The Role of Transcranial Direct Current Stimulation in Chronic Shoulder Pain: A Scoping Review
Abstract
1. Introduction
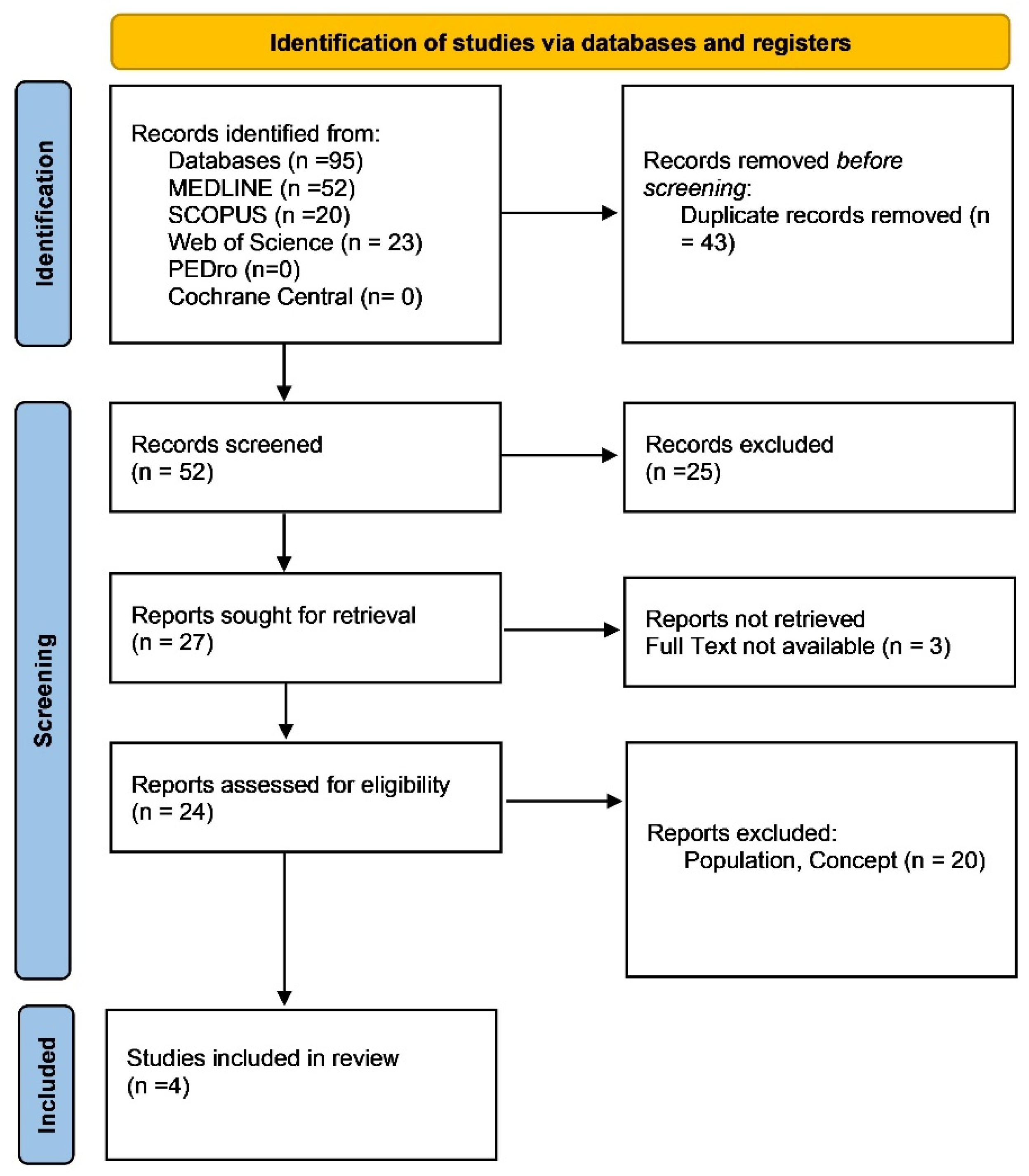
2. Methods
2.1. Review Question
2.2. Eligibility Criteria
2.2.1. Population
- Rotator cuff disorders, such as tendinopathy or tears.
- Myofascial pain syndrome (MPS) in the shoulder region, characterized by trigger points and referred pain.
- Subacromial pain syndrome (SAPS), including subacromial bursitis and impingement syndromes.
2.2.2. Concept
- They investigated the efficacy of tDCS in reducing pain intensity.
