Safety, Feasibility, and Tolerability of Ten Days of At-Home, Remotely Supervised tDCS During Gamified Attention Training in Children with Acquired Brain Injury: An Open-Label, Dose-Controlled Pilot Trial
Abstract
1. Introduction
2. Materials and Methods
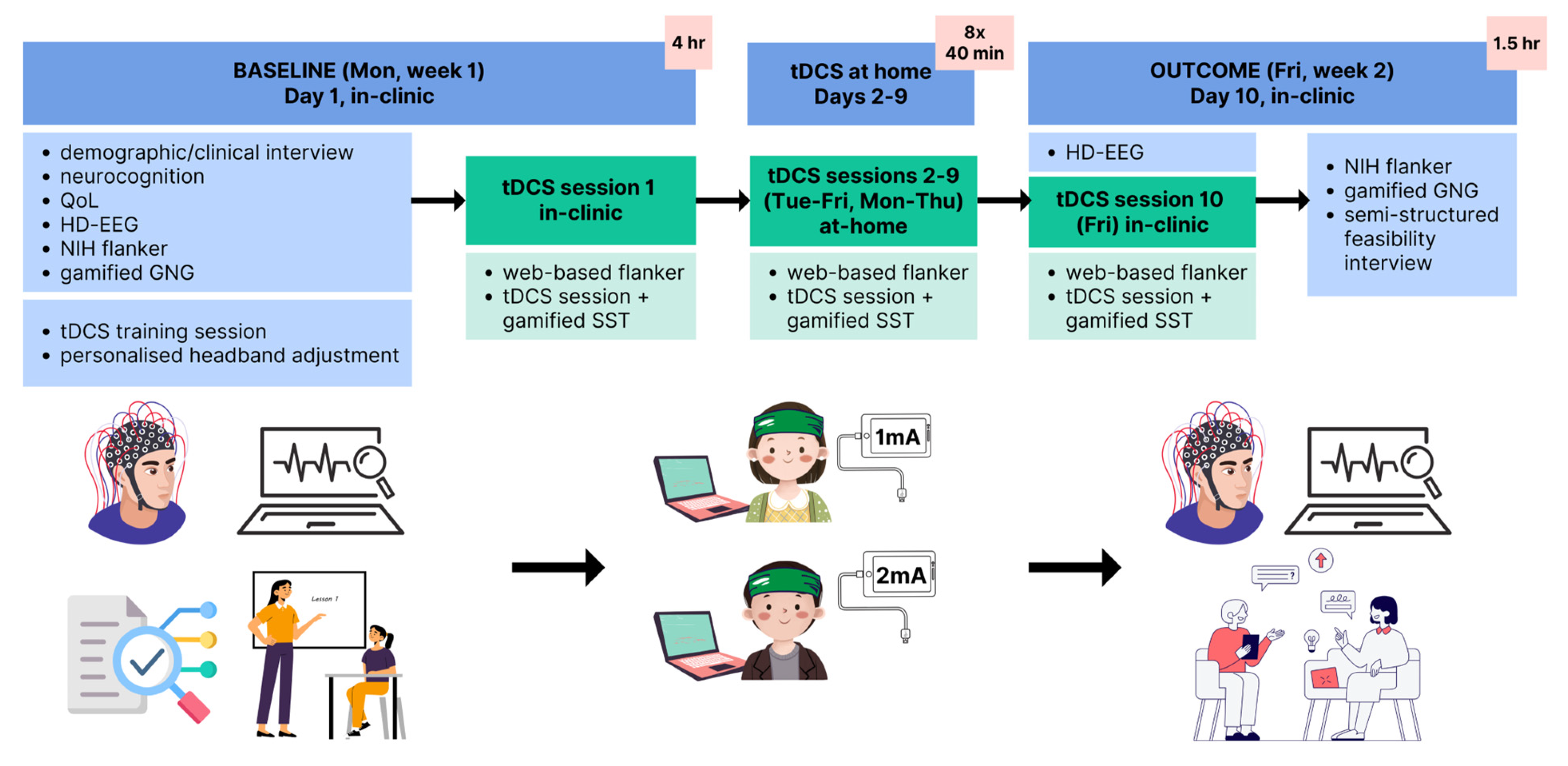
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Sample Size, Randomization, and Blinding
2.4. Primary Outcomes
Safety, Feasibility, and Tolerability
2.5. Secondary Outcome Measures
2.6. Intervention
2.7. Home-Based Protocol
2.8. Baseline Assessments
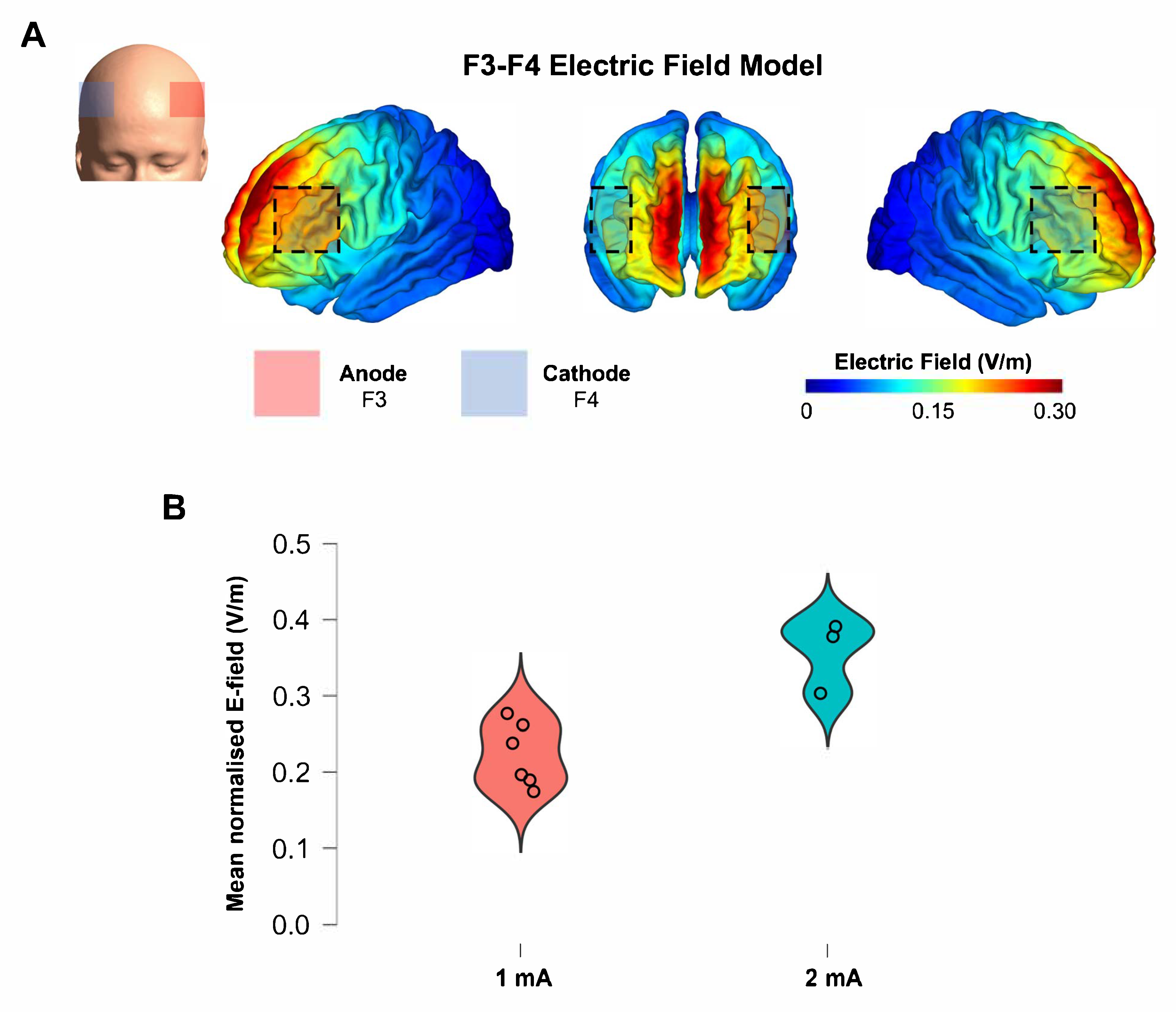
2.9. Electric Field Modeling
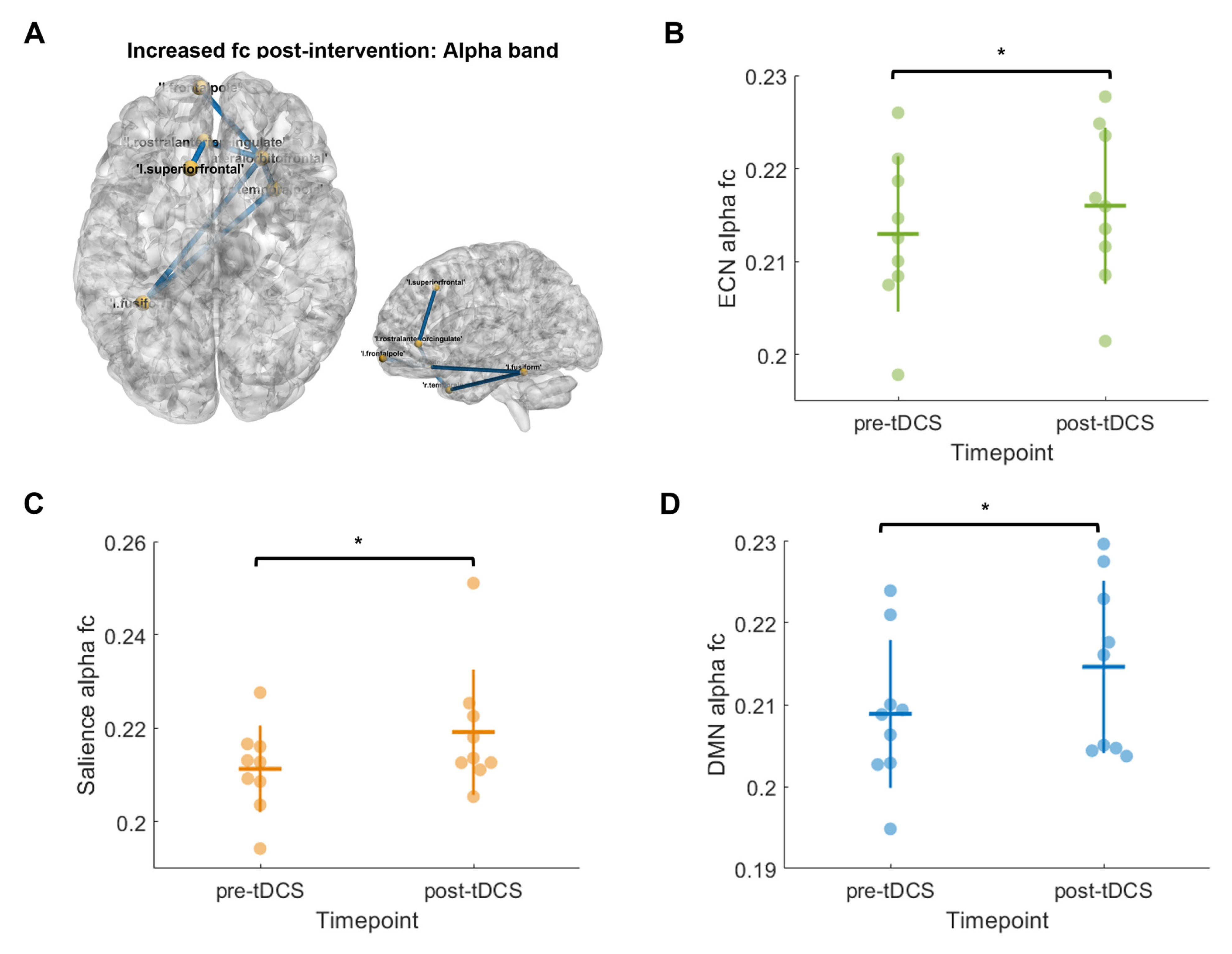
2.10. HD-EEG Functional Connectivity
2.11. Statistical Analysis
3. Results
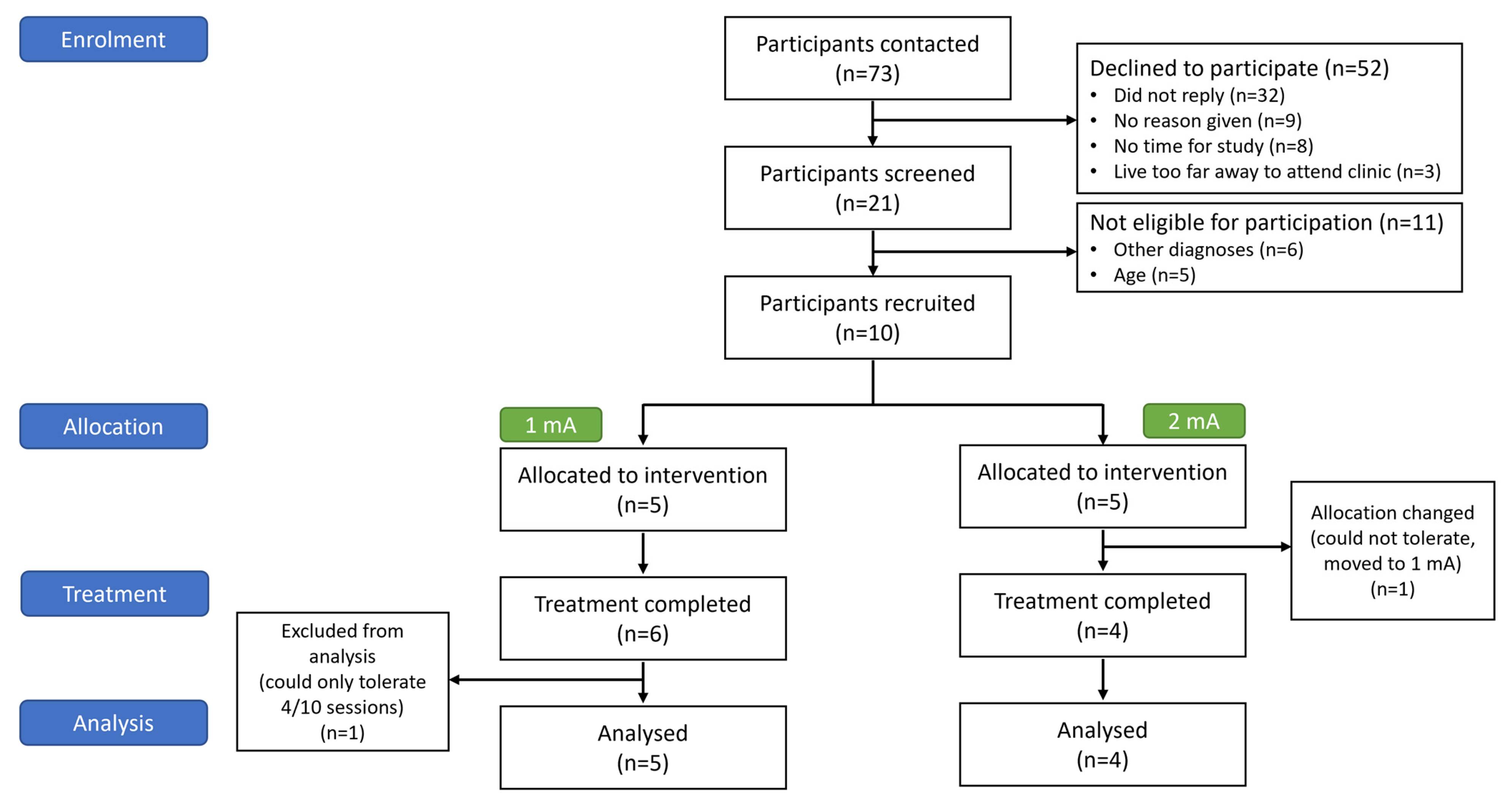
3.1. Participants
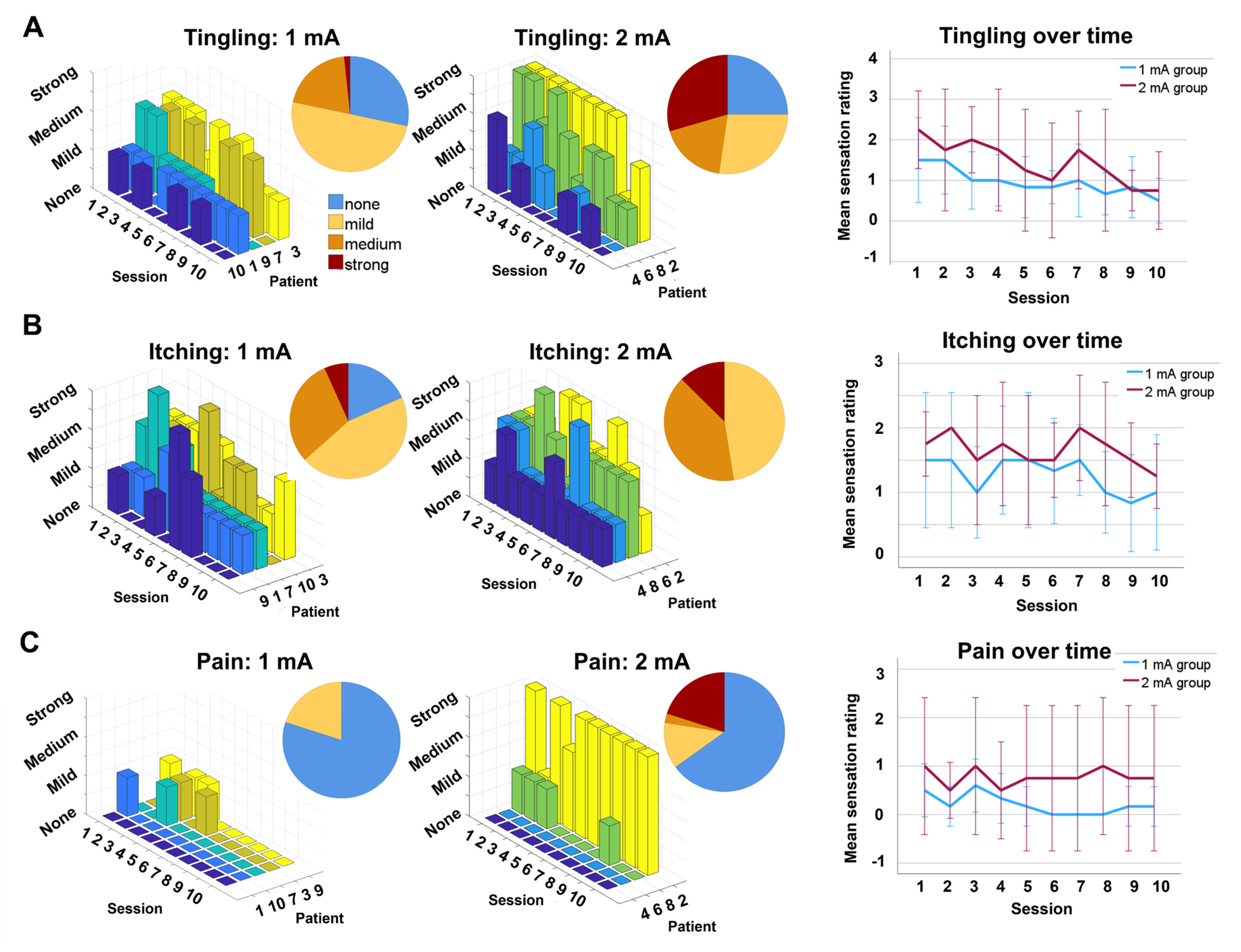
3.2. Feasibility and Tolerability
3.2.1. Feasibility
3.2.2. Tolerability
3.3. Qualitative Feedback
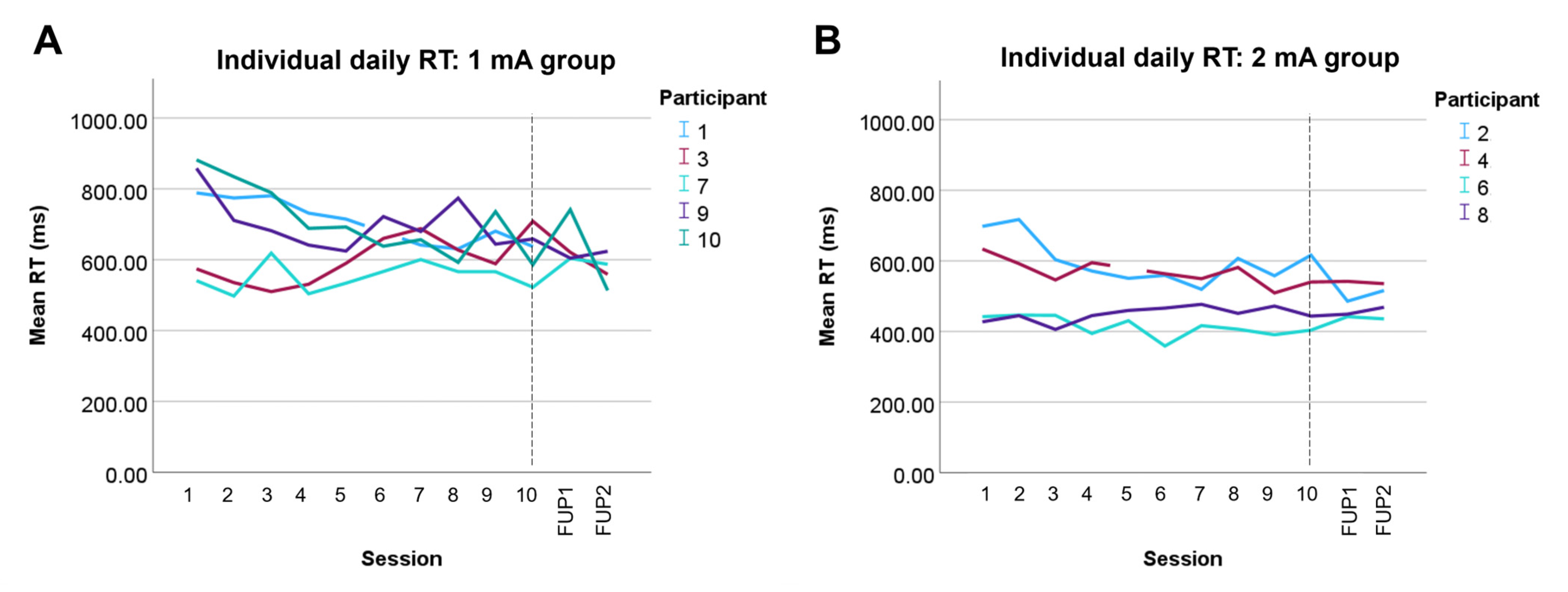
3.4. Attention
| ID | Age at Study Baseline (Years) | Time Post-ABI (Years) | Sex | Injury Severity | Medication with Effect on Cortical Excitability ◇ | tDCS Dosage Assigned | tDCS Dosage Received | Pre-tDCS Flanker RT (ms) | Post-tDCS Flanker RT (ms) | RT Change Following Intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9.7 | 8.6 | M | Severe | Non-stimulant ADHD medication (D) | 1 mA | 1 mA | 766.29 | 637.54 | Faster |
| 3 | 16.5 | 0.3 | F | Mild | Amitriptyline (D) | 1 mA | 1 mA | 604.26 | 623.34 | Slower |
| 5 | 7.4 | 2.0 | M | Mild | None | 1 mA | 1 mA * | 853.74 | 924.45 | Slower + |
| 7 | 12.5 | 10.0 | M | Severe | SSRI (I) Stimulant (I) | 1 mA | 1 mA | 545.41 | 422.13 | Faster |
| 9 | 9.4 | 0.8 ++ | F | Severe | None | 1 mA | 1 mA | 722.04 | 596.68 | Faster |
| 10 | 10.8 | 4.1 | M | Mild | Atypical antipsychotic (I) Stimulant (I) | 2 mA | 1 mA ** | 856.20 | 685.61 | Faster |
| 2 | 11.9 | 9.6 | F | Severe | None | 2 mA | 2 mA | 484.59 | 382.13 | Faster |
| 4 | 15.8 | 2.0 | F | Mild | Amitriptyline (D) Topiramate (D) | 2 mA | 2 mA | 625.85 | 525.21 | Faster |
| 6 | 12.8 | 2.8 | M | Mild | None | 2 mA | 2 mA | 389.14 | 322.12 | Faster |
| 8 | 14.3 | 1.3 | F | Mild | Stimulant (I) | 2 mA | 2 mA | 361.25 | 383.45 | Slower |
3.5. Simulated E-Fields
3.6. HD-EEG Functional Connectivity
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| tDCS | Transcranial direct current stimulation |
| TBI | Traumatic brain injury |
| HD-EEG | High-density electroencephalography |
| ABI | Acquired brain injury |
| ECN | Executive control network |
| SN | Salience network |
| DMN | Default mode network |
| dlPFC | Dorsolateral prefrontal cortex |
| NBS | Network-based statistics |
| E-field | Electric field |
| ADHD | Attention deficit/hyperactivity disorder |
| ROI | Region of interest |
| CAT | Computational anatomy toolbox |
References
- Ahmed, S.; Venigalla, H.; Mekala, H.M.; Dar, S.; Hassan, M.; Ayub, S. Traumatic brain injury and neuropsychiatric complications. Indian J. Psychol. Med. 2017, 39, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yeates, K.O.; Armstrong, K.; Janusz, J.; Taylor, H.G.; Wade, S.; Stancin, T.; Drotar, D. Long-term attention problems in children with traumatic brain injury. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 574–584. [Google Scholar] [CrossRef]
- Charach, A.; Skyba, A.; Cook, L.; Antle, B.J. Using stimulant medication for children with ADHD: What do parents say? A brief report. J. Can. Acad. Child Adolesc. Psychiatry 2006, 15, 75–83. [Google Scholar]
- Bedell, G.M.; Dumas, H.M. Social participation of children and youth with acquired brain injuries discharged from inpatient rehabilitation: A follow-up study. Brain Inj. 2004, 18, 65–82. [Google Scholar] [CrossRef]
- Aitken, M.E.; McCarthy, M.L.; Slomine, B.S.; Ding, R.; Durbin, D.R.; Jaffe, K.M.; Paidas, C.N.; Dorsch, A.M.; Christensen, J.R.; MacKenzie, E.J. Family burden after traumatic brain injury in children. Pediatrics 2009, 123, 199–206. [Google Scholar] [CrossRef]
