Neurotechnological Approaches to Cognitive Rehabilitation in Mild Cognitive Impairment: A Systematic Review of Neuromodulation, EEG, Virtual Reality, and Emerging AI Applications
Abstract
1. Introduction
2. Literature Review
2.1. Mild Cognitive Impairment (MCI): Pathophysiology and Neural Mechanisms
2.2. Rehabilitation Approaches for MCI
2.3. Neurophysiological and Neuroimaging Methodologies in MCI
2.4. AI Applications in MCI Management
2.5. Research Questions
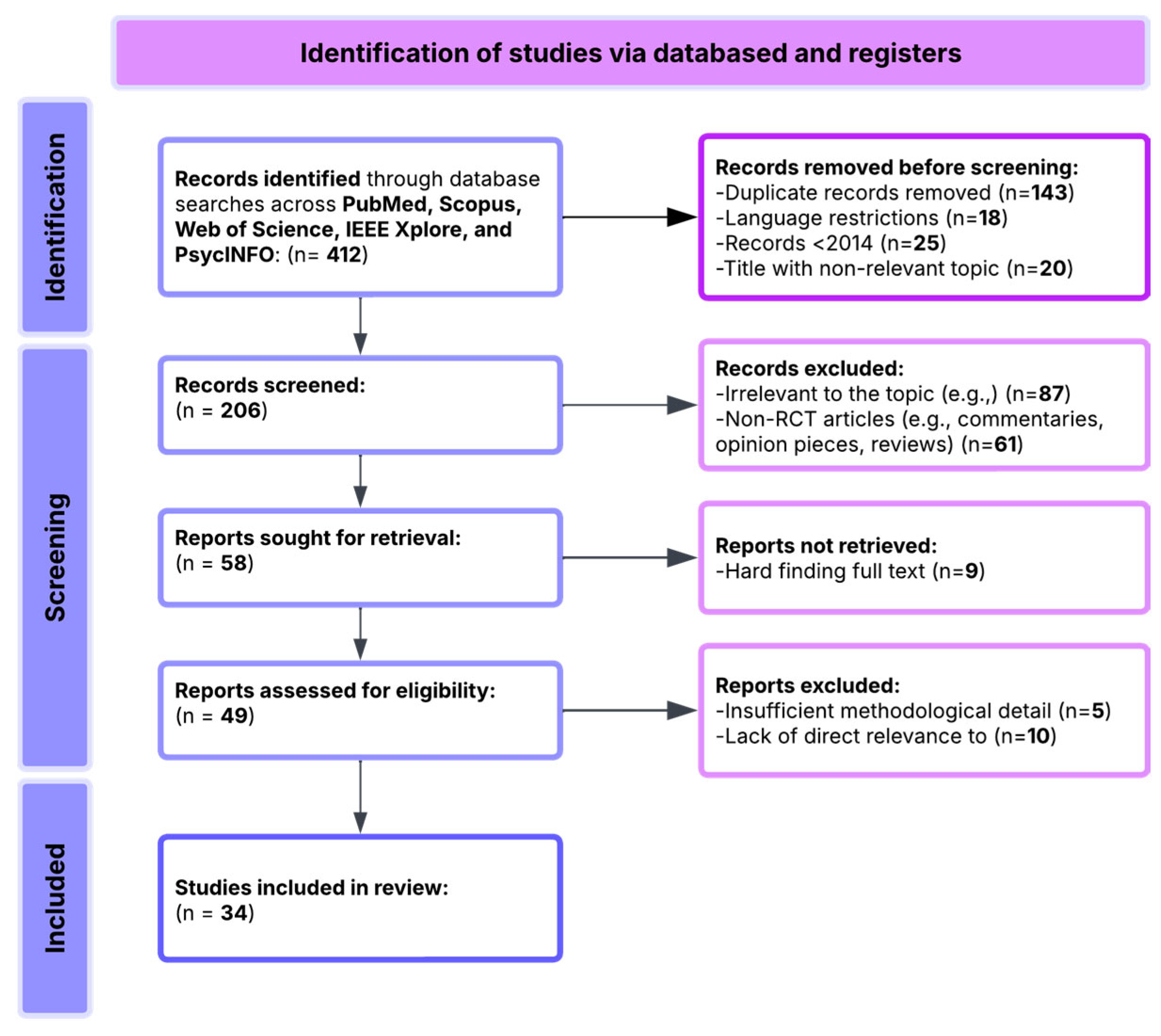
3. Materials and Methods
3.1. Search Sources and Databases
- 143 duplicate records;
- 18 non-English-language publications;
- 25 studies published before 2014;
- 20 records with clearly irrelevant titles.
- 87 articles unrelated to MCI or technological interventions for cognitive rehabilitation;
- 61 articles excluded as reviews, editorials, protocols, or theoretical papers lacking empirical data;
- 9 articles excluded due to difficulty accessing the full text.
- 25 studies were excluded due to insufficient methodological detail;
- 19 studies were excluded for lacking alignment with the primary research questions focusing on MCI.
3.2. Search Strategy
3.3. Inclusion and Exclusion Criteria
3.4. Risk of Bias Assessment
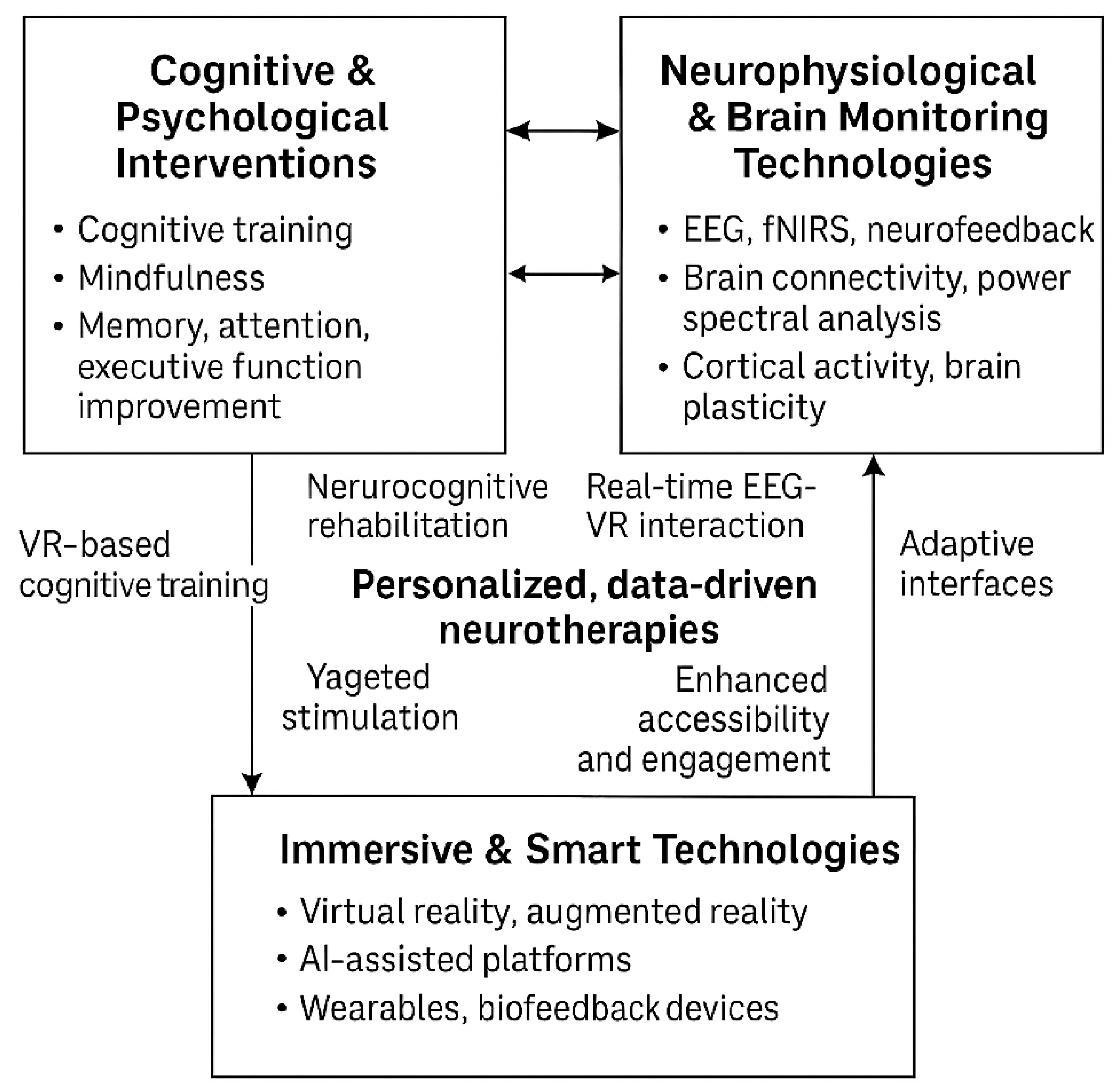
4. Results
- The Cognitive & Psychological Interventions domain encompasses traditional and digital approaches such as cognitive training, mindfulness-based therapies, and strategies targeting improvements in memory, attention, and executive function.
- The Neurophysiological and Brain Monitoring Technologies domain utilizes EEG, fNIRS, and neurofeedback techniques to monitor and modulate brain activity, focusing on connectivity and cortical dynamics.
- The Immersive and smart Technologies domain includes implementing VR/AR environments, wearable devices, and AI-powered platforms that enhance interactivity and enable real-time biofeedback.
4.1. [RQ1] How Effective Are Neuromodulation Techniques (tDCS, TMS) in Enhancing Cognitive Function and Improving Outcomes in Individuals with MCI?
4.2. [RQ2] What Neurophysiological Markers Identified Through EEG Analysis Characterize MCI and Predict Progression of Cognitive Decline?
4.3. [RQ3] How Can Virtual Reality Technologies Enhance Assessment, Cognitive Training, and Rehabilitation Outcomes in Individuals with MCI?
4.4. [RQ4] What Cognitive Training Interventions Show Efficacy for Improving Cognitive Performance and Functional Outcomes in MCI Populations?
