The Use of Griffiths III in the Appraisal of the Developmental Profile in Autism: A Systematic Search and Review
Abstract
1. The Child’s Developmental Profile
2. The Child’s Developmental Profile in Autism
3. The Current Study: The Research Questions
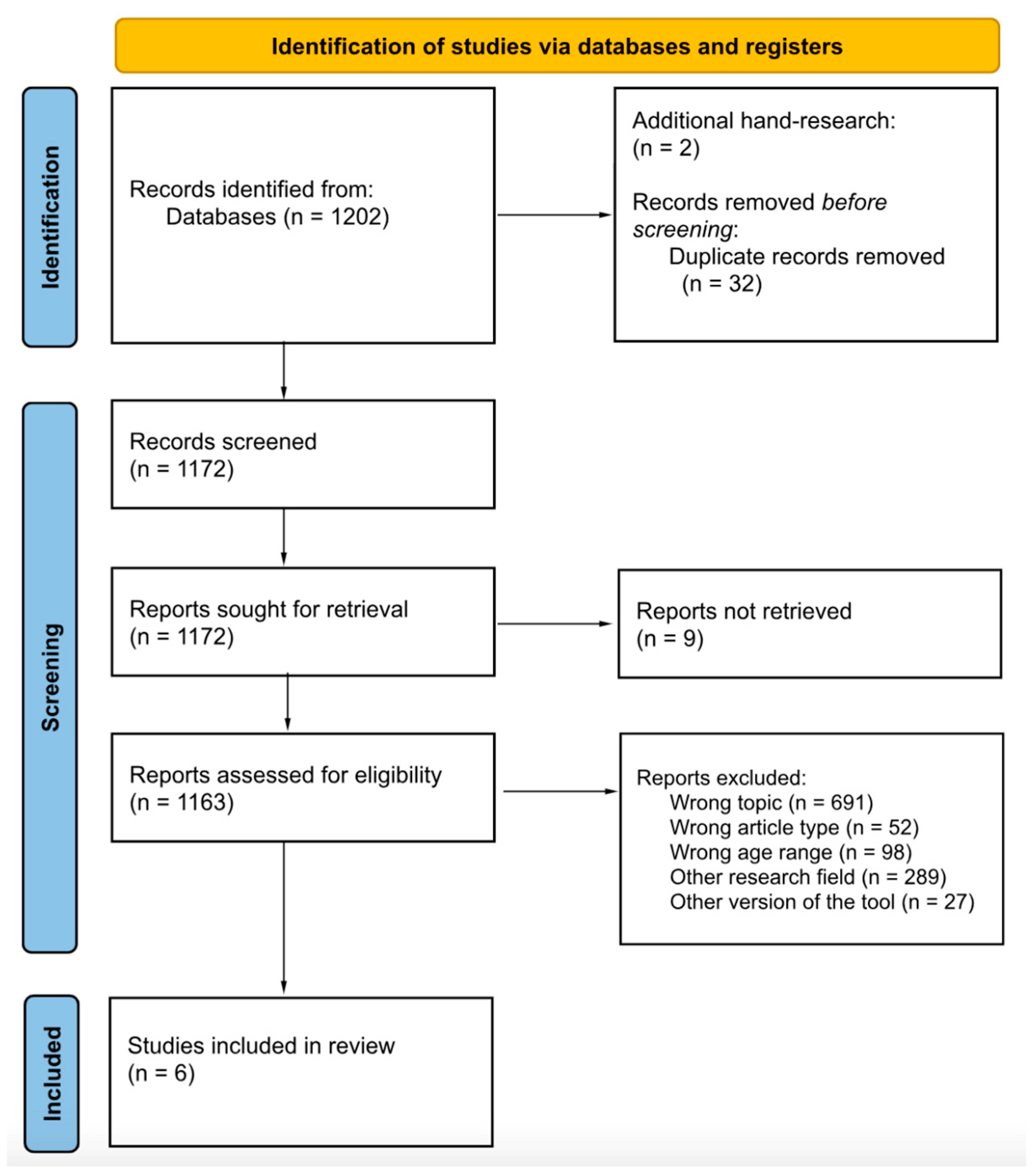
4. Method
4.1. Phrase Search Syntax
4.2. Pilot Search Strategy and Select Eligible Papers
4.3. Methodological Quality Appraisal of Reviewed Studies
5. Results
5.1. Summary of the Reviewed Studies
5.1.1. Case–Control and Case Report Studies
5.1.2. Intervention Studies
5.2. Methodology Quality Appraisal
5.2.1. Quality Appraisal: STROBE Statement
5.2.2. Quality Appraisal: JBI Critical Appraisal Tool
5.2.3. Quality Appraisal: CEC Protocol
6. Discussion
7. Strengths, Limitations, and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jansen, J.M.; Green, E.; Stroud, L.A.; Watson, M.B. Using the Griffiths III and Quartile Charts in Assessing Autism Spectrum Disorder: A Case Study. J. Educ. Lear. 2020, 9, 30–40. [Google Scholar] [CrossRef]
- Marlow, N. Outcomes of preterm birth and evidence synthesis. Dev. Med. Child. Neurol. 2018, 60, 330. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Developmental examination: Birth to 5 years. Arch. Dis. Child. 2011, 96, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Fernell, E.; Eriksson, M.A.; Gillberg, C. Early diagnosis of autism and impact on prognosis: A narrative review. Clin. Epidemiol. 2013, 5, 33–43. [Google Scholar] [CrossRef]
- Newborg, J. Battelle Developmental Inventory, 2nd ed.; Examiner’s manual; Riverside Publishing: Rolling Meadows, IL, USA, 2006. [Google Scholar]
- Bayley, N.; Aylward, G. Bayley Scales of Infant and Toddler Development: Technical Manual, 4th ed.; NCS Pearson: Bloomington, MN, USA, 2019. [Google Scholar]
- Griffiths, R. The Abilities of Babies: A Study in Mental Measurement; McGraw-Hill: New York, NY, USA, 1954. [Google Scholar]
- Mullen, E.M. Mullen Scales of Early Learning; Pearson: Circle Pines, MN, USA, 1995. [Google Scholar]
- Huntley, M. Griffiths Mental Development Scales from Birth to 2 Years—Manual; ARICD: Manchester, UK, 1996. [Google Scholar]
- Luiz, D.M.; Faragher, B.; Barnard, A.; Knoesen, N.; Kotras, N.; Burns, L.E.; Challis, D. GMDS-ER: Griffiths Mental Development Scales—Extended Revised Analysis Manual; Hogrefe: Oxford, UK, 2006. [Google Scholar]
