Naringenin Exhibits Antiglioma Activity Related to Aryl Hydrocarbon Receptor Activity and IL-6, CCL2, and TNF-α Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Cell Cultures
2.2. Spheroid Formation
2.3. Drugs and Treatments
2.4. Cell Viability Assays
2.4.1. MTT Assay
2.4.2. Sulforhodamine B (SRB) Assay
2.5. Migration Assays
2.5.1. Monolayer Scratch Assay
2.5.2. Spheroid-Based Migration Assay
2.6. RT-qPCR for mRNA
2.7. Statistical Analysis
3. Results
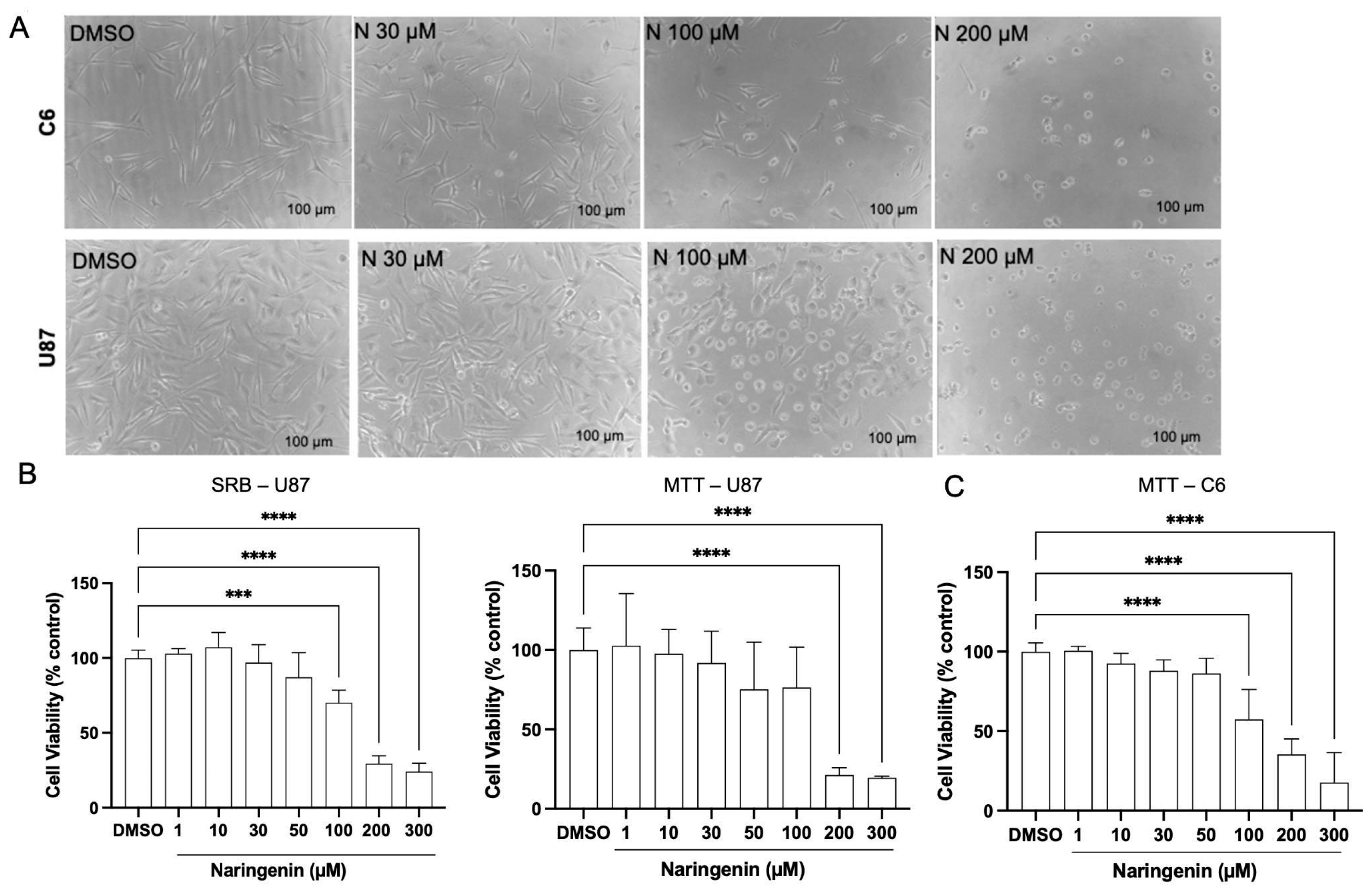
3.1. Naringenin Alters the Viability of Glioma Cell Lines U87 and C6
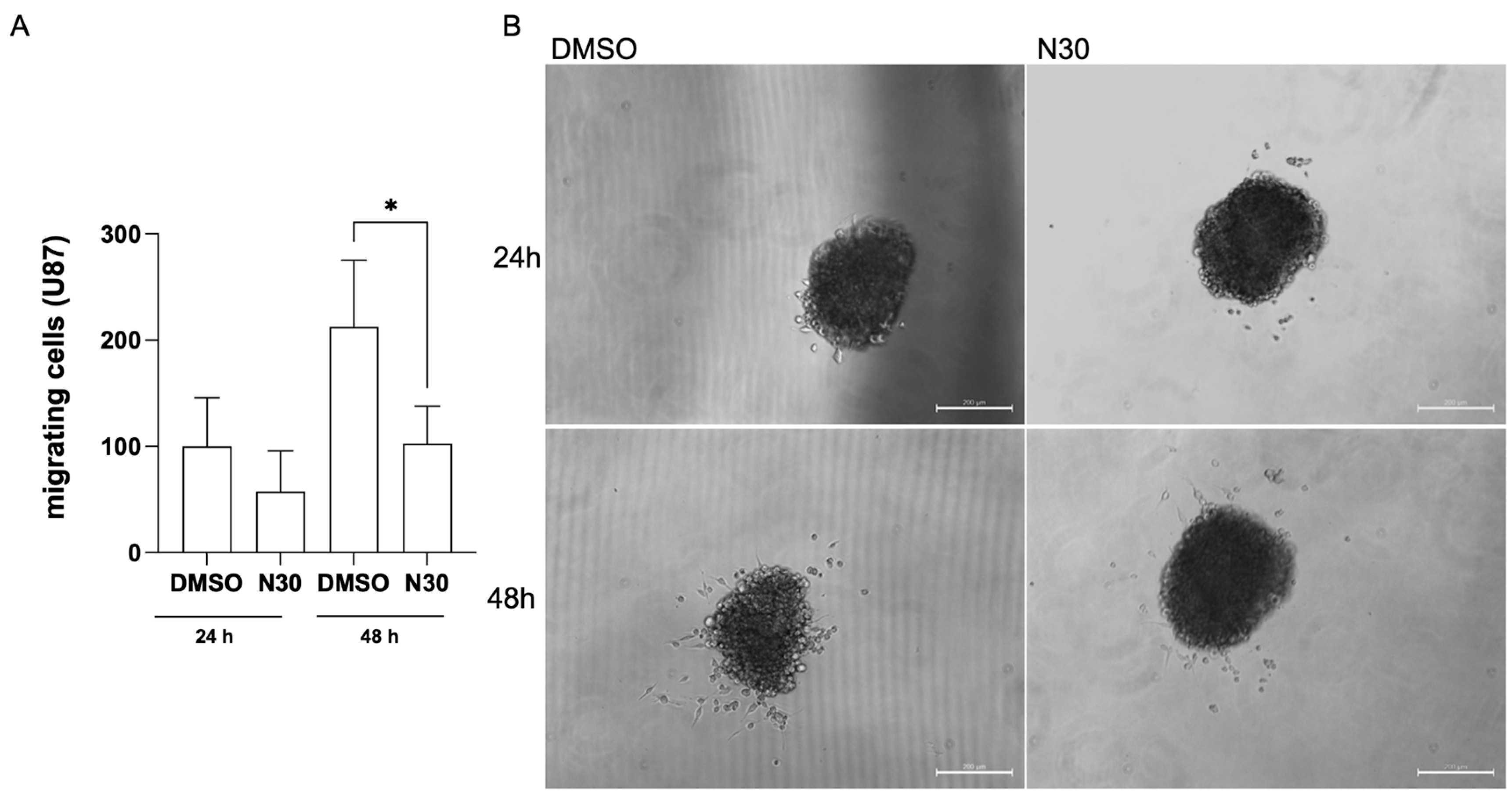
3.2. Naringenin Inhibits the Migration of Glioma Cells
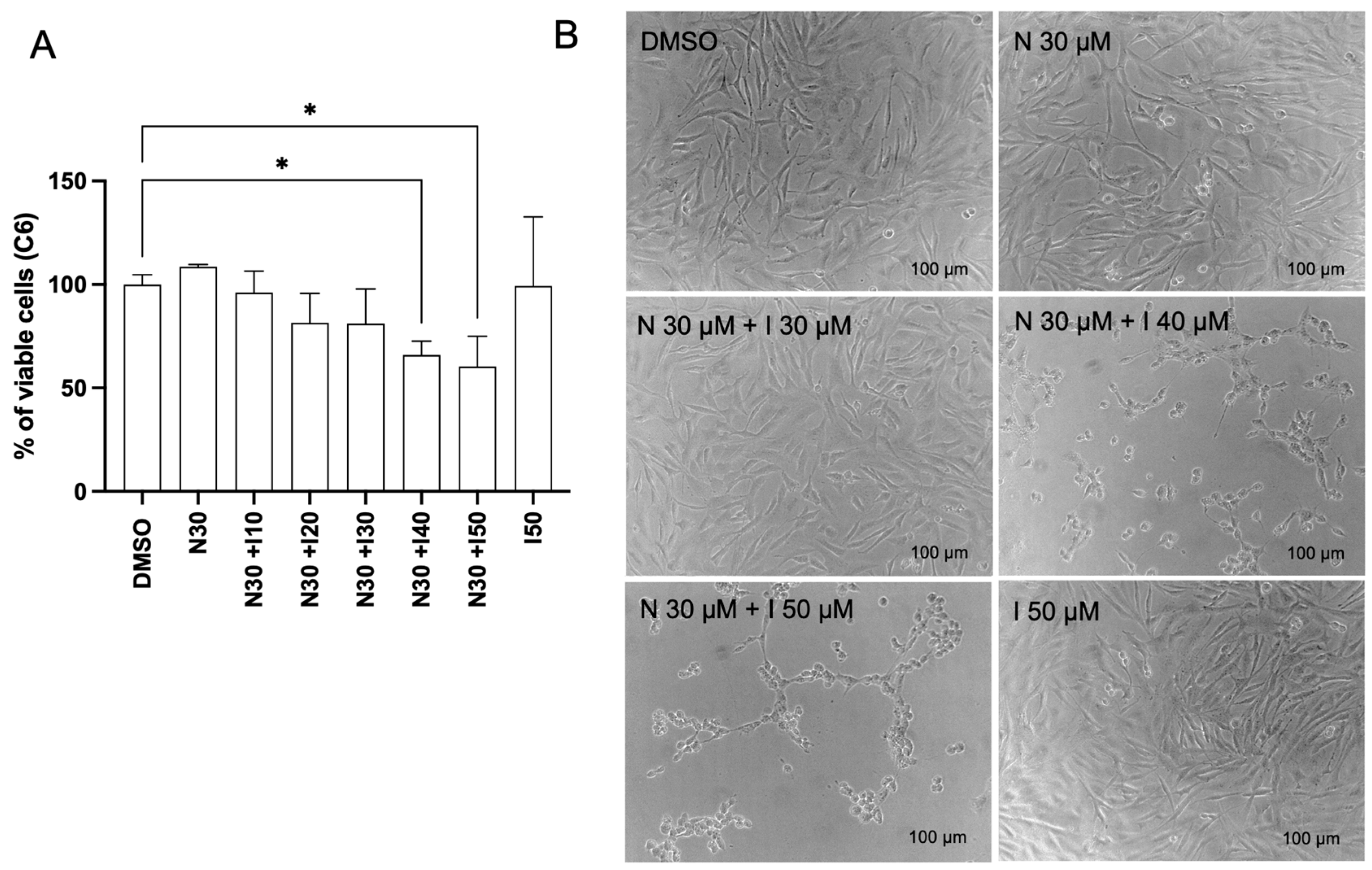
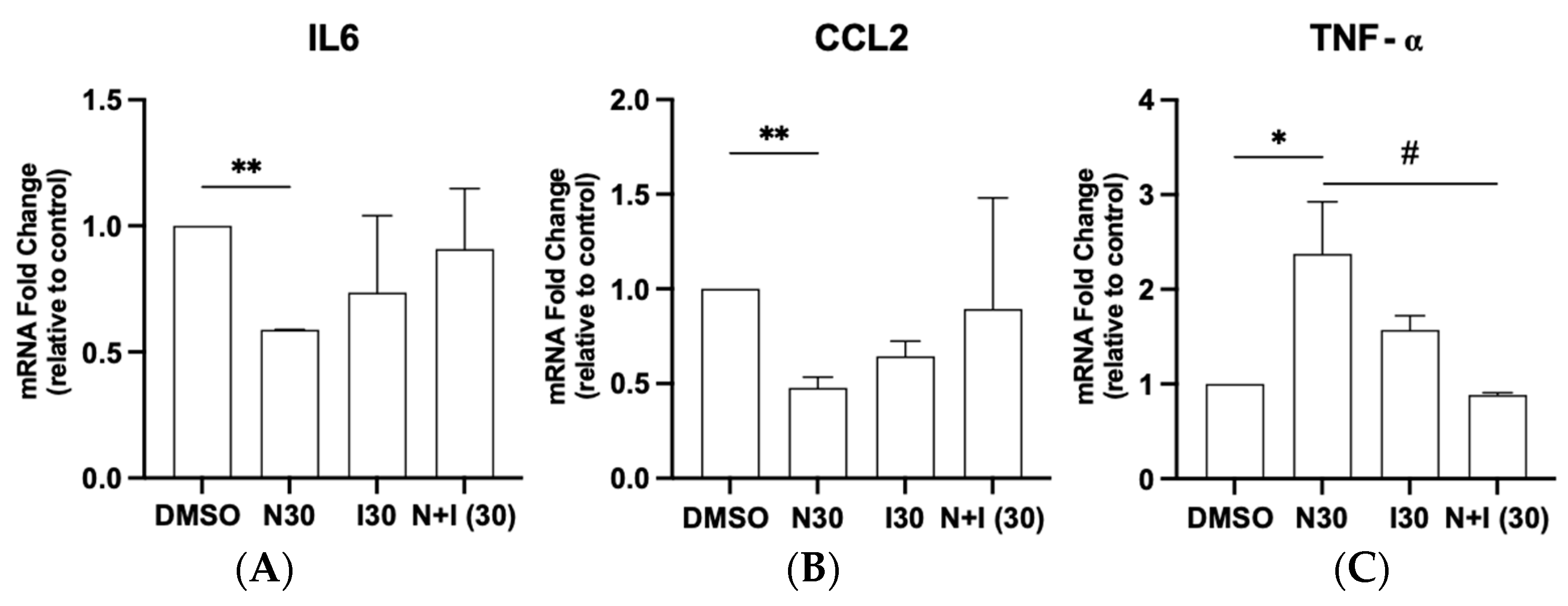
3.3. Antagonistic Potential of Naringenin in Regulating AhR Target Gene Expression in Glioma Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics. CA A Cancer J. Clin. 2021, 71, 381–406. [Google Scholar]
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro Oncol. 2024, 26, vi1–vi85. [Google Scholar]
- Du, L.; Xing, Z.; Tao, B.; Li, T.; Yang, D.; Li, W.; Zheng, Y.; Kuang, C.; Yang, Q. Both IDO1 and TDO contribute to the malignancy of gliomas via the Kyn–AhR–AQP4 signaling pathway. Signal Transduct. Target. Ther. 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; López-Blanch, R.; Pineda, B.; Gutiérrez-Arroyo, J.L.; Loras, A.; Gonzalez-Bonet, L.G.; Martinez-Cadenas, C.; Estrela, J.M.; et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chavarro, V.S.; Gerstl, J.V.E.; Blitz, S.E.; Spanehl, L.; Dubinski, D.; Valdes, P.A.; Tran, L.N.; Gupta, S.; Esposito, L.; et al. Recurrent Glioblastoma—Molecular Underpinnings and Evolving Treatment Paradigms. Int. J. Mol. Sci. 2024, 25, 6733. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.V.R.; Batista, C.; Afonso, B.d.H.; Alexandre-Moreira, M.S.; Dubois, L.G.; Pontes, B.; Neto, V.M.; de Mendes, F.A. Obstacles to Glioblastoma Treatment Two Decades after Temozolomide. Cancers 2022, 14, 3203. [Google Scholar] [CrossRef]
- Erices, J.I.; Bizama, C.; Niechi, I.; Uribe, D.; Rosales, A.; Fabres, K.; Navarro-Martínez, G.; Torres, Á.; San Martín, R.; Roa, J.C.; et al. Glioblastoma Microenvironment and Invasiveness: New Insights and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 7047. [Google Scholar] [CrossRef]
- Mescher, M.; Haarmann-Stemmann, T. Modulation of CYP1A1 metabolism: From adverse health effects to chemoprevention and therapeutic options. Pharmacol. Ther. 2018, 187, 71–87. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Sha, R.; Li, Y.; Xu, T.; Hu, X.; Xu, L.; Xie, Q.; Zhao, B. A new insight into the role of aryl hydrocarbon receptor (AhR) in the migration of glioblastoma by AhR-IL24 axis regulation. Environ. Int. 2021, 154, 106658. [Google Scholar] [CrossRef] [PubMed]
- Paris, A.; Tardif, N.; Galibert, M.D.; Corre, S. AhR and cancer: From gene profiling to targeted therapy. Int. J. Mol. Sci. 2021, 22, 752. [Google Scholar] [CrossRef]
- Soltani-asl, M.; Azimnasab-sorkhabi, P.; Yoshinaga, T.T.; de Oliveira Massoco, C.; Kfoury, J.R. The combination of IDO and AHR blockers reduces the migration and clonogenicity of breast cancer cells. Immunol. Res. 2024, 72, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Kim, S.B.; Safe, S. Omeprazole Inhibits Pancreatic Cancer Cell Invasion through a Nongenomic Aryl Hydrocarbon Receptor Pathway. Chem. Res. Toxicol. 2015, 28, 907–918. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Obara-Michlewska, M. The tryptophan metabolism, kynurenine pathway and oxidative stress—Implications for glioma pathobiology. Neurochem. Int. 2022, 158, 105363. [Google Scholar] [CrossRef]