- Greenwald, B.D.; Burnett, D.M.; Miller, M.A. Congenital and acquired brain injury. 1. Brain injury: Epidemiology and pathophysiology. Arch. Phys. Med. Rehabil. 2003, 84, S3–S7. [Google Scholar] [CrossRef]
- World Health Organization. Neurological Disorders: Public Health Challenges; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Levin, H.; Troyanskaya, M.; Petrie, J.; Wilde, E.A.; Hunter, J.V.; Abildskov, T.J.; Scheibel, R.S. Methylphenidate Treatment of Cognitive Dysfunction in Adults After Mild to Moderate Traumatic Brain Injury: Rationale, Efficacy, and Neural Mechanisms. Front. Neurol. 2019, 10, 925. [Google Scholar] [CrossRef]
- Kuczynski, A.; Crawford, S.; Bodell, L.; Dewey, D.; Barlow, K.M. Characteristics of post-traumatic headaches in children following mild traumatic brain injury and their response to treatment: A prospective cohort. Dev. Med. Child Neurol. 2013, 55, 636–641. [Google Scholar] [CrossRef]
- Nejati, V.; Estaji, R. The impact of transcranial direct current stimulation on attention bias modification in children with ADHD. J. Neural Transm. 2024, 131, 823–832. [Google Scholar] [CrossRef]
- Krauel, K.; Brauer, H.; Breitling-Ziegler, C.; Freitag, C.M.; Luckhardt, C.; Mühlherr, A.; Schütz, M.; Boxhoorn, S.; Ecker, C.; Castelo-Branco, M. Prefrontal Transcranial Direct Current Stimulation in Pediatric Attention-Deficit/Hyperactivity Disorder: A Randomized Clinical Trial. JAMA Netw. Open 2025, 8, e2460477. [Google Scholar] [CrossRef]
- Sierawska, A.; Splittgerber, M.; Moliadze, V.; Siniatchkin, M.; Buyx, A. Transcranial Direct Current Stimulation (tDCS) in Pediatric Populations—Voices from Typically Developing Children and Adolescents and their Parents. Neuroethics 2023, 16, 3. [Google Scholar] [CrossRef]
- Stein, A.; Caulfield, K.A.; Singh, M.; Riddle, J.; Friehs, M.A.; Craven, M.P.; Groom, M.J.; Iyer, K.K.; Barlow, K.M. The effect of a single session of tDCS on attention in pediatric acquired brain injury: Characterising inter-individual structural and functional network response variability. medRxiv 2025. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Filmer, H.L.; Dux, P.E.; Mattingley, J.B. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014, 37, 742–753. [Google Scholar] [CrossRef]
- Ulam, F.; Shelton, C.; Richards, L.; Davis, L.; Hunter, B.; Fregni, F.; Higgins, K. Cumulative effects of transcranial direct current stimulation on EEG oscillations and attention/working memory during subacute neurorehabilitation of traumatic brain injury. Clin. Neurophysiol. 2015, 126, 486–496. [Google Scholar] [CrossRef]
- Lesniak, M.; Polanowska, K.; Seniów, J.; Czlonkowska, A. Effects of repeated anodal tDCS coupled with cognitive training for patients with severe traumatic brain injury: A pilot randomized controlled trial. J. Head Trauma Rehabil. 2014, 29, E20–E29. [Google Scholar] [CrossRef]
- Li, L.M.; Violante, I.R.; Zimmerman, K.; Leech, R.; Hampshire, A.; Patel, M.; Opitz, A.; McArthur, D.; Jolly, A.; Carmichael, D.W. Traumatic axonal injury influences the cognitive effect of non-invasive brain stimulation. Brain 2019, 142, 3280–3293. [Google Scholar] [CrossRef]
- Stein, A.; Iyer, K.K.; Barlow, K.M. The effect of pediatric traumatic brain injury on simulated tDCS e-field. In Proceedings of the New York Neuroergonomics and Neuromodulation Meeting 2022, New York, NY, USA, 28 July–1 August 2022. [Google Scholar]
- Alekseichuk, I.; Wischnewski, M.; Opitz, A. A minimum effective dose for (transcranial) alternating current stimulation. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 2022, 15, 1221–1222. [Google Scholar] [CrossRef]
- Datta, A.; Truong, D.; Minhas, P.; Parra, L.C.; Bikson, M. Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front. Psychiatry 2012, 3, 91. [Google Scholar] [CrossRef]