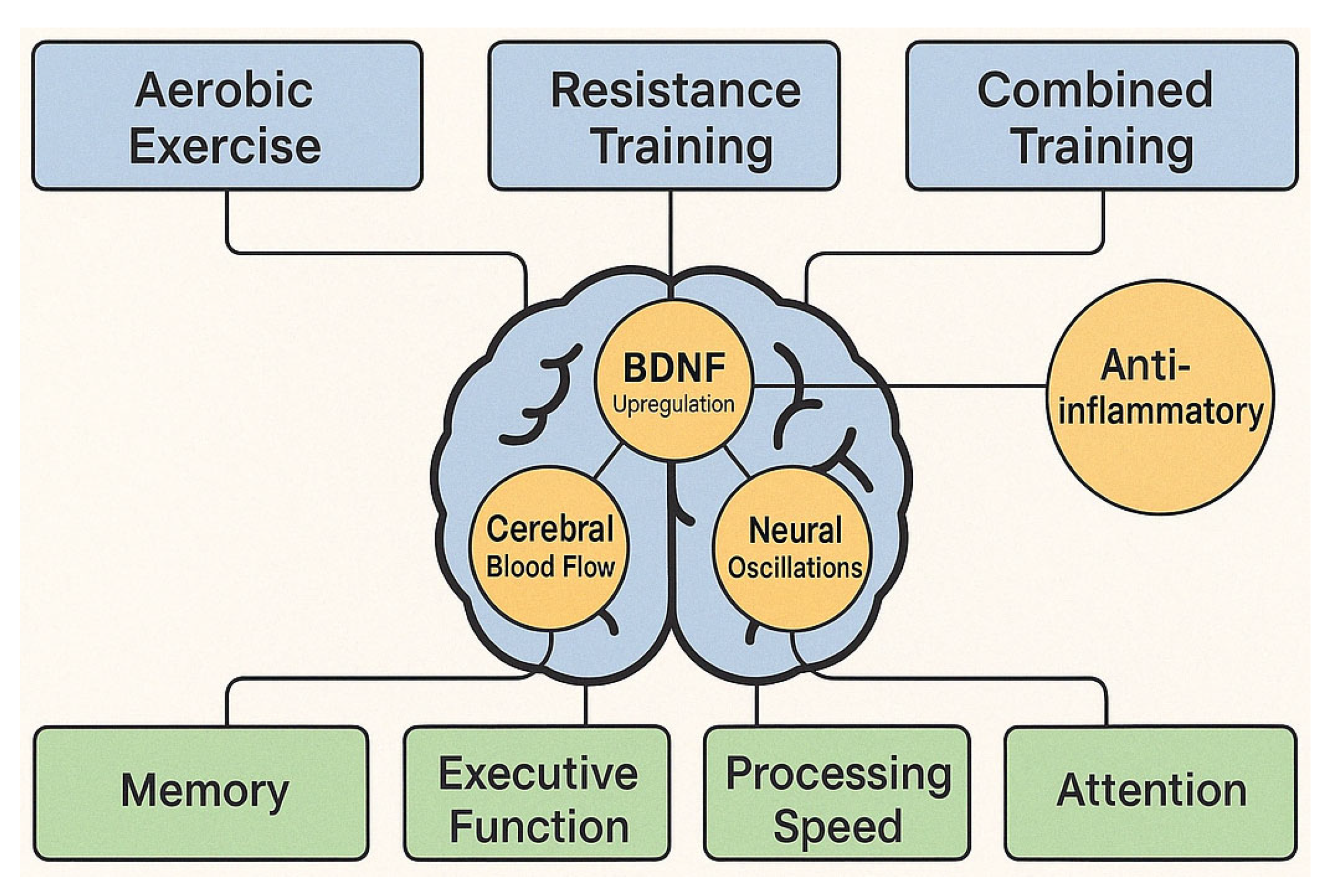
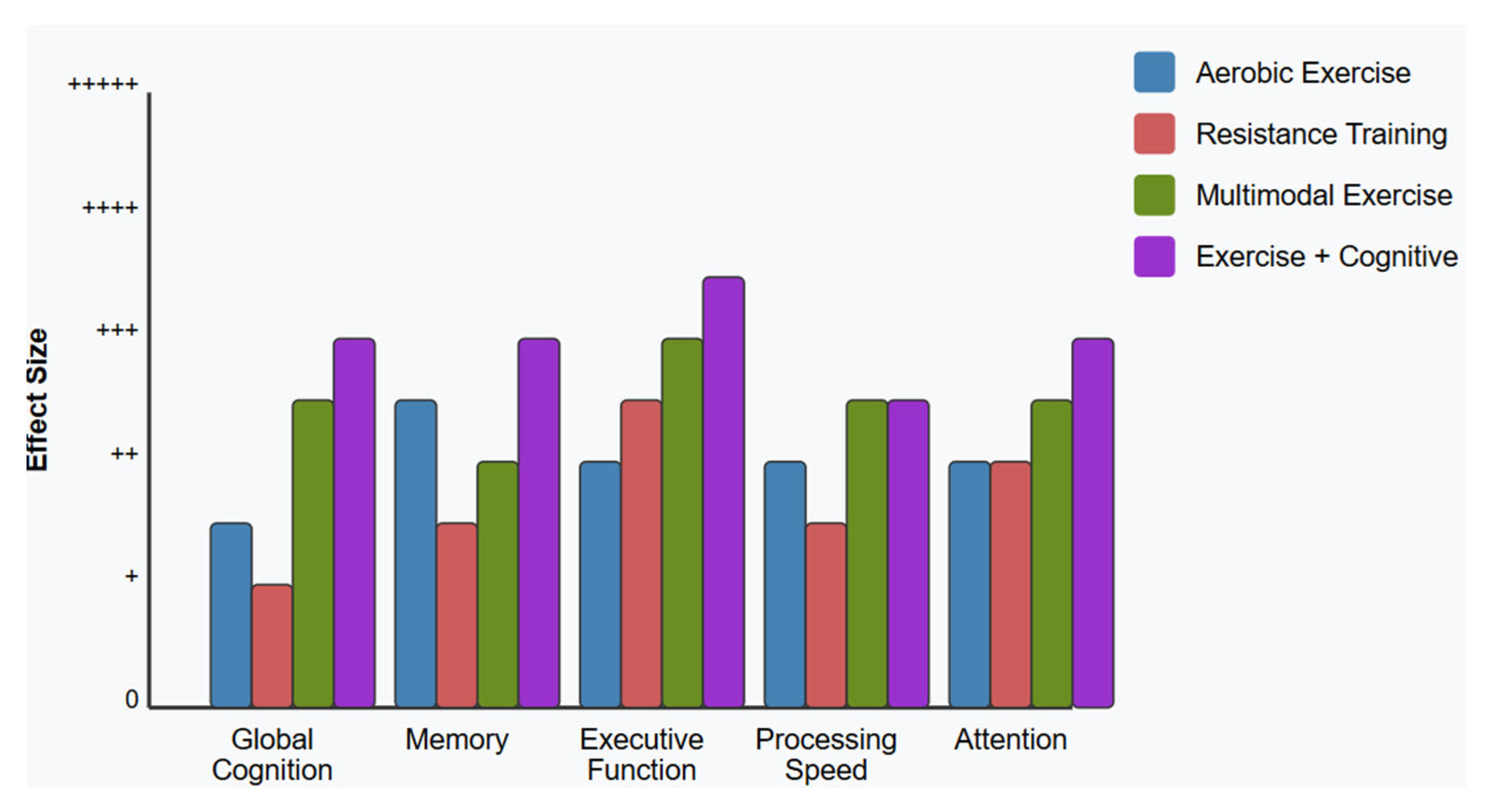
4.5. [RQ5] How Does Physical Exercise, Alone or Combined with Cognitive Intervention, Affect Cognitive Function and Brain Health in People with MCI?
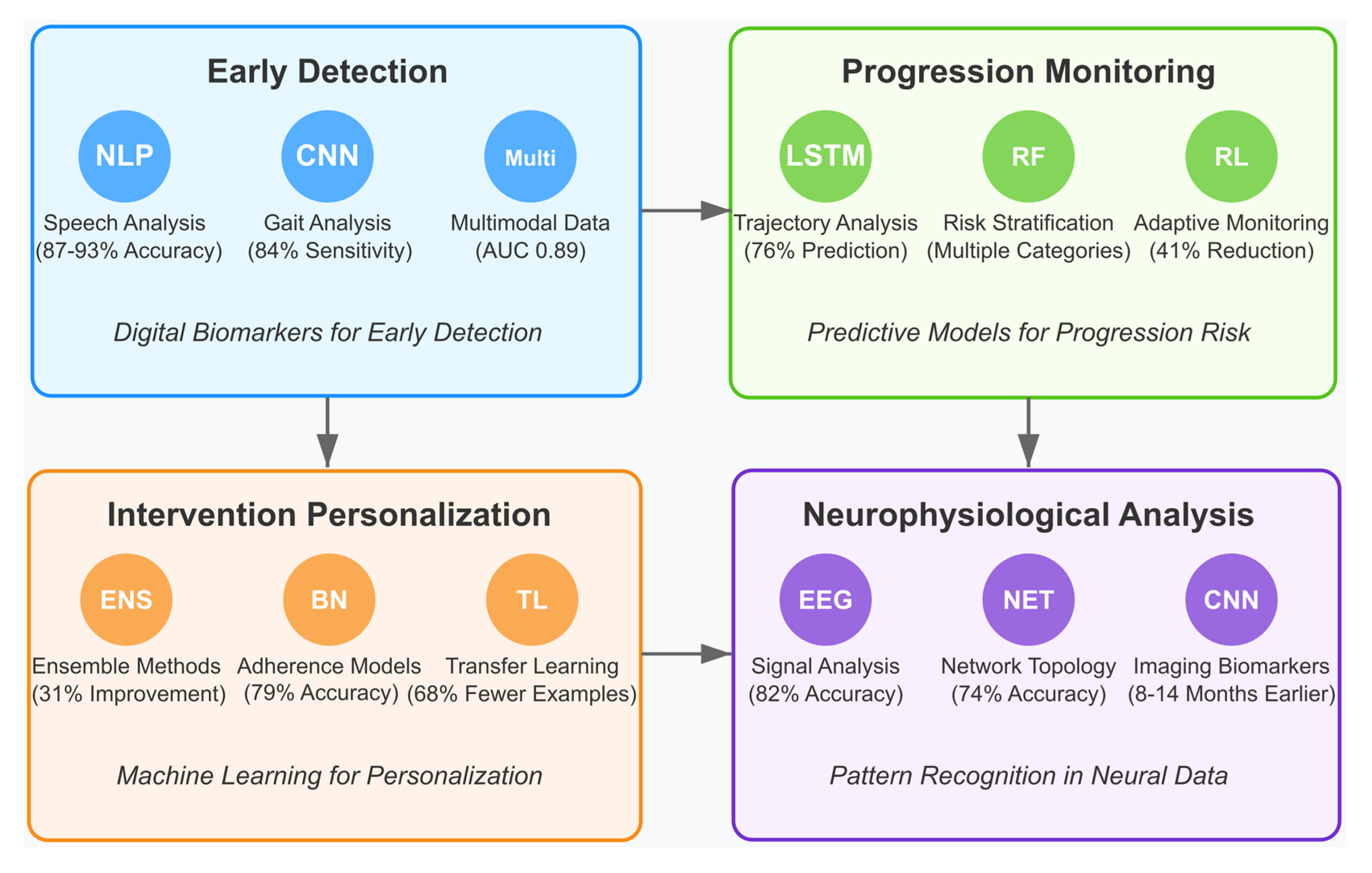
4.6. [RQ6] How Are Artificial Intelligence and Machine Learning Beginning to Transform MCI Detection, Progression Monitoring, and Intervention Personalization?
