008, 1, 363–369. [Google Scholar] [CrossRef]
- Hoyer, E.H.; Celnik, P.A. Understanding and Enhancing Motor Recovery after Stroke Using Transcranial Magnetic Stimulation. Restor. Neurol. Neurosci. 2011, 29, 395–409. [Google Scholar] [CrossRef]
- Bonnì, S.; Motta, C.; Pellicciari, M.C.; Casula, E.P.; Cinnera, A.M.; Maiella, M.; Picazio, S.; Tramontano, M.; Sallustio, F.; Koch, G. Intermittent Cerebellar Theta Burst Stimulation Improves Visuo-Motor Learning in Stroke Patients: A Pilot Study. Cerebellum 2020, 19, 739–743. [Google Scholar] [CrossRef]
- Vidoni, E.D.; Boyd, L.A. Preserved Motor Learning after Stroke Is Related to the Degree of Proprioceptive Deficit. Behav. Brain Funct. 2009, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Gowland, C.; Stratford, P.; Ward, M.; Moreland, J.; Torresin, W.; Van Hullenaar, S.; Sanford, J.; Barreca, S.; Vanspall, B.; Plews, N. Measuring Physical Impairment and Disability with the Chedoke-Mcmaster Stroke Assessment. Stroke 1993, 24, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.H.; di Bella, P.; Johnson, G. Reliability and Validity of Arm Function Assessment with Standardized Guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A Multicentre Study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [CrossRef]
- Kwakkel, G. Standardised Measurement of Sensorimotor Recovery in Stroke Trials: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable (SRRR). Neurorehabil. Neural Repair. 2017, 31, 784–792. [Google Scholar] [CrossRef]
- Coderre, A.M.; Zeid, A.A.; Dukelow, S.P.; Demmer, M.J.; Moore, K.D.; Demers, M.J.; Bretzke, H.; Herter, T.M.; Glasgow, J.I.; Norman, K.E.; et al. Assessment of Upper-Limb Sensorimotor Function of Subacute Stroke Patients Using Visually Guided Reaching. Neurorehabil. Neural Repair. 2010, 24, 528–541. [Google Scholar] [CrossRef]
- Scott, S.H.; Dukelow, S.P. Potential of Robots as Next-Generation Technology for Clinical Assessment of Neurological Disorders and Upper-Limb Therapy. J. Rehabil. Res. Dev. 2011, 48, 335. [Google Scholar] [CrossRef]
- Smith, D.B.; Scott, S.H.; Semrau, J.A.; Dukelow, S.P. Impairments of the Ipsilesional Upper-Extremity in the First 6-Months Post-Stroke. J. Neuroeng. Rehabil. 2023, 20, 106. [Google Scholar] [CrossRef]
- Semrau, J.A.; Herter, T.M.; Scott, S.H.; Dukelow, S.P. Robotic Identification of Kinesthetic Deficits after Stroke. Stroke 2013, 44, 3414–3421. [Google Scholar] [CrossRef] [PubMed]
- Dukelow, S.P.; Herter, T.M.; Moore, K.D.; Demers, M.J.; Glasgow, J.I.; Bagg, S.D.; Norman, K.E.; Scott, S.H. Quantitative Assessment of Limb Postion Sense Following Stroke. Neurorehabil. Neural Repair. 2010, 24, 178–187. [Google Scholar] [CrossRef]
- Hossain, D.; Scott, S.H.; Cluff, T.; Dukelow, S.P. The Use of Machine Learning and Deep Learning Techniques to Assess Proprioceptive Impairments of the Upper Limb after Stroke. J. Neuroeng. Rehabil. 2023, 20, 15. [Google Scholar] [CrossRef]
- Van Heugten, C.M.; Dekker, J.; Deelman, B.G.; Stehmann-Saris, F.C.; Kinebanian, A. A Diagnostic Test for Apraxia in Stroke Patients: Internal Consistency and Diagnostic Value. Clin. Neuropsychol. 1999, 13, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.J.; Poh, E.; De Rugy, A. New Visuomotor Maps Are Immediately Available to the Opposite Limb. J. Neurophysiol. 2014, 111, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Salomonczyk, D.; Henriques, D.Y.P.; Cressman, E.K. Proprioceptive Recalibration in the Right and Left Hands Following Abrupt Visuomotor Adaptation. Exp. Brain Res. 2012, 217, 187–196. [Google Scholar] [CrossRef]
