The Influence of Transcranial Alternating Current Stimulation on the Excitability of the Unstimulated Contralateral Primary Motor Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
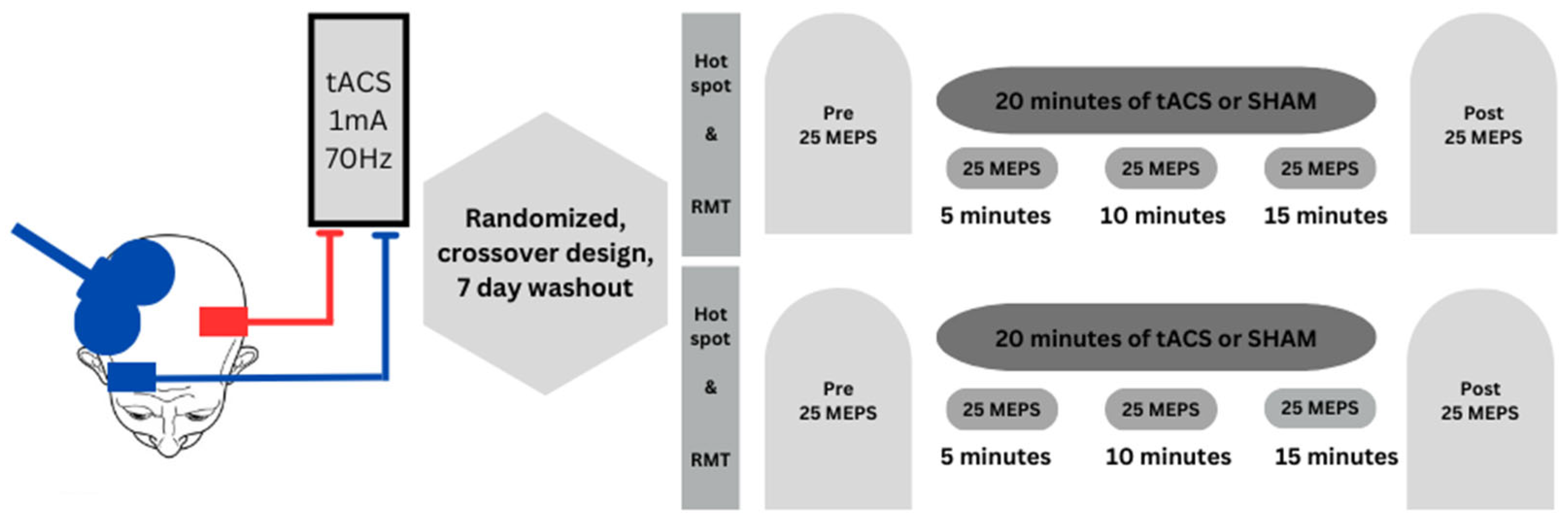
2.2. Experimental Design
2.3. Experimental Procedures
2.3.1. TMS Administration and Surface EMG Recording
2.3.2. tACS Administration and Electrode Placement
2.4. Data and Statistical Analyses
3. Results
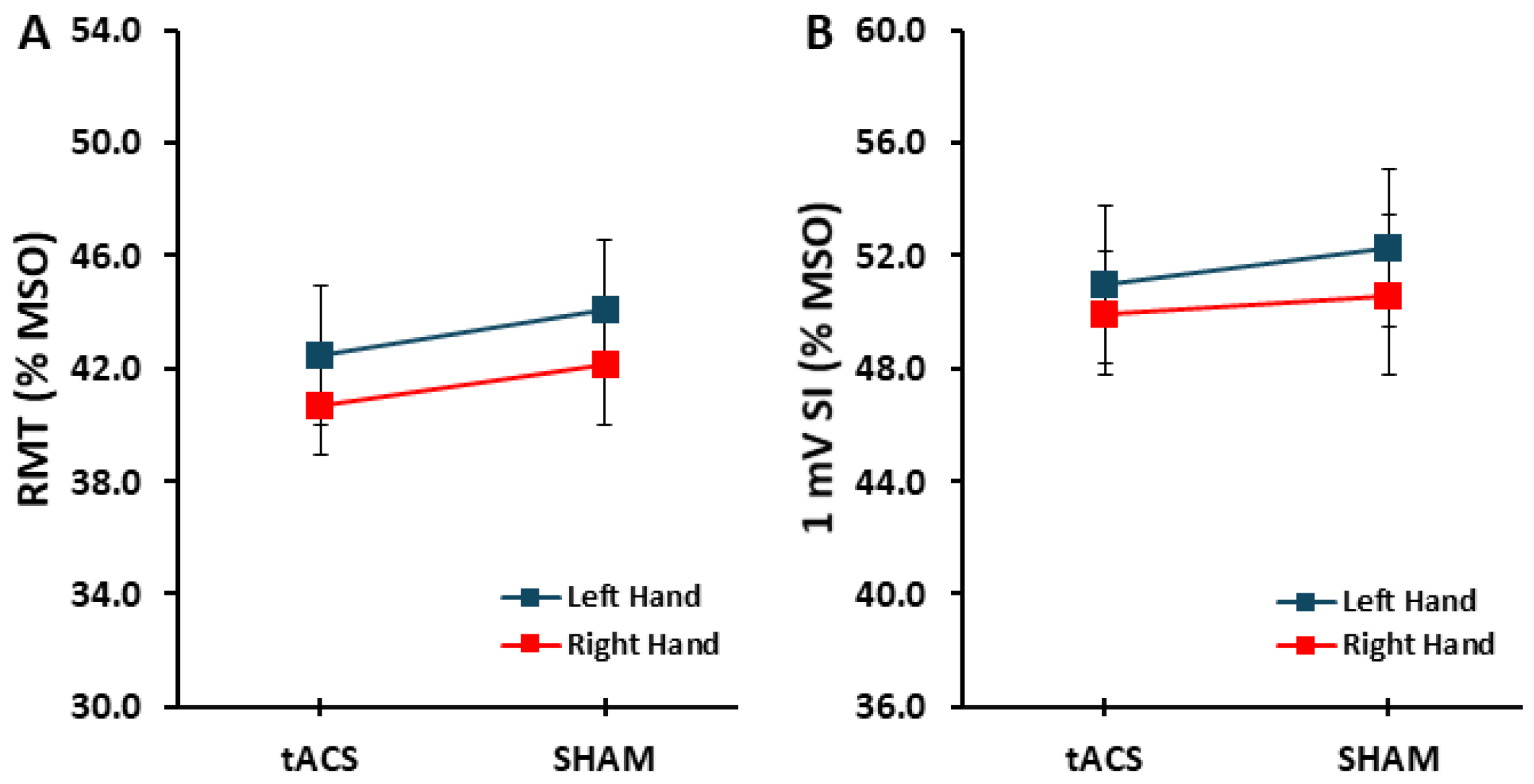
3.1. Control Variables: RMT and 1 mV SI
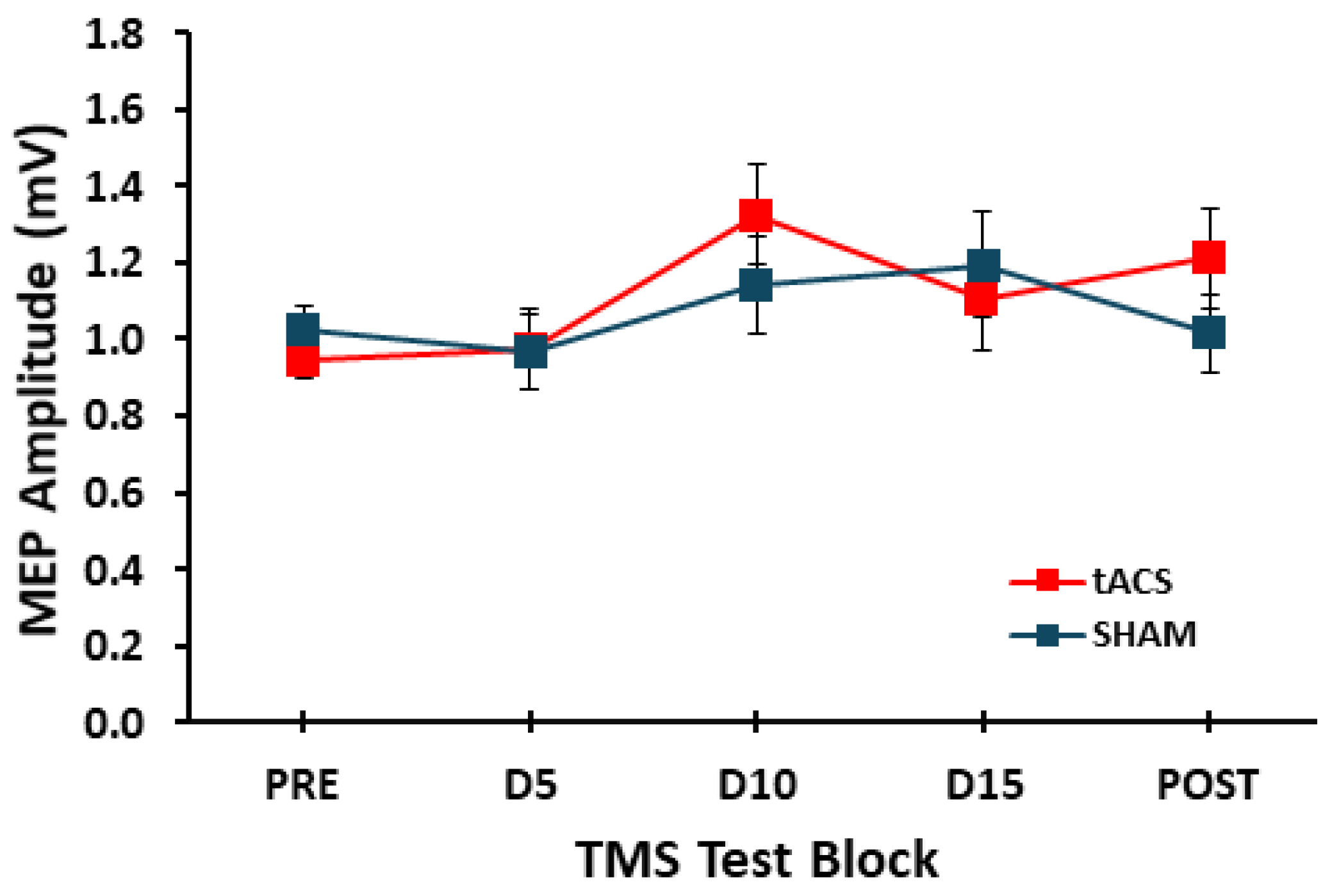
3.2. MEP Amplitude
4. Discussion
4.1. Effects of tACS on Excitability of the Contralateral Non-Dominant M1
4.2. Potential Explanations for the Absence of Left M1-tACS Effects on Right M1
4.3. Study Limitations
4.4. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moliadze, V.; Antal, A.; Paulus, W. Boosting Brain Excitability by Transcranial High Frequency Stimulation in the Ripple Range. J. Physiol. 2010, 588 Pt 24, 4891–4904. [Google Scholar] [CrossRef] [PubMed]
- Sugata, H.; Yagi, K.; Yazawa, S.; Nagase, Y.; Tsuruta, K.; Ikeda, T.; Matsushita, K.; Hara, M.; Kawakami, K.; Kawakami, K. Modulation of Motor Learning Capacity by Transcranial Alternating Current Stimulation. Neuroscience 2018, 391, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yi, Y.G.; Chang, M.C. The Effect of Transcranial Alternating Current Stimulation on Functional Recovery in Patients with Stroke: A Narrative Review. Front. Neurol. 2023, 14, 1327383. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhang, L.; Wu, Y.; Tang, L.; Chen, X.; Li, Y.; Shan, C. Exploring the Therapeutic Effects and Mechanisms of Transcranial Alternating Current Stimulation on Improving Walking Ability in Stroke Patients via Modulating Cerebellar Gamma Frequency Band-a Narrative Review. Cerebellum 2023, 23, 1593–1603. [Google Scholar] [CrossRef]
- Hu, K.; Wan, R.; Liu, Y.; Niu, M.; Guo, J.; Guo, F. Effects of Transcranial Alternating Current Stimulation on Motor Performance and Motor Learning for Healthy Individuals: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 1064584. [Google Scholar] [CrossRef]
- Wessel, M.J.; Draaisma, L.R.; Hummel, F.C. Mini-Review: Transcranial Alternating Current Stimulation and the Cerebellum. Cerebellum 2023, 22, 120–128. [Google Scholar] [CrossRef]
- Pollok, B.; Boysen, A.C.; Krause, V. The Effect of Transcranial Alternating Current Stimulation (tACS) at Alpha and Beta Frequency on Motor Learning. Behav. Brain Res. 2015, 293, 234–240. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial Direct Current Stimulation: State of the Art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef]