- Caulfield, K.A.; Indahlastari, A.; Nissim, N.R.; Lopez, J.W.; Fleischmann, H.H.; Woods, A.J.; George, M.S. Electric field strength from prefrontal transcranial direct current stimulation determines degree of working memory response: A potential application of reverse-calculation modeling? Neuromodul. Technol. Neural Interface 2022, 25, 578–587. [Google Scholar] [CrossRef]
- Bergmann, T.O.; Hartwigsen, G. Inferring causality from noninvasive brain stimulation in cognitive neuroscience. J. Cogn. Neurosci. 2021, 33, 195–225. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.J.; Scott, G.; Leech, R. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 2014, 10, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.J. High density EEG—What do we have to lose? J. Neurophysiol. 2015, 126, 433–434. [Google Scholar] [CrossRef]
- Marquetand, J.; Vannoni, S.; Carboni, M.; Li Hegner, Y.; Stier, C.; Braun, C.; Focke, N.K. Reliability of magnetoencephalography and high-density electroencephalography resting-state functional connectivity metrics. Brain Connect. 2019, 9, 539–553. [Google Scholar] [CrossRef]
- Rolle, C.E.; Narayan, M.; Wu, W.; Toll, R.; Johnson, N.; Caudle, T.; Yan, M.; El-Said, D.; Watts, M.; Eisenberg, M.; et al. Functional connectivity using high density EEG shows competitive reliability and agreement across test/retest sessions. J. Neurosci. Methods 2022, 367, 109424. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef]
- Filmer, H.L.; Varghese, E.; Hawkins, G.E.; Mattingley, J.B.; Dux, P.E. Improvements in attention and decision-making following combined behavioral training and brain stimulation. Cereb. Cortex 2017, 27, 3675–3682. [Google Scholar] [CrossRef]
- Alonzo, A.; Brassil, J.; Taylor, J.L.; Martin, D.; Loo, C.K. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul. 2012, 5, 208–213. [Google Scholar] [CrossRef]
- Charvet, L.E.; Kasschau, M.; Datta, A.; Knotkova, H.; Stevens, M.C.; Alonzo, A.; Loo, C.; Krull, K.R.; Bikson, M. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: Guidelines for technology and protocols. Front. Syst. Neurosci. 2015, 9, 26. [Google Scholar] [CrossRef]
- Cappon, D.; den Boer, T.; Jordan, C.; Yu, W.; Lo, A.; LaGanke, N.; Biagi, M.C.; Skorupinski, P.; Ruffini, G.; Morales, O. Safety and feasibility of tele-supervised home-based transcranial direct current stimulation for major depressive disorder. Front. Aging Neurosci. 2022, 13, 765370. [Google Scholar] [CrossRef]
- Cappon, D.; den Boer, T.; Yu, W.; LaGanke, N.; Fox, R.; Brozgol, M.; Hausdorff, J.M.; Manor, B.; Pascual-Leone, A. An educational program for remote training and supervision of home-based transcranial electrical stimulation: Feasibility and preliminary effectiveness. Neuromodul. Technol. Neural Interface 2024, 27, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, G.; Vogel-Eyny, A.; Lustberg, M.; Best, P.; Malik, M.; Walton-Masters, L.; George, A.; Mirza, I.; Zhovtis, L.; Datta, A. Tolerability and feasibility of at-home remotely supervised transcranial direct current stimulation (RS-tDCS): Single-center evidence from 6,779 sessions. Brain Stimul. 2022, 15, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Charvet, L.E.; Shaw, M.T.; Bikson, M.; Woods, A.J.; Knotkova, H. Supervised transcranial direct current stimulation (tDCS) at home: A guide for clinical research and practice. Brain Stimul. 2020, 13, 686–693. [Google Scholar] [CrossRef]
- ACRM. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993, 8, 86–87. [Google Scholar] [CrossRef]
- Stein, A. Using Non-Invasive Brain Stimulation to Improve Attention Following Childhood Acquired Brain Injury. Ph.D. Thesis, The University of Queensland, Brisbane, QLD, Australia, 2024. [Google Scholar]