- Stockinger, C.; Thürer, B.; Focke, A.; Stein, T. Intermanual Transfer Characteristics of Dynamic Learning: Direction, Coordinate Frame, and Consolidation of Interlimb Generalization. J. Neurophysiol. 2015, 114, 3166–3176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reuter, E.M.; Cunnington, R.; Mattingley, J.B.; Riek, S.; Carroll, T.J. Feedforward Compensation for Novel Dynamics Depends on Force Field Orientation but Is Similar for the Left and Right Arms. J. Neurophysiol. 2016, 116, 2260–2271. [Google Scholar] [CrossRef]
- Sainburg, R.L.; Wang, J. Interlimb Transfer of Visuomotor Rotations: Independence of Direction and Final Position Information. Exp. Brain Res. 2002, 145, 437–447. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.; Wong, W.; Armstrong, I.T.; Flanagan, J.R. Relation between Reaction Time and Reach Errors during Visuomotor Adaptation. Behav. Brain Res. 2011, 219, 8–14. [Google Scholar] [CrossRef]
- Moore, R.T.; Cluff, T. Individual Differences in Sensorimotor Adaptation Are Conserved over Time and across Force-Field Tasks. Front. Hum. Neurosci. 2021, 15, 712. [Google Scholar] [CrossRef]
- Telgen, S.; Parvin, D.; Diedrichsen, J. Mirror Reversal and Visual Rotation Are Learned and Consolidated via Separate Mechanisms: Recalibrating or Learning de Novo? J. Neurosci. 2014, 34, 13768–13779. [Google Scholar] [CrossRef]
- Michel, C.; Bonnetain, L.; Amoura, S.; White, O. Force Field Adaptation Does Not Alter Space Representation. Sci. Rep. 2018, 8, 10982. [Google Scholar] [CrossRef]
- Dukelow, S.P.; Herter, T.M.; Bagg, S.D.; Scott, S.H. The Independence of Deficits in Position Sense and Visually Guided Reaching Following Stroke. J. Neuroeng. Rehabil. 2012, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- McCrea, P.H.; Eng, J.J. Consequences of Increased Neuromotor Noise for Reaching Movements in Persons with Stroke. Exp. Brain Res. 2005, 162, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Otaka, E.; Otaka, Y.; Kasuga, S.; Nishimoto, A.; Yamazaki, K.; Kawakami, M.; Ushiba, J.; Liu, M. Clinical Usefulness and Validity of Robotic Measures of Reaching Movement in Hemiparetic Stroke Patients. J. Neuroeng. Rehabil. 2015, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, M.J.; Low, T.A.; Dukelow, S.P. Beyond the Dorsal Column Medial Lemniscus in Proprioception and Stroke: A White Matter Investigation. Brain Sci. 2022, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Simmatis, L.E.R.; Early, S.; Moore, K.D.; Appaqaq, S.; Scott, S.H. Statistical Measures of Motor, Sensory and Cognitive Performance across Repeated Robot-Based Testing. J. Neuroeng. Rehabil. 2020, 17, 86. [Google Scholar] [CrossRef]
- Findlater, S.E.; Hawe, R.L.; Semrau, J.A.; Kenzie, J.M.; Yu, A.Y.; Scott, S.H.; Dukelow, S.P. Lesion Locations Associated with Persistent Proprioceptive Impairment in the Upper Limbs after Stroke. Neuroimage Clin. 2018, 20, 955–971. [Google Scholar] [CrossRef]