- Tavakoli, A.V.; Yun, K. Transcranial Alternating Current Stimulation (tACS) Mechanisms and Protocols. Front. Cell Neurosci. 2017, 11, 214. [Google Scholar] [CrossRef]
- Wach, C.; Krause, V.; Moliadze, V.; Paulus, W.; Schnitzler, A.; Pollok, B. Effects of 10 Hz and 20 Hz Transcranial Alternating Current Stimulation (tACS) on Motor Functions and Motor Cortical Excitability. Behav. Brain Res. 2013, 241, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Buch, E.R.; Santarnecchi, E.; Antal, A.; Born, J.; Celnik, P.A.; Classen, J.; Gerloff, C.; Hallett, M.; Hummel, F.C.; Nitsche, M.A.; et al. Effects of tDCS on Motor Learning and Memory Formation: A Consensus and Critical Position Paper. Clin. Neurophysiol. 2017, 128, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, T.; Zoghi, M.; Farrell, M.; Egan, G.F.; Jaberzadeh, S. Does Transcranial Electrical Stimulation Enhance Corticospinal Excitability of the Motor Cortex in Healthy Individuals? A Systematic Review and Meta-Analysis. Eur. J. Neurosci. 2017, 46, 1968–1990. [Google Scholar] [CrossRef]
- Horvath, J.C.; Forte, J.D.; Carter, O. Evidence That Transcranial Direct Current Stimulation (tDCS) Generates Little-to-No Reliable Neurophysiologic Effect Beyond MEP Amplitude Modulation in Healthy Human Subjects: A Systematic Review. Neuropsychologia 2015, 66, 213–236. [Google Scholar] [CrossRef]
- Klees-Themens, G.; Theoret, H. The Effects of Transcranial Direct Current Stimulation on Corticospinal Excitability: A Systematic Review of Nonsignificant Findings. Eur. J. Neurosci. 2023, 58, 3074–3097. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained Excitability Elevations Induced by Transcranial DC Motor Cortex Stimulation in Humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Transcranial Direct Current Stimulation—Update 2011. Restor. Neurol. Neurosci. 2011, 29, 463–492. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC Stimulation (tDCS): A Tool for Double-Blind Sham-Controlled Clinical Studies in Brain Stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Qi, S.; Liang, Z.; Wei, Z.; Liu, Y.; Wang, X. Effects of Transcranial Direct Current Stimulation on Motor Skills Learning in Healthy Adults Through the Activation of Different Brain Regions: A Systematic Review. Front. Hum. Neurosci. 2022, 16, 1021375. [Google Scholar] [CrossRef]
- Meek, A.W.; Greenwell, D.R.; Nishio, H.; Poston, B.; Riley, Z.A. Anodal M1 tDCS Enhances Online Learning of Rhythmic Timing Videogame Skill. PLoS ONE 2024, 19, e0295373. [Google Scholar] [CrossRef] [PubMed]
