The Effects of Deep Brain Stimulation on Balance in Parkinson’s Disease as Measured Using Posturography—A Narrative Review
Abstract
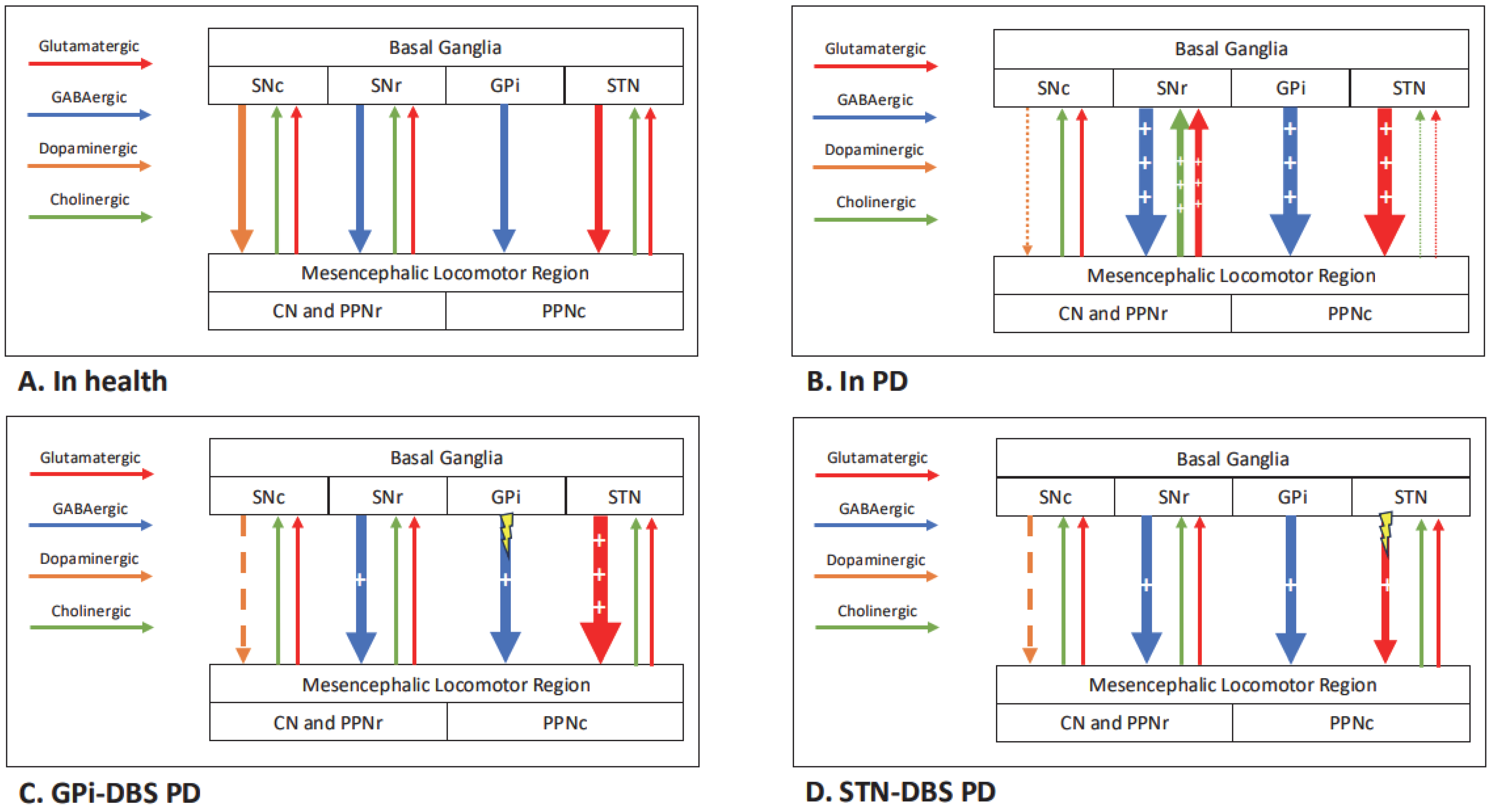
1. Introduction
1.1. Static Posturography
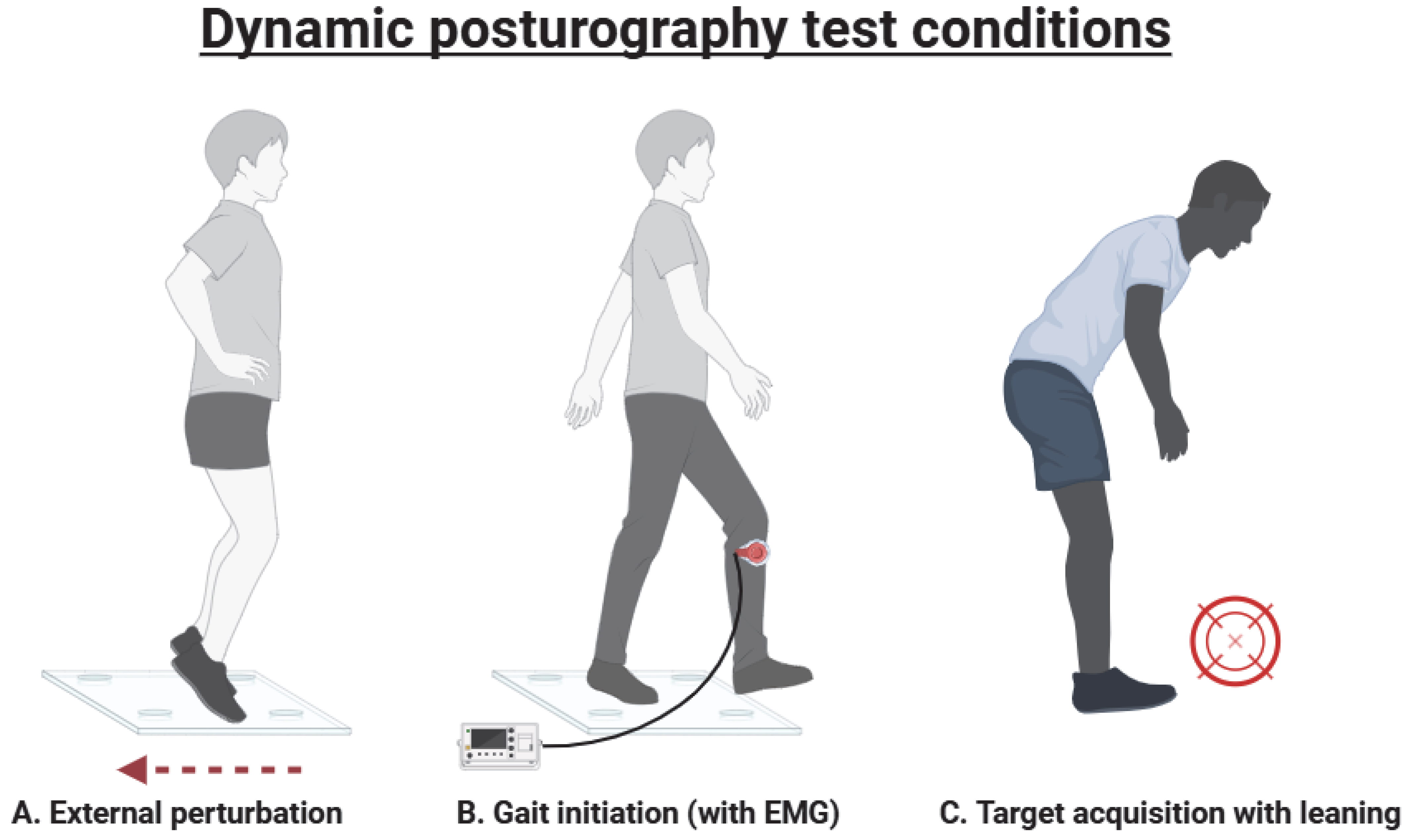
1.2. Dynamic Posturography
2. Materials and Methods
3. Results
3.1. Subthalamic Nucleus (STN)
3.2. Globus Pallidus Internus (GPi)
3.3. GPi vs. STN
3.4. Ventral Intermediate (VIM) Nucleus and Thalamic Tracts
3.5. Pedunculopontine Nucleus (PPN)
4. Discussion
4.1. Effects of DBS on Static Posturography
4.2. Effects of DBS on Dynamic Posturography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APA | Anticipatory postural adjustment |
| APR | Automatic postural response |
| CoM | Centre of mass |
| CoP | Centre of pressure |
| CN | Cuneiform nucleus |
| DBS | Deep brain stimulation |
| EMG | Electromyography |
| ET | Essential tremor |
| GPi | Globus pallidus internus |
| IMU | Inertial measurement unit |
| ISPGR | International Society for Posture and Gait Research |
| LOS | Limit of stability |
| PD | Parkinson’s disease |
| PPN | Pedunculopontine nucleus |
| SOT | Sensory Organisation Test |
| STN | Subthalamic nucleus |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VIM | Ventral intermediate nucleus |
References
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: Modelling study of Global Burden of Disease Study. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Glial reactions in Parkinson’s disease. Mov. Disord. 2008, 23, 474–483. [Google Scholar] [CrossRef]
- Blaylock, R.L. Parkinson’s disease: Microglial/macrophage-induced immunoexcitotoxicity as a central mechanism of neurodegeneration. Surg. Neurol. Int. 2017, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Madetko-Alster, N.; Otto-Ślusarczyk, D.; Migda, A.; Migda, B.; Struga, M.; Friedman, A. Role of orexin in pathogenesis of neurodegenerative parkinsonisms. Neurol. Neurochir. Pol. 2023, 57, 335–343. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.X.; Zhu, Y.Y.; Yu, Q.Y.; Msigwa, S.S.; Zeng, Z.H.; Zhang, X.; Wu, H.M.; Zhu, J.H. Epidemiologic Risk and Prevention and Interventions in Parkinson Disease: From a Nutrition-Based Perspective. J. Nutr. 2025, 155, 1019–1030. [Google Scholar] [CrossRef]
- Lee, J.W.; Song, Y.S.; Kim, H.; Ku, B.D.; Lee, W.W. Alteration of Tremor Dominant and Postural Instability Gait Difficulty Subtypes During the Progression of Parkinson’s Disease: Analysis of the PPMI Cohort. Front. Neurol. 2019, 10, 471. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Mason, S.L.; Evans, J.R.; Foltynie, T.; Brayne, C.; Robbins, T.W.; Barker, R.A. The CamPaIGN Study of Parkinson’s Disease: 10-Year Outlook in an Incident Population-Based Cohort. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1258–1264. [Google Scholar] [CrossRef]
- Visser, J.E.; Bloem, B.R. Role of the Basal Ganglia in Balance Control. Neural Plast. 2005, 12, 161–174. [Google Scholar] [CrossRef]