- Zhong, C.; Peng, L.; Tao, B.; Yin, S.; Lyu, L.; Ding, H.; Yang, X.; Peng, T.; He, H.; Zhou, P. TDO2 and tryptophan metabolites promote kynurenine/AhR signals to facilitate glioma progression and immunosuppression. Am. J. Cancer Res. 2022, 12, 2558–2575. [Google Scholar]
- Ma, W.; Ye, L.; Zhong, C.; Li, J.; Ye, F.; Lv, L.; Yu, Y.; Jiang, S.; Zhou, P. Kynurenine produced by tryptophan 2,3-dioxygenase metabolism promotes glioma progression through an aryl hydrocarbon receptor-dependent signaling pathway. Cell Biol. Int. 2022, 46, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Pereira do Nascimento, R.; Lino dos Santos, B.; Alves Oliveira Amparo, J.; Ribeiro Pereira Soares, J.; Costa da Silva, K.; Reis Santana, M.; Maria Alves Nunes Almeida, Á.; Diógenes Amaral da Silva, V.; de Fátima Dias Costa, M.; Ulrich, H.; et al. Neuroimmunomodulatory Properties of Flavonoids and Derivates: A Potential Action as Adjuvants for the Treatment of Glioblastoma. Pharmaceutics 2021, 14, 116. [Google Scholar] [CrossRef]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Flavonoids on High-Grade Adult-Type Diffuse Gliomas. Nutrients 2023, 15, 797. [Google Scholar] [CrossRef]
- Roszkowski, S. Application of Polyphenols and Flavonoids in Oncological Therapy. Molecules 2023, 28, 4080. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.L.; Yu, C.T.R.; Lien, H.M.; Sheu, G.T.; Cherng, S.H. Cytotoxicity of naringenin induces Bax-mediated mitochondrial apoptosis in human lung adenocarcinoma A549 cells. Environ. Toxicol. 2020, 35, 1386–1394. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, G.; Ge, Y.; Guo, Z. Naringenin inhibits migration of breast cancer cells via inflammatory and apoptosis cell signaling pathways. Inflammopharmacology 2019, 27, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Kong, S.; Zhao, S.; Tang, Q. Naringenin inhibits human breast cancer cells (MDA-MB-231) by inducing programmed cell death, caspase stimulation, G2/M phase cell cycle arrest and suppresses cancer metastasis. Cell. Mol. Biol. 2021, 67, 8–13. [Google Scholar] [CrossRef]
- Choi, J.; Lee, D.H.; Jang, H.; Park, S.Y.; Seol, J.W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int. J. Med. Sci. 2020, 17, 3049–3057. [Google Scholar] [CrossRef]
- Jayalakshmi, J.; Vanisree, A.J. Naringenin Sensitizes Resistant C6 Glioma Cells with a Repressive Impact on the Migrating Ability. Ann. Neurosci. 2020, 27, 114–123. [Google Scholar] [CrossRef]
- Stompor, M.; Uram, Ł.; Podgórski, R. In vitro effect of 8-prenylnaringenin and naringenin on fibroblasts and glioblastoma cells-cellular accumulation and cytotoxicity. Molecules 2017, 22, 1092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chang, Y.M.; Wang, K.Y.; Chen, P.N.; Hseu, Y.C.; Chen, K.M.; Yeh, K.T.; Chen, C.J.; Hsu, L.S. Naringenin inhibited migration and invasion of glioblastoma cells through multiple mechanisms. Environ. Toxicol. 2019, 34, 233–239. [Google Scholar] [CrossRef]
- Sargazi, M.L.; Juybari, K.B.; Tarzi, M.E.; Amirkhosravi, A.; Nematollahi, M.H.; Mirzamohammdi, S.; Mehrbani, M.; Mehrabani, M.; Mehrabani, M. Naringenin attenuates cell viability and migration of C6 glioblastoma cell line: A possible role of hedgehog signaling pathway. Mol. Biol. Rep. 2021, 48, 6413–6421. [Google Scholar] [CrossRef]