4.6.1. AI-Enabled Early Detection and Digital Biomarkers
4.6.2. AI for Monitoring Disease Progression
4.6.3. Personalization of Interventions Through Machine Learning
4.6.4. Neurophysiological Data Analysis and Pattern Recognition
4.6.5. Large Language Models for MCI Intervention
5. Discussion
5.1. Neuromodulation Efficacy and Mechanisms in MCI [RQ1]
5.2. EEG as a Neurophysiological Assessment and Monitoring Tool [RQ2]
5.3. Virtual Reality Applications for MCI [RQ3]
5.4. Cognitive Training Approaches and Response Patterns [RQ4]
5.5. Physical Exercise and Multimodal Interventions [RQ5]
5.6. Emerging AI Applications in MCI Management [RQ6]
5.7. Limitations and Ethical Considerations
5.7.1. Methodological and Representation Limitations
5.7.2. Data Privacy and Algorithmic Bias
5.7.3. Cultural Considerations and Technology Design
5.7.4. Access and Implementation Barriers
5.8. Future Research Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| AI | Artificial Intelligence |
| ANN | Artificial Neural Network |
| AR | Augmented Reality |
| BAN | Body Area Network |
| BCI | Brain–Computer Interface |
| CNN | Convolutional Neural Network |
| DLPFC | Dorsolateral Prefrontal Cortex |
| DL | Deep Learning |
| DNN | Deep Neural Network |
| DMN | Default Mode Network |
| ECG | Electrocardiography |
| EEG | Electroencephalography |
| EOG | Electrooculography |
| EMG | Electromyography |
| ERP | Event-Related Potential |
| ERSP | Event-Related Spectral Perturbation |
| ESCARF | Enhanced Simultaneous Cognitive-Physical Dual-Task Training Based on Fairy Tales |
| FCcANN | Fully Connected Cascade Artificial Neural Network |
| fMRI | Functional Magnetic Resonance Imaging |
| fNIRS | Functional Near-Infrared Spectroscopy |
| HGS | Hand Grip Strength |
| ICA | Independent Component Analysis |
| ITC | Inter-Trial Coherence |
| ITT | Intention-to-Treat |
| LLTM | Long Short-Term Memory |
| MCI | Mild Cognitive Impairment |
| ML | Machine Learning |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| MSC | Magnitude-Squared Coherence |
| NIBS | Non-Invasive Brain Stimulation |
| NF | Neurofeedback |
| NIRS | Near-Infrared Spectroscopy |
| NOS | Newcastle–Ottawa Scale |
| PCC | Posterior Cingulate Cortex |
| PCu | Precuneus |
| PLV | Phase Locking Value |
| PSG | Polysomnography |
| RBANS | Repeatable Battery for the Assessment of Neuropsychological Status |
| RoB | Risk of Bias |
| rs-fMRI | Resting-State Functional MRI |
| SDST | Symbol Digit Substitution Test |
| SCD | Subjective Cognitive Decline |
| SMR | Sensorimotor Rhythm |
| SoP | Speed of Processing |
| TES | Transcranial Electrical Stimulation |
| tACS | Transcranial Alternating Current Stimulation |
| TMT | Trail Making Test |
| tDCS | Transcranial Direct Current Stimulation |
| TMS | Transcranial Magnetic Stimulation |
| TR | Telerehabilitation |
| UFOV | Useful Field of View |
| VR | Virtual Reality |
| VRCT | Virtual Reality-Based Cognitive Training |
| VMR | Vasomotor Reactivity |
| WM | Working Memory |
References
- You, Y.; Liu, Z.; Chen, Y.; Xu, Y.; Qin, J.; Guo, S.; Huang, J.; Tao, J. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Acta Diabetol. 2021, 58, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Manly, J.J.; Jones, R.N.; Langa, K.M.; Ryan, L.H.; Levine, D.A.; McCammon, R.; Heeringa, S.G.; Weir, D. Estimating the prevalence of dementia and mild cognitive impairment in the US: The 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022, 79, 1242–1249. [Google Scholar] [CrossRef]
- Maruta, M.; Makizako, H.; Ikeda, Y.; Miyata, H.; Nakamura, A.; Han, G.; Shimokihara, S.; Tokuda, K.; Kubozono, T.; Ohishi, M.; et al. Association between apathy and satisfaction with meaningful activities in older adults with mild cognitive impairment: A population-based cross-sectional study. Int. J. Geriatr. Psychiatry 2021, 36, 1065–1074. [Google Scholar] [CrossRef]
- Chuang, Y.-F.; Liu, Y.-C.; Tseng, H.-Y.; Lin, P.-X.; Li, C.-Y.; Shih, M.-H.; Lin, K.-C.; Yang, T.O.; Yan, S.-H.; Chiu, Y.-L. Urban-rural differences in the prevalence and correlates of mild cognitive impairment in community-dwelling older adults in Taiwan: The EMCIT study. J. Formos. Med. Assoc. 2021, 120, 1749–1757. [Google Scholar] [CrossRef]
- Mattke, S.; Jun, H.; Chen, E.; Liu, Y.; Becker, A.; Wallick, C. Expected and diagnosed rates of mild cognitive impairment and dementia in the US. Medicare population: Observational analysis. Alzheimer’s Res. Ther. 2023, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.S.; Teixeira-Santos, A.C.; Leist, A.K. The prevalence of mild cognitive impairment in Latin America and the Caribbean: A systematic review and meta-analysis. Aging Ment. Health 2022, 26, 1710–1720. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Z.; Huang, F.; Su, C.; Du, W.; Jiang, H.; Wang, H.; Wang, J.; Wang, F.; Su, W.; et al. A comparison of the Mini-Mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: A cross-sectional study. BMC Psychiatry 2021, 21, 485. [Google Scholar] [CrossRef]
- Jung, A.-R.; Kim, D.; Park, E.-A. Cognitive intervention using information and communication technology for older adults with mild cognitive impairment: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 11535. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Somoza, L.M.; Irazoki, E.; Toribio-Guzmán, J.M.; de la Torre-Díez, I.; Diaz-Baquero, A.A.; Parra-Vidales, E.; Perea-Bartolomé, M.V.; Franco-Martín, M.Á. Usability and user experience of cognitive intervention technologies for elderly people with mci or dementia: A systematic review. Front. Psychol. 2021, 12, 636116. [Google Scholar] [CrossRef]
- Lydon, E.A.; Nguyen, L.T.; Nie, Q.; Rogers, W.A.; Mudar, R.A. An Integrative framework to guide social engagement interventions and technology design for persons with mild cognitive impairment. Front. Public Health 2022, 9, 750340. [Google Scholar] [CrossRef]
- Zuschnegg, J.; Schoberer, D.; Häussl, A.; Herzog, S.A.; Russegger, S.; Ploder, K.; Fellner, M.; Hofmarcher-Holzhacker, M.M.; Roller-Wirnsberger, R.; Paletta, L.; et al. Effectiveness of computer-based interventions for community-dwelling people with cognitive decline: A systematic review with meta-analyses. BMC Geriatr. 2023, 23, 229. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, H.; Yan, M.; Ding, Y.; Wang, Y.; Chen, L.; Yin, H. Virtual reality technology in the detection of mild cognitive impairment: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 87, 101889. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Next-Generation Cognitive-Behavioral Therapy for Depression: Integrating Digital Tools, Teletherapy, and Personalization for Enhanced Mental Health Outcomes. Medicina 2025, 61, 431. [Google Scholar] [CrossRef]
- MacRitchie, J.; Floridou, G.A.; Christensen, J.; Timmers, R.; de Witte, L. The use of technology for arts-based activities in older adults living with mild cognitive impairment or dementia: A scoping review. Dementia 2023, 22, 252–280. [Google Scholar] [CrossRef] [PubMed]
- Halkiopoulos, C.; Gkintoni, E. The Role of Machine Learning in AR/VR-Based Cognitive Therapies: A Systematic Review for Mental Health Disorders. Electronics 2025, 14, 1110. [Google Scholar] [CrossRef]
- Chen, R.; Huang, L.; Wang, R.; Fei, J.; Wang, H.; Wang, J. Advances in non-invasive neuromodulation techniques for improving cognitive function: A review. Brain Sci. 2024, 14, 354. [Google Scholar] [CrossRef]
- Palacino, F.; Manganotti, P.; Benussi, A. Targeting neural oscillations for cognitive enhancement in Alzheimer’s disease. Medicina 2025, 61, 547. [Google Scholar] [CrossRef]
- Shu, I.-W.; Lin, Y.; Granholm, E.L.; Singh, F. A focused review of gamma neuromodulation as a therapeutic target in Alzheimer’s spectrum disorders. J. Psychiatry Brain Sci. 2024, 9, e240001. [Google Scholar] [CrossRef]
- Lanni, I.; Chiacchierini, G.; Papagno, C.; Santangelo, V.; Campolongo, P. Treating Alzheimer’s disease with brain stimulation: From preclinical models to non-invasive stimulation in humans. Neurosci. Biobehav. Rev. 2024, 152, 105831. [Google Scholar] [CrossRef]
- Chan, J.J.; Cho, Y.; Lee, J.-H. Transcranial direct current stimulation for global cognition in mild cognitive impairment. Chonnam Med. J. 2025, 61, 1–8. [Google Scholar] [CrossRef]
- Moulaei, K.; Sharifi, H.; Bahaadinbeigy, K.; Dinari, F. Efficacy of virtual reality-based training programs and games on the improvement of cognitive disorders in patients: A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 55. [Google Scholar] [CrossRef] [PubMed]
- Tortora, C.; Di Crosta, A.; La Malva, P.; Prete, G.; Ceccato, I.; Mammarella, N.; Di Domenico, A.; Palumbo, R. Virtual reality and cognitive rehabilitation for older adults with mild cognitive impairment: A systematic review. Ageing Res. Rev. 2024, 93, 102146. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G.; Boutsinas, B. Digital and AI-Enhanced Cognitive Behavioral Therapy for Insomnia: Neurocognitive Mechanisms and Clinical Outcomes. J. Clin. Med. 2024, 14, 2265. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, D.E.; Rengifo, C.F.; Guzmán, J.D.; Cena, C.E.G. Virtual reality games for cognitive rehabilitation of older adults: A review of adaptive games, domains and techniques. Virtual Real. 2025, 28, 92. [Google Scholar] [CrossRef]
- Yi, Q.; Liu, Z.; Zhong, F.; Selvanayagam, V.S.; Cheong, J.P.G. Cognitive and physical impact of combined exercise and cognitive intervention in older adults with mild cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0308466. [Google Scholar] [CrossRef]
- Reparaz-Escudero, I.; Izquierdo, M.; Bischoff-Ferrari, H.A.; Martínez-Lage, P.; de Asteasu, M.L.S. Effect of long-term physical exercise and multidomain interventions on cognitive function and the risk of mild cognitive impairment and dementia in older adults: A systematic review with meta-analysis. Ageing Res. Rev. 2024, 93, 102463. [Google Scholar] [CrossRef]
- Cardona, M.I.; Afi, A.; Lakicevic, N.; Thyrian, J.R. Physical activity interventions and their effects on cognitive function in people with dementia: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 8753. [Google Scholar] [CrossRef]
- Kale, M.; Wankhede, N.; Pawar, R.; Ballal, S.; Kumawat, R.; Goswami, M.; Khalid, M.; Taksande, B.; Upaganlawar, A.; Umekar, M.; et al. AI-driven innovations in Alzheimer’s disease: Integrating early diagnosis, personalized treatment, and prognostic modelling. Ageing Res. Rev. 2024, 101, 102497. [Google Scholar] [CrossRef]
- Chudzik, A.; Śledzianowski, A.; Przybyszewski, A.W. Machine learning and digital biomarkers can detect early stages of neurodegenerative diseases. Sensors 2024, 24, 1572. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; Milne, R.; Curlewis, K. Ethical issues when using digital biomarkers and artificial intelligence for the early detection of dementia. WIREs Data Min. Knowl. Discov. 2023, 13, e1492. [Google Scholar] [CrossRef]