- Kenzie, J.M.; Semrau, J.A.; Findlater, S.E.; Yu, A.Y.; Desai, J.A.; Herter, T.M.; Hill, M.D.; Scott, S.H.; Dukelow, S.P. Localization of Impaired Kinesthetic Processing Post-Stroke. Front. Hum. Neurosci. 2016, 10, 505. [Google Scholar] [CrossRef]
- Karnath, H.O.; Rorden, C.; Ticini, L.F. Damage to White Matter Fiber Tracts in Acute Spatial Neglect. Cereb. Cortex 2009, 19, 2331–2337. [Google Scholar] [CrossRef]
- Rorden, C.; Karnath, H.O.; Bonilha, L. Improving Lesion-Symptom Mapping. J. Cogn. Neurosci. 2007, 19, 1081–1088. [Google Scholar] [CrossRef]
- Rorden, C.; Bonilha, L.; Fridriksson, J.; Bender, B.; Karnath, H.O. Age-Specific CT and MRI Templates for Spatial Normalization. Neuroimage 2012, 61, 957–965. [Google Scholar] [CrossRef]
- Friston, K.J.; Holmes, A.P.; Worsley, K.J.; Poline, J.-P.; Frith, C.D.; Frackowiak, R.S.J. Statistical Parametric Maps in Functional Imaging: A General Linear Approach. Hum. Brain Mapp. 1994, 2, 189–210. [Google Scholar] [CrossRef]
- Brett, M.; Leff, A.P.; Rorden, C.; Ashburner, J. Spatial Normalization of Brain Images with Focal Lesions Using Cost Function Masking. Neuroimage 2001, 14, 486–500. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S. The Post-Stroke Hemiplegic Patient. 1. a Method for Evaluation of Physical Performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Tiffin, J.; Asher, E.J. The Purdue Pegboard: Norms and Studies of Reliability and Validity. J. Appl. Psychol. 1948, 32, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Paternostro-Sluga, T.; Grim-Stieger, M.; Posch, M.; Schuhfried, O.; Vacariu, G.; Mittermaier, C.; Bittner, C.; Fialka-Moser, V. Reliability and Validity of the Medical Research Council (MRC) Scale and a Modified Scale for Testing Muscle Strength in Patients with Radial Palsy. J. Rehabil. Med. 2008, 40, 665–671. [Google Scholar] [CrossRef]
- Charalambous, C.P. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. In Classic Papers in Orthopaedics; Springer: London, UK, 2014; pp. 415–417. ISBN 9781447154518. [Google Scholar]
- Hirayama, K.; Fukutake, T.; Kawamura, M. “Thumb Localizing Test” for Detecting a Lesion in the Posterior Column-Medial Lemniscal System. J. Neurol. Sci. 1999, 167, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.A.; Granger, C.V.; Hamilton, B.B.; Sherwin, F.S. The Functional Independence Measure: A New Tool for Rehabilitation. Adv. Clin. Rehabil. 1987, 1, 6–18. [Google Scholar] [PubMed]
- Boos, D.D. Introduction to the Bootstrap World. Stat. Sci. 2003, 18, 168–174. [Google Scholar] [CrossRef]
- Wilcox, R.R. Comparing Pearson Correlations: Dealing with Heteroscedasticity and Nonnormality. Commun. Stat. Simul. Comput. 2009, 38, 2220–2234. [Google Scholar] [CrossRef]
- Ferguson, C.J. An Effect Size Primer: A Guide for Clinicians and Researchers. Prof. Psychol. Res. Pr. 2009, 40, 532–538. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the p Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Barnard, G.A. Significance Tests for 2 × 2 Tables. Biometrika 1947, 34, 123. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Schaefer, S.Y.; Haaland, K.Y.; Sainburg, R.L. Hemispheric Specialization and Functional Impact of Ipsilesional Deficits in Movement Coordination and Accuracy. Neuropsychologia 2009, 47, 2953–2966. [Google Scholar] [CrossRef]