- Harbinson, M.; Zarshenas, S.; Cullen, N.K. Long-term functional and psychosocial outcomes after hypoxic-ischemic brain injury: A case-controlled comparison to traumatic brain injury. PM&R 2017, 9, 1200–1207. [Google Scholar]
- Nemanich, S.T.; Lench, D.H.; Sutter, E.N.; Kowalski, J.L.; Francis, S.M.; Meekins, G.D.; Krach, L.E.; Feyma, T.; Gillick, B.T. Safety and feasibility of transcranial direct current stimulation stratified by corticospinal organization in children with hemiparesis. Eur. J. Paediatr. Neurol. 2023, 43, 27–35. [Google Scholar] [CrossRef]
- Gillick, B.T.; Feyma, T.; Menk, J.; Usset, M.; Vaith, A.; Wood, T.J.; Worthington, R.; Krach, L.E. Safety and feasibility of transcranial direct current stimulation in pediatric hemiparesis: Randomized controlled preliminary study. Phys. Ther. 2015, 95, 337–349. [Google Scholar] [CrossRef]
- Ryan, J.L.; Beal, D.S.; Fehlings, D.L.; Levac, D.E.; Tendera, A.; Wright, F.V. Evaluating Transcranial Direct Current Stimulation as an Adjunct to Inpatient Physiotherapy in Paediatric Acquired Brain Injury: A Randomized Feasibility Trial. Physiother. Can. 2023, e20230015. [Google Scholar] [CrossRef]
- Pearson, N.; Naylor, P.-J.; Ashe, M.C.; Fernandez, M.; Yoong, S.L.; Wolfenden, L. Guidance for conducting feasibility and pilot studies for implementation trials. Pilot Feasibility Stud. 2020, 6, 167. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Schroeder, P.A.; Dignath, D.; Janczyk, M. Individual differences in uncertainty tolerance are not associated with cognitive control functions in the flanker task. Exp. Psychol. 2018, 65, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Craven, M.P.; Groom, M.J. Computer games for user engagement in Attention Deficit Hyperactivity Disorder (ADHD) monitoring and therapy. In Proceedings of the 2015 International Conference on Interactive Technologies and Games, Nottingham, UK, 22–23 October 2015; pp. 34–40. [Google Scholar]
- Donders, F.C. On the speed of mental processes. Acta Psychol. 1969, 30, 412–431. [Google Scholar] [CrossRef]
- Young, Z.; Craven, M.P.; Groom, M.; Crowe, J. Snappy App: A mobile continuous performance test with physical activity measurement for assessing Attention Deficit Hyperactivity Disorder. In Human-Computer Interaction. Applications and Services, Proceedings of the 16th International Conference, HCI International 2014, Heraklion, Crete, Greece, 22–27 June 2014; Proceedings, Part III 16; Springer: Cham, Switzerland, 2014; pp. 363–373. [Google Scholar]
- Gershon, R.C.; Wagster, M.V.; Hendrie, H.C.; Fox, N.A.; Cook, K.F.; Nowinski, C.J. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013, 80, S2–S6. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Bauer, P.J. National Institutes of Health Toolbox Cognition Battery (NIH Toolbox CB): Validation for Children Between 3 and 15 Years; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Friehs, M.A.; Dechant, M.; Vedress, S.; Frings, C.; Mandryk, R.L. Effective gamification of the stop-signal task: Two controlled laboratory experiments. JMIR Serious Games 2020, 8, e17810. [Google Scholar] [CrossRef]
- Schroeder, P.A.; Lohmann, J.; Ninaus, M. Preserved Inhibitory Control Deficits of Overweight Participants in a Gamified Stop-Signal Task: Experimental Study of Validity. JMIR Serious Games 2021, 9, e25063. [Google Scholar] [CrossRef]
- Fertonani, A.; Rosini, S.; Cotelli, M.; Rossini, P.M.; Miniussi, C. Naming facilitation induced by transcranial direct current stimulation. Behav. Brain Res. 2010, 208, 311–318. [Google Scholar] [CrossRef]
- Brooks, B.L.; Sherman, E.M. Computerized neuropsychological testing to rapidly evaluate cognition in pediatric patients with neurologic disorders. J. Child Neurol. 2012, 27, 982–991. [Google Scholar] [CrossRef]
- Conners, C.K.; Sitarenios, G.; Parker, J.D.; Epstein, J.N. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J. Abnorm. Child Psychol. 1998, 26, 257–268. [Google Scholar] [CrossRef]