- They assessed improvements in functional recovery, such as range of motion, strength, and daily activity levels.
- They focused on tDCS applied over motor cortex regions relevant to shoulder pain (e.g., primary motor cortex M1).
2.2.3. Context
- Physical therapy clinics.
- Pain management centres.
- Research laboratories conducting experimental neuromodulation studies.
2.3. Exclusion Criteria
2.4. Search Strategy
2.5. Data Extraction and Data Synthesis
3. Results
3.1. Pain Reduction
- Larrivée et al. (2021) [27]: Patients in both the real and sham tDCS groups experienced substantial pain relief, with a mean reduction of 3.5 points on the VAS in the real tDCS group and 3.3 points in the sham group. These reductions indicate that pain relief was primarily due to the corticosteroid injections rather than the tDCS intervention.
- Choi et al. (2014) [29]: In this study, pain intensity significantly decreased in the DLPFC group by an average of 2.5 points on the VAS. This reduction was observed after the second stimulation session and was maintained throughout the treatment period. The pain reduction in the M1 and sham groups was less pronounced, highlighting the potential role of targeting the DLPFC for chronic pain management.
- Belley et al. (2018) [28]: Pain-related disability, measured using the DASH score, improved from a baseline of 45.6 to 29.3 in both the real and sham tDCS groups after 12 weeks. The WORC index showed similar improvements, with both groups reporting significant reductions in shoulder-related disability. However, the study did not find any additional benefits of real tDCS over sham stimulation.
- Sakrajai et al. (2014) [30]: Patients in the active tDCS group reported a mean pain reduction of 4 points on the NRS, compared to a 2-point reduction in the sham group. Pain relief in the active tDCS group was more sustained, with improvements maintained at the 1-week and 4-week follow-ups.
3.2. Functional Recovery
- Larrivée et al. (2021) [27]: The SANE scores improved significantly, from an average of 65% to 85% post-treatment. Both the tDCS and sham groups showed similar levels of improvement, suggesting that the corticosteroid injection was the primary driver of functional recovery.
- Choi et al. (2014) [29]: Patients in the DLPFC group demonstrated better improvements in range of motion and pain thresholds compared to the M1 and sham groups. The study reported a 15% increase in functional recovery scores in the DLPFC group, highlighting the potential of tDCS to enhance physical performance.
- Belley et al. (2018) [28]: Functional outcomes were measured using the DASH and WORC indices. Both groups showed improvements from baseline to week 12, with the DASH score decreasing by approximately 16 points and the WORC score increasing by 25 points. However, no additional benefits were observed in the real tDCS group.
- Sakrajai et al. (2014) [30]: Shoulder adduction PROM improved by an average of 15 degrees in the active tDCS group, compared to a 7-degree improvement in the sham group. The active tDCS group also reported faster recovery times, with improvements seen as early as the second treatment session.
3.3. Cortical Modulation and Neuroplasticity
3.4. Quality of Life and Patient Satisfaction
- Belley et al. (2018) [28]: The study reported an 85% patient satisfaction rate at the end of the 12-week intervention. Participants in both the real and sham tDCS groups reported positive outcomes, including reduced pain and improved shoulder function.
- Sakrajai et al. (2014) [30]: The study highlighted patient-reported outcomes such as decreased reliance on pain medications and increased satisfaction with the treatment. These findings suggest that tDCS may enhance patients’ overall treatment experience and quality of life.