- Green, E.; Stroud, L.; O’Connell, R.; Bloomfield, S.; Cronje, J.; Foxcroft, C.; Hurter, K.; Lane, H.; Marais, R.; Marx, C.; et al. Griffiths Scales of Child Development, 3rd ed.; Part II: Administration and Scoring; Hogrefe: Oxford, UK, 2016. [Google Scholar]
- Stroud, L.; Green, E.; Cronje, J. A Revision Process That Bridges Qualitative and Quantitative Assessment. Psychology 2020, 11, 436–444. [Google Scholar] [CrossRef]
- Barnett, A.L.; Guzzetta, A.; Mercuri, E.; E Henderson, S.; Haataja, L.; Cowan, F.; Dubowitz, L. Can the Griffiths scales predict neuromotor and perceptual-motor impairment in term infants with neonatal encephalopathy? Arch. Dis. Child. 2004, 89, 637–643. [Google Scholar] [CrossRef]
- Green, E.M.; Stroud, L.; Marx, C.; Cronje, J. Child development assessment: Practitioner input in the revision for Griffiths III. Child. Care Health Dev. 2020, 46, 682–691. [Google Scholar] [CrossRef]
- Li, H.H.; Wang, C.X.; Feng, J.Y.; Wang, B.; Li, C.L.; Jia, F.Y. A Developmental Profile of Children With Autism Spectrum Disorder in China Using the Griffiths Mental Development Scales. Front. Psychol. 2020, 11, 570923. [Google Scholar] [CrossRef]
- Scandurra, V.; Emberti Gialloreti, L.; Barbanera, F.; Scordo, M.R.; Pierini, A.; Canitano, R. Neurodevelopmental Disorders and Adaptive Functions: A Study of Children with Autism Spectrum Disorders (ASD) and/or Attention Deficit and Hyperactivity Disorder (ADHD). Front. Psychiatry 2019, 10, 443074. [Google Scholar] [CrossRef]
- Bertamini, G.; Bentenuto, A.; Perzolli, S.; Paolizzi, E.; Furlanello, C.; Venuti, P. Quantifying the Child–Therapist Interaction in ASD Intervention: An Observational Coding System. Brain Sci. 2021, 11, 366. [Google Scholar] [CrossRef]
- Lerna, A.; Esposito, D.; Conson, M.; Massagli, A. Long-term effects of PECS on social–communicative skills of children with autism spectrum disorders: A follow-up study. Int. J. Lang. Commun. Disord. 2014, 49, 478–485. [Google Scholar] [CrossRef]
- Marino, F.; Chilà, P.; Sfrazzetto, S.T.; Carrozza, C.; Crimi, I.; Failla, C.; Busà, M.; Bernava, G.; Tartarisco, G.; Vagni, D.; et al. Outcomes of a Robot-Assisted Social-Emotional Understanding Intervention for Young Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2020, 50, 1973–1987. [Google Scholar] [CrossRef]
- The Association for Research in Infant and Child Development. Griffiths III Year Four Items Grouped by Quartile Level of Difficulty. 2018. Available online: https://www.aricd.ac.uk/ (accessed on 15 July 2024).