- de Santana, M.R.; dos Santos, Y.B.; Santos, K.S.; Junior, M.C.S.; Victor, M.M.; Ramos, G.d.S.; Nascimento, R.P.D.; Costa, S.L. Differential Interactions of Flavonoids with the Aryl Hydrocarbon Receptor In Silico and Their Impact on Receptor Activity In Vitro. Pharmaceuticals 2024, 17, 980. [Google Scholar] [CrossRef]
- Yan, X.; Lin, T.; Zhu, Q.; Zhang, Y.; Song, Z.; Pan, X. Naringenin protects against acute pancreatitis-associated intestinal injury by inhibiting NLRP3 inflammasome activation via AhR signaling. Front. Pharmacol. 2023, 14, 1090261. [Google Scholar] [CrossRef] [PubMed]
- West, A.J.; Tsui, V.; Stylli, S.S.; Nguyen, H.P.T.; Morokoff, A.P.; Kaye, A.H.; Luwor, R.B. The role of interleukin-6-STAT3 signalling in glioblastoma. Oncol. Lett. 2018, 16, 4095–4104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shi, G.; Lu, J.; Qian, X.; Wang, D. Potential regulatory mechanism of TNF-α/TNFR1/ANXA1 in glioma cells and its role in glioma cell proliferation. Open Life Sci. 2022, 17, 208–220. [Google Scholar] [CrossRef]
- Chang, A.L.; Miska, J.; Wainwright, D.A.; Dey, M.; Rivetta, C.V.; Yu, D.; Kanojia, D.; Pituch, K.C.; Qiao, J.; Pytel, P.; et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory t cells and myeloid-derived suppressor cells. Cancer Res. 2016, 76, 5671–5682. [Google Scholar] [CrossRef]
- Santos, B.L.; Oliveira, M.N.; Coelho, P.L.C.; Pitanga, B.P.S.; da Silva, A.B.; Adelita, T.; Silva, V.D.A.; Costa, M.D.F.D.; El-Bachá, R.S.; Tardy, M.; et al. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem.-Biol. Interact. 2015, 242, 123–138. [Google Scholar] [CrossRef]
