The Diagnostic and Prognostic Role of Biomarkers in Mild Traumatic Brain Injury: An Umbrella Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection and Data Sources
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Statistical Analysis
2.4. Subgroup and Sensitivity Analyses
- Comparisons between high and low biomarker cutoff values;
- Differences between pediatric and adult populations;
- Impact of time from injury to biomarker measurement.
2.5. Risk of Bias and Quality Assessment
2.6. Final Study Selection
3. Results
3.1. Selection Process (PRISMA Guidelines)
3.2. Umbrella Systematic Review
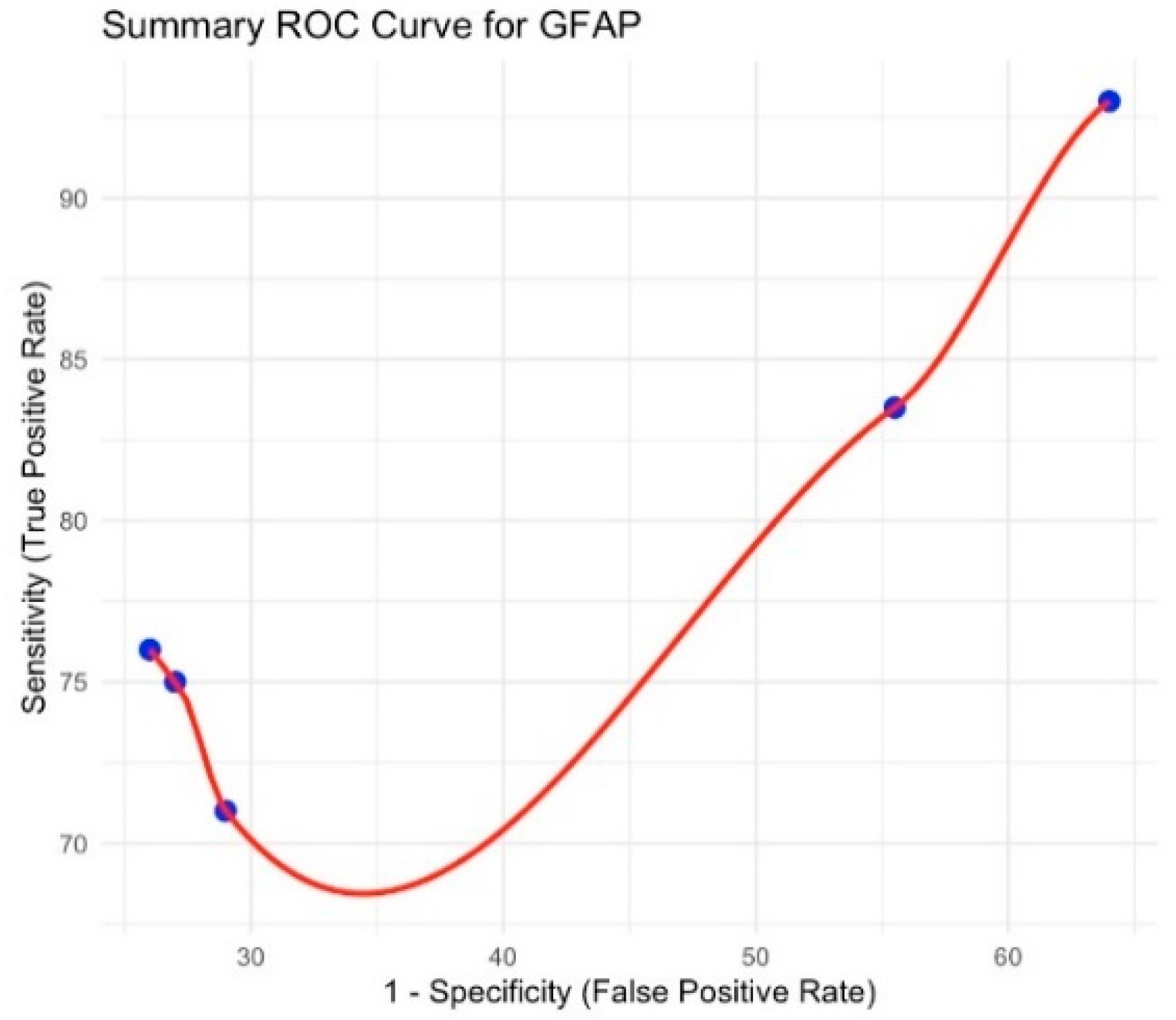
3.3. Glial Fibrillary Acidic Protein (GFAP)
3.4. Ubiquitin C-Terminal Hydrolase L1 (UCH-L1)
3.5. S100B Protein
3.6. Tau Protein
3.7. Neurofilament Light Chain
4. Discussion
5. Summary of Findings
- S100B had a pooled sensitivity of 91.6% (95% CI: 84.3–99.0) and specificity of 42.4% (95% CI: 31.1–53.6). This confirms its value as a screening tool for ruling out mTBI, particularly in adult and pediatric populations [13,22]. However, its low specificity limits its utility in avoiding unnecessary CT scans.