- Wang, L.A.L.; Petrulla, V.; Zampella, C.J.; Waller, R.; Schultz, R.T. Gross Motor Impairment and Its Relation to Social Skills in Autism Spectrum Disorder: A Systematic Review and Two Meta-Analyses. Psychol. Bull. 2022, 148, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Tager-Flusberg, H.; Rogers, S.; Cooper, J.; Landa, R.; Lord, C.; Paul, R.; Rice, M.; Stoel-Gammon, C.; Wetherby, A.; Yoder, P. Defining Spoken Language Benchmarks and Selecting Measures of Expressive Language Development for Young Children With Autism Spectrum Disorders. J. Speech Lang. Hear. Res. 2009, 52, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.; Dounavi, K. The Emergence of Autism Symptoms Prior to 18 Months of Age: A Systematic Literature Review. J. Autism Dev. Disord. 2021, 51, 973–993. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Müller, R.A.; Fishman, I. Brain Connectivity and Neuroimaging of Social Networks in Autism. Trends Cogn. Sci. 2018, 22, 1103–1116. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Fang, P.; Zhou, Q. Facial expression recognition: Can. preschoolers with cochlear implants and hearing aids catch it? Res. Dev. Disabil. 2011, 32, 2583–2588. [Google Scholar] [CrossRef]
- Lawson, L.P.; Joshi, R.; Barbaro, J.; Dissanayake, C. Gender Differences During Toddlerhood in Autism Spectrum Disorder: A Prospective Community-Based Longitudinal Follow-Up Study. J. Autism Dev. Disord. 2018, 48, 2619–2628. [Google Scholar] [CrossRef]
- Alshurman, W.; Alsreaa, I. The Efficiency of Peer Teaching of Developing Non Verbal Communication to Children with Autism Spectrum Disorder (ASD). J. Educ. Pract. 2015, 6, 33–38. [Google Scholar]
- Backer van Ommeren, T.; Koot, H.M.; Begeer, S. Reciprocity in autistic and typically developing children and adolescents with and without mild intellectual disabilities. J. Intellect. Disabil. 2017, 61, 810–817. [Google Scholar] [CrossRef]
- Camargo, S.P.H.; Rispoli, M.; Ganz, J.; Hong, E.R.; Davis, H.; Mason, R. A review of the quality of behaviorally-based intervention research to improve social interaction skills of children with ASD in inclusive settings. J. Autism Dev. Disord. 2014, 44, 2096–2116. [Google Scholar] [CrossRef]
- Baranek, G.T.; David, F.J.; Poe, M.D.; Stone, W.L.; Watson, L.R. Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. J. Child Psychol. Psychiatry 2006, 47, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Bolton, P.F.; Golding, J.; Emond, A.; Steer, C.D. Autism Spectrum Disorder and Autistic Traits in the Avon Longitudinal Study of Parents and Children: Precursors and Early Signs. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 249–260.e25. [Google Scholar] [CrossRef]
- Network, I.; Estes, A.; Zwaigenbaum, L.; Gu, H.; John, T.S.; Paterson, S.; Elison, J.T.; Hazlett, H.; Botteron, K.; Dager, S.R.; et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J. Neurodev. Disord. 2015, 7, 24. [Google Scholar] [CrossRef]
- Flanagan, J.E.; Landa, R.; Bhat, A.; Bauman, M. Head Lag in Infants at Risk for Autism: A Preliminary Study. Am. J. Occup. Ther. 2012, 66, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Levante, A.; Martis, C.; Antonioli, G.; Dima, M.; Duma, L.; Perrone, M.; Russo, L.; Lecciso, F. The Effect of Sports Activities on Motor and Social Skills in Autistic Children and Adolescents: A Systematic Narrative Review. Curr. Dev. Disord. Rep. 2023, 10, 155–174. [Google Scholar] [CrossRef]
- Pellicano, E. Do Autistic Symptoms Persist Across Time? Evidence of Substantial Change in Symptomatology Over a 3-year Period in Cognitively Able Children With Autism. Am. J. Intellect. Dev. Disabil. 2012, 117, 156–166. [Google Scholar] [CrossRef]
- Simonoff, E.; Kent, R.; Stringer, D.; Lord, C.; Briskman, J.; Lukito, S.; Pickles, A.; Charman, T.; Baird, G. Trajectories in Symptoms of Autism and Cognitive Ability in Autism From Childhood to Adult Life: Findings From a Longitudinal Epidemiological Cohort. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 1342–1352. [Google Scholar] [CrossRef]