- French, I.T.; Muthusamy, K.A. A Review of the Pedunculopontine Nucleus in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Karachi, C.; Grabli, D.; Bernard, F.A.; Tandé, D.; Wattiez, N.; Belaid, H.; Bardinet, E.; Prigent, A.; Nothacker, H.P.; Hunot, S.; et al. Cholinergic Mesencephalic Neurons Are Involved in Gait and Postural Disorders in Parkinson Disease. J. Clin. Investig. 2010, 120, 2745–2754. [Google Scholar] [CrossRef]
- Bohnen, N.; Albin, R. Cholinergic Denervation Occurs Early in Parkinson Disease. Neurology 2009, 73, 256–257. [Google Scholar] [CrossRef]
- Jordan, L.M.; Liu, J.; Hedlund, P.B.; Akay, T.; Pearson, K.G. Descending Command Systems for the Initiation of Locomotion in Mammals. Brain Res. Rev. 2008, 57, 183–191. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical Control of Postural Responses. J. Neural Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef]
- Cardin, V.; Smith, A.T. Sensitivity of Human Visual and Vestibular Cortical Regions to Egomotion-Compatible Visual Stimulation. Cereb. Cortex 2010, 20, 1964–1973. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Brontë-Stewart, H. High Frequency Deep Brain Stimulation and Neural Rhythms in Parkinson’s Disease. Neuropsychol. Rev. 2015, 25, 384–397. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Anderson, R.W. Deep Brain Stimulation Mechanisms: The Control of Network Activity via Neurochemistry Modulation. J. Neurochem. 2016, 139, 338–345. [Google Scholar] [CrossRef]
- Kogan, M.; McGuire, M.; Riley, J. Deep Brain Stimulation for Parkinson Disease. Neurosurg. Clin. 2019, 30, 137–146. [Google Scholar] [CrossRef]
- Dostrovsky, J.O.; Lozano, A.M. Mechanisms of Deep Brain Stimulation. Mov. Disord. 2002, 17, S63–S68. [Google Scholar] [CrossRef]
- Pollak, P.; Benabid, A.L.; Gross, C.; Gao, D.M.; Laurent, A.; Benazzouz, A.; Hoffmann, D.; Gentil, M.; Perret, J. Effects of the Stimulation of the Subthalamic Nucleus in Parkinson Disease. Rev. Neurol. 1993, 149, 175–176. [Google Scholar]
- Weaver, F.M.; Follett, K.; Stern, M.; Hur, K.; Harris, C.; Marks, W.J.; Rothlind, J.; Sagher, O.; Reda, D.; Moy, C.S.; et al. Bilateral Deep Brain Stimulation vs Best Medical Therapy for Patients with Advanced Parkinson Disease: A Randomized Controlled Trial. JAMA 2009, 301, 63–73. [Google Scholar] [CrossRef]
- Fasano, A.; Aquino, C.C.; Krauss, J.K.; Honey, C.R.; Bloem, B.R. Axial Disability and Deep Brain Stimulation in Patients with Parkinson Disease. Nat. Rev. Neurol. 2015, 11, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Zampogna, A.; Cavallieri, F.; Bove, F.; Suppa, A.; Castrioto, A.; Meoni, S.; Pélissier, P.; Schmitt, E.; Bichon, A.; Lhommée, E.; et al. Axial Impairment and Falls in Parkinson’s Disease: 15 Years of Subthalamic Deep Brain Stimulation. NPJ Park. Dis. 2022, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Rolland, A.S.; Karachi, C.; Muriel, M.P.; Hirsch, E.C.; François, C. Internal Pallidum and Substantia Nigra Control Different Parts of the Mesopontine Reticular Formation in Primate. Mov. Disord. 2011, 26, 1648–1656. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhang, Y.Q.; Zhang, X.H.; Wang, Y.P.; Li, J.P.; Li, Y.J. Deep Brain Stimulation of Pedunculopontine Nucleus for Postural Instability and Gait Disorder After Parkinson Disease: A Meta-Analysis of Individual Patient Data. World Neurosurg. 2017, 102, 72–78. [Google Scholar] [CrossRef]