- UCH-L1 yielded a pooled sensitivity of 86.7% (95% CI: 77.8–95.6) and specificity of 37.3% (95% CI: 30.4–44.2), suggesting that it is not as effective in ruling out mTBI as S100B but may have a role in combination with other biomarkers [13].
- Tau protein was evaluated in a single meta-analysis, limiting the ability to perform an umbrella meta-analysis. The available study indicated that tau may have potential as a diagnostic marker, but more research is needed to validate its clinical applicability [20].
- NfL was primarily associated with sports-related concussions, showing significantly elevated levels in affected individuals [15]. However, it did not exhibit a strong diagnostic utility in military-related TBI, highlighting variability in its effectiveness across different populations.
6. Interpretation of Findings and Clinical Implications
7. Subgroup Analysis and Heterogeneity
- Time-dependent changes: S100B and GFAP levels peak within 3–6 h post-injury, whereas UCH-L1 and tau protein may remain elevated for longer durations. This suggests a potential temporal window for optimal biomarker assessment [11].
- Population-specific differences: NfL was most predictive in athletes suffering from concussions, but it was not significantly elevated in military-related TBIs, possibly due to differences in injury mechanisms [15].
8. Risk of Bias and Limitations
- Heterogeneity across studies: Differences in biomarker cutoff values, sample collection methods, and patient demographics contributed to substantial variability in reported sensitivity and specificity [23].
- Publication bias: Funnel plot analysis indicated a potential bias in studies reporting higher diagnostic accuracy, suggesting that negative or inconclusive findings may be underreported.
- Limited data for tau protein and NfL: The scarcity of meta-analyses on these biomarkers prevented a more robust statistical synthesis, underscoring the need for further research.
9. Clinical Perspectives and Future Directions
9.1. Biomarker-Based Diagnostic Algorithm for Mild Traumatic Brain Injury (mTBI)
9.2. Clinical Decision Support System (CDSS) for Biomarker-Based Diagnosis
10. Interpreting the CDSS Score
- 0–2 Points → Low risk: No CT scan is required; monitor symptoms.
- 3–5 Points → Moderate risk: Consider a CT scan and monitor the symptoms closely.
- 6+ Points → High risk: An immediate CT scan is needed; consider hospitalization.
Future Directions
- Standardization of cutoff values: Future studies should establish consensus guidelines for biomarker thresholds, ensuring uniformity in clinical practice.
- Longitudinal studies on prognostic value: While most studies focus on acute diagnosis, biomarkers like NfL and tau protein may have significant prognostic value in predicting long-term cognitive outcomes [20].
- Machine learning applications: Emerging research suggests that AI-driven algorithms could optimize biomarker interpretation, facilitating real-time decision-making in emergency settings [23].
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild traumatic brain injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, e0174847. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Surveillance Report on Traumatic Brain Injury-Related Hospitalizations and Deaths. 2021. Available online: https://www.cdc.gov/traumatic-brain-injury/data-research/index.html (accessed on 11 April 2025).