- Bigoni, C.; Zandvliet, S.B.; Beanato, E.; Crema, A.; Coscia, M.; Espinosa, A.; Henneken, T.; Hervé, J.; Oflar, M.; Evangelista, G.G.; et al. A novel patient-tailored, cumulative neurotechnology-based therapy for upper-limb rehabilitation in severely impaired chronic stroke patients: The AVANCER study protocol. Front. Neurol. 2022, 13, 919511. [Google Scholar] [CrossRef] [PubMed]
- Halkiopoulos, C.; Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H. Advances in Neuroimaging and Deep Learning for Emotion Detection: A Systematic Review of Cognitive Neuroscience and Algorithmic Innovations. Diagnostics 2025, 15, 456. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Skokou, M.; Gourzis, P. Integrating Clinical Neuropsychology and Psychotic Spectrum Disorders: A Systematic Analysis of Cognitive Dynamics, Interventions, and Underlying Mechanisms. Medicina 2024, 60, 645. [Google Scholar] [CrossRef]
- Corbo, I.; Marselli, G.; Di Ciero, V.; Casagrande, M. The protective role of cognitive reserve in mild cognitive impairment: A systematic review. J. Clin. Med. 2023, 12, 1759. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, L.; Zhang, H.; Lv, X.; Tu, L.; Zhang, M.; Zhang, Y.; Yan, C.; Yu, X.; Wang, H. Apathy as a risky neuropsychiatric syndrome of progression from normal aging to mild cognitive impairment and dementia: A systematic review and meta-analysis. Front. Psychiatry 2021, 12, 792168. [Google Scholar] [CrossRef]
- Jiao, B.; Li, R.; Zhou, H.; Qing, K.; Liu, H.; Pan, H.; Lei, Y.; Fu, W.; Wang, X.; Xiao, X.; et al. Neural biomarker diagnosis and prediction to mild cognitive impairment and Alzheimer’s disease using EEG technology. Alzheimer’s Res. Ther. 2023, 15, 32. [Google Scholar] [CrossRef]
- Trinh, T.-T.; Tsai, C.-F.; Hsiao, Y.-T.; Lee, C.-Y.; Wu, C.-T.; Liu, Y.-H. Identifying individuals with mild cognitive impairment using working memory-induced intra-subject variability of resting-state EEGS. Front. Comput. Neurosci. 2021, 15, 700467. [Google Scholar] [CrossRef] [PubMed]
- Meghdadi, A.H.; Salat, D.; Hamilton, J.; Hong, Y.; Boeve, B.F.; Louis, E.K.S.; Verma, A.; Berka, C. EEG and ERP biosignatures of mild cognitive impairment for longitudinal monitoring of early cognitive decline in Alzheimer’s disease. PLoS ONE 2024, 19, e0308137. [Google Scholar] [CrossRef]
- Del Percio, C.; Lopez, S.; Noce, G.; Lizio, R.; Tucci, F.; Soricelli, A.; Ferri, R.; Nobili, F.; Arnaldi, D.; Famà, F.; et al. What a single electroencephalographic (EEG) channel can tell us about Alzheimer’s disease patients with mild cognitive impairment. Clin. EEG Neurosci. 2023, 54, 21–35. [Google Scholar] [CrossRef]
- Keller, S.M.; Reyneke, C.; Gschwandtner, U.; Fuhr, P. Information contained in EEG allows characterization of cognitive decline in neurodegenerative disorders. Clin. EEG Neurosci. 2023, 54, 391–398. [Google Scholar] [CrossRef]
- Cavedoni, S.; Chirico, A.; Pedroli, E.; Cipresso, P.; Riva, G. Digital Biomarkers for the Early Detection of Mild Cognitive Impairment: Artificial Intelligence Meets Virtual Reality. Front. Hum. Neurosci. 2020, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Penalba-Sánchez, L.; Oliveira-Silva, P.; Sumich, A.L.; Cifre, I. Increased functional connectivity patterns in mild Alzheimer’s disease: A rsfMRI study. Front. Aging Neurosci. 2023, 14, 1037347. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, H.; Song, Y.; Chen, S.; Ge, H.; Yan, Z.; Yuan, Q.; Liang, X.; Lin, X.; Chen, J. Functional MRI-specific alterations in frontoparietal network in mild cognitive impairment: An ALE meta-analysis. Front. Aging Neurosci. 2023, 15, 1165908. [Google Scholar] [CrossRef]
- Yue, J.; Han, S.-W.; Wang, S.; Zhao, W.-W.; Cai, L.-N.; Cao, D.-N.; Mah, J.Z.; Hou, Y.; Cui, X.; Wang, Y.; et al. Functional brain activity in patients with amnestic mild cognitive impairment: An rs-fMRI study. Front. Neurol. 2023, 14, 1244696. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X.; Liu, N.; Sun, H.; Gong, Q.; Yao, L.; Lui, S. Convergent and distinct neural structural and functional patterns of mild cognitive impairment: A multimodal meta-analysis. Cereb. Cortex 2023, 33, 8876–8889. [Google Scholar] [CrossRef]
- Zhu, Z.; Zeng, Q.; Kong, L.; Luo, X.; Li, K.; Xu, X.; Zhang, M.; Huang, P.; Yang, Y. Altered spontaneous brain activity in subjects with different cognitive states of biologically defined Alzheimer’s Disease: A surface-based functional brain imaging study. Front. Aging Neurosci. 2021, 13, 683783. [Google Scholar] [CrossRef]
- Yuan, Q.; Qi, W.; Xue, C.; Ge, H.; Hu, G.; Chen, S.; Xu, W.; Song, Y.; Zhang, X.; Xiao, C.; et al. Convergent functional changes of default mode network in mild cognitive impairment using activation likelihood estimation. Front. Aging Neurosci. 2021, 13, 708687. [Google Scholar] [CrossRef]
- Hu, A.-M.; Ma, Y.-L.; Li, Y.-X.; Han, Z.-Z.; Yan, N.; Zhang, Y.-M. Association between changes in white matter microstructure and cognitive impairment in white matter lesions. Brain Sci. 2022, 12, 482. [Google Scholar] [CrossRef]
- Bergamino, M.; Schiavi, S.; Daducci, A.; Walsh, R.R.; Stokes, A.M. Analysis of brain structural connectivity networks and white matter integrity in patients with mild cognitive impairment. Front. Aging Neurosci. 2022, 14, 793991. [Google Scholar] [CrossRef]
- Kim, T.; Aizenstein, H.J.; Snitz, B.E.; Cheng, Y.; Chang, Y.-F.; Roush, R.E.; Huppert, T.J.; Cohen, A.; Doman, J.; Becker, J.T. Tract specific white matter lesion load affects white matter microstructure and their relationships with functional connectivity and cognitive decline. Front. Aging Neurosci. 2022, 13, 760663. [Google Scholar] [CrossRef]
- Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H.; Halkiopoulos, C. From Neural Networks to Emotional Networks: A Systematic Review of EEG-Based Emotion Recognition in Cognitive Neuroscience and Real-World Applications. Brain Sci. 2025, 15, 220. [Google Scholar] [CrossRef]
- Zdanovskis, N.; Platkājis, A.; Kostiks, A.; Karelis, G.; Grigorjeva, O. Brain structural connectivity differences in patients with normal cognition and cognitive impairment. Brain Sci. 2021, 11, 943. [Google Scholar] [CrossRef]
- Huang, C.; Kritikos, M.; Clouston, S.A.; Deri, Y.; Serrano-Sosa, M.; Bangiyev, L.; Santiago-Michels, S.; Gandy, S.; Sano, M.; Bromet, E.J.; et al. White matter connectivity in incident mild cognitive impairment: A Diffusion Spectrum Imaging Study of World Trade Center Responders at midlife. J. Alzheimer’s Dis. 2021, 80, 1209–1219. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Song, Z.; Fan, Y.; Gao, T.; Tang, X. Abnormal white matter changes in Alzheimer’s disease based on diffusion tensor imaging: A systematic review. Ageing Res. Rev. 2023, 87, 101911. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Murphy, K.; Andrews, G. A game a day keeps cognitive decline away? A systematic review and meta-analysis of commercially-available brain training programs in healthy and cognitively impaired older adults. Neuropsychol. Rev. 2022, 32, 601–630. [Google Scholar] [CrossRef]
- Guglietti, B.; Hobbs, D.; Collins-Praino, L.E. Optimizing cognitive training for the treatment of cognitive dysfunction in Parkinson’s disease: Current limitations and future directions. Front. Aging Neurosci. 2021, 13, 709484. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Plakias, S.; Vlotinou, P.; Athanasouli, P.; Terzoudi, A.; Kyriazidou, S.; Serdari, A.; Karakitsiou, G.; Megari, K.; Aggelousis, N.; et al. Ιnnovative Health Promotion Strategies: A 6-Month Longitudinal Study on Computerized Cognitive Training for Older Adults with Minor Neurocognitive Disorders. Eur. J. Investig. Health Psychol. Educ. 2025, 15, 34. [Google Scholar] [CrossRef]
- Ben Ayed, I.; Aouichaoui, C.; Ammar, A.; Naija, S.; Tabka, O.; Jahrami, H.; Trabelsi, K.; Trabelsi, Y.; El Massioui, N.; El Massioui, F. Mid-term and long-lasting psycho–cognitive benefits of bidomain training intervention in elderly individuals with mild cognitive impairment. Eur. J. Investig. Health Psychol. Educ. 2024, 14, 284–298. [Google Scholar] [CrossRef]
- Reynolds, G.O.; Willment, K.; Gale, S.A. Mindfulness and cognitive training interventions in mild cognitive impairment: Impact on cognition and mood. Am. J. Med. 2021, 134, 444–455. [Google Scholar] [CrossRef]
- Tse, Z.C.K.; Cao, Y.; Ogilvie, J.M.; Chau, B.K.H.; Ng, D.H.C.; Shum, D.H.K. Correction to: Prospective memory training in older adults: A systematic review and meta-analysis. Neuropsychol. Rev. 2023, 33, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Potvin, M.-J.; Labelle, V.; Chasles, M.-J.; Kergoat, M.-J.; Villalpando, J.M.; Joubert, S.; Rouleau, I. Effectiveness of a visual imagery training program to improve prospective memory in older adults with and without mild cognitive impairment: A randomized controlled study. Neuropsychol. Rehabil. 2022, 32, 1576–1604. [Google Scholar] [CrossRef] [PubMed]
- Corvalan, N.; Crivelli, L.; Allegri, R.F.; Pedreira, M.E.; Fernández, R.S. The impact of reward and punishment sensitivity on memory and executive performance in individuals with Amnestic Mild Cognitive Impairment. Behav. Brain Res. 2024, 471, 115099. [Google Scholar] [CrossRef] [PubMed]
- Baquero, A.A.D.; Dröes, R.-M.; Bartolomé, M.V.P.; Irazoki, E.; Toribio-Guzmán, J.M.; Franco-Martín, M.A.; van der Roest, H. Methodological designs applied in the development of computer-based training programs for the cognitive rehabilitation in people with mild cognitive impairment (MCI) and mild dementia. systematic review. J. Clin. Med. 2021, 10, 1222. [Google Scholar] [CrossRef]
- Nousia, A.; Martzoukou, M.; Siokas, V.; Aretouli, E.; Aloizou, A.-M.; Folia, V.; Peristeri, E.; Messinis, L.; Nasios, G.; Dardiotis, E. Beneficial effect of computer-based multidomain cognitive training in patients with mild cognitive impairment. Appl. Neuropsychol. Adult 2021, 28, 717–726. [Google Scholar] [CrossRef]
- Chae, H.J.; Lee, S.H. Effectiveness of online-based cognitive intervention in community-dwelling older adults with cognitive dysfunction: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2023, 38, 5853. [Google Scholar] [CrossRef]
- Chan, J.Y.C.; Liu, J.; Chan, A.T.C.; Tsoi, K.K.F. Computerized cognitive training for memory functions in mild cognitive impairment or dementia: A systematic review and meta-analysis. Medicine 2024, 7, 1. [Google Scholar] [CrossRef]
- Boller, B.; Ouellet, É.; Belleville, S. Using virtual reality to assess and promote transfer of memory training in older adults with memory complaints: A randomized controlled trial. Front. Psychol. 2021, 12, 627242. [Google Scholar] [CrossRef]