- Bieleninik, Ł.; Posserud, M.B.; Geretsegger, M.; Thompson, G.; Elefant, C.; Gold, C. Tracing the temporal stability of autism spectrum diagnosis and severity as measured by the Autism Diagnostic Observation Schedule: A systematic review and meta-analysis. PLoS ONE. 2017, 12, e0183160. [Google Scholar] [CrossRef]
- Ildiz, G.O.; Bayari, S.; Karadag, A.; Kaygisiz, E.; Fausto, R. Fourier Transform Infrared Spectroscopy Based Complementary Diagnosis Tool for Autism Spectrum Disorder in Children and Adolescents. Molecules 2020, 25, 2079. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ozonoff, S.; Iosif, A.M. Changing conceptualizations of regression: What prospective studies reveal about the onset of autism spectrum disorder. Neurosci. Biobehav. Rev. 2019, 100, 296–304. [Google Scholar] [CrossRef]
- Miller, L.E.; Dai, Y.G.; Fein, D.A.; Robins, D.L. Characteristics of toddlers with early versus later diagnosis of autism spectrum disorder. Autism 2020, 25, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Shumway, S.; Thurm, A.; Swedo, S.E.; Deprey, L.; Barnett, L.A.; Amaral, D.G.; Rogers, S.J.; Ozonoff, S. Brief report: Symptom onset patterns and functional outcomes in young children with autism spectrum disorders. J. Autism Dev. Disord. 2011, 41, 1727–1732. [Google Scholar] [CrossRef]
- Petrocchi, S.; Levante, A.; Lecciso, F. Systematic Review of Level 1 and Level 2 Screening Tools for Autism Spectrum Disorders in Toddlers. Brain Sci. 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Levante, A.; Petrocchi, S.; Lecciso, F. The Criterion Validity of the First Year Inventory and the Quantitative-CHecklist for Autism in Toddlers: A Longitudinal Study. Brain Sci. 2020, 10, 729. [Google Scholar] [CrossRef]
- Levante, A.; Petrocchi, S.; Massagli, A.; Filograna, M.R.; De Giorgi, S.; Lecciso, F. Early Screening of the Autism Spectrum Disorders: Validity Properties and Cross-Cultural Generalizability of the First Year Inventory in Italy. Brain Sci. 2020, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Lecciso, F.; Levante, A.; Signore, F.; Petrocchi, S. Preliminary evidence of the structural validity and measurement invariance of the Quantitative-CHecklist for Autism in Toddler (Q-CHAT) on Italian unselected children. Electron. J. Appl. Stat. Anal. 2019, 12, 320–340. [Google Scholar] [CrossRef]
- Levante, A.; Petrocchi, S.; Lecciso, F. Test-retest Reliability and Measurement Invariance across time of the Quantitative-CHecklist for Autism in Toddler. Electron. J. Appl. Stat. Anal. 2021, 14, 146–166. [Google Scholar] [CrossRef]
- Towle, P.O.; Patrick, P.A. Autism Spectrum Disorder Screening Instruments for Very Young Children: A Systematic Review. Autism Res. Treat. 2016, 2016, 4624829. [Google Scholar] [CrossRef]
- Thabtah, F.; Peebles, D. Early Autism Screening: A Comprehensive Review. Int. J. Environ. Res. Public Health 2019, 16, 3502. [Google Scholar] [CrossRef] [PubMed]
- Magiati, I.; Goh, D.A.; Lim, S.J.; Gan, D.Z.Q.; Leong, J.C.L.; Allison, C.; Baron-Cohen, S.; Rifkin-Graboi, A.; Broekman, B.F.P.; Saw, S.M.; et al. The psychometric properties of the Quantitative-Checklist for Autism in Toddlers (Q-CHAT) as a measure of autistic traits in a community sample of Singaporean infants and toddlers. Mol. Autism 2015, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Allison, C.; Matthews, F.E.; Ruta, L.; Pasco, G.; Soufer, R.; Brayne, C.; Charman, T.; Baron-Cohen, S. Quantitative Checklist for Autism in Toddlers (Q-CHAT). A population screening study with follow-up: The case for multiple time-point screening for autism. BMJ Paediatr. Open 2021, 5, e000700. [Google Scholar] [CrossRef]
- Magán-Maganto, M.; Canal-Bedia, R.; Hernández-Fabián, A.; Bejarano-Martín, Á.; Fernández-Álvarez, C.J.; Martínez-Velarte, M.; Martín-Cilleros, M.V.; Flores-Robaina, N.; Roeyers, H.; de la Paz, M.P. Spanish Cultural Validation of the Modified Checklist for Autism in Toddlers, Revised. J. Autism Dev. Disord. 2020, 50, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Ruta, L.; Chiarotti, F.; Arduino, G.M.; Apicella, F.; Leonardi, E.; Maggio, R.; Carrozza, C.; Chericoni, N.; Costanzo, V.; Turco, N.; et al. Validation of the Quantitative CHecklist for Autism in Toddlers (Q-CHAT) in an Italian clinical sample of young children with Autism and Other Developmental Disorders. Front. Psychiatry 2019, 10, 442144. [Google Scholar] [CrossRef]