- Maas, A.I.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Kutcher, J.S.; Ashwal, S.; Barth, J.; Getchius, T.S.; Gioia, G.A.; Gronseth, G.S.; Guskiewicz, K.; Mandel, S.; Manley, G.; et al. Summary of evidence-based guideline update: Evaluation and management of concussion in sports. Neurology 2014, 82, 2250–2255. [Google Scholar] [CrossRef]
- Laker, S.R. Return to play decisions. Phys. Med. Rehabil. Clin. N. Am. 2011, 22, 619–634. [Google Scholar] [CrossRef]
- Blennow, K.; Hardy, J.; Zetterberg, H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2016, 98, 886–899. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Panenka, W.J.; Iverson, G.L.; McCulloch, K.L. Attention and processing speed in post-concussion syndrome: Systematic review and meta-analysis. Neurology 2020, 95, e662–e672. [Google Scholar]
- Theadom, A.; Starkey, N.J.; Barker-Collo, S.; Jones, K.; Ameratunga, S.; Feigin, V.L. Population-based cohort study of the impacts of mild traumatic brain injury in adults four years post-injury. PLoS ONE 2016, 11, e0145927. [Google Scholar] [CrossRef] [PubMed]
- Shlosberg, D.; Benifla, M.; Sheleg, S.V.; Fattal-Valevski, A. Blood biomarkers in traumatic brain injury. J. Pediatr. Neurosci. 2010, 5, 11–16. [Google Scholar]
- Undén, J.; Calcagnile, O.; Undén, M.; Reinstrup, P.; Bellander, B.M. Validation of the Scandinavian guidelines for initial management of minimal, mild, and moderate head injuries in adults. BMC Med. 2013, 11, 50. [Google Scholar] [CrossRef]
- Papa, L.; Slobounov, S.M.; Breiter, H.C.; Walter, A.; Bream, T.; Seidenberg, P.; Bailes, J.E.; Bravo, S.; Johnson, B.; Kaufman, D.; et al. Elevations in microRNA biomarkers in serum are associated with measures of concussion, neurocognitive performance, and head impact exposure. J. Neurotrauma 2016, 33, 1247–1254. [Google Scholar]
- Shahim, P.; Politis, A.; van der Merwe, A.; Moore, B.; Ekanayake, V.; Lippa, S.M.; Chou, Y.-Y.; Pham, D.L.; Butman, J.A.; Diaz-Arrastia, R.; et al. Time course and diagnostic utility of NfL, tau, GFAP, and UCH-L1 in subacute and chronic TBI. Neurology 2018, 90, e1811–e1819. [Google Scholar] [CrossRef] [PubMed]
- Mondello, S.; Sorinola, A.; Czeiter, E.; Vámos, Z.; Amrein, K.; Synnot, A.; Donoghue, E.; Sándor, J.; Wang, K.; Diaz-Arrastia, R.; et al. Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: A living systematic review and meta-analysis. J. Neurotrauma 2020, 38, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Kawata, K.; Liu, C.Y.; Merkel, S.F.; Ramirez, S.H.; Tierney, R.T. Blood biomarkers for brain injury: What are we measuring? Neurosci. Biobehav. Rev. 2016, 68, 460–473. [Google Scholar] [CrossRef]
- Karantali, E.; Kazis, D.; Chatzikonstantinou, S.; Petridis, F. Serum neurofilament light chain (NfL) as a biomarker in sports-related concussion and mild traumatic brain injury: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 1327–1334. [Google Scholar]
- Olivera, A.; Lejbman, N.; Jeromin, A.; Devoto, C.; Simkins, T. A single concussion exposure increases tau in the brain and blood of rats as detected by immuno-MALDI mass spectrometry and ELISA. J. Neurochem. 2021, 156, 47–57. [Google Scholar]
- McMahon, P.J.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Beers, S.R.; Gordon, W.A.; Valadka, A.B.; Manley, G.T. Symptomatology and functional outcome in mild traumatic brain injury: Results from the TRACK-TBI study. J. Neurotrauma 2014, 31, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.D.; Duhaime, A.C.; Iaccarino, M.A. Mild traumatic brain injury in 2019–2020. J. Am. Med. Assoc. (JAMA) 2019, 323, 177–178. [Google Scholar] [CrossRef]
- McCrea, M.A.; Nelson, L.D.; Guskiewicz, K.M. Diagnosis and management of acute concussion: A review. J. Am. Med. Assoc. (JAMA) 2020, 323, 795–810. [Google Scholar]
- Zetterberg, H.; Blennow, K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat. Rev. Neurol. 2016, 12, 563–574. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Oris, C.; Pereira, B.; Durif, J.; Simon-Pimmel, J.; Castellani, C.; Manzano, S.; Sapin, V.; Bouvier, D. The biomarker S100B and mild traumatic brain injury: A meta-analysis. Pediatrics 2018, 141, e20180037. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Edwards, D.; Ramia, M. Exploring Serum Biomarkers for Mild Traumatic Brain Injury [Internet]; Kobeissy, F.H., Ed.; PubMed; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK299199/ (accessed on 11 April 2025).