- Fresnoza, S.; Christova, M.; Feil, T.; Gallasch, E.; Korner, C.; Zimmer, U.; Ischebeck, A. The Effects of Transcranial Alternating Current Stimulation (tACS) at Individual Alpha Peak Frequency (iAPF) on Motor Cortex Excitability in Young and Elderly Adults. Exp. Brain Res. 2018, 236, 2573–2588. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Bramanti, A.; Leo, A.; Manuli, A.; Sciarrone, F.; Russo, M.; Bramanti, P.; Calabro, R.S. Effects of Cerebellar Transcranial Alternating Current Stimulation on Motor Cortex Excitability and Motor Function. Brain Struct. Funct. 2017, 222, 2891–2906. [Google Scholar] [CrossRef]
- Lang, N.; Nitsche, M.A.; Paulus, W.; Rothwell, J.C.; Lemon, R.N. Effects of Transcranial Direct Current Stimulation over the Human Motor Cortex on Corticospinal and Transcallosal Excitability. Exp. Brain Res. 2004, 156, 439–443. [Google Scholar] [CrossRef]
- Wilkins, E.W.; Young, R.J.; Houston, D.; Kawana, E.; Lopez Mora, E.; Sunkara, M.S.; Riley, Z.A.; Poston, B. Non-Dominant Hemisphere Excitability Is Unaffected During and after Transcranial Direct Current Stimulation of the Dominant Hemisphere. Brain Sci. 2024, 14, 694. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Castro, L.O.; Savagim, E.A.; Braite, R.; Cruz, V.C.; Rocha, R.R.; Rigonatti, S.P.; Silva, M.T.; Fregni, F. Enhancement of Non-Dominant Hand Motor Function by Anodal Transcranial Direct Current Stimulation. Neurosci. Lett. 2006, 404, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.K.; Kim, B.O.; Song, H.T. Effect of Stimulation Polarity of Transcranial Direct Current Stimulation on Non-Dominant Hand Function. Ann. Rehabil. Med. 2012, 36, 1–7. [Google Scholar] [CrossRef]
- Vines, B.W.; Nair, D.G.; Schlaug, G. Contralateral and Ipsilateral Motor Effects after Transcranial Direct Current Stimulation. Neuroreport 2006, 17, 671–674. [Google Scholar] [CrossRef]
- Reis, J.; Fischer, J.T.; Prichard, G.; Weiller, C.; Cohen, L.G.; Fritsch, B. Time- but Not Sleep-Dependent Consolidation of tDCS-Enhanced Visuomotor Skills. Cereb. Cortex 2013, 25, 109–117. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive Cortical Stimulation Enhances Motor Skill Acquisition over Multiple Days through an Effect on Consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef]
- Pantovic, M.; Albuquerque, L.L.; Mastrantonio, S.; Pomerantz, A.S.; Wilkins, E.W.; Riley, Z.A.; Guadagnoli, M.A.; Poston, B. Transcranial Direct Current Stimulation of Primary Motor Cortex over Multiple Days Improves Motor Learning of a Complex Overhand Throwing Task. Brain Sci. 2023, 13, 1441. [Google Scholar] [CrossRef]