4. Discussion
5. Limitations
6. Clinical Practice Implications
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pozzi, F.; Sousa, C.O.; Plummer, H.A.; Andrade, B.; Awokuse, D.; Kono, N.; Mack, W.J.; Roll, S.C.; Michener, L.A. Development of Shoulder Pain with Job-Related Repetitive Load: Mechanisms of Tendon Pathology and Anxiety. J. Shoulder Elb. Surg. 2022, 31, 225–234. [Google Scholar] [CrossRef]
- Linaker, C.H.; Walker-Bone, K. Shoulder Disorders and Occupation. Best. Pr. Res. Clin. Rheumatol. 2015, 29, 405–423. [Google Scholar] [CrossRef]
- Luime, J.J.; Koes, B.W.; Hendriksen, I.J.M.; Burdorf, A.; Verhagen, A.P.; Miedema, H.S.; Verhaar, J.A.N. Prevalence and Incidence of Shoulder Pain in the General Population; a Systematic Review. Scand. J. Rheumatol. 2004, 33, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ostergren, P.-O.; Hanson, B.S.; Balogh, I.; Ektor-Andersen, J.; Isacsson, A.; Orbaek, P.; Winkel, J.; Isacsson, S.-O.; Malmö Shoulder Neck Study Group. Incidence of Shoulder and Neck Pain in a Working Population: Effect Modification between Mechanical and Psychosocial Exposures at Work? Results from a One Year Follow up of the Malmö Shoulder and Neck Study Cohort. J. Epidemiol. Community Health 2005, 59, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Adebajo, A.; Hay, E.; Carr, A. Shoulder Pain: Diagnosis and Management in Primary Care. BMJ 2005, 331, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R. Reevaluating the Drucebo Effect: Implications for Physiotherapy Practice. J. Psychosoc. Rehabil. Ment. Health 2024, 11, 391–393. [Google Scholar] [CrossRef]
- Littlewood, C.; Ashton, J.; Chance-Larsen, K.; May, S.; Sturrock, B. Exercise for Rotator Cuff Tendinopathy: A Systematic Review. Physiotherapy 2012, 98, 101–109. [Google Scholar] [CrossRef]
- Tedeschi, R.; Berti, L.; Platano, D. Transcranial Direct Current Stimulation (tDCS) in Managing Pain and Recovery: A Clinical Case of Radial Capitellum Fracture. Int. J. Surg. Case Rep. 2024, 114, 109120. [Google Scholar] [CrossRef]
- Tedeschi, R.; Benedetti, M.G.; Berti, L.; Donati, D.; Platano, D. Transcranial Direct Current Stimulation in the Treatment of Chronic Knee Pain: A Scoping Review. Appl. Sci. 2024, 14, 7100. [Google Scholar] [CrossRef]
- Tedeschi, R.; Platano, D.; Donati, D.; Giorgi, F. Functional Approaches in Tendinopathy Rehabilitation: Exploring the Role of Tendon Neuroplastic Training. Man. Med. 2024, 1–7. [Google Scholar] [CrossRef]
- Buchbinder, R.; Green, S.; Youd, J.M. Corticosteroid Injections for Shoulder Pain. Cochrane Database Syst. Rev. 2003, 2003, CD004016. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, D.A.; Koes, B.W.; de Jong, B.A.; Bouter, L.M. Shoulder Disorders in General Practice: Incidence, Patient Characteristics, and Management. Ann. Rheum. Dis. 1995, 54, 959–964. [Google Scholar] [CrossRef]
- Lewis, J.S. Rotator Cuff Tendinopathy: A Model for the Continuum of Pathology and Related Management. Br. J. Sports Med. 2010, 44, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.-A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic Pain: Towards an Understanding of Prevalent Pain Conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central Sensitization: Implications for the Diagnosis and Treatment of Pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Woolf, C.J.; Doubell, T.P. The Pathophysiology of Chronic Pain--Increased Sensitivity to Low Threshold A Beta-Fibre Inputs. Curr. Opin. Neurobiol. 1994, 4, 525–534. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial Direct Current Stimulation: State of the Art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low Intensity Transcranial Electric Stimulation: Safety, Ethical, Legal Regulatory and Application Guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]
- Mylius, V.; Jung, M.; Menzler, K.; Haag, A.; Khader, P.H.; Oertel, W.H.; Rosenow, F.; Lefaucheur, J.-P. Effects of Transcranial Direct Current Stimulation on Pain Perception and Working Memory. Eur. J. Pain. 2012, 16, 974–982. [Google Scholar] [CrossRef]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef]