- Yu, K.; Ren, Z.; Hu, Y.; Guo, S.; Ye, X.; Li, J.; Li, Y. Efficacy of Caudal Pedunculopontine Nucleus Stimulation on Postural Instability and Gait Disorders in Parkinson’s Disease. Acta Neurochir. 2022, 164, 575–585. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Berardelli, A. Sensorimotor Integration in Movement Disorders. Mov. Disord. 2003, 18, 231–240. [Google Scholar] [CrossRef]
- Versace, V.; Campostrini, S.; Sebastianelli, L.; Saltuari, L.; Valls-Solé, J.; Kofler, M. Influence of Posture on Blink Reflex Prepulse Inhibition Induced by Somatosensory Inputs from Upper and Lower Limbs. Gait Posture 2019, 73, 120–125. [Google Scholar] [CrossRef]
- Fetsch, C.R.; Deangelis, G.C.; Angelaki, D.E. Bridging the Gap between Theories of Sensory Cue Integration and the Physiology of Multisensory Neurons. Nat. Rev. Neurosci. 2013, 14, 429–442. [Google Scholar] [CrossRef]
- DiFrancisco-Donoghue, J.; Jung, M.K.; Geisel, P.; Werner, W.G. Learning Effects of the Sensory Organization Test as a Measure of Postural Control and Balance in Parkinson’s Disease. Park. Relat. Disord. 2015, 21, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Difrancisco-Donoghue, J.; Jung, M.-K.; Apoznanski, T.; Werner, W.G.; Yao, S. The Reliability of the Sensory Organization Test in Parkinson’s Disease to Identify Fall Risk. Int. J. Neurol. Phys. Ther. 2016, 2, 39–43. [Google Scholar]
- Waterston, J.A.; Hawken, M.B.; Tanyeri, S.; Jantti, P.; Kennard, C. Influence of Sensory Manipulation on Postural Control in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1993, 56, 1276–1281. [Google Scholar] [CrossRef]
- Carpenter, M.G.; Allum, J.H.J.; Honegger, F.; Adkin, A.L.; Bloem, B.R. Postural Abnormalities to Multidirectional Stance Perturbations in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1245–1254. [Google Scholar] [CrossRef]
- Bloem, B.R.; Visser, J.E.; Allum, J.H.J. Posturography. In Handbook of Clinical Neurophysiology; Hallett, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 1. [Google Scholar]
- Visser, J.E.; Carpenter, M.G.; van der Kooij, H.; Bloem, B.R. The Clinical Utility of Posturography. Clin. Neurophysiol. 2008, 119, 2424–2436. [Google Scholar] [CrossRef]
- Fransson, P.A.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Patel, M. Deep Brain Stimulation in the Subthalamic Nuclei Alters Postural Alignment and Adaptation in Parkinson’s Disease. PLoS ONE 2021, 16, e0259862. [Google Scholar] [CrossRef]
- Nashner, L.M.; Owen Black, F.; Wall, C.; Good, S. Adaptation to Altered Support and Visual Conditions During Stance: Patients with Vestibular Deficits. J. Neurosci. 1982, 2, 536–544. [Google Scholar] [CrossRef]
- Mackey, D.C.; Robinovitch, S.N. Mechanisms Underlying Age-Related Differences in Ability to Recover Balance with the Ankle Strategy. Gait Posture 2006, 23, 59–68. [Google Scholar] [CrossRef]
- Baston, C.; Mancini, M.; Rocchi, L.; Horak, F. Effects of Levodopa on Postural Strategies in Parkinson’s Disease. Gait Posture 2016, 46, 26–29. [Google Scholar] [CrossRef]