- Allison, C.; Baron-Cohen, S.; Wheelwright, S.; Charman, T.; Richler, J.; Pasco, G.; Brayne, C. The Q-CHAT (Quantitative CHecklist for Autism in Toddlers): A normally distributed quantitative measure of autistic traits at 18–24 months of age: Preliminary report. J. Autism Dev. Disord. 2008, 38, 1414–1425. [Google Scholar] [CrossRef]
- Watkins, E.E.; Zimmermann, Z.J.; Poling, A. The gender of participants in published research involving people with autism spectrum disorders. Res. Autism Spectr. Disord. 2014, 8, 143–146. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Cook, J.; Hull, L.; Crane, L.; Mandy, W. Camouflaging in autism: A systematic review. Clin. Psychol. Rev. 2021, 89, 102080. [Google Scholar] [CrossRef]
- McQuaid, G.A.; Lee, N.R.; Wallace, G.L. Camouflaging in autism spectrum disorder: Examining the roles of sex, gender identity, and diagnostic timing. Autism 2021, 26, 552–559. [Google Scholar] [CrossRef]
- Randall, M.; Egberts, K.J.; Samtani, A.; Scholten, R.J.P.M.; Hooft, L.; Livingstone, N.; Sterling-Levis, K.; Woolfenden, S.; Williams, K. Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Akshoomoff, N. Use of the Mullen Scales of Early Learning for the Assessment of Young Children with Autism Spectrum Disorders. Child Neuropsychol. 2006, 12, 269–277. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Bryson, S.; Lord, C.; Rogers, S.; Carter, A.; Carver, L.; Chawarska, K.; Constantino, J.; Dawson, G.; Dobkins, K.; et al. Clinical Assessment and Management of Toddlers With Suspected Autism Spectrum Disorder: Insights From Studies of High-Risk Infants. Pediatrics 2009, 123, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Filipek, P.A.; Accardo, P.J.; Ashwal, S.; Baranek, G.T.; Cook, E.H.; Dawson, G.; Gordon, B.; Gravel, J.; Johnson, C.; Kallen, R.; et al. Practice parameter: Screening and diagnosis of autism. Report of the quality standards subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology 2000, 55, 468–479. [Google Scholar] [CrossRef]
- Lecciso, F.; Petrocchi, S.; Marchetti, A. Hearing mothers and oral deaf children: An atypical relational context for theory of mind. Eur. J. Psychol. Educ. 2013, 28, 903–922. [Google Scholar] [CrossRef]
- Lecciso, F.; Petrocchi, S.; Savazzi, F.; Marchetti, A.; Nobile, M.; Molteni, M. The association between maternal resolution of the diagnosis of autism, maternal mental representations of the relationship with the child, and children’s attachment. Life Span Disab. 2013, 16, 21–38. [Google Scholar]
- Sher-Censor, E.; Shahar-Lahav, R. Parents’ resolution of their child’s diagnosis: A scoping review. Attach. Hum. Dev. 2022, 24, 580–604. [Google Scholar] [CrossRef]
- Naicker, V.V.; Bury, S.M.; Hedley, D. Factors associated with parental resolution of a child’s autism diagnosis: A systematic review. Front. Psychiatry 2023, 13, 1079371. [Google Scholar] [CrossRef]
- Warren, Z.; McPheeters, M.L.; Sathe, N.; Foss-Feig, J.H.; Glasser, A.; Veenstra-VanderWeele, J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics 2011, 127, e1303–e1311. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Autism Spectrum Disorder in Under 19s: Support and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2013. [Google Scholar]
- Eikeseth, S. Outcome of comprehensive psycho-educational interventions for young children with autism. Res. Dev. Disabil. 2009, 30, 158–178. [Google Scholar] [CrossRef]