- Varela-Aldás, J.; Buele, J.; Amariglio, R.; García-Magariño, I.; Palacios-Navarro, G. The cupboard task: An immersive virtual reality-based system for everyday memory assessment. Int. J. Hum. Comput. Stud. 2022, 167, 102885. [Google Scholar] [CrossRef]
- Szczepocka, E.; Mokros, Ł.; Kaźmierski, J.; Nowakowska, K.; Łucka, A.; Antoszczyk, A.; Oltra-Cucarella, J.; Werzowa, W.; Hellevik, M.; Skouras, S.; et al. Virtual reality-based training may improve visual memory and some aspects of sustained attention among healthy older adults—Preliminary results of a randomized controlled study. BMC Psychiatry 2024, 24, 347. [Google Scholar] [CrossRef]
- Moll, B.; Sykes, E. Optimized virtual reality-based Method of Loci memorization techniques through increased immersion and effective memory palace designs: A feasibility study. Virtual Real. 2023, 27, 941–966. [Google Scholar] [CrossRef]
- Gulin, W.; Oziemblewska, M.; Zajac-Lamparska, L. Use of Virtual Reality to Improve Spatial Orientation in Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review. Curr. Alzheimer Res. 2025, 21, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Tuena, C.; Mancuso, V.; Stramba-Badiale, C.; Pedroli, E.; Stramba-Badiale, M.; Riva, G.; Repetto, C. Egocentric and allocentric spatial memory in mild cognitive impairment with real-world and virtual navigation tasks: A systematic review. J. Alzheimer’s Dis. 2021, 79, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Tuena, C.; Serino, S.; Pedroli, E.; Stramba-Badiale, C.; Goulene, K.M.; Stramba-Badiale, M.; Riva, G. Embodied Spatial Navigation Training in Mild Cognitive Impairment: A Proof-of-Concept Trial. J. Alzheimer’s Dis. 2024, 100, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Catania, V.; Rundo, F.; Panerai, S.; Ferri, R. Virtual reality for the rehabilitation of acquired cognitive disorders: A narrative review. Bioengineering 2023, 11, 35. [Google Scholar] [CrossRef]
- Liu, Q.; Vaci, N.; Koychev, I.; Kormilitzin, A.; Li, Z.; Cipriani, A.; Nevado-Holgado, A. Personalised treatment for cognitive impairment in dementia: Development and validation of an artificial intelligence model. BMC Med. 2022, 20, 45. [Google Scholar] [CrossRef]
- Tuena, C.; Serino, S.; Goulene, K.M.; Pedroli, E.; Stramba-Badiale, M.; Riva, G. Bodily and Visual-Cognitive Navigation Aids to Enhance Spatial Recall in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2024, 99, 899–910. [Google Scholar] [CrossRef]
- Andrieu-Devilly, P.-A.; Gandit, M.; Schwab, D.; Quillion-Dupré, L.; Monfort, E. Unlocking Cognitive Potential: Exploring a Virtual Environment for Cognitive Training in Healthy Aging and Mild Cognitive Impairment. IRBM 2025, 46, 100885. [Google Scholar] [CrossRef]
- Yu, D.J.; Yu, A.P.; Bernal, J.D.K.; Fong, D.Y.; Chan, D.K.C.; Cheng, C.P.; Siu, P.M. Effects of exercise intensity and frequency on improving cognitive performance in middle-aged and older adults with mild cognitive impairment: A pilot randomized controlled trial on the minimum physical activity recommendation from WHO. Front. Physiol. 2022, 13, 1021428. [Google Scholar] [CrossRef]
- Ribarič, S. Physical exercise, a potential non-pharmacological intervention for attenuating neuroinflammation and Cognitive decline in Alzheimer’s disease patients. Int. J. Mol. Sci. 2022, 23, 3245. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, X.; Li, B.; Cai, Y.; Zhang, S.; Yu, F.; Wan, Q. Biomarkers for evaluating the effects of exercise interventions in patients with MCI or dementia: A systematic review and meta-analysis. Exp. Gerontol. 2021, 151, 111424. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Gao, S. Effects of mind-body exercises on cognitive impairment in people with Parkinson’s disease: A mini-review. Front. Neurol. 2022, 13, 931460. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.K.; Andel, R.; Small, B.J. Effects of yoga-related mind-body therapies on cognitive function in older adults: A systematic review with meta-analysis. Arch. Gerontol. Geriatr. 2021, 93, 104319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cao, Y.; Yan, Z. Mindfulness, mind-body exercises, and health promotion. Frontiers in Psychology. Front. Psychol. 2025, 16, 1559535. [Google Scholar] [CrossRef]
- Gilliam, E.A.; Cheung, T.; Kraemer, K.; Litrownik, D.; Wayne, P.M.; Moy, M.L.; Yeh, G.Y. The impact of Tai Chi and mind-body breathing in COPD: Insights from a qualitative sub-study of a randomized controlled trial. PLoS ONE 2021, 16, e0249263. [Google Scholar] [CrossRef]
- Fan, M.; Li, Q.; Yang, T.; Yang, Y.; Chen, Z.; Xuan, G.; Ruan, Y.; Sun, S.; Wang, M.; Chen, X.; et al. Effect of Multimodal Intervention in Individuals with Mild Cognitive Impairment: A Randomized Clinical Trial in Shanghai. J. Alzheimer’s Dis. 2024, 101, 235–248. [Google Scholar] [CrossRef]
- Adarsh, V.; Gangadharan, G.R.; Fiore, U.; Zanetti, P. Multimodal classification of Alzheimer’s disease and mild cognitive impairment using custom MKSCDDL kernel over CNN with transparent decision-making for explainable diagnosis. Sci. Rep. 2024, 14, 1774. [Google Scholar] [CrossRef] [PubMed]
- Grässler, B.; Herold, F.; Dordevic, M.; Gujar, T.A.; Darius, S.; Böckelmann, I.; Müller, N.G.; Hökelmann, A. Multimodal measurement approach to identify individuals with mild cognitive impairment: Study protocol for a cross-sectional trial. BMJ Open 2021, 11, e046879. [Google Scholar] [CrossRef]
- Fadzil, N.H.M.; Shahar, S.; Rajikan, R.; Singh, D.K.A.; Ludin, A.F.M.; Subramaniam, P.; Ibrahim, N.; Vanoh, D.; Ali, N.M. A Scoping review for usage of telerehabilitation among older adults with mild cognitive impairment or cognitive frailty. Int. J. Environ. Res. Public Health 2022, 19, 4000. [Google Scholar] [CrossRef]
- Formica, C.; Bonanno, M.; Sorbera, C.; Quartarone, A.; Giambò, F.M.; Marra, A.; Calabrò, R.S. Smartphone-Based Cognitive Telerehabilitation: A Usability and Feasibility Study Focusing on Mild Cognitive Impairment. Sensors 2024, 24, 525. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, D.Y.; Park, S.-W.; Lee, B.-S.; Han, H.-W.; Jeon, N.; Kim, M.; Kang, M.; Kim, S. A systematic review of cognitive telerehabilitation in patients with cognitive dysfunction. Front. Neurol. 2025, 15, 1450977. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Mindfulness-Based Cognitive Therapy in Clinical Practice: A Systematic Review of Neurocognitive Outcomes and Applications for Mental Health and Well-Being. J. Clin. Med. 2025, 14, 1703. [Google Scholar] [CrossRef]
- Lassi, M.; Fabbiani, C.; Mazzeo, S.; Burali, R.; Vergani, A.A.; Giacomucci, G.; Moschini, V.; Morinelli, C.; Emiliani, F.; Scarpino, M.; et al. Degradation of EEG microstates patterns in subjective cognitive decline and mild cognitive impairment: Early biomarkers along the Alzheimer’s Disease continuum? NeuroImage Clin. 2023, 38, 103407. [Google Scholar] [CrossRef]
- Tomasello, L.; Carlucci, L.; Laganà, A.; Galletta, S.; Marinelli, C.V.; Raffaele, M.; Zoccolotti, P. Neuropsychological evaluation and quantitative EEG in patients with frontotemporal dementia, Alzheimer’s disease, and mild cognitive impairment. Brain Sci. 2023, 13, 930. [Google Scholar] [CrossRef]
- Olichney, J.; Xia, J.; Church, K.J.; Moebius, H.J. Predictive power of cognitive biomarkers in neurodegenerative disease drug development: Utility of the P300 event-related potential. Neural Plast. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Mohamed, N.; Kim, J.G. P300 Latency with Memory Performance: A Promising Biomarker for Preclinical Stages of Alzheimer’s Disease. Biosensors 2024, 14, 616. [Google Scholar] [CrossRef]
- Roy, O.; Moshfeghi, Y.; Ibanez, A.; Lopera, F.; Parra, M.A.; Smith, K.M. FAST functional connectivity implicates P300 connectivity in working memory deficits in Alzheimer’s disease. Netw. Neurosci. 2024, 8, 1467–1490. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Cutrona, C.; Leodori, G.; Malimpensa, L.; D’antonio, F.; Conte, A.; Belvisi, D. Exploring easily accessible neurophysiological biomarkers for predicting Alzheimer’s disease progression: A systematic review. Alzheimer’s Res. Ther. 2024, 16, 244. [Google Scholar] [CrossRef]
- Maruya, K.; Arai, T.; Fujita, H. Brain Activity in the Prefrontal Cortex during Cognitive Tasks and Dual Tasks in Community-Dwelling Elderly People with Pre-Frailty: A Pilot Study for Early Detection of Cognitive Decline. Healthcare 2021, 9, 1250. [Google Scholar] [CrossRef]
- Perez, V.; Garrido-Chaves, R.; Zapater-Fajarí, M.; Pulopulos, M.M.; Barbosa, F.; Hidalgo, V.; Salvador, A. Deficits in facial emotional valence processing in older people with subjective memory complaints: Behavioral and electrophysiological evidence. Psychophysiology 2021, 59, e13989. [Google Scholar] [CrossRef]
- Youssef, N.; Xiao, S.; Liu, M.; Lian, H.; Li, R.; Chen, X.; Zhang, W.; Zheng, X.; Li, Y.; Li, Y. Functional brain networks in mild cognitive impairment based on resting electroencephalography signals. Front. Comput. Neurosci. 2021, 15, 698386. [Google Scholar] [CrossRef]
- Adebisi, A.T.; Veluvolu, K.C. Brain network analysis for the discrimination of dementia disorders using electrophysiology signals: A systematic review. Front. Aging Neurosci. 2023, 15, 1039496. [Google Scholar] [CrossRef] [PubMed]
- Paitel, E.R.; Otteman, C.B.D.; Polking, M.C.; Licht, H.J.; Nielson, K.A. Functional and effective EEG connectivity patterns in Alzheimer’s disease and mild cognitive impairment: A systematic review. Front. Aging Neurosci. 2025, 17, 1496235. [Google Scholar] [CrossRef]
- Sedghizadeh, M.J.; Aghajan, H.; Vahabi, Z.; Fatemi, S.N.; Afzal, A. Network synchronization deficits caused by dementia and Alzheimer’s disease serve as topographical biomarkers: A pilot study. Brain Struct. Funct. 2022, 227, 2957–2969. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.; Zhang, Y.; Liu, X.; Qing, K. EEG biomarkers analysis in different cognitive impairment after stroke: An exploration study. Front. Neurol. 2024, 15, 1358167. [Google Scholar] [CrossRef]
- Sanches, C.; Stengel, C.; Godard, J.; Mertz, J.; Teichmann, M.; Migliaccio, R.; Valero-Cabré, A. Past, Present, and future of non-invasive brain stimulation approaches to treat cognitive impairment in neurodegenerative diseases: Time for a comprehensive critical review. Front. Aging Neurosci. 2021, 12, 578339. [Google Scholar] [CrossRef] [PubMed]
- Menardi, A.; Rossi, S.; Koch, G.; Hampel, H.; Vergallo, A.; Nitsche, M.A.; Stern, Y.; Borroni, B.; Cappa, S.F.; Cotelli, M.; et al. Toward noninvasive brain stimulation 2.0 in Alzheimer’s disease. Ageing Res. Rev. 2022, 75, 101555. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Wang, J.-Y.; Qin, J.-Y.; Qin, J.-Y.; Ye, J.-Y.; Ye, J.-Y.; Li, W.-T.; Li, W.-T.; Tong, M.-Q.; Tong, M.-Q.; et al. The therapeutic effects of noninvasive brain stimulation combined with cognitive training in elders with Alzheimer’s disease or amnesic mild cognitive impairment. J. Prev. Alzheimer’s Dis. 2024, 11, 222–229. [Google Scholar] [CrossRef]