- Santing, J.A.L.; Hopman, J.H.; Verheul, R.J.; van der Naalt, J.; van den Brand, C.L.; Jellema, K. Clinical value of S100B in detecting intracranial injury in elderly patients with mild traumatic brain injury. Injury 2024, 55, 111313. [Google Scholar] [CrossRef] [PubMed]





| Meta-Analysis (Ref) | Biomarker | No. of Studies Included | Pooled Sensitivity (%) | 95% CI | Pooled Specificity (%) | 95% CI | Notes |
|---|---|---|---|---|---|---|---|
| Mondello et al., 2020 [13] | GFAP | 3 | 84.5 | 74.9–94.2 | 42.4 | 44.5–77.6 | Increased heterogenicity |
| Mondello et al., 2020 [13] | UCH–L1 | 2 | 86.7 | 77.8–95.6 | 61.1 | 30.4–44.2 | Low heterogenicity |
| Oris et al., 2018 [22] | S100B | 3 | 91.7 | 84.3–99.1 | 37.3 | 31.2–53.7 | No heterogenicity |
| Mondello et al., 2020 [13] | Tau protein | 1 | - | - | - | - | Only a meta-analysis (Mondello et al., 2020) reported variable data > lower sensitivity than GFAP/S100B and moderate specificity |
| Karantali et al., 2022 [15] | NfL | 1 | - | - | - | - | A single quantitative meta-analysis (Karantali et al., 2022 [15]) showed significant increases in sports concussion (p = 0.0023) and p = 0.0015 for athletes; no specificity/sensitivity |
| Risk Category | Clinical and Biomarker Criteria | Recommended Action |
|---|---|---|
| Low Risk | S100B < 0.1 μg/L, GFAP within normal limits; no neurological symptoms | No CT scan required; safe discharge with symptom monitoring |
| Moderate Risk | GFAP > 626 pg/mL, UCH-L1 > 225 pg/mL; mild neurological symptoms | Consider observation, repeat clinical assessment, and a CT scan if symptoms worsen |
| High Risk | S100B significantly elevated, GFAP and UCH-L1 highly elevated; persistent or worsening neurological symptoms | Immediate CT scan and possible hospitalization |
| Long-Term Prognosis | Persistent symptoms beyond four weeks; elevated NfL and tau protein levels | Neurology referral, cognitive rehabilitation, and long-term monitoring |
| Biomarker | Low Risk (0 Points) | Moderate Risk (1 Point) | High Risk (2 Points) |
|---|---|---|---|
| S100B | <0.1 μg/L | 0.1–0.5 μg/L | >0.5 μg/L |
| GFAP | <500 pg/mL | 500–1000 pg/mL | >1000 pg/mL |
| UCH-L1 | <200 pg/mL | 200–400 pg/mL | >400 pg/mL |
| NfL | <20 pg/mL | 20–70 pg/mL | >70 pg/mL |
| Tau | <5 pg/mL | 5–15 pg/mL | >15 pg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroudis, I.; Petridis, F.; Kazis, D.; Ciobica, A.; Dăscălescu, G.; Petroaie, A.D.; Dobrin, I.; Novac, O.; Vata, I.; Novac, B. The Diagnostic and Prognostic Role of Biomarkers in Mild Traumatic Brain Injury: An Umbrella Meta-Analysis. Brain Sci. 2025, 15, 581. https://doi.org/10.3390/brainsci15060581
Mavroudis I, Petridis F, Kazis D, Ciobica A, Dăscălescu G, Petroaie AD, Dobrin I, Novac O, Vata I, Novac B. The Diagnostic and Prognostic Role of Biomarkers in Mild Traumatic Brain Injury: An Umbrella Meta-Analysis. Brain Sciences. 2025; 15(6):581. https://doi.org/10.3390/brainsci15060581
Chicago/Turabian StyleMavroudis, Ioannis, Foivos Petridis, Dimitrios Kazis, Alin Ciobica, Gabriel Dăscălescu, Antoneta Dacia Petroaie, Irina Dobrin, Otilia Novac, Ioana Vata, and Bogdan Novac. 2025. "The Diagnostic and Prognostic Role of Biomarkers in Mild Traumatic Brain Injury: An Umbrella Meta-Analysis" Brain Sciences 15, no. 6: 581. https://doi.org/10.3390/brainsci15060581
APA StyleMavroudis, I., Petridis, F., Kazis, D., Ciobica, A., Dăscălescu, G., Petroaie, A. D., Dobrin, I., Novac, O., Vata, I., & Novac, B. (2025). The Diagnostic and Prognostic Role of Biomarkers in Mild Traumatic Brain Injury: An Umbrella Meta-Analysis. Brain Sciences, 15(6), 581. https://doi.org/10.3390/brainsci15060581