- Magiati, I.; Charman, T.; Howlin, P. A two-year prospective follow-up study of community-based early intensive behavioural intervention and specialist nursery provision for children with autism spectrum disorders. J. Child Psychol. Psychiatry 2007, 48, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Wu, W.; Dai, M.; Zeng, J.; Luo, J.; Cai, L.; Wan, B.; Jing, J. Cognitive, Language, and Behavioral Outcomes in Children With Autism Spectrum Disorders Exposed to Early Comprehensive Treatment Models: A Meta-Analysis and Meta-Regression. Front. Psychiatry 2021, 12, 691148. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.V.; Busuoli, E.M.; Schreibman, L.; Stahmer, A.C.; Pramparo, T.; Landi, I.; Mandelli, V.; Bertelsen, N.; Barnes, C.C.; Gazestani, V.; et al. Pre-treatment clinical and gene expression patterns predict developmental change in early intervention in autism. Mol. Psychiatry 2021, 26, 7641–7651. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Cirnigliaro, L.; Valle, M.S.; Casabona, A.; Randazzo, M.; La Bruna, F.; Pettinato, F.; Narzisi, A.; Rizzo, R.; Barone, R. The Griffiths Autism Early Screening (GAES): A Novel Developmental Test for Screening Autism Spectrum Disorder. J. Autism. Dev. Disord. 2023, 55, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Taddei, M.; Bulgheroni, S.; Toffalini, E.; Pantaleoni, C.; Lanfranchi, S. Developmental profiles of young children with autism spectrum disorder and global developmental delay: A study with the Griffiths III scales. Autism Res. 2023, 16, 1344–1359. [Google Scholar] [CrossRef]
- Colombi, C.; Chericoni, N.; Bargagna, S.; Costanzo, V.; Devescovi, R.; Lecciso, F.; Pierotti, C.; Prosperi, M.; Contaldo, A. Case report: Preemptive intervention for an infant with early signs of autism spectrum disorder during the first year of life. Front. Psychiatry 2023, 14, 1105253. [Google Scholar] [CrossRef]
- Colombi, C.; Narzisi, A.; Ruta, L.; Cigala, V.; Gagliano, A.; Pioggia, G.; Siracusano, R.; Rogers, S.J.; Muratori, F.; Prima Pietra Team. Implementation of the Early Start Denver Model in an Italian community. Autism 2016, 22, 126–133. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob. Adv. Integr. Med. Health 2013, 20, 385–386. [Google Scholar] [CrossRef]
- Council for Exceptional Children: Standards for Evidence-Based Practices in Special Education. Teach. Except. Child. 2014, 46, 206–212. [CrossRef]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Luyster, R.; Gotham, K.; Guthrie, W. Autism Diagnostic Observation Schedule, 2nd ed.; (ADOS-2) Manual (Part II): Toddler Module; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Reznick, J.S.; Baranek, G.T.; Reavis, S.; Watson, L.R.; Crais, E.R. A parent-report instrument for identifying one-year-olds at risk for an eventual diagnosis of autism: The first year inventory. J. Autism Dev. Disord. 2007, 37, 1691–1710. [Google Scholar] [CrossRef]
- Achenbach, T.; Rescorla, L. Manual for the ASEBA Preschool Forms and Profiles; University of Vermont: Burlington, VM, USA, 2000. [Google Scholar]
- Schopler, E.; Reichler, R.J.; DeVellis, R.F.; Daly, K. Childhood Autism Rating Scale; PsycTests: Washington, DC, USA, 1980. [Google Scholar] [CrossRef]
- Conners, C.K. Conners 3—Parent and Teacher Surveys, Long Form (Conners 3), 3rd ed.; Manual; Multi-Health System; MHS Publishing: Toronto, ON, Canada, 2008. [Google Scholar]
- Goodenough, F.; Harris, D.B. The Goodenough-Harris Drawing Test; Harcourt, Brace, and World: New York, NY, USA, 1963. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.; Balla, D.A. Vineland Adaptive Behavior Scales, 2nd ed.; (Vineland-II); PsycTests: Circle Pines, MN, USA, 2005. [Google Scholar]
- Barbaro, J.; Dissanayake, C. Early markers of autism spectrum disorders in infants and toddlers prospectively identified in the Social Attention and Communication Study. Autism 2013, 17, 64–86. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, J.; Sadka, N.; Gilbert, M.; Beattie, E.; Li, X.; Ridgway, L.; Lawson, L.P.; Dissanayake, C. Diagnostic Accuracy of the Social Attention and Communication Surveillance–Revised with Preschool Tool for Early Autism Detection in Very Young Children. JAMA Netw. Open. 2022, 5, e2146415. [Google Scholar] [CrossRef]
- Dong, H.Y.; Miao, C.Y.; Zhang, Y.; Shan, L.; Feng, J.Y.; Jia, F.Y.; Jia, F.-Y.; Du, L. Risk factors for developmental quotients in ASD children: A cross-sectional study. Front. Psychol. 2023, 14, 1126622. [Google Scholar] [CrossRef]
- Muratori, F.; Narzisi, A. Exploratory study describing 6 month outcomes for young children with autism who receive treatment as usual in Italy. Neuropsychiatr. Dis. Treat. 2014, 10, 577–586. [Google Scholar] [CrossRef]