- Baston, C.; Mancini, M.; Schoneburg, B.; Horak, F.; Rocchi, L. Postural Strategies Assessed with Inertial Sensors in Healthy and Parkinsonian Subjects. Gait Posture 2014, 40, 70–75. [Google Scholar] [CrossRef]
- Patel, M.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Fransson, P.A. Strategic Alterations of Posture Are Delayed in Parkinson’s Disease Patients during Deep Brain Stimulation. Sci. Rep. 2021, 11, 23550. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.S.; Najafi, T.; Schuck, L.; Wang, T.; Ivry, R.B. Implicit Sensorimotor Adaptation Is Preserved in Parkinson’s Disease. Brain Commun. 2022, 4, fcac303. [Google Scholar] [CrossRef]
- Liu, W.; McIntire, K.; Kim, S.H.; Zhang, J.; Dascalos, S.; Lyons, K.E.; Pahwa, R. Bilateral Subthalamic Stimulation Improves Gait Initiation in Patients with Parkinson’s Disease. Gait Posture 2006, 23, 492–498. [Google Scholar] [CrossRef]
- Jian, Y.; Winter, D.A.; Ishac, M.G.; Gilchrist, L. Trajectory of the Body COG and COP during Initiation and Termination of Gait. Gait Posture 1993, 1, 9–22. [Google Scholar] [CrossRef]
- Elble, R.J.; Moody, C.; Leffler, K.; Sinha, R. The Initiation of Normal Walking. Mov. Disord. 1994, 9, 139–146. [Google Scholar] [CrossRef]
- Breniere, Y.; Cuong Do, M.; Bouisset, S. Are Dynamic Phenomena Prior to Stepping Essential to Walking? J. Mot. Behav. 1987, 19, 62–76. [Google Scholar] [CrossRef]
- Brecl Jakob, G.; Pelykh, O.; Plate, A.; Košutzká, Z.; Pirtošek, Z.; Trošt, M.; Ilmberger, J.; Valkovic, P.; Mehrkens, J.H.; Bötzel, K. Hypometric Anticipatory Postural Adjustments in Dystonia Are Not Affected by Deep Brain Stimulation of Globus Pallidus Internus. Neurosci. Lett. 2017, 636, 151–157. [Google Scholar] [CrossRef]
- Rocchi, L.; Carlson-Kuhta, P.; Chiari, L.; Burchiel, K.J.; Hogarth, P.; Horak, F.B. Effects of Deep Brain Stimulation in the Subthalamic Nucleus or Globus Pallidus Internus on Step Initiation in Parkinson Disease: Laboratory Investigation. J. Neurosurg. 2012, 117, 1141–1149. [Google Scholar] [CrossRef]
- Ng, T.H.B.; Sowman, P.F.; Brock, J.; Johnson, B.W. Neuromagnetic Imaging Reveals Timing of Volitional and Anticipatory Motor Control in Bimanual Load Lifting. Behav. Brain Res. 2013, 247, 182–192. [Google Scholar] [CrossRef]
- Horak, F.B.; Dimitrova, D.; Nutt, J.G. Direction-Specific Postural Instability in Subjects with Parkinson’s Disease. Exp. Neurol. 2005, 193, 504–521. [Google Scholar] [CrossRef]
- Juras, G.; Słomka, K.; Fredyk, A.; Sobota, G.; Bacik, B. Evaluation of the Limits of Stability (LOS) Balance Test. J. Hum. Kinet. 2008, 19, 39–52. [Google Scholar] [CrossRef]
- McIntyre, C.; Richardson, S.; Frankemolle, A.; Varga, G.; Noecker, A.; Alberts, J. Improving Postural Stability via Computational Modelling Approach to Deep Brain Stimulation Programming. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 1 December 2011. [Google Scholar]