- Victor, M.M.; David, J.M.; Cortez, M.V.M.; Leite, J.L.; da Silva, G.S.B. A High-Yield Process for Extraction of Hesperidin from Orange (Citrus sinensis L. osbeck) Peels Waste, and Its Transformation to Diosmetin, A Valuable and Bioactive Flavonoid. Waste Biomass Valorization 2021, 12, 313–320. [Google Scholar] [CrossRef]
- Victor, M.M.; David, J.M.; Sakukuma, M.C.; França, E.L.; Nunes, A.V. A simple and efficient process for the extraction of naringin from grapefruit peel waste. Green. Process. Synth. 2018, 7, 524–529. [Google Scholar]
- Lima, I.S.; Soares, É.N.; Nonaka, C.K.V.; Souza, B.S.d.F.; dos Santos, B.L.; Costa, S.L. Flavonoid Rutin Presented Anti-Glioblastoma Activity Related to the Modulation of Onco miRNA-125b Expression and STAT3 Signaling and Impact on Microglia Inflammatory Profile. Brain Sci. 2024, 14, 90. [Google Scholar] [CrossRef]
- Voigt, W. Sulforhodamine B assay and chemosensitivity. Methods Mol. Med. 2005, 110, 39–48. [Google Scholar] [CrossRef]
- Papadimitriou, M.; Hatzidaki, E.; Papasotiriou, I. Linearity Comparison of Three Colorimetric Cytotoxicity Assays. J. Cancer Ther. 2019, 10, 580–590. [Google Scholar] [CrossRef]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Rev. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Pohjanvirta, R.; Niittynen, M.; Lindén, J.; Boutros, P.C.; Moffat, I.D.; Okey, A.B. Evaluation of various housekeeping genes for their applicability for normalization of mRNA expression in dioxin-treated rats. Chem.-Biol. Interact. 2006, 160, 134–149. [Google Scholar] [CrossRef]
- Prokopec, S.D.; Buchner, N.B.; Fox, N.S.; Chong, L.C.; Mak, D.Y.F.; Watson, J.D.; Petronis, A.; Pohjanvirta, R.; Boutros, P.C. Validating reference genes within a mouse model system of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxicity. Chem.-Biol. Interact. 