- Chen, R.; Cohen, L.G.; Hallett, M. Role of the Ipsilateral Motor Cortex in Voluntary Movement. Can. J. Neurol. Sci. 1997, 24, 284–291. [Google Scholar] [CrossRef]
- Chen, R.; Gerloff, C.; Hallett, M.; Cohen, L.G. Involvement of the Ipsilateral Motor Cortex in Finger Movements of Different Complexities. Ann. Neurol. 1997, 41, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Swayne, O.B.; Vandermeeren, Y.; Camus, M.; Dimyan, M.A.; Harris-Love, M.; Perez, M.A.; Ragert, P.; Rothwell, J.C.; Cohen, L.G. Contribution of Transcranial Magnetic Stimulation to the Understanding of Cortical Mechanisms Involved in Motor Control. J. Physiol. 2008, 586, 325–351. [Google Scholar] [CrossRef] [PubMed]
- Murase, N.; Duque, J.; Mazzocchio, R.; Cohen, L.G. Influence of Interhemispheric Interactions on Motor Function in Chronic Stroke. Ann. Neurol. 2004, 55, 400–409. [Google Scholar] [CrossRef]
- Duque, J.; Murase, N.; Celnik, P.; Hummel, F.; Harris-Love, M.; Mazzocchio, R.; Olivier, E.; Cohen, L.G. Intermanual Differences in Movement-Related Interhemispheric Inhibition. J. Cogn. Neurosci. 2007, 19, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, L.; Larsen, M.N.; Grey, M.J.; Nielsen, J.B.; Lundbye-Jensen, J. Long-Term Progressive Motor Skill Training Enhances Corticospinal Excitability for the Ipsilateral Hemisphere and Motor Performance of the Untrained Hand. Eur. J. Neurosci. 2017, 45, 1490–1500. [Google Scholar] [CrossRef]
- Waters, S.; Wiestler, T.; Diedrichsen, J. Cooperation Not Competition: Bihemispheric tDCS and fMRI Show Role for Ipsilateral Hemisphere in Motor Learning. J. Neurosci. 2017, 37, 7500–7512. [Google Scholar] [CrossRef]
- Ruddy, K.L.; Carson, R.G. Neural Pathways Mediating Cross Education of Motor Function. Front. Hum. Neurosci. 2013, 7, 397. [Google Scholar] [CrossRef]
- Carroll, T.J.; Lee, M.; Hsu, M.; Sayde, J. Unilateral Practice of a Ballistic Movement Causes Bilateral Increases in Performance and Corticospinal Excitability. J. Appl. Physiol. 2008, 104, 1656–1664. [Google Scholar] [CrossRef]
- Hortobagyi, T.; Richardson, S.P.; Lomarev, M.; Shamim, E.; Meunier, S.; Russman, H.; Dang, N.; Hallett, M. Interhemispheric Plasticity in Humans. Med. Sci. Sports Exerc. 2011, 43, 1188–1199. [Google Scholar] [CrossRef]
- Bachtiar, V.; Johnstone, A.; Berrington, A.; Lemke, C.; Johansen-Berg, H.; Emir, U.; Stagg, C.J. Modulating Regional Motor Cortical Excitability with Noninvasive Brain Stimulation Results in Neurochemical Changes in Bilateral Motor Cortices. J. Neurosci. 2018, 38, 7327–7336. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Screening Questionnaire before TMS: An Update. Clin. Neurophysiol. 2011, 122, 1686. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Uehara, K.; Hanakawa, T. The Contribution of Interindividual Factors to Variability of Response in Transcranial Direct Current Stimulation Studies. Front. Cell Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef]