- Nantel, J.; McDonald, J.C.; Bronte-Stewart, H. Effect of Medication and STN-DBS on Postural Control in Subjects with Parkinson’s Disease. Park. Relat. Disord. 2012, 18, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Shivitz, N.; Koop, M.M.; Fahimi, J.; Heit, G.; Bronte-Stewart, H.M. Bilateral Subthalamic Nucleus Deep Brain Stimulation Improves Certain Aspects of Postural Control in Parkinson’s Disease, Whereas Medication Does Not. Mov. Disord. 2006, 21, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Colnat-Coulbois, S.; Gauchard, G.C.; Maillard, L.; Barroche, G.; Vespignani, H.; Auque, J.; Perrin, P.P. Bilateral Subthalamic Nucleus Stimulation Improves Balance Control in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 780–787. [Google Scholar] [CrossRef]
- Guehl, D.; Dehail, P.; De Sèze, M.P.; Cuny, E.; Faux, P.; Tison, F.; Barat, M.; Bioulac, B.; Burbaud, P. Evolution of Postural Stability after Subthalamic Nucleus Stimulation in Parkinson’s Disease: A Combined Clinical and Posturometric Study. Exp. Brain Res. 2006, 170, 206–215. [Google Scholar] [CrossRef]
- De la Casa-Fages, B.; Alonso-Frech, F.; Grandas, F. Effect of Subthalamic Nucleus Deep Brain Stimulation on Balance in Parkinson’s Disease: A Static Posturographic Analysis. Gait Posture 2017, 52, 374–380. [Google Scholar] [CrossRef]
- Liu, W.; McIntire, K.; Kim, S.H.; Zhang, J.; Dascalos, S.; Lyons, K.E.; Pahwa, R. Quantitative Assessments of the Effect of Bilateral Subthalamic Stimulation on Multiple Aspects of Sensorimotor Function for Patients with Parkinson’s Disease. Park. Relat. Disord. 2005, 11, 503–508. [Google Scholar] [CrossRef]
- Oz, F.; Yucekeya, B.; Huzmeli, I.; Yilmaz, A. Does Subthalamic Nucleus Deep Brain Stimulation Affect the Static Balance at Different Frequencies? Neurocirugia 2023, 34, 60–66. [Google Scholar] [CrossRef]
- Vallabhajosula, S.; Haq, I.U.; Hwynn, N.; Oyama, G.; Okun, M.; Tillman, M.D.; Hass, C.J. Low-Frequency versus High-Frequency Subthalamic Nucleus Deep Brain Stimulation on Postural Control and Gait in Parkinson’s Disease: A Quantitative Study. Brain Stimul. 2015, 8, 64–75. [Google Scholar] [CrossRef]
- Cani, I.; D’Ascanio, I.; Baldelli, L.; Lopane, G.; Ranciati, S.; Mantovani, P.; Conti, A.; Cortelli, P.; Calandra-Buonaura, G.; Chiari, L.; et al. Evaluating gait and postural responses to subthalamic stimulation and levodopa: A prospective study using wearable technology. Eur. J. Neurol. 2025, 32, e16580. [Google Scholar] [CrossRef]
- Szlufik, S.; Kloda, M.; Friedman, A.; Potrzebowska, I.; Gregier, K.; Mandat, T.; Przybyszewski, A.; Dutkiewicz, J.; Figura, M.; Habela, P.; et al. The Neuromodulatory Impact of Subthalamic Nucleus Deep Brain Stimulation on Gait and Postural Instability in Parkinson’s Disease Patients: A Prospective Case Controlled Study. Front. Neurol. 2018, 9, 906. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Aita, N.; Hokari, Y.; Kitahara, E.; Tani, M.; Izawa, N.; Hatori, K.; Nakamura, R.; Sasaki, F.; Sekimoto, S.; et al. Balance and Gait Improvements of Postoperative Rehabilitation in Patients with Parkinson’s Disease Treated with Subthalamic Nucleus Deep Brain Stimulation (STN-DBS). Park. Dis. 2019, 2019, 7104071. [Google Scholar] [CrossRef] [PubMed]
- Collomb-Clerc, A.; Welter, M.L. Effects of Deep Brain Stimulation on Balance and Gait in Patients with Parkinson’s Disease: A Systematic Neurophysiological Review. Neurophysiol. Clin. 2015, 45, 371–388. [Google Scholar] [CrossRef]