2013, 205, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Chen, X.J.; Wu, M.Y.; Li, D.H.; You, J. Apigenin inhibits glioma cell growth through promoting microRNA-16 and suppression of BCL-2 and nuclear factor-κB/MMP-9. Mol. Med. Rep. 2016, 14, 2352–2358. [Google Scholar] [CrossRef]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Michelhaugh, S.K.; Polin, L.A.; Shrestha, R.; Mittal, S.; Safe, S. Omeprazole inhibits glioblastoma cell invasion and tumor growth. Cancers 2020, 12, 2097. [Google Scholar] [CrossRef]
- Bellail, A.C.; Hunter, S.B.; Brat, D.J.; Tan, C.; van Meir, E.G. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int. J. Biochem. Cell Biol. 2004, 36, 1046–1069. [Google Scholar] [CrossRef]
- de Gooijer, M.C.; Guillén Navarro, M.; Bernards, R.; Wurdinger, T.; van Tellingen, O. An Experimenter’s Guide to Glioblastoma Invasion Pathways. Trends Mol. Med. 2018, 24, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Litzenburger, U.M.; Opitz, C.A.; Sahm, F.; Rauschenbach, K.J.; Trump, S.; Winter, M.; Ott, M.; Ochs, K.; Lutz, C.; Liu, X.; et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 2014, 5, 1038–1051. [Google Scholar] [PubMed]
- Takacs, G.P.; Kreiger, C.J.; Luo, D.; Tian, G.; Garcia, J.S.; Deleyrolle, L.P.; Mitchell, D.A.; Harrison, J.K. Glioma-derived CCL2 and CCL7 mediate migration of immune suppressive CCR2+/CX3CR1+ M-MDSCs into the tumor microenvironment in a redundant manner. Front. Immunol. 2023, 13, 993444. [Google Scholar] [CrossRef]
- Qian, Y.; Dong, J.; Zhang, W.; Xue, X.; Xiong, Z.; Zeng, W.; Wang, Q.; Fan, Z.; Zuo, Z.; Huang, Z.; et al. Deguelin inhibits the glioblastoma progression through suppressing CCL2/NFκB signaling pathway. Neuropharmacology 2024, 259, 110109. [Google Scholar] [CrossRef]