- Nielsen, J.D.; Madsen, K.H.; Puonti, O.; Siebner, H.R.; Bauer, C.; Madsen, C.G.; Saturnino, G.B.; Thielscher, A. Automatic skull segmentation from MR images for realistic volume conductor models of the head: Assessment of the state-of-the-art. Neuroimage 2018, 174, 587–598. [Google Scholar] [CrossRef]
- Thielscher, A.; Antunes, A.; Saturnino, G.B. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 222–225. [Google Scholar]
- Saturnino, G.B.; Puonti, O.; Nielsen, J.D.; Antonenko, D.; Madsen, K.H.; Thielscher, A. SimNIBS 2.1: A comprehensive pipeline for individualized electric field modelling for transcranial brain stimulation. In Brain Human Body Modeling; Springer: Cham, Switzerland, 2019; pp. 3–25. [Google Scholar]
- Vanderwal, T.; Kelly, C.; Eilbott, J.; Mayes, L.C.; Castellanos, F.X. Inscapes: A movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage 2015, 122, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef] [PubMed]
- Fonov, V.; Evans, A.C.; Botteron, K.; Almli, C.R.; McKinstry, R.C.; Collins, D.L.; Group, B.D.C. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011, 54, 313–327. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Richards, J.E.; Almli, C.R. Age-specific MRI templates for pediatric neuroimaging. Dev. Neuropsychol. 2012, 37, 379–399. [Google Scholar] [CrossRef]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Calbi, M.; Siri, F.; Heimann, K.; Barratt, D.; Gallese, V.; Kolesnikov, A.; Umiltà, M.A. How context influences the interpretation of facial expressions: A source localization high-density EEG study on the “Kuleshov effect”. Sci. Rep. 2019, 9, 2107. [Google Scholar] [CrossRef]
- Gaser, C.; Dahnke, R.; Thompson, P.M.; Kurth, F.; Luders, E. CAT—A computational anatomy toolbox for the analysis of structural MRI data. bioRxiv 2022. [Google Scholar] [CrossRef]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef]
- Dale, A.M.; Liu, A.K.; Fischl, B.R.; Buckner, R.L.; Belliveau, J.W.; Lewine, J.D.; Halgren, E. Dynamic statistical parametric mapping: Combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 2000, 26, 55–67. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar]
- Hilbert, D. Grundzüge Einer Allgemeinen Theorie der Linearen Integralgleichungen; BG Teubner: Stuttgart, Germany, 1912. [Google Scholar]
- Zalesky, A.; Fornito, A.; Bullmore, E.T. Network-based statistic: Identifying differences in brain networks. Neuroimage 2010, 53, 1197–1207. [Google Scholar] [CrossRef]
- Stein, A.; Thorstensen, J.R.; Ho, J.M.; Ashley, D.P.; Iyer, K.K.; Barlow, K.M. Attention Please! Unravelling the link between brain network connectivity and cognitive attention following acquired brain injury: A systematic review of structural and functional measures. Brain Connect. 2024, 14, 4–38. [Google Scholar] [CrossRef] [PubMed]
- Bastani, A.; Jaberzadeh, S. Within-session repeated a-tDCS: The effects of repetition rate and inter-stimulus interval on corticospinal excitability and motor performance. Clin. Neurophysiol. 2014, 125, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Monte-Silva, K.; Kuo, M.-F.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). J. Neurophysiol. 2010, 103, 1735–1740. [Google Scholar] [CrossRef]
- Hordacre, B. The role of telehealth to assist in-home tDCS: Opportunities, promising results and acceptability. Brain Sci. 2018, 8, 102. [Google Scholar] [CrossRef]
- Buchanan, D.M.; D’Angiulli, A.; Samson, A.; Maisonneuve, A.R.; Robaey, P. Acceptability of transcranial direct current stimulation in children and adolescents with ADHD: The point of view of parents. J. Health Psychol. 2022, 27, 36–46. [Google Scholar] [CrossRef]
- Datta, A.; Baker, J.M.; Bikson, M.; Fridriksson, J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul. 2011, 4, 169–174. [Google Scholar] [CrossRef]