- Patel, M.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Fransson, P.A. Spectral Analysis of Body Movement during Deep Brain Stimulation in Parkinson’s Disease. Gait Posture 2021, 86, 217–225. [Google Scholar] [CrossRef]
- Patel, M.; Nilsson, M.H.; Rehncrona, S.; Tjernström, F.; Magnusson, M.; Johansson, R.; Fransson, P.A. Effects of Deep Brain Stimulation on Postural Control in Parkinson’s Disease. Comput. Biol. Med. 2020, 122, 103828. [Google Scholar] [CrossRef]
- Temiz, G.; Santin, M.D.N.; Olivier, C.; Collomb-Clerc, A.; Fernandez-Vidal, S.; Hainque, E.; Bardinet, E.; Lau, B.; François, C.; Karachi, C.; et al. Freezing of Gait Depends on Cortico-Subthalamic Network Recruitment Following STN-DBS in PD Patients. Park. Relat. Disord. 2022, 104, 49–57. [Google Scholar] [CrossRef]
- Muniz, A.M.S.; Liu, H.; Lyons, K.E.; Pahwa, R.; Liu, W.; Nadal, J. Quantitative Evaluation of the Effects of Subthalamic Stimulation on Gait in Parkinson’s Disease Patients Using Principal Component Analysis. Int. J. Neurosci. 2010, 120, 609–616. [Google Scholar] [CrossRef]
- Muniz, A.M.S.; Nadal, J.; Lyons, K.E.; Pahwa, R.; Liu, W. Long-Term Evaluation of Gait Initiation in Six Parkinson’s Disease Patients with Bilateral Subthalamic Stimulation. Gait Posture 2012, 35, 452–457. [Google Scholar] [CrossRef]
- Cherif, S.; Tempier, N.; Yeche, M.; Temiz, G.; Perrière, J.; Romanato, M.; Ziri, D.; Fernandez-Vidal, S.; Hainque, E.; Maltête, D.; et al. Directional Subthalamic Deep Brain Stimulation Better Improves Gait and Balance Disorders in Parkinson’s Disease Patients: A Randomized Controlled Study. Ann. Neurol. 2024, 97, 149–162. [Google Scholar] [CrossRef]
- Leodori, G.; Santilli, M.; Modugno, N.; D’Avino, M.; De Bartolo, M.; Fabbrini, A.; Rocchi, L.; Conte, A.; Fabbrini, G.; Belvisi, D. Postural Instability and Risk of Falls in Patients with Parkinson’s Disease Treated with Deep Brain Stimulation: A Stabilometric Platform Study. Brain Sci. 2023, 13, 1243. [Google Scholar] [CrossRef]
- Heß, T.; Oehlwein, C.; Milani, T.L. Anticipatory Postural Adjustments and Compensatory Postural Responses to Multidirectional Perturbations—Effects of Medication and Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease. Brain Sci. 2023, 13, 454. [Google Scholar] [CrossRef]
- Krishnamurthi, N.; Mulligan, S.; Mahant, P.; Samanta, J.; Abbas, J.J. Deep Brain Stimulation Amplitude Alters Posture Shift Velocity in Parkinson’s Disease. Cogn. Neurodyn. 2012, 6, 325–332. [Google Scholar] [CrossRef]
- Li, H.; Liang, S.; Yu, Y.; Wang, Y.; Cheng, Y.; Yang, H.; Tong, X. Effect of Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) on Balance Performance in Parkinson’s Disease. PLoS ONE 2020, 15, e0238936. [Google Scholar] [CrossRef]
- Li, H.; Liang, S.; Yu, Y.; Wang, Y.; Cheng, Y.; Yang, H.; Tong, X. Clinical Experience of Comprehensive Treatment on the Balance Function of Parkinson’s Disease. Medicine 2020, 99, e20154. [Google Scholar] [CrossRef]
- Johnson, L.; Rodrigues, J.; Teo, W.-P.; Walters, S.; Stell, R.; Thickbroom, G.; Mastaglia, F.; Johnson, L. Interactive Effects of GPI Stimulation and Levodopa on Postural Control in Parkinson’s Disease. Gait Posture 2015, 41, 929–934. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. Neurol. Neurosurg. Psychiatry 2002, 73, 267–274. [Google Scholar] [CrossRef]