- Pellegrini, M.; Zoghi, M.; Jaberzadeh, S. Biological and Anatomical Factors Influencing Interindividual Variability to Noninvasive Brain Stimulation of the Primary Motor Cortex: A Systematic Review and Meta-Analysis. Rev. Neurosci. 2018, 29, 199–222. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; McGlory, C.; Gibala, M.J.; Phillips, S.M. Investigating Human Skeletal Muscle Physiology with Unilateral Exercise Models: When One Limb Is More Powerful Than Two. Appl. Physiol. Nutr. Metab. 2017, 42, 563–570. [Google Scholar] [CrossRef]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.G.; Fischer, K.M.; Jalene, S.; Landers, M.R.; Mari, Z.; Poston, B. Long-Term Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Learning in Parkinson’s Disease. Cerebellum 2022, 21, 333–349. [Google Scholar] [CrossRef]
- Lima de Albuquerque, L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. An Acute Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Performance in Parkinson’s Disease. Brain Sci. 2020, 10, 735. [Google Scholar] [CrossRef]
- Proessl, F.; Poston, B.; Rudroff, T. Does a Single Application of Anodal tDCS Improve Knee Extensor Fatigability in People with Multiple Sclerosis? Brain Stimul. 2018, 11, 1388–1390. [Google Scholar] [CrossRef]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines 2023, 11, 2219. [Google Scholar] [CrossRef] [PubMed]
- Pantovic, M.; Lidstone, D.E.; de Albuquerque, L.L.; Wilkins, E.W.; Munoz, I.A.; Aynlender, D.G.; Morris, D.; Dufek, J.S.; Poston, B. Cerebellar Transcranial Direct Current Stimulation Applied over Multiple Days Does Not Enhance Motor Learning of a Complex Overhand Throwing Task in Young Adults. Bioengineering 2023, 10, 1265. [Google Scholar] [CrossRef]
- Dominici, F.; Popa, T.; Ginanneschi, F.; Mazzocchio, R.; Rossi, A. Cortico-Motoneuronal Output to Intrinsic Hand Muscles Is Differentially Influenced by Static Changes in Shoulder Positions. Exp. Brain Res. 2005, 164, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Ginanneschi, F.; Del Santo, F.; Dominici, F.; Gelli, F.; Mazzocchio, R.; Rossi, A. Changes in Corticomotor Excitability of Hand Muscles in Relation to Static Shoulder Positions. Exp. Brain Res. 2005, 161, 374–382. [Google Scholar] [CrossRef]