- Ågård, A.; Lomborg, K.; Tønnesen, E.; Egerod, I. Rehabilitation activities, out-patient visits and employment in patients and partners the first year after ICU: A descriptive study. Intensive Crit. Care Nurs. 2014, 30, 101–110. [Google Scholar] [CrossRef]
- Rimmer, R. Community-Based Tdcs Treatment for Depression: Acceptability and Neuropsychological Correlates. Ph.D. Thesis, University of East London, London, UK, 2025. [Google Scholar]
- Martin, D.M.; Liu, R.; Alonzo, A.; Green, M.; Loo, C.K. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: Effect of timing of stimulation. Exp. Brain Res. 2014, 232, 3345–3351. [Google Scholar] [CrossRef]
- Szafir, D.; Mutlu, B. Pay attention! Designing adaptive agents that monitor and improve user engagement. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Austin, TX, USA, 5–10 May 2012; pp. 11–20. [Google Scholar]
- Draheim, C.; Tsukahara, J.S.; Martin, J.D.; Mashburn, C.A.; Engle, R.W. A toolbox approach to improving the measurement of attention control. J. Exp. Psychol. 2021, 150, 242–275. [Google Scholar] [CrossRef]
- Kirton, A.; Ciechanski, P.; Zewdie, E.; Andersen, J.; Nettel-Aguirre, A.; Carlson, H.; Carsolio, L.; Herrero, M.; Quigley, J.; Mineyko, A. Transcranial direct current stimulation for children with perinatal stroke and hemiparesis. Neurology 2017, 88, 259–267. [Google Scholar] [CrossRef]
- Razza, L.B.; De Smet, S.; Van Hoornweder, S.; De Witte, S.; Luethi, M.S.; Baeken, C.; Brunoni, A.R.; Vanderhasselt, M.-A. Investigating the variability of prefrontal tDCS effects on working memory: An individual E-field distribution study. Cortex 2024, 172, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Nikolin, S.; Martin, D.; Loo, C.K.; Boonstra, T.W. Effects of TDCS dosage on working memory in healthy participants. Brain Stimul. 2018, 11, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, S.E.; Filmer, H.L.; Wards, Y.; Mattingley, J.B.; Dux, P.E. The influence of tDCS intensity on decision-making training and transfer outcomes. J. Neurophysiol. 2021, 125, 385–397. [Google Scholar] [CrossRef]
- Hoy, K.E.; Emonson, M.R.; Arnold, S.L.; Thomson, R.H.; Daskalakis, Z.J.; Fitzgerald, P.B. Testing the limits: Investigating the effect of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia 2013, 51, 1777–1784. [Google Scholar] [CrossRef]
- Moliadze, V.; Schmanke, T.; Andreas, S.; Lyzhko, E.; Freitag, C.M.; Siniatchkin, M. Stimulation intensities of transcranial direct current stimulation have to be adjusted in children and adolescents. Clin. Neurophysiol. 2015, 126, 1392–1399. [Google Scholar] [CrossRef]
- Levin, H.S.; Goldstein, F.C. Organization of verbal memory after severe closed-head injury. J. Clin. Exp. Neuropsychol. 1986, 8, 643–656. [Google Scholar] [CrossRef]
- Slomine, B.; Locascio, G. Cognitive rehabilitation for children with acquired brain injury. Dev. Disabil. Res. Rev. 2009, 15, 133–143. [Google Scholar] [CrossRef]
- Samogin, J.; Marino, M.; Porcaro, C.; Wenderoth, N.; Dupont, P.; Swinnen, S.P.; Mantini, D. Frequency-dependent functional connectivity in resting state networks. Hum. Brain Mapp. 2020, 41, 5187–5198. [Google Scholar] [CrossRef]
- Hillebrand, A.; Barnes, G.R.; Bosboom, J.L.; Berendse, H.W.; Stam, C.J. Frequency-dependent functional connectivity within resting-state networks: An atlas-based MEG beamformer solution. Neuroimage 2012, 59, 3909–3921. [Google Scholar] [CrossRef]
- Wu, C.W.; Gu, H.; Lu, H.; Stein, E.A.; Chen, J.-H.; Yang, Y. Frequency specificity of functional connectivity in brain networks. Neuroimage 2008, 42, 1047–1055. [Google Scholar] [CrossRef]
- Deiber, M.-P.; Hasler, R.; Colin, J.; Dayer, A.; Aubry, J.-M.; Baggio, S.; Perroud, N.; Ros, T. Linking alpha oscillations, attention and inhibitory control in adult ADHD with EEG neurofeedback. NeuroImage Clin. 2020, 25, 102145. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Iyer, K.K.; Khetani, A.M.; Barlow, K.M. Changes in working memory-related cortical responses following pediatric mild traumatic brain injury: A longitudinal fMRI study. J. Concussion 2021, 5, 20597002211006541. [Google Scholar] [CrossRef]