- Jeffery, D.T.; Norton, J.A.; Roy, F.D.; Gorassini, M.A. Effects of Transcranial Direct Current Stimulation on the Excitability of the Leg Motor Cortex. Exp. Brain Res. 2007, 182, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Doemkes, S.; Karaköse, T.; Antal, A.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Shaping the Effects of Transcranial Direct Current Stimulation of the Human Motor Cortex. J. Neurophysiol. 2007, 97, 3109–3117. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Nitsche, M.A.; Paulus, W.; Rothwell, J.C.; Lemon, R.N. Effects of Transcranial Direct Current Stimulation over the Human Motor Cortex on Corticospinal and Transcallosal Excitability. Exp. Brain Res. 2004, 156, 439–443. [Google Scholar] [CrossRef]
- Meinzer, M.; Shahbabaie, A.; Antonenko, D.; Blankenburg, F.; Fischer, R.; Hartwigsen, G.; Nitsche, M.A.; Li, S.-C.; Thielscher, A.; Timmann, D.; et al. Investigating the Neural Mechanisms of Transcranial Direct Current Stimulation Effects on Human Cognition: Current Issues and Potential Solutions. Front. Neurosci. 2024, 18, 1389651. [Google Scholar] [CrossRef]
- Zhang, N.; Nitsche, M.A.; Miao, Y.; Xiong, Z.; Vicario, C.M.; Qi, F. Transcranial Direct-Current Stimulation Over the Primary Motor Cortex and Cerebellum Improves Balance and Shooting Accuracy in Elite Ice Hockey Players. Int. J. Sports Physiol. Perform. 2024, 19, 1107–1114. [Google Scholar] [CrossRef]
- Larrivée, S.; Balg, F.; Léonard, G.; Bédard, S.; Tousignant, M.; Boissy, P. Transcranial Direct Current Stimulation (a-tCDS) after Subacromial Injections in Patients with Subacromial Pain Syndrome: A Randomized Controlled Pilot Study. BMC Musculoskelet. Disord. 2021, 22, 265. [Google Scholar] [CrossRef]
- Belley, A.F.; Mercier, C.; Bastien, M.; Léonard, G.; Gaudreault, N.; Roy, J.-S. Anodal Transcranial Direct-Current Stimulation to Enhance Rehabilitation in Individuals with Rotator Cuff Tendinopathy: A Triple-Blind Randomized Controlled Trial. J. Orthop. Sports Phys. Ther. 2018, 48, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-H.; Jung, S.-J.; Lee, C.H.; Lee, S.-U. Additional Effects of Transcranial Direct-Current Stimulation and Trigger-Point Injection for Treatment of Myofascial Pain Syndrome: A Pilot Study with Randomized, Single-Blinded Trial. J. Altern. Complement. Med. 2014, 20, 698–704. [Google Scholar] [CrossRef]
- Sakrajai, P.; Janyacharoen, T.; Jensen, M.P.; Sawanyawisuth, K.; Auvichayapat, N.; Tunkamnerdthai, O.; Keeratitanont, K.; Auvichayapat, P. Pain Reduction in Myofascial Pain Syndrome by Anodal Transcranial Direct Current Stimulation Combined with Standard Treatment: A Randomized Controlled Study. Clin. J. Pain. 2014, 30, 1076–1083. [Google Scholar] [CrossRef]
- Peters: Joanna Briggs Institute Reviewer’s Manual, JBI-Google Scholar. Available online: https://scholar-google-com.ezproxy.unibo.it/scholar_lookup?hl=en&publication_year=2020&author=MDJ+Peters&author=C+Godfrey&author=P+McInerney&author=Z+Munn&author=AC+Tricco&author=H+Khalil&title=Joanna+Briggs+Institute+Reviewer%27s+Manual%2C+JBI (accessed on 9 June 2022).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Jog, M.A.; Anderson, C.; Kubicki, A.; Boucher, M.; Leaver, A.; Hellemann, G.; Iacoboni, M.; Woods, R.; Narr, K. Transcranial Direct Current Stimulation (tDCS) in Depression Induces Structural Plasticity. Sci. Rep. 2023, 13, 2841. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, and Study Type | Methods | Results | Outcomes Achieved |
|---|---|---|---|
| Larrivée et al., 2021 (Randomized Controlled Pilot Study) [27] | A total of 38 patients with SAPS received corticosteroid injections followed by either real, sham, or no tDCS. Pain and function were measured using the VAS and SANE. | All groups showed significant improvement in pain and function. No additional benefits of tDCS were observed compared to sham or no intervention. | Improvement in pain and upper limb function was observed; however, tDCS did not provide additional benefits. |