- Bréchet, L.; Yu, W.; Biagi, M.C.; Ruffini, G.; Gagnon, M.; Manor, B.; Pascual-Leone, A. Patient-tailored, home-based non-invasive Brain stimulation for memory deficits in dementia due to Alzheimer’s disease. Front. Neurol. 2021, 12, 598135. [Google Scholar] [CrossRef] [PubMed]
- Carrarini, C.; Pappalettera, C.; Le Pera, D.; Rossini, P.M. Non-invasive brain stimulation in cognitive sciences and Alzheimer’s disease. Front. Hum. Neurosci. 2025, 18, 1500502. [Google Scholar] [CrossRef]
- Aderinto, N.; Olatunji, G.; Muili, A.; Kokori, E.; Edun, M.; Akinmoju, O.; Yusuf, I.; Ojo, D. A narrative review of non-invasive brain stimulation techniques in neuropsychiatric disorders: Current applications and future directions. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 50. [Google Scholar] [CrossRef]
- Esposito, S.; Trojsi, F.; Cirillo, G.; de Stefano, M.; Di Nardo, F.; Siciliano, M.; Caiazzo, G.; Ippolito, D.; Ricciardi, D.; Buonanno, D.; et al. Repetitive transcranial magnetic stimulation (rTMS) of dorsolateral prefrontal cortex may influence semantic fluency and functional connectivity in fronto-parietal network in mild cognitive impairment (MCI). Biomedicines 2022, 10, 994. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, L.; Sun, X.; Wang, L.; Xu, Q.; Li, Q.; Huang, W.; Xiao, Y. Effect of high-frequency (5Hz) rTMS stimulating left DLPFC combined with galantamine on cognitive impairment after ischemic stroke and serum homocysteine and neuron-specific enolase. Front. Neurol. 2024, 15, 1345832. [Google Scholar] [CrossRef] [PubMed]
- Sharbafshaaer, M.; Gigi, I.; Lavorgna, L.; Esposito, S.; Bonavita, S.; Tedeschi, G.; Esposito, F.; Trojsi, F. Repetitive transcranial magnetic stimulation (rtms) in mild cognitive impairment: Effects on cognitive functions—A systematic review. J. Clin. Med. 2023, 12, 6190. [Google Scholar] [CrossRef]
- Liu, M.; Ren-Li, R.; Sun, J.; Yeo, J.S.Y.; Ma, J.; Yan, J.; Er, B.M.Y.L.M.X.K.; Tu, Z.; Li, Y. High-Frequency rTMS Improves Visual Working Memory in Patients With aMCI: A Cognitive Neural Mechanism Study. CNS Neurosci. Ther. 2025, 31, e70301. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, L.; Zhu, W.; Xiu, Y.; Liu, Y. Differential effects of high-frequency repetitive transcranial magnetic stimulation over the left and right dorsolateral prefrontal cortex for post-stroke cognitive impairment. Neurol. Sci. 2025. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Christie, G.J.; Cosco, T.D.; Arab, A.; Mansouri, M.; Wagner, K.R.; DiPaola, S.; Moreno, S. Neuroimaging and machine learning for studying the pathways from mild cognitive impairment to alzheimer’s disease: A systematic review. BMC Neurol. 2023, 23, 309. [Google Scholar] [CrossRef]
- Li, Y.; Shao, Y.; Wang, J.; Liu, Y.; Yang, Y.; Wang, Z.; Xi, Q. Machine learning based on functional and structural connectivity in mild cognitive impairment. Magn. Reson. Imaging 2024, 109, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, G.; Elahi, R.; Hasankhani, Z.; Rad, H.S.; Shalbaf, A. Deep Learning Approaches for Early Prediction of Conversion from MCI to AD using MRI and Clinical Data: A Systematic Review. Arch. Comput. Methods Eng. 2024, 32, 1229–1298. [Google Scholar] [CrossRef]
- Uysal, G.; Ozturk, M. Comparative analysis of different brain regions using machine learning for prediction of EMCI and LMCI stages of Alzheimer’s disease. Multimedia Tools Appl. 2024, 83, 21455–21470. [Google Scholar] [CrossRef]
- Formica, C.; Bonanno, L.; Giambò, F.M.; Maresca, G.; Latella, D.; Marra, A.; Cucinotta, F.; Bonanno, C.; Lombardo, M.; Tomarchio, O.; et al. Paving the Way for Predicting the Progression of Cognitive Decline: The Potential Role of Machine Learning Algorithms in the Clinical Management of Neurodegenerative Disorders. J. Pers. Med. 2023, 13, 1386. [Google Scholar] [CrossRef]
- Sajid, M.; Sharma, R.; Beheshti, I.; Tanveer, M. Alzheimer’s Disease Neuroimaging Initiative. Decoding cognitive health using machine learning: A comprehensive evaluation for diagnosis of significant memory concern. WIREs Data Min. Knowl. Discov. 2024, 14, e1546. [Google Scholar] [CrossRef]
- Noroozi, M.; Gholami, M.; Sadeghsalehi, H.; Behzadi, S.; Habibzadeh, A.; Erabi, G.; Sadatmadani, S.-F.; Diyanati, M.; Rezaee, A.; Dianati, M.; et al. Machine and deep learning algorithms for classifying different types of dementia: A literature review. Appl. Neuropsychol. Adult 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Karaman, B.K.; Mormino, E.C.; Sabuncu, M.R. Alzheimer’s Disease Neuroimaging Initiative. Machine learning based multi-modal prediction of future decline toward Alzheimer’s disease: An empirical study. PLoS ONE 2022, 17, e0277322. [Google Scholar] [CrossRef]
- Gkintoni, E.; Nikolaou, G. The Cross-Cultural Validation of Neuropsychological Assessments and Their Clinical Applications in Cognitive Behavioral Therapy: A Scoping Analysis. Int. J. Environ. Res. Public Health 2024, 21, 1110. [Google Scholar] [CrossRef]
- Castellano, G.; Esposito, A.; Lella, E.; Montanaro, G.; Vessio, G. Automated detection of Alzheimer’s disease: A multi-modal approach with 3D MRI and amyloid PET. Sci. Rep. 2024, 14, 5210. [Google Scholar] [CrossRef]
- Yuan, C.; Linn, K.A.; Hubbard, R.A. Algorithmic fairness of machine learning models for Alzheimer disease progression. JAMA Netw. Open 2023, 6, e2342203. [Google Scholar] [CrossRef] [PubMed]
- Sadegh-Zadeh, S.-A.; Nazari, M.-J.; Aljamaeen, M.; Yazdani, F.; Mousavi, S.; Vahabi, Z. Predictive models for Alzheimer’s disease diagnosis and MCI identification: The use of cognitive scores and artificial intelligence algorithms. NPG Neurol. Psychiatr. Gériatrie 2024, 24, 194–211. [Google Scholar] [CrossRef]
- Pang, Y.; Kukull, W.; Sano, M.; Albin, R.L.; Shen, C.; Zhou, J.; Dodge, H.H. Predicting progression from normal to MCI and from MCI to AD using clinical variables in the national Alzheimer’s coordinating center uniform data set version 3: Application of machine learning models and a probability calculator. J. Prev. Alzheimer’s Dis. 2023, 10, 301–313. [Google Scholar] [CrossRef]
- Grueso, S.; Viejo-Sobera, R. Machine learning methods for predicting progression from mild cognitive impairment to Alzheimer’s disease dementia: A systematic review. Alzheimer’s Res. Ther. 2021, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Richter-Laskowska, M.; Sobotnicka, E.; Bednorz, A. Cognitive performance classification of older patients using machine learning and electronic medical records. Sci. Rep. 2025, 15, 6564. [Google Scholar] [CrossRef]
- Panesar, K.; de Alba, M.B.P.C. Natural language processing-driven framework for the early detection of language and cognitive decline. Lang. Health 2023, 1, 20–35. [Google Scholar] [CrossRef]
- Gagliardi, G. Natural language processing techniques for studying language in pathological ageing: A scoping review. Int. J. Lang. Commun. Disord. 2024, 59, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaibani, M.; Fard, A.P.; Sun, J.; Poor, F.F.; Pressman, P.S.; Mahoor, M.H. A Review of Machine Learning Approaches for Non-Invasive Cognitive Impairment Detection. IEEE Access 2025, 13, 56355–56384. [Google Scholar] [CrossRef]
- Lima, M.R.; Capstick, A.; Geranmayeh, F.; Nilforooshan, R.; Matarić, M.; Vaidyanathan, R.; Barnaghi, P. Evaluating Spoken Language as a Biomarker for Automated Screening of Cognitive Impairment. arXiv 2025, arXiv:2501.18731. [Google Scholar]
- Subramanian, K.; Hajamohideen, F.; Viswan, V.; Shaffi, N.; Mahmud, M. Exploring intervention techniques for Alzheimer’s disease: Conventional methods and the role of AI in advancing care. Artif. Intell. Appl. 2024, 2, 59–77. [Google Scholar] [CrossRef]
- Halkiopoulos, C.; Gkintoni, E. Leveraging AI in e-learning: Personalized learning and adaptive assessment through cognitive neuropsychology—A systematic analysis. Electronics 2024, 13, 3762. [Google Scholar] [CrossRef]
- Khalid, U.B.; Naeem, M.; Stasolla, F.; Syed, M.H.; Abbas, M.; Coronato, A. Impact of AI-powered solutions in rehabilitation process: Recent improvements and future trends. Int. J. Gen. Med. 2024, 17, 943–969. [Google Scholar] [CrossRef]
- Hasan, W.U.; Zaman, K.T.; Shalan, M.; Wang, X.; Li, J.; Xie, B.; Tao, C. CareCompanion: A Personalized Virtual Assistant for Enhancing Support and Independence in ADRD Patients and Older Adults. In Proceedings of the 2024 International Conference on Smart Applications, Communications and Networking (SmartNets), Washington, DC, USA, 28–30 May 2024; pp. 1–10. [Google Scholar] [CrossRef]
- Qi, X.; Wu, B. ChatGPT: A Promising Tool to Combat Social Isolation and Loneliness in Older Adults with Mild Cognitive Impairment. Neurol. Live 2023, 6, 23–25. [Google Scholar]
- Azami, H.; Mirjalili, M.; Rajji, T.K.; Wu, C.-T.; Humeau-Heurtier, A.; Jung, T.-P.; Wei, C.-S.; Trinh, T.-T.; Liu, Y.-H. Electroencephalogram and Event-Related Potential in Mild Cognitive Impairment: Recent Developments in Signal Processing, Machine Learning, and Deep Learning. IEEE J. Sel. Areas Sensors 2025, 2, 162–184. [Google Scholar] [CrossRef]
- Narasimhan, R.; Gopalan, M.; Sikkandar, M.Y.; Alassaf, A.; AlMohimeed, I.; Alhussaini, K.; Aleid, A.; Sheik, S.B. Employing deep-learning approach for the early detection of mild cognitive impairment transitions through the analysis of digital biomarkers. Sensors 2023, 23, 8867. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Akker, O.R.v.D.; Peters, G.-J.Y.; Bakker, C.J.; Carlsson, R.; Coles, N.A.; Corker, K.S.; Feldman, G.; Moreau, D.; Nordström, T.; Pickering, J.S.; et al. Increasing the transparency of systematic reviews: Presenting a generalized registration form. Syst. Rev. 2023, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Amjad, I.; Toor, H.; Niazi, I.K.; Pervaiz, S.; Jochumsen, M.; Shafique, M.; Haavik, H.; Ahmed, T. Xbox 360 Kinect Cognitive Games Improve Slowness, Complexity of EEG, and Cognitive Functions in Subjects with Mild Cognitive Impairment: A Randomized Control Trial. Games Health J. 2019, 8, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Amjad, I.; Toor, H.; Niazi, I.K.; Afzal, H.; Jochumsen, M.; Shafique, M.; Allen, K.; Haavik, H.; Ahmed, T. Therapeutic effects of aerobic exercise on EEG parameters and higher cognitive functions in mild cognitive impairment patients. Int. J. Neurosci. 2019, 129, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Vecchio, F.; Altavilla, R.; Tibuzzi, F.; Lizio, R.; Altamura, C.; Palazzo, P.; Maggio, P.; Ursini, F.; Ercolani, M.; et al. Hypercapnia affects the functional coupling of resting state electroencephalographic rhythms and cerebral haemodynamics in healthy elderly subjects and in patients with amnestic mild cognitive impairment. Clin. Neurophysiol. 2014, 125, 685–693. [Google Scholar] [CrossRef]