- Caeyenberghs, K.; Wenderoth, N.; Smits-Engelsman, B.; Sunaert, S.; Swinnen, S. Neural correlates of motor dysfunction in children with traumatic brain injury: Exploration of compensatory recruitment patterns. Brain 2009, 132, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, B.T.; Urban, K.; Da Costa, L.; Wong, S.M.; Pang, E.W.; Taylor, M.J. Default mode network oscillatory coupling is increased following concussion. Front. Neurol. 2018, 9, 280. [Google Scholar] [CrossRef]
- Stein, A.; To, X.V.; Nasrallah, F.; Barlow, K.M. Evidence of ongoing cerebral microstructural reorganization in children with persisting symptoms following mild traumatic brain injury: A NODDI DTI analysis. J. Neurotrauma 2024, 41, 41–58. [Google Scholar] [CrossRef]
- Gallagher, R.; Kessler, K.; Bramham, J.; Dechant, M.; Friehs, M.A. A proof-of-concept study exploring the effects of impulsivity on a gamified version of the stop-signal task in children. Front. Psychol. 2023, 14, 1068229. [Google Scholar] [CrossRef]
- Friehs, M.A.; Dechant, M.; Vedress, S.; Frings, C.; Mandryk, R.L. Shocking advantage! Improving digital game performance using non-invasive brain stimulation. Int. J. Hum.-Comput. Stud. 2021, 148, 102582. [Google Scholar] [CrossRef]
- Okamoto, M.; Dan, H.; Sakamoto, K.; Takeo, K.; Shimizu, K.; Kohno, S.; Oda, I.; Isobe, S.; Suzuki, T.; Kohyama, K.; et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 2004, 21, 99–111. [Google Scholar] [CrossRef]
- Caulfield, K.A.; George, M.S. Optimized APPS-tDCS electrode position, size, and distance doubles the on-target stimulation magnitude in 3000 electric field models. Sci. Rep. 2022, 12, 20116. [Google Scholar] [CrossRef]
- Geuzaine, C.; Remacle, J.F. Gmsh: A 3-D finite element mesh generator with built-in pre-and post-processing facilities. Int. J. Numer. Methods Eng. 2009, 79, 1309–1331. [Google Scholar] [CrossRef]
- Brainard, D.H.; Vision, S. The psychophysics toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D.G.; Vision, S. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M.; Brainard, D.; Pelli, D.; Ingling, A.; Murray, R.; Broussard, C. What’s new in psych-toolbox-3. Perception 2007, 36, 1–16. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Angelini, M.; Calbi, M.; Ferrari, A.; Sbriscia-Fioretti, B.; Franca, M.; Gallese, V.; Umiltà, M.A. Proactive control strategies for overt and covert go/nogo tasks: An electrical neuroimaging study. PLoS ONE 2016, 11, e0152188. [Google Scholar]
- Fraschini, M.; Demuru, M.; Crobe, A.; Marrosu, F.; Stam, C.J.; Hillebrand, A. The effect of epoch length on estimated EEG functional connectivity and brain network organisation. J. Neural Eng. 2016, 13, 036015. [Google Scholar] [CrossRef]
- Roig-Herrero, A.; Planchuelo-Gómez, Á.; Hernández-García, M.; de Luis-García, R.; Fernández-Linsenbarth, I.; Molina, V. Default mode network components and its relationship with anomalous self-experiences in schizophrenia: A rs-fMRI exploratory study. Psychiatry Res. Neuroimaging 2022, 324, 111495. [Google Scholar] [CrossRef]
- Pimontel, M.A.; Solomonov, N.; Oberlin, L.; Kanellopoulos, T.; Bress, J.N.; Hoptman, M.J.; Alexopoulos, G.S.; Gunning, F.M. Cortical thickness of the salience network and change in apathy following antidepressant treatment for late-life depression. Am. J. Geriatr. Psychiatry 2021, 29, 241–248. [Google Scholar] [CrossRef]
- Metzler-Baddeley, C.; Caeyenberghs, K.; Foley, S.; Jones, D.K. Task complexity and location specific changes of cortical thickness in executive and salience networks after working memory training. Neuroimage 2016, 130, 48–62. [Google Scholar] [CrossRef]