- Tsay, J.S.; Tan, S.; Chu, M.A.; Ivry, R.B.; Cooper, E.A. Low Vision Impairs Implicit Sensorimotor Adaptation in Response to Small Errors, but Not Large Errors. J. Cogn. Neurosci. 2023, 35, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Anguera, J.A.; Reuter-Lorenz, P.A.; Willingham, D.T.; Seidler, R.D. Contributions of Spatial Working Memory to Visuomotor Learning. J. Cogn. Neurosci. 2010, 22, 1917–1930. [Google Scholar] [CrossRef]
- Galea, J.M.; Vazquez, A.; Pasricha, N.; Orban De Xivry, J.J.; Celnik, P. Dissociating the Roles of the Cerebellum and Motor Cortex during Adaptive Learning: The Motor Cortex Retains What the Cerebellum Learns. Cereb. Cortex 2011, 21, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Krakauer, J.W.; Ivry, R.B. Explicit and Implicit Contributions to Learning in a Sensorimotor Adaptation Task. J. Neurosci. 2014, 34, 3023–3032. [Google Scholar] [CrossRef]
- Tsay, J.S.; Kim, H.E.; Parvin, D.E.; Stover, A.R.; Ivry, R.B. Individual Differences in Proprioception Predict the Extent of Implicit Sensorimotor Adaptation. J. Neurophysiol. 2021, 125, 1307–1321. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, J. The Effect of Proprioceptive Acuity Variability on Motor Adaptation in Older Adults. Exp. Brain Res. 2018, 236, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Binyamin-Netser, R.; Goldhamer, N.; Avni, I.; Ressel Zviely, A.; Shmuelof, L. Cognitive Impairments after Stroke Do Ot Attenuate Explicit Visuomotor Adaptation in Reaching and Savings with the Unaffected Arm. Neurorehabil. Neural Repair. 2023, 37, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Stark-Inbar, A.; Raza, M.; Taylor, J.A.; Ivry, R.B. Individual Differences in Implicit Motor Learning: Task Specificity in Sensorimotor Adaptation and Sequence Learning. J. Neurophysiol. 2017, 117, 412–428. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Liang, Y.; Abdollahi, F.; Fisher Bittmann, M.; Kording, K.; Wei, K. The Statistical Determinants of the Speed of Motor Learning. PLoS Comput. Biol. 2016, 12, 1005023. [Google Scholar] [CrossRef]
- Krakauer, J.W. Motor Learning and Consolidation: The Case of Visuomotor Rotation. Adv. Exp. Med. Biol. 2009, 629, 405–421. [Google Scholar] [CrossRef]
- Kasuga, S.; Heming, E.; Lowrey, C.; Scott, S.H. High Intra-Task and Low Inter-Task Correlations of Motor Skills in Humans Creates an Individualized Behavioural Pattern. Sci. Rep. 2022, 12, 20156. [Google Scholar] [CrossRef]
- Logan, L.M.; Semrau, J.A.; Debert, C.T.; Kenzie, J.M.; Scott, S.H.; Dukelow, S.P. Using Robotics to Quantify Impairments in Sensorimotor Ability, Visuospatial Attention, Working Memory, and Executive Function after Traumatic Brain Injury. J. Head Trauma Rehabil. 2018, 33, E61–E73. [Google Scholar] [CrossRef]
- Kitchen, N.M.; Kim, K.S.; Wang, P.Z.; Hermosillo, R.J.; Max, L. Individual Sensorimotor Adaptation Characteristics Are Independent across Orofacial Speech Movements and Limb Reaching Movements. J. Neurophysiol. 2022, 128, 696–710. [Google Scholar] [CrossRef]
- Zhang, A.; Ruitenberg, M.F.L.; Warburton, M.; Scott, S.; Tsay, J.S. Large Reaching Datasets Quantify the Impact of Age, Sex/Gender, and Experience on Motor Control. bioRxiv 2025. [Google Scholar] [CrossRef]
- Donchin, O.; Rabe, K.; Diedrichsen, J.; Lally, N.; Schoch, B.; Gizewski, E.R.; Timmann, D. Cerebellar Regions Involved in Adaptation to Force Field and Visuomotor Perturbation. J. Neurophysiol. 2011, 107, 134–147. [Google Scholar] [CrossRef]