- Levante, A.; Martis, C.; Antonioli, G.; Dima, M.; Duma, L.; Perrone, M.; Lecciso, F. A Protocol for Basketball as Inclusive Sport to Boost Motor and Social Skills in Autistic Preschoolers. Disabilities 2024, 4, 955–971. [Google Scholar] [CrossRef]
- Gabriels, R.L.; Pan, Z.; Dechant, B.; Agnew, J.A.; Brim, N.; Mesibov, G. Randomized Controlled Trial of Therapeutic Horseback Riding in Children and Adolescents with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 541–549. [Google Scholar] [CrossRef]
- Turygin, N.; Matson, J.L.; Konst, M.; Williams, L. The relationship of early communication concerns to developmental delay and symptoms of autism spectrum disorders. Dev. Neurorehabil. 2013, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Ballarotto, G.; Murray, L.; Bozicevic, L.; Marzilli, E.; Cerniglia, L.; Cimino, S.; Tambelli, R. Parental sensitivity to toddler’s need for autonomy: An empirical study on mother-toddler and father-toddler interactions during feeding and play. Infant Behav. Dev. 2023, 73, 101892. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Mandy, W.; Elliott, D.; White, R.; Pittwood, T.; Ford, T. Selection bias on intellectual ability in autism research: A cross-sectional review and meta-analysis. Mol. Autism 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.; Martins Filho, J. Family relationships with pediatricians: The maternal views. Rev. Paul. Pediatr. 2016, 34, 330–335. [Google Scholar] [CrossRef]
| Domains (Subscale) | Skills Assessed | Item Example |
|---|---|---|
| Foundations of Learning (subscale A) | Assess the child’s ability to learn (e.g., including attention, problem-solving abilities, sequential reasoning, processing speed, visuospatial skills, and memory). | Ask the child to build a bridge with blocks after the demonstration. |
| Language and Communication (subscale B) | Evaluate the child’s receptive and expressive language and communication abilities. | Ask the child to name objects and pictures, define objects by their use, and follow instructions. |
| Eye and Hand Coordination (subscale C) | Assess the child’s visual perception and fine motor skills. | Ask the child to stack or build a tower with blocks. |
| Personal–Social–Emotional (subscale D) | Evaluate the child’s ability to adapt, his or her independence, and early socio-emotional abilities. Assess the child’s imitation, joint attention, emotional recognition, and empathy. | The child identifies body parts, participates in group games, and pronounces their name. |
| Gross Motor (subscale E) | Assess the child’s early development of postural control, gross body coordination, balance, and visual–spatial coordination. | Ask the child to run, jump, or walk in a straight line. |
| Search Date | Search Strategy | Filters | Sources |
|---|---|---|---|
| 5 June 2024 | “autis*” OR “ASD” OR “autism spectrum condition” OR “ASC” OR “develop* disorder” OR “neurodevelop* disorder” OR “pervasive disorder” OR “autistic spectrum” OR “autism spectrum disorder” | Subject area: psychology; social sciences; health professions; multidisciplinary; neurosciences. | SCOPUS |
| AND | Document type: peer-reviewed articles. | Web of Science | |
| “diagnos*” OR “early diagnos*” OR “assess*” OR “evaluat*” OR “measur*” | Source type: academic journals. | MEDLINE | |
| AND | Language: English. | PsycINFO | |
| “Griffiths” OR “Griffiths scales of child development” OR “Griffiths-III” OR “Griffiths III” OR “GSCD” OR “GSCD-III” OR “Griffiths development scale” OR “Griffiths 3rd” OR “Griffiths 3rd edition” | Age group: children aged 0–6 years. | CINAHL |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| (1) Empirical studies (i.e., cross-sectional, case–control study, intervention studies). (2) Studies administering Griffiths III. (3) Children with a low likelihood or increased likelihood for autism; autistic children. (4) Children aged ≥ 0 and ≤6 years. (5) Papers published between 1 January 2016 and 6 May 2024. | (1) Studies using previous versions of the Griffiths Scales. (2) Participants aged ≥ 6 years. (3) Papers that did not report the participants’ age or mean scores of assessment measures. (4) Non-empirical studies (e.g., dissertations, conference abstracts and/or papers, editorials, opinions, commentaries, recommendations, letters, books, and book chapters; other systematic, non-systematic reviews, and meta-analyses). |