- Bae, J.-H.; Choi, M.; Lee, J.J.; Lee, K.H.; Kim, J.U. Connectivity changes in two-channel prefrontal ERP associated with early cognitive decline in the elderly population: Beta band responses to the auditory oddball stimuli. Front. Aging Neurosci. 2024, 16, 1456169. [Google Scholar] [CrossRef]
- Cai, Z.-Z.; Lin, R.; Wang, X.-X.; Yan, Y.-J.; Li, H. Effects of mindfulness in patients with mild cognitive impairment with insomnia: A double-blind randomized controlled trial. Geriatr. Nurs. 2022, 47, 239–246. [Google Scholar] [CrossRef]
- Caminiti, S.P.; Bernini, S.; Bottiroli, S.; Mitolo, M.; Manca, R.; Grillo, V.; Avenali, M.; De Icco, R.; Capellari, S.; Carlesimo, G.A.; et al. Exploring the neural and behavioral correlates of cognitive telerehabilitation in mild cognitive impairment with three distinct approaches. Front. Aging Neurosci. 2024, 16, 1425784. [Google Scholar] [CrossRef]
- Emonson, M.; Fitzgerald, P.; Rogasch, N.; Hoy, K. Neurobiological effects of transcranial direct current stimulation in younger adults, older adults and mild cognitive impairment. Neuropsychologia 2019, 125, 51–61. [Google Scholar] [CrossRef]
- Han, S.-H.; Youn, Y.C. Quantitative Electroencephalography Changes in Patients with Mild Cognitive Impairment after Choline Alphoscerate Administration. J. Clin. Neurosci. 2022, 102, 42–48. [Google Scholar] [CrossRef]
- Hathaway, E.; Morgan, K.; Carson, M.; Shusterman, R.; Fernandez-Corazza, M.; Luu, P.; Tucker, D.M. Transcranial Electrical Stimulation targeting limbic cortex increases the duration of human deep sleep. Sleep Med. 2021, 81, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-G.; Kim, J.-H.; Jun, T.-W. Effects of 12-Week Resistance Exercise on Electroencephalogram Patterns and Cognitive Function in the Elderly with Mild Cognitive Impairment: A Randomized Controlled Trial. Clin. J. Sport Med. 2018, 28, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, S.; Wang, L.; Liu, X. An Investigation of Limbs Exercise as a Treatment in Improving the Psychomotor Speed in Older Adults with Mild Cognitive Impairment. Brain Sci. 2019, 9, 277. [Google Scholar] [CrossRef]
- Jung, E.-S.; Choi, Y.-Y.; Lee, K.-H. Effects of Integrative Cognitive Function Improvement Program on Cognitive Function, Oral Health, and Mental Health in Older People: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 14339. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Kim, H.; Roh, D.; Kim, D.H. Effects of Phytoncide Fragrance on Resting-State Brain Activity in Mild Cognitive Impairment: A Randomized Double-Blind Controlled Study. J. Integr. Complement. Med. 2024, 30, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.; Yang, H. Effectiveness of an enhanced simultaneous cognitive-physical dual-task training based on fairy tales (ESCARF) in older adults with mild cognitive impairment. Geriatr. Nurs. 2023, 53, 57–65. [Google Scholar] [CrossRef]
- Klados, M.A.; Styliadis, C.; Frantzidis, C.A.; Paraskevopoulos, E.; Bamidis, P.D. Beta-Band Functional Connectivity is Reorganized in Mild Cognitive Impairment after Combined Computerized Physical and Cognitive Training. Front. Neurosci. 2016, 10, 55. [Google Scholar] [CrossRef]
- Knoefel, F.; Gaudet, C.; Zunini, R.L.; Breau, M.; Sweet, L.; Wallace, B.; Goubran, R.; Taler, V. Implementation of a Brain Training Pilot Study for People with Mild Cognitive Impairment. Can. Geriatr. J. 2018, 21, 264–268. [Google Scholar] [CrossRef]
- Lavy, Y.; Dwolatzky, T.; Kaplan, Z.; Guez, J.; Todder, D. Mild Cognitive Impairment and Neurofeedback: A Randomized Controlled Trial. Front. Aging Neurosci. 2021, 13, 657646. [Google Scholar] [CrossRef]
- Leite, J.; Gonçalves, Ó.F.; Carvalho, S. Speed of Processing (SoP) Training Plus α-tACS in People with Mild Cognitive Impairment: A Double Blind, Parallel, Placebo Controlled Trial Study Protocol. Front. Aging Neurosci. 2022, 14, 880510. [Google Scholar] [CrossRef]
- Makmee, P.; Wongupparaj, P. VR Cognitive-based Intervention for Enhancing Cognitive Functions and Well-being in Older Adults with Mild Cognitive Impairment: Behavioral and EEG Evidence. Psychosoc. Interv. 2025, 34, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Marlats, F.; Bao, G.; Chevallier, S.; Boubaya, M.; Djabelkhir-Jemmi, L.; Wu, Y.-H.; Lenoir, H.; Rigaud, A.-S.; Azabou, E. SMR/Theta Neurofeedback Training Improves Cognitive Performance and EEG Activity in Elderly with Mild Cognitive Impairment: A Pilot Study. Front. Aging Neurosci. 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Marlats, F.; Djabelkhir-Jemmi, L.; Azabou, E.; Boubaya, M.; Pouwels, S.; Rigaud, A.-S. Comparison of effects between SMR/delta-ratio and beta1/theta-ratio neurofeedback training for older adults with Mild Cognitive Impairment: A protocol for a randomized controlled trial. Trials 2019, 20, 88. [Google Scholar] [CrossRef] [PubMed]
- McNett, S.D.; Vyshedskiy, A.; Savchenko, A.; Durakovic, D.; Heredia, G.; Cahn, R.; Kogan, M. A Feasibility Study of AlzLife 40 Hz Sensory Therapy in Patients with MCI and Early AD. Healthcare 2023, 11, 2040. [Google Scholar] [CrossRef] [PubMed]
- Mudar, R.A.; Nguyen, L.T.; Eroh, J.; Chiang, H.-S.; Rackley, A.; Chapman, S.B. Event-related neural oscillation changes following reasoning training in individuals with Mild Cognitive Impairment. Brain Res. 2019, 1704, 229–240. [Google Scholar] [CrossRef]
- Oh, W.; Park, H.; Hallett, M.; You, J.H. The Effectiveness of a Multimodal Brain Empowerment Program in Mild Cognitive Impairment: A Single-Blind, Quasi-Randomized Experimental Study. J. Clin. Med. 2023, 12, 4895. [Google Scholar] [CrossRef]
- Rosales-Lagarde, A.; Rodriguez-Torres, E.E.; Itzá-Ortiz, B.A.; Miramontes, P.; Vázquez-Tagle, G.; Enciso-Alva, J.C.; García-Muñoz, V.; Cubero-Rego, L.; Pineda-Sánchez, J.E.; Martínez-Alcalá, C.I.; et al. The Color of Noise and Weak Stationarity at the NREM to REM Sleep Transition in Mild Cognitive Impaired Subjects. Front. Psychol. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Steiner, G.Z.; Bensoussan, A.; Liu, J.; Hohenberg, M.I.; Chang, D.H. Study protocol for a randomised, double-blind, placebo-controlled 12-week pilot phase II trial of Sailuotong (SLT) for cognitive function in older adults with mild cognitive impairment. Trials 2018, 19, 522. [Google Scholar] [CrossRef]
- Styliadis, C.; Kartsidis, P.; Paraskevopoulos, E.; Ioannides, A.A.; Bamidis, P.D. Neuroplastic Effects of Combined Computerized Physical and Cognitive Training in Elderly Individuals at Risk for Dementia: An eLORETA Controlled Study on Resting States. Neural Plast. 2015, 1–12. [Google Scholar] [CrossRef]
- Thapa, N.; Park, H.J.; Yang, J.-G.; Son, H.; Jang, M.; Lee, J.; Kang, S.W.; Park, K.W.; Park, H. The Effect of a Virtual Reality-Based Intervention Program on Cognition in Older Adults with Mild Cognitive Impairment: A Randomized Control Trial. J. Clin. Med. 2020, 9, 1283. [Google Scholar] [CrossRef]
- Trauberg, P.; Trenado, C.; Elben, S.; Dimenshteyn, K.; Boschheidgen, M.; Rübenach, J.; Wojtecki, L. P 41. Resting-state EEG as biomarker of cognitive training and movement training in Parkinson’s Disease (PD) with mild cognitive impairment (MCI). Clin. Neurophysiol. 2021, 132, e18. [Google Scholar] [CrossRef]
- Trenado, C.; Trauberg, P.; Elben, S.; Dimenshteyn, K.; Folkerts, A.-K.; Witt, K.; Weiss, D.; Liepelt-Scarfone, I.; Kalbe, E.; Wojtecki, L. Resting state EEG as biomarker of cognitive training and physical activity’s joint effect in Parkinson’s patients with mild cognitive impairment. Neurol. Res. Pract. 2023, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-G.; Thapa, N.; Park, H.-J.; Bae, S.; Park, K.W.; Park, J.-H.; Park, H. Virtual Reality and Exercise Training Enhance Brain, Cognitive, and Physical Health in Older Adults with Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2022, 19, 13300. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, H.; Pei, Z.; Lian, C.; Su, X.; Lan, X.; Chen, C.; Lei, Y.; Li, B.; Guo, Y. Dual-targeted repetitive transcranial magnetic stimulation modulates brain functional network connectivity to improve cognition in mild cognitive impairment patients. Front. Physiol. 2022, 13, 1066290. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Ge, C.; Liu, X.; Chen, M.; Zhang, C. Effect of Process-Based Multi-Task Cognitive Training Program on Executive Function in Older Adults with Mild Cognitive Impairment: Study Rationale and Protocol Design for a Randomized Controlled Trial. Front. Psychiatry 2020, 11, 655. [Google Scholar] [CrossRef]
- Ziloochi, F.; Niazi, I.K.; Amjad, I.; Cade, A.; Duehr, J.; Ghani, U.; Holt, K.; Haavik, H.; Shalchyan, V. Investigating the effects of chiropractic care on resting-state EEG of MCI patients. Front. Aging Neurosci. 2024, 16, 1406664. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Kumar, A.; Singh, G. Navigating the Neuromarketing Landscape: Valuable Insights for Businesses in a Post-Pandemic World. J. Bus. Bus. Mark. 2024, 31, 261–279. [Google Scholar] [CrossRef]
- Albarracin, D.; Schwarz, N. The Psychological Science of Pandemics: Contributions to and Recommendations for Social, Educational, and Health Policy. Perspect. Psychol. Sci. 2024, 19, 607–611. [Google Scholar] [CrossRef]
- Ironsi, C.S. Navigating learners towards technology-enhanced learning during post COVID-19 semesters. Trends Neurosci. Educ. 2022, 29, 100189. [Google Scholar] [CrossRef]
- Bradbury, A. Ability, Inequality and Post-Pandemic Schools: Rethinking Contemporary Myths of Meritocracy; Bristol University Press: Bristol, UK, 2021. [Google Scholar] [CrossRef]
- Panda, S.; Padhy, P.C. Bridging the Gap: Intersecting Perspectives on Digital and Human Consciousness. In Comparative Analysis of Digital Consciousness and Human Consciousness: Bridging the Divide in AI Discourse; IGI Global: New York, NY, USA, 2024; pp. 65–88. [Google Scholar] [CrossRef]
- Leena, A.M.N.; Maheswari, M.G. Designing Tomorrow’s Minds: A Design Thinking Approach to AI Enabled Brain based Learning for Enhanced Cognitive Development; CiiT Publications: Ramnagar, India, 2024. [Google Scholar]
- Mantello, P.; Ho, M.-T.; Nguyen, M.-H.; Vuong, Q.-H. Bosses without a heart: Socio-demographic and cross-cultural determinants of attitude toward Emotional AI in the workplace. AI Soc. 2023, 38, 97–119. [Google Scholar] [CrossRef]
- Balanagu, S.S.N.; Boppisetty, J. The Brain as a Market: Understanding Neurological Consumer Responses. In The Quantum AI Era of Neuromarketing; IGI Global: New York, NY, USA, 2025; pp. 61–92. [Google Scholar] [CrossRef]