- Rabe, K.; Livne, O.; Gizewski, E.R.; Aurich, V.; Beck, A.; Timmann, D.; Donchin, O. Adaptation to Visuomotor Rotation and Force Field Perturbation Is Correlated to Different Brain Areas in Patients with Cerebellar Degeneration. J. Neurophysiol. 2009, 101, 1961–1971. [Google Scholar] [CrossRef]
- Kuczynski, A.M.; Kirton, A.; Semrau, J.A.; Dukelow, S.P. Relative Independence of Upper Limb Position Sense and Reaching in Children with Hemiparetic Perinatal Stroke. J. Neuroeng. Rehabil. 2021, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Semrau, J.A.; Herter, T.M.; Scott, S.H.; Dukelow, S.P. Examining Differences in Patterns of Sensory and Motor Recovery after Stroke with Robotics. Stroke 2015, 46, 3459–3469. [Google Scholar] [CrossRef] [PubMed]
- Tyryshkin, K.; Coderre, A.M.; Glasgow, J.I.; Herter, T.M.; Bagg, S.D.; Dukelow, S.P.; Scott, S.H. A Robotic Object Hitting Task to Quantify Sensorimotor Impairments in Participants with Stroke. J. Neuroeng. Rehabil. 2014, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Haggard, P.; Richardson, J. Spatial Patterns in the Control of Human Arm Movement. J. Exp. Psychol. Hum. Percept. Perform. 1996, 22, 42–62. [Google Scholar] [CrossRef]
- Bastian, A.; Riehle, A.; Erlhagen, W.; Schöner, G. Prior Information Preshapes the Population Representation of Movement Direction in Motor Cortex. Neuroreport 1998, 9, 315–319. [Google Scholar] [CrossRef]
- Chapman, C.S.; Gallivan, J.P.; Wood, D.K.; Milne, J.L.; Culham, J.C.; Goodale, M.A. Reaching for the Unknown: Multiple Target Encoding and Real-Time Decision-Making in a Rapid Reach Task. Cognition 2010, 116, 168–176. [Google Scholar] [CrossRef]

| Demographics | Control | Stroke |
|---|---|---|
| N | 41 | 41 |
| Age | 62 [33–77] | 65 [27–88] |
| Sex (F:M) | 22:19 | 17:24 |
| Handedness (L:R) | 6:35 | 2:39 |
| Clinical Measures | ||
| Affected Arm (Dominant:Non-dominant) | 26:15 | |
| Stroke Type (Ischemic:Hemorrhagic) | 36:5 | |
| Days from Stroke to Robotic Assessment | 31 [3–1102] | |
| Lesion Volume (mL) | 8.72 [0.25–97.10] | |
| FMA (/66) ƗƗƗ | 62 [35–66] | |
| CMSA Arm (/7) Ɨ | 6 [3–7] | |
| PPT ƗƗ | 6 [0–15] | |
| MRC Arm Strength composite (/45) Ɨ | 43 [20–45] | |
| MAS (/4) Ɨ | 0 [0–2] | |
| TLT (/3) Ɨ | 0 [0–3] | |
| FIM (/126) ƗƗƗƗ | 115 [87–126] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, R.T.; Piitz, M.A.; Singh, N.; Dukelow, S.P.; Cluff, T. Movement Impairments May Not Preclude Visuomotor Adaptation After Stroke. Brain Sci. 2025, 15, 619. https://doi.org/10.3390/brainsci15060619
Moore RT, Piitz MA, Singh N, Dukelow SP, Cluff T. Movement Impairments May Not Preclude Visuomotor Adaptation After Stroke. Brain Sciences. 2025; 15(6):619. https://doi.org/10.3390/brainsci15060619
Chicago/Turabian StyleMoore, Robert Taylor, Mark Andrew Piitz, Nishita Singh, Sean Peter Dukelow, and Tyler Cluff. 2025. "Movement Impairments May Not Preclude Visuomotor Adaptation After Stroke" Brain Sciences 15, no. 6: 619. https://doi.org/10.3390/brainsci15060619
APA StyleMoore, R. T., Piitz, M. A., Singh, N., Dukelow, S. P., & Cluff, T. (2025). Movement Impairments May Not Preclude Visuomotor Adaptation After Stroke. Brain Sciences, 15(6), 619. https://doi.org/10.3390/brainsci15060619