- Rossini, P.M.; Desiato, M.T.; Lavaroni, F.; Caramia, M.D. Brain Excitability and Electroencephalographic Activation: Non-Invasive Evaluation in Healthy Humans via Transcranial Magnetic Stimulation. Brain Res. 1991, 567, 111–119. [Google Scholar] [CrossRef]
- Eckert, N.R.; Poston, B.; Riley, Z.A. Modulation of the Cutaneous Silent Period in the Upper-Limb with Whole-Body Instability. PLoS ONE 2016, 11, e0151520. [Google Scholar] [CrossRef]
- Eckert, N.R.; Poston, B.; Riley, Z.A. Differential Processing of Nociceptive Input within Upper Limb Muscles. PLoS ONE 2018, 13, e0196129. [Google Scholar] [CrossRef]
- Albuquerque, L.L.; Fischer, K.M.; Pauls, A.L.; Pantovic, M.; Guadagnoli, M.A.; Riley, Z.A.; Poston, B. An Acute Application of Transcranial Random Noise Stimulation Does Not Enhance Motor Skill Acquisition or Retention in a Golf Putting Task. Hum. Mov. Sci. 2019, 66, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Labruna, L.; Jamil, A.; Fresnoza, S.; Batsikadze, G.; Kuo, M.F.; Vanderschelden, B.; Ivry, R.B.; Nitsche, M.A. Efficacy of Anodal Transcranial Direct Current Stimulation is Related to Sensitivity to Transcranial Magnetic Stimulation. Brain Stimul. 2016, 9, 8–15. [Google Scholar] [CrossRef]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in Response to Transcranial Direct Current Stimulation of the Motor Cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Ammann, C.; Guida, P.; Caballero-Insaurriaga, J.; Pineda-Pardo, J.A.; Oliviero, A.; Foffani, G. A Framework to Assess the Impact of Number of Trials on the Amplitude of Motor Evoked Potentials. Sci. Rep. 2020, 10, 21422. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, K.A.; Young, R.J.; Contini, V.; Clinton, E.; Hitchcock, A.; Riley, Z.A.; Poston, B. The Influence of Transcranial Alternating Current Stimulation on Fatigue Resistance. Brain Sci. 2023, 13, 1225. [Google Scholar] [CrossRef]
- Dissanayaka, T.D.; Zoghi, M.; Farrell, M.; Egan, G.F.; Jaberzadeh, S. Sham Transcranial Electrical Stimulation and Its Effects on Corticospinal Excitability: A Systematic Review and Meta-Analysis. Rev. Neurosci. 2018, 29, 223–232. [Google Scholar] [CrossRef]
- Stagg, C.J.; Best, J.G.; Stephenson, M.C.; O’Shea, J.; Wylezinska, M.; Kincses, Z.T.; Morris, P.G.; Matthews, P.M.; Johansen-Berg, H. Polarity-Sensitive Modulation of Cortical Neurotransmitters by Transcranial Stimulation. J. Neurosci. 2009, 29, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Brunoni, A.R.; Charvet, L.E.; Clark, V.P.; Cohen, L.G.; Deng, Z.D.; Dmochowski, J.; Edwards, D.J.; Frohlich, F.; Kappenman, E.S.; et al. Rigor and Reproducibility in Research with Transcranial Electrical Stimulation: An NIMH-Sponsored Workshop. Brain Stimul. 2018, 11, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, N.; Lin, Y.; Chen, R.; Zhang, J. Hemispheric Differences in Functional Interactions between the Dorsal Lateral Prefrontal Cortex and Ipsilateral Motor Cortex. Front. Hum. Neurosci. 2020, 14, 202. [Google Scholar] [CrossRef]