- Brandmeir, N.J.; Brandmeir, C.L.; Carr, D.; Kuzma, K.; McInerney, J. Deep Brain Stimulation for Parkinson Disease Does Not Worsen or Improve Postural Instability: A Prospective Cohort Trial. Clin. Neurosurg. 2018, 83, 1173–1181. [Google Scholar] [CrossRef]
- St George, R.; Carlson-Kuhta, P.; King, L.; Burchiel, K.; Horak, F. Compensatory Stepping in Parkinson’s Disease Is Still a Problem after Deep Stimulation Randomized to STN or GPi. Neurobiol. Deep. Brain Stimul. 2015, 114, 1417–1423. [Google Scholar] [CrossRef]
- St George, R.J.; Carlson-Kuhta, P.; Burchiel, K.J.; Hogarth, P.; Frank, N.; Horak, F.B. The Effects of Subthalamic and Pallidal Deep Brain Stimulation on Postural Responses in Patients with Parkinson Disease: Laboratory Investigation. J. Neurosurg. 2012, 116, 1347–1356. [Google Scholar] [CrossRef]
- Ondo, W.G.; Almaguer, M.; Cohen, H. Computerized Posturography Balance Assessment of Patients with Bilateral Ventralis Intermedius Nuclei Deep Brain Stimulation. Mov. Disord. 2006, 21, 2243–2247. [Google Scholar] [CrossRef]
- Rocha, M.S.G.; De Freitas, J.L.; Costa, C.D.M.; De Oliveira, M.O.; Terzian, P.R.; Queiroz, J.W.M.; Ferraz, J.B.; Tatsch, J.F.S.; Soriano, D.C.; Hamani, C.; et al. Fields of Forel Brain Stimulation Improves Levodopa-Unresponsive Gait and Balance Disorders in Parkinson’s Disease. Neurosurgery 2021, 89, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.; Vilela Filho, O.; Viselli, F.; Insola, A.; Sposato, S.; Vitale, F.; Scarnati, E. Our First Decade of Experience in Deep Brain Stimulation of the Brainstem: Elucidating the Mechanism of Action of Stimulation of the Ventrolateral Pontine Tegmentum. J. Neural Transm. 2016, 123, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Welter, M.L.; Demain, A.; Ewenczyk, C.; Czernecki, V.; Lau, B.; El Helou, A.; Belaid, H.; Yelnik, J.; François, C.; Bardinet, E.; et al. PPNa-DBS for Gait and Balance Disorders in Parkinson’s Disease: A Double-Blind, Randomised Study. J. Neurol. 2015, 262, 1515–1525. [Google Scholar] [CrossRef]
- Yousif, N.; Bhatt, H.; Bain, P.G.; Nandi, D.; Seemungal, B.M. The Effect of Pedunculopontine Nucleus Deep Brain Stimulation on Postural Sway and Vestibular Perception. Eur. J. Neurol. 2016, 23, 668–670. [Google Scholar] [CrossRef]
- Bourilhon, J.; Olivier, C.; You, H.; Collomb-Clerc, A.; Grabli, D.; Belaid, H.; Mullie, Y.; François, C.; Czernecki, V.; Lau, B.; et al. Pedunculopontine and Cuneiform Nuclei Deep Brain Stimulation for Severe Gait and Balance Disorders in Parkinson’s Disease: Interim Results from a Randomized Double-Blind Clinical Trial. J. Park. Dis. 2022, 12, 639–653. [Google Scholar] [CrossRef]
- Bourilhon, J.; Mullie, Y.; Olivier, C.; Cherif, S.; Belaid, H.; Grabli, D.; Czernecki, V.; Karachi, C.; Welter, M.L. Stimulation of the Pedunculopontine and Cuneiform Nuclei for Freezing of Gait and Falls in Parkinson Disease: Cross-over Single-Blinded Study and Long-Term Follow-Up. Park. Relat. Disord. 2022, 96, 13–17. [Google Scholar] [CrossRef]