- Nochaiwong, S.; Ruengorn, C.; Thavorn, K.; Hutton, B.; Awiphan, R.; Phosuya, C.; Ruanta, Y.; Wongpakaran, N.; Wongpakaran, T. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 10173. [Google Scholar] [CrossRef] [PubMed]
- Mental Disorders Collaborators, G.B.D. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar]
- Moitra, M.; Owens, S.; Hailemariam, M.; Wilson, K.S.; Mensa-Kwao, A.; Gonese, G.; Kamamia, C.K.; White, B.; Young, D.M.; Collins, P.Y. Global mental health: Where we are and where we are going. Curr. Psychiatry Rep. 2023, 25, 301–311. [Google Scholar] [CrossRef]
- Gkintoni, E.; Antonopoulou, H.; Sortwell, A.; Halkiopoulos, C. Challenging cognitive load theory: The role of educational neuroscience and artificial intelligence in redefining learning efficacy. Brain Sci. 2025, 15, 203. [Google Scholar] [CrossRef]
- Al-Hamzawi, A.; Alonso, J.; Bruffaerts, R.; de Almeida, J.M.C.; Chardoul, S.; Chiu, W.T.; Degenhardt, L.; Demler, O.V.; Ferry, F.; Gureje, O.; et al. Age of onset and cumulative risk of mental disorders: A cross-national analysis of population surveys from 29 countries. Lancet Psychiatry 2023, 10, 668–681. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Correll, C.U.; Arango, C.; Berk, M.; Patel, V.; Ioannidis, J.P. Preventive psychiatry: A blueprint for improving the mental health of young people. World Psychiatry 2021, 20, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Zulfiker, S.; Kabir, N.; Biswas, A.A.; Nazneen, T.; Uddin, M.S. An in-depth analysis of machine learning approaches to predict depression. Curr. Res. Behav. Sci. 2021, 2, 100044. [Google Scholar] [CrossRef]
- Piltch-Loeb, R.; Merdjanoff, A.; Meltzer, G. Anticipated mental health consequences of COVID-19 in a nationally-representative sample: Context, coverage, and economic consequences. Prev. Med. 2021, 145, 106441. [Google Scholar] [CrossRef]











| Inclusion Criteria | Details |
|---|---|
| Research Focus | Investigates the efficacy, feasibility, or applicability of technological interventions (neuromodulation, EEG applications, virtual reality, cognitive training, physical exercise, or AI approaches) for MCI assessment, monitoring, or rehabilitation. |
| Study Design | Randomized Controlled Trials (RCTs), quasi-experimental studies, controlled trials, pre-post designs, or other empirical studies using validated methodologies with quantitative outcomes. |
| Target Population | Adults with diagnosed MCI using established clinical criteria (e.g., Petersen criteria, NIA-AA criteria, DSM-5), including amnestic and non-amnestic subtypes. |
| Intervention Types | Studies evaluating at least one of the following: (1) neuromodulation techniques (tDCS, TMS), (2) EEG-based assessment or interventions, (3) virtual reality applications, (4) cognitive training programs, (5) physical exercise interventions, or (6) AI applications for assessment, prediction, or personalization. |
| Outcome Measures | Reports quantitative measures of at least one of the following: cognitive function, neurophysiological measures, functional outcomes, or biomarkers of disease progression relevant to MCI. |
| Publication Source | Peer-reviewed journal articles published between 2014 and 2024. |
| Language | Published in English to ensure consistent analysis and interpretation. |
| Full-Text Access | Studies must have full-text availability for comprehensive review, coding, and extraction. |
| Exclusion Criteria | Details |
|---|---|
| n-Relevant Focus | Studies have not addressed technological interventions for MCI or reported outcomes related to cognitive function, neurophysiological measures, or functional abilities. |
| Inappropriate Population | Studies focused solely on healthy older adults without MCI, or exclusively on populations with established dementia or other neurodegenerative disorders. |
| Intervention Type | Studies evaluating only pharmacological interventions or conventional non-technological rehabilitation approaches without any specified technological components. |
| Study Type | Systematic reviews, meta-analyses, protocols, editorials, commentaries, case studies with n < 5, or non-empirical opinion pieces. |
| Language Restriction | Studies published in languages other than English. |
| Methodological Limitations | Studies with significant limitations, such as the absence of validated MCI diagnostic criteria, lack of validated outcome measures, or insufficient methodological detail to evaluate quality. |
| Publication Type | Conference abstracts, book chapters, dissertations, or non-peer-reviewed publications. |
| Publication Date | Studies published before 2014 were excluded to ensure a focus on the most recent decade of technological interventions for MCI. |
| Authors | Population Characteristics | Study Objectives | Methodology | Main Findings |
|---|---|---|---|---|
| Amjad et al. (2019a) [144] |
|
|
|
|
| Amjad et al. (2019b) [145] |
|
|
|
|
| Babiloni et al. (2014) [146] |
| The study objectives were to investigate the relationship between cerebral vasomotor reactivity (VMR) and coherence of resting state electroencephalographic (EEG) rhythms in normal elderly (Nold) subjects and amnesic MCI patients |
|
|
| Bae et al. (2024) [147] |
|
|
|
|
| Cai et al. (2022) [148] |
|
|
|
|
| Caminiti et al. (2024) [149] |
|
|
|
|
| Emonson et al. (2019) [150] |
|
|
|
|
| Han and Youn (2023) [151] |
|
|
|
|
| Hathaway et al. (2021) [152] |
|
|
|
|
| Hong et al. (2018) [153] |
|
|
|
|
| Jiang et al. (2019) [154] |
|
| The study used a quasi-experimental design with a randomized control trial and questionnaire. A total of 44 participants with MCI were randomly assigned to either an experimental group (n = 22) or a control group (n = 22). The inclusion criteria were based on MCI diagnosis, including a chief complaint of memory impairment. The outcome measures used were psychomotor speed tests (Finger Tapping Test, Purdue Pegboard Test) and cognitive assessments (Montreal Cognitive Assessment, EEG). |
|
| Jung et al. (2022) [155] |
|
|
|
|
| Kim et al. (2024) [156] |
|
|
|
|
| Kim et al. (2023) [157] |
|
|
|
|
| Klados et al. (2016) [158] |
|
| The methodology involved dividing 50 MCI participants into an experimental (LLM) and active control (AC) group, recording resting-state EEG before and after the intervention, estimating functional connectivity using magnitude-squared coherence between cortical sources computed with sLORETA, forming characteristic weighted graphs for each group using a statistical model, and assessing the effects of the interventions using network density and node strength. |
|
| Knoefel et al. (2018) [159] |
| To determine the feasibility of recruiting patients with MCI to test cognitive interventions |
|
|
| Lavy et al. (2021) [160] |
|
| The study used a randomized controlled trial design with 30 participants diagnosed with MCI. Participants were randomly assigned to either an experimental group that received neurofeedback training to increase upper alpha power at the Pz electrode or a sham group that received random feedback from different electrodes. All participants underwent cognitive assessment using the NeuroTrax computerized battery before and after the 10 training sessions, as well as at a 30-day follow-up. EEG was recorded during the sessions using a 19-channel system. |
|
| Leite et al. (2022) [161] |
|
|
The primary outcome is the Useful Field of View (UFOV) test, which measures speed of processing and attention. Secondary outcomes include the NIH EXAMINER battery to assess transfer effects to other cognitive domains, and EEG measures of brain connectivity and coherence. | |
| Makmee and Wongupparaj (2025) [162] |
|
|
|
|
| Marlats et al. (2020 [163] |
|
|
| |
| Marlats et al. (2019) [164] |
|
|
|
|
| McNett et al. (2023) [165] |
|
|
|
|
| Mudar et al. (2019) [166] |
| The study objectives were to examine the effects of Gist Reasoning training versus New Learning training on event-related neural oscillations (theta and alpha band power) during Go/NoGo tasks involving basic and superordinate semantic categorization in older adults with amnestic MCI. |
|
|
| Oh et al. (2023) [167] |
|
|
|
|
| Rosales-Lagarde et al. (2018) [168] |
|
|
|
|
| Steiner et al. (2018) [169] |
|
|
| |
| Styliadis et al. (2015) [170] |
|
|
|
|
| Thapa et al. (2020) [171] |
|
|
|
|
| Trauberg et al. (2021) [172] |
|
|
|
|
| Trenado et al. (2023) [173] |
|
|
|
|
| Yang et al. (2022) [174] |
| The study objectives were to investigate the effectiveness of virtual-reality-based cognitive training (VRCT) and exercise on the brain, and the cognitive and physical activity of older adults with MCI. Specific outcomes measured included global cognitive function (MMSE), brain activity (resting-state EEG), and physical function (handgrip strength, gait speed). |
|
|
| Zhang et al. (2022) [175] |
|
|
|
|
| Zhao et al. (2020) [176] |
|
| The study used a randomized controlled trial design with 90 participants with MCI randomly assigned to a cognitive training group or a wait-list control group. The cognitive training group received 10 weeks of process-based multi-task cognitive training and health education, while the control group received only health education. The primary outcome was executive function, with secondary outcomes of neuropsychological assessments and EEG measures, assessed at baseline, after 10 weeks, and 3-month follow-up. | |
| Ziloochi et al. (2024) [177] |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G.; Vantarakis, A. Neurotechnological Approaches to Cognitive Rehabilitation in Mild Cognitive Impairment: A Systematic Review of Neuromodulation, EEG, Virtual Reality, and Emerging AI Applications. Brain Sci. 2025, 15, 582. https://doi.org/10.3390/brainsci15060582
Gkintoni E, Vassilopoulos SP, Nikolaou G, Vantarakis A. Neurotechnological Approaches to Cognitive Rehabilitation in Mild Cognitive Impairment: A Systematic Review of Neuromodulation, EEG, Virtual Reality, and Emerging AI Applications. Brain Sciences. 2025; 15(6):582. https://doi.org/10.3390/brainsci15060582
Chicago/Turabian StyleGkintoni, Evgenia, Stephanos P. Vassilopoulos, Georgios Nikolaou, and Apostolos Vantarakis. 2025. "Neurotechnological Approaches to Cognitive Rehabilitation in Mild Cognitive Impairment: A Systematic Review of Neuromodulation, EEG, Virtual Reality, and Emerging AI Applications" Brain Sciences 15, no. 6: 582. https://doi.org/10.3390/brainsci15060582
APA StyleGkintoni, E., Vassilopoulos, S. P., Nikolaou, G., & Vantarakis, A. (2025). Neurotechnological Approaches to Cognitive Rehabilitation in Mild Cognitive Impairment: A Systematic Review of Neuromodulation, EEG, Virtual Reality, and Emerging AI Applications. Brain Sciences, 15(6), 582. https://doi.org/10.3390/brainsci15060582