- Hehl, M.; Van Malderen, S.; Geraerts, M.; Meesen, R.L.J.; Rothwell, J.C.; Swinnen, S.P.; Cuypers, K. Probing Intrahemispheric Interactions with a Novel Dual-Site TMS setup. Clin. Neurophysiol. 2024, 158, 180–195. [Google Scholar] [CrossRef]
- Dum, R.P.; Strick, P.L. Frontal Lobe Inputs to the Digit Representations of the Motor Areas on the Lateral Surface of the Hemisphere. J. Neurosci. 2005, 25, 1375–1386. [Google Scholar] [CrossRef]
- Marconi, B.; Genovesio, A.; Giannetti, S.; Molinari, M.; Caminiti, R. Callosal Connections of Dorso-Lateral Premotor Cortex. Eur. J. Neurosci. 2003, 18, 775–788. [Google Scholar] [CrossRef]
- Lopez-Alonso, V.; Cheeran, B.; Fernandez-del-Olmo, M. Relationship Between Non-Invasive Brain Stimulation-Induced Plasticity and Capacity for Motor Learning. Brain Stimul. 2015, 8, 1209–1219. [Google Scholar] [CrossRef]
- Jonker, Z.D.; Gaiser, C.; Tulen, J.H.M.; Ribbers, G.M.; Frens, M.A.; Selles, R.W. No effect of anodal tDCS on Motor Cortical Excitability and no Evidence for Responders in a Large Double-Blind Placebo-Controlled trial. Brain Stimul. 2021, 14, 100–109. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially Non-Linear Stimulation Intensity-Dependent Effects of Direct Current Stimulation on Motor Cortex Excitability in Humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Picciotto, M. Consideration of Sample Size in Neuroscience Studies. J. Neurosci. 2020, 40, 4076–4077. [Google Scholar]
- Szucs, D.; Ioannidis, J.P. Sample Size Evolution in Neuroimaging Research: An Evaluation of Highly-Cited Studies (1990–2012) and of Latest Practices (2017–2018) in High-Impact Journals. NeuroImage 2020, 221, 117164. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.H.; Delvendahl, I.; Kuhnke, N.G.; Hauschke, D.; Stolle, S.; Mall, V. Navigated Transcranial Magnetic Stimulation Does Not Decrease the Variability of Motor-Evoked Potentials. Brain Stimul. 2010, 3, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Santos, M.C.; Lima, M.; Vieira, A.L.; Rigonatti, S.P.; Silva, M.T.; Barbosa, E.R.; Nitsche, M.A.; Pascual-Leone, A. Noninvasive Cortical Stimulation with Transcranial Direct Current Stimulation in Parkinson’s Disease. Mov. Disord. 2006, 21, 1693–1702. [Google Scholar] [CrossRef]