| Choi et al., 2014 (Randomized, Single-Blind Trial) [29] | A total of 21 patients with MPS were randomized into three groups to receive TPI and tDCS over different brain areas (M1, DLPFC). Pain was assessed using the VAS and the McGill Pain Questionnaire. | Pain reduction was observed in all groups, with significant changes in the DLPFC group starting after the second session. | Significant pain reduction in the DLPFC group was observed; tDCS showed promise as an adjunct to TPI. |
| Belley et al., 2018 (Triple-Blind Randomized Controlled Trial) [28] | A total of 40 patients with rotator cuff tendinopathy were divided into two groups receiving sensorimotor training with either real or sham tDCS. Outcomes were measured using DASH and WORC. | Both groups showed improvements in DASH and WORC scores over 12 weeks. No added effects of tDCS were found. | Functional improvements in both groups were found; no additional benefits of tDCS were noted. |
| Sakrajai et al., 2014 (Randomized Controlled Study) [30] | A total of 31 patients with chronic myofascial pain were randomized to receive either active or sham tDCS over M1 combined with standard care. Pain intensity and PROM were measured. | The active tDCS group reported greater reductions in pain intensity and improvement in shoulder adduction PROM compared to the sham group. | Pain intensity reduction and improvements in PROM were achieved faster in the active tDCS group. |
| Author, Year | Diagnosis | Sample Size | Anode/Cathode Montage | Current Intensity (mA) | Session Duration (min) | No. of Sessions | Follow-Up Period | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Larrivée et al., 2021 [27] | SAPS | 38 | M1/Contralateral supraorbital | 2.0 | 20 | 1 | 4 weeks | No added effect of tDCS vs. sham |
| Choi et al., 2014 [29] | MPS | 21 | DLPFC/Contralateral | 2.0 | 20 | 5 | Post 5 sessions | Greater pain reduction in DLPFC group |
| Belley et al., 2018 [28] | Rotator Cuff Tendinopathy | 40 | M1/Contralateral | 2.0 | 20 | 10 | 12 weeks | No additional benefits of tDCS |
| Sakrajai et al., 2014 [30] | MPS | 31 | M1/Contralateral | 1.5 | 20 | 5 | 1 and 4 weeks | Improved pain and ROM in tDCS group |
| Clinical Condition | Cortical Target | Pain Effect | Function Effect |
|---|---|---|---|
| Myofascial Pain Syndrome (MPS) | DLPFC | ✓ | ✓ |
| Myofascial Pain Syndrome (MPS) | M1 | ✓ | ✓ |
| Subacromial Pain Syndrome (SAPS) | M1 | ✗ | ✗ |
| Rotator Cuff Tendinopathy | M1 | ✗ | ✗ |
| Study | Diagnosis | Group | Baseline VAS (Mean ± SD) | Post-Treatment VAS (Mean ± SD) | Pain Reduction (Mean) |
|---|---|---|---|---|---|
| Choi et al., 2014 [29] | Myofascial Pain Syndrome | Active tDCS | 4.22 ± 0.52 | 2.56 ± 0.73 | ↓ 1.66 |
| Sham | 4.37 ± 0.48 | 3.50 ± 0.63 | ↓ 0.87 | ||
| Sakrajai et al., 2014 [30] | Myofascial Pain Syndrome | Active tDCS | 6.5 ± 1.2 | 3.2 ± 1.0 | ↓ 3.3 |
| Sham | 6.4 ± 1.3 | 5.1 ± 1.1 | ↓ 1.3 | ||
| Larrivée et al., 2021 [27] | Subacromial Pain Syndrome | Active tDCS | 6.8 ± 1.0 | 3.3 ± 0.9 | ↓ 3.5 |
| Sham | 6.7 ± 1.1 | 4.5 ± 1.0 | ↓ 2.2 | ||
| Belley et al., 2018 [28] | Rotator Cuff Tendinopathy | Active tDCS | ~5.5 ± 1.0 | ~3.5 ± 1.2 | ↓ ~2.0 |
| Sham | ~5.5 ± 1.0 | ~3.5 ± 1.2 | ↓ ~2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, R.; Giorgi, F.; Donati, D. The Role of Transcranial Direct Current Stimulation in Chronic Shoulder Pain: A Scoping Review. Brain Sci. 2025, 15, 584. https://doi.org/10.3390/brainsci15060584
Tedeschi R, Giorgi F, Donati D. The Role of Transcranial Direct Current Stimulation in Chronic Shoulder Pain: A Scoping Review. Brain Sciences. 2025; 15(6):584. https://doi.org/10.3390/brainsci15060584
Chicago/Turabian StyleTedeschi, Roberto, Federica Giorgi, and Danilo Donati. 2025. "The Role of Transcranial Direct Current Stimulation in Chronic Shoulder Pain: A Scoping Review" Brain Sciences 15, no. 6: 584. https://doi.org/10.3390/brainsci15060584
APA StyleTedeschi, R., Giorgi, F., & Donati, D. (2025). The Role of Transcranial Direct Current Stimulation in Chronic Shoulder Pain: A Scoping Review. Brain Sciences, 15(6), 584. https://doi.org/10.3390/brainsci15060584