- Hwang, J.S.; Jung, E.H.; Kwon, M.Y.; Han, I.O. Glioma-secreted soluble factors stimulate microglial activation: The role of interleukin-1β and tumor necrosis factor-α. J. Neuroimmunol. 2016, 298, 165–171. [Google Scholar] [CrossRef]

| Molecule | Structure |
|---|---|
| Naringenin |  |
| Indole-3-carbinol |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, M.R.d.; Argolo, D.S.; Lima, I.S.; Santos, C.C.d.; Victor, M.M.; Ramos, G.d.S.; Nascimento, R.P.d.; Ulrich, H.; Costa, S.L. Naringenin Exhibits Antiglioma Activity Related to Aryl Hydrocarbon Receptor Activity and IL-6, CCL2, and TNF-α Expression. Brain Sci. 2025, 15, 325. https://doi.org/10.3390/brainsci15030325
Santana MRd, Argolo DS, Lima IS, Santos CCd, Victor MM, Ramos GdS, Nascimento RPd, Ulrich H, Costa SL. Naringenin Exhibits Antiglioma Activity Related to Aryl Hydrocarbon Receptor Activity and IL-6, CCL2, and TNF-α Expression. Brain Sciences. 2025; 15(3):325. https://doi.org/10.3390/brainsci15030325
Chicago/Turabian StyleSantana, Monique Reis de, Deivison Silva Argolo, Irlã Santos Lima, Cleonice Creusa dos Santos, Maurício Moraes Victor, Gabriel dos Santos Ramos, Ravena Pereira do Nascimento, Henning Ulrich, and Silvia Lima Costa. 2025. "Naringenin Exhibits Antiglioma Activity Related to Aryl Hydrocarbon Receptor Activity and IL-6, CCL2, and TNF-α Expression" Brain Sciences 15, no. 3: 325. https://doi.org/10.3390/brainsci15030325
APA StyleSantana, M. R. d., Argolo, D. S., Lima, I. S., Santos, C. C. d., Victor, M. M., Ramos, G. d. S., Nascimento, R. P. d., Ulrich, H., & Costa, S. L. (2025). Naringenin Exhibits Antiglioma Activity Related to Aryl Hydrocarbon Receptor Activity and IL-6, CCL2, and TNF-α Expression. Brain Sciences, 15(3), 325. https://doi.org/10.3390/brainsci15030325