- Hummel, F.; Celnik, P.; Giraux, P.; Floel, A.; Wu, W.H.; Gerloff, C.; Cohen, L.G. Effects of Non-Invasive Cortical Stimulation on Skilled Motor Function in Chronic Stroke. Brain 2005, 128 Pt 3, 490–499. [Google Scholar] [CrossRef]
- Horvath, J.C.; Carter, O.; Forte, J.D. Transcranial Direct Current Stimulation: Five Important Issues We Aren’t Discussing (but Probably Should be). Front. Syst. Neurosci. 2014, 8, 2. [Google Scholar] [CrossRef]
- Bestmann, S.; Krakauer, J.W. The Uses and Interpretations of the Motor-Evoked Potential for Understanding Behaviour. Exp. Brain Res. 2015, 233, 679–689. [Google Scholar] [CrossRef]
- Buccolieri, A.; Abbruzzese, G.; Rothwell, J.C. Relaxation from a Voluntary Contraction Is Preceded by Increased Excitability of Motor Cortical Inhibitory Circuits. J. Physiol. 2004, 558 Pt 2, 685–695. [Google Scholar] [CrossRef]
- Mooney, R.A.; Cirillo, J.; Byblow, W.D. Neurophysiological Mechanisms Underlying Motor Skill Learning in Young and Older Adults. Exp. Brain Res. 2019, 237, 2331–2344. [Google Scholar] [CrossRef] [PubMed]
- Coxon, J.P.; Peat, N.M.; Byblow, W.D. Primary Motor Cortex Disinhibition During Motor Skill Learning. J. Neurophysiol. 2014, 112, 156–164. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkins, E.W.; Young, R.J.; Davidson, R.; Krider, R.; Alhwayek, G.; Park, J.A.; Parikh, A.C.; Riley, Z.A.; Poston, B. The Influence of Transcranial Alternating Current Stimulation on the Excitability of the Unstimulated Contralateral Primary Motor Cortex. Brain Sci. 2025, 15, 512. https://doi.org/10.3390/brainsci15050512
Wilkins EW, Young RJ, Davidson R, Krider R, Alhwayek G, Park JA, Parikh AC, Riley ZA, Poston B. The Influence of Transcranial Alternating Current Stimulation on the Excitability of the Unstimulated Contralateral Primary Motor Cortex. Brain Sciences. 2025; 15(5):512. https://doi.org/10.3390/brainsci15050512
Chicago/Turabian StyleWilkins, Erik W., Richard J. Young, Ryder Davidson, Reese Krider, George Alhwayek, Jonathan A. Park, Armaan C. Parikh, Zachary A. Riley, and Brach Poston. 2025. "The Influence of Transcranial Alternating Current Stimulation on the Excitability of the Unstimulated Contralateral Primary Motor Cortex" Brain Sciences 15, no. 5: 512. https://doi.org/10.3390/brainsci15050512
APA StyleWilkins, E. W., Young, R. J., Davidson, R., Krider, R., Alhwayek, G., Park, J. A., Parikh, A. C., Riley, Z. A., & Poston, B. (2025). The Influence of Transcranial Alternating Current Stimulation on the Excitability of the Unstimulated Contralateral Primary Motor Cortex. Brain Sciences, 15(5), 512. https://doi.org/10.3390/brainsci15050512