- Shen, K.k.; Welton, T.; Lyon, M.; McCorkindale, A.N.; Sutherland, G.T.; Burnham, S.; Fripp, J.; Martins, R.; Grieve, S.M. Structural core of the executive control network: A high angular resolution diffusion MRI study. Hum. Brain Mapp. 2020, 41, 1226–1236. [Google Scholar] [CrossRef]
- Whelan, R. Effective analysis of reaction time data. Psychol. Rec. 2008, 58, 475–482. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci. Biobehav. Rev. 2009, 33, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Aron, A.R.; Band, G.P.; Beste, C.; Bissett, P.G.; Brockett, A.T.; Brown, J.W.; Chamberlain, S.R.; Chambers, C.D.; Colonius, H. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 2019, 8, e46323. [Google Scholar] [CrossRef]
- Lo, S.; Andrews, S. To transform or not to transform: Using generalized linear mixed models to analyse reaction time data. Front. Psychol. 2015, 6, 1171. [Google Scholar] [CrossRef]
| 1 mA Group | 2 mA Group | |
|---|---|---|
| Age at baseline (years), mean (95% CI) | 11.9 (8.2, 15.4) | 13.7 (11.0, 16.5) |
| Age at injury (years), mean (95% CI) | 7.0 (−0.4, 14.5) | 9.6 (1.4, 17.8) |
| Time post-injury (years), mean (95% CI) | 4.8 (−0.7, 10.2) | 3.9 (−2.2, 10.0) |
| Sex, n males (%) | 4 (66.7) | 1 (25.0) |
| Handedness, n right (%) | 5 (83.3) | 4 (100.0) |
| Special help at school, n (%) | 4 (66.7) | 4 (100.0) |
| Medication, n yes (%) | 4 (66.7) | 2 (50.0) |
| TBI severity | ||
| Mild | 4 (66.6%) | 2 (33.3%) |
| Severe | 2 (33.3%) | 2 (33.3%) |
| CNSVS NCI, mean (95% CI) | 91.4 (72.2, 110.6) | 100.0 (79.1, 120.9) |
| CNSVS RT, mean (95% CI) | 104.8 (53.1, 156.5) | 99.5 (53.6, 145.4) |
| CNSVS complex attention, mean (95% CI) | 74.8 (32.9, 116.7) | 104.5 (93.6, 115.4) |
| BRIEF2 inhibit, mean (95% CI) | 65.4 (47.8, 83.0) | 59.5 (52.6, 66.4) |
| BRIEF2 shift, mean (95% CI) | 66.6 (45.1, 88.1) | 69.5 (40.7, 98.3) |
| Conners 4 inattention, mean (95% CI) | 61.2 (41.2, 81.2) | 68.7 (47.7, 89.8) |
| Conners 4 hyperactivity, mean (95% CI) | 62.2 (39.9, 84.5) | 57.8 (35.0, 80.5) |
| Conners 4 impulsivity, mean (95% CI) | 65.6 (46.2, 85.0) | 59.5 (34.9, 84.1) |
| Conners 4 ADHD score, mean (SD) | 17.2 (12.8) | 21.3 (9.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, A.; Riddle, J.; Caulfield, K.A.; Dux, P.E.; Friehs, M.A.; Schroeder, P.A.; Craven, M.P.; Groom, M.J.; Iyer, K.K.; Barlow, K.M. Safety, Feasibility, and Tolerability of Ten Days of At-Home, Remotely Supervised tDCS During Gamified Attention Training in Children with Acquired Brain Injury: An Open-Label, Dose-Controlled Pilot Trial. Brain Sci. 2025, 15, 561. https://doi.org/10.3390/brainsci15060561
Stein A, Riddle J, Caulfield KA, Dux PE, Friehs MA, Schroeder PA, Craven MP, Groom MJ, Iyer KK, Barlow KM. Safety, Feasibility, and Tolerability of Ten Days of At-Home, Remotely Supervised tDCS During Gamified Attention Training in Children with Acquired Brain Injury: An Open-Label, Dose-Controlled Pilot Trial. Brain Sciences. 2025; 15(6):561. https://doi.org/10.3390/brainsci15060561
Chicago/Turabian StyleStein, Athena, Justin Riddle, Kevin A. Caulfield, Paul E. Dux, Maximilian A. Friehs, Philipp A. Schroeder, Michael P. Craven, Madeleine J. Groom, Kartik K. Iyer, and Karen M. Barlow. 2025. "Safety, Feasibility, and Tolerability of Ten Days of At-Home, Remotely Supervised tDCS During Gamified Attention Training in Children with Acquired Brain Injury: An Open-Label, Dose-Controlled Pilot Trial" Brain Sciences 15, no. 6: 561. https://doi.org/10.3390/brainsci15060561
APA StyleStein, A., Riddle, J., Caulfield, K. A., Dux, P. E., Friehs, M. A., Schroeder, P. A., Craven, M. P., Groom, M. J., Iyer, K. K., & Barlow, K. M. (2025). Safety, Feasibility, and Tolerability of Ten Days of At-Home, Remotely Supervised tDCS During Gamified Attention Training in Children with Acquired Brain Injury: An Open-Label, Dose-Controlled Pilot Trial. Brain Sciences, 15(6), 561. https://doi.org/10.3390/brainsci15060561