- Błaszczyk, J.W. The Use of Force-Plate Posturography in the Assessment of Postural Instability. Gait Posture 2016, 44, 1–6. [Google Scholar] [CrossRef]
- Gagey, P.-M. International Standardization of Clinical Stabilometry (Minutes of the Meeting of Posturologists, Paris 07.10.2015). Man. Ther. Posturology Rehabil. J. 2020, 1–3. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ishikawa, K.; Aoki, M.; Mizuta, K.; Ito, Y.; Asai, M.; Shojaku, H.; Yamanaka, T.; Fujimoto, C.; Murofushi, T.; et al. Japanese Standard for Clinical Stabilometry Assessment: Current Status and Future Directions. Auris Nasus Larynx 2018, 45, 201–206. [Google Scholar] [CrossRef]
- Carrick, F.; Hankir, A.; Zaman, R.; Wright, C. Metrological Performance of Instruments Used in Clinical Evaluation of Balance. Psychiatr. Danub. 2019, 31, 324–330. [Google Scholar]
- Ciocca, M.; Hosli, S.; Hadi, Z.; Mahmud, M.; Tai, Y.F.; Seemungal, B.M. Vestibular Prepulse Inhibition of the Human Blink Reflex. Clin. Neurophysiol. 2024, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Alam, M.; Schwabe, K.; Krauss, J.K. The Pedunculopontine Nucleus Area: Critical Evaluation of Interspecies Differences Relevant for Its Use as a Target for Deep Brain Stimulation. Brain 2011, 134, 11–23. [Google Scholar] [CrossRef]
- Lizárraga, K.J.; Gnanamanogaran, B.; Al-Ozzi, T.M.; Cohn, M.; Tomlinson, G.; Boutet, A.; Elias, G.J.B.; Germann, J.; Soh, D.; Kalia, S.K.; et al. Lateralized Subthalamic Stimulation for Axial Dysfunction in Parkinson’s Disease: A Randomized Trial. Mov. Disord. 2022, 37, 1079–1087. [Google Scholar] [CrossRef]
- Devos, D.; Derambure, P.; Bourriez, J.L.; Cassim, D.F.; Blond, S.; Guieu, J.D.; Destée, A.; Defebvre, L. Influence of Internal Globus Pallidus Stimulation on Motor Cortex Activation Pattern in Parkinson’s Disease. Clin. Neurophysiol. 2002, 113, 1110–1120. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L.; Cappello, A. Feature Selection of Stabilometric Parameters Based on Principal Component Analysis. Med. Biol. Eng. Comput. 2004, 42, 71–79. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lonergan, B.; Seemungal, B.M.; Ciocca, M.; Tai, Y.F. The Effects of Deep Brain Stimulation on Balance in Parkinson’s Disease as Measured Using Posturography—A Narrative Review. Brain Sci. 2025, 15, 535. https://doi.org/10.3390/brainsci15050535
Lonergan B, Seemungal BM, Ciocca M, Tai YF. The Effects of Deep Brain Stimulation on Balance in Parkinson’s Disease as Measured Using Posturography—A Narrative Review. Brain Sciences. 2025; 15(5):535. https://doi.org/10.3390/brainsci15050535
Chicago/Turabian StyleLonergan, Bradley, Barry M. Seemungal, Matteo Ciocca, and Yen F. Tai. 2025. "The Effects of Deep Brain Stimulation on Balance in Parkinson’s Disease as Measured Using Posturography—A Narrative Review" Brain Sciences 15, no. 5: 535. https://doi.org/10.3390/brainsci15050535
APA StyleLonergan, B., Seemungal, B. M., Ciocca, M., & Tai, Y. F. (2025). The Effects of Deep Brain Stimulation on Balance in Parkinson’s Disease as Measured Using Posturography—A Narrative Review. Brain Sciences, 15(5), 535. https://doi.org/10.3390/brainsci15050535