| References [Study Authors (Year)], | Country | Study Design (Cross-Sectional vs. Longitudinal) | Sample Characteristics: Sample Size, Mean Age and Standard Deviation, Age Range | Measures | Study Purpose(s) | Relevant Results | ||
| Case–Control studies | ||||||||
| 1 | Cirnigliaro et al., [77] | Italy | Cross-sectional study | Total sample age range: 18–48 months. Study 1 N = 78 children. Specifically: ASD group: N = 26 children with ASD (males: 76.92%). M(SD) = 39.46 (12.48) months. DD group: N = 26 children with DD (males: 84.62%). M(SD) = 34.07 (7.88) months. TD group: N = 26 TD children (males: 69.23%). M(SD) = 26.38 (7.48) months. Study 2 N = 219 children. Specifically: ASD group: N = 57 children with ASD (males: 75%). M(SD) = 32 (8.00) months. DD group: N = 61 children with DD (males: 82%). M(SD) = 34 (7.5) months. TD group: N = 101 TD children (males: 51%). M(SD) = 22 (8.5) months. | Child’s developmental profile: Griffith Scales of Child Development (Griffiths III [11]). Child’s risky behaviours for autism: Developmental Autism Early Screening (DAES [77]). Autistic symptoms: Autism Diagnostic Interview-Revised (ADI-R [84]). Autism Diagnostic Observation Schedule, second edition (ADOS-2 [85]). | Study 1: Development of a screener for detecting at-risk children for autism according to the Griffiths Scales of Child Development III. Study 2: Assessment of the reliability and validity of the screener. | Study 1: The B (Language and Communication) and D (Personal–Social–Emotional) subscales of Griffiths III were the most sensitive in capturing differences between groups: differences between the ASD and DD/TD were found on the B (Language and Communication) and D (Personal–Social–Emotional) subscale. Study 2: Analyses supported the diagnostic accuracy and criterion validity. Gender differences were not computed. | |
| 2 | Levante et al., [46] | Italy | Longitudinal study | T1 N = 12 children (males: 50%). M(SD) = 12.5 (0.54) months. Age range: 11–13 months. T2 N = 11 children (males: 72%). M(SD) = 19.6 (3.6) months. Age range: 18–21 months. T3 N = 16 children (males. 66%). M(SD) = 12.11 (0.92) months. Age range: n.s. | Child’s developmental profile: Griffith Scales of Child Development (Griffiths III [11]). Child’s risky behaviours for autism: First Year Inventory (FYI [86]). Quantitative-CHecklist for Autism in Toddlers (Q-CHAT [56]). Autistic symptoms: Autism Diagnostic Observation Schedule, second edition (ADOS-2 [85]). Child’ internalising/externalising symptoms: Child Behaviour Checklist (CBCL [87]). | Examine the criterion validity of two screeners, i.e., FYI and the Q-CHAT. | The criterion validity is supported by results. Preliminary accuracy data have been provided. Differences were found between both at-risk groups and the typically developing (TD) group across all subscales except gross motor. At-risk groups had the lowest scores in Griffiths III subscales B (Language and Communication) and D (Personal–Social–Emotional). Furthermore, the severity of ASD symptoms negatively correlated with DP (with subscale D at 11–13 months and with subscales B, C, and D during the second year of life). Gender differences were not computed. | |
| 3 | Taddei et al., [78] | Italy | Cross-sectional study | Total sample age range: 6–68 months. N = 74 children. Specifically: ASD + DD group: N = 39 children with ASD + DD (males = 82.05%). M(SD) = 42.6 (15.5) months. DD group: N = 35 children with DD (males = 68.57%). M(SD) = 31.7 (16.5) months. | Child’s developmental profile: Griffith Scales of Child Development (Griffiths III [11]). | Identify developmental profiles associated with Autism Spectrum Disorder and global developmental delay in preschool-aged Italian children using Griffiths III. | Both groups showed developmental delays across all subscales according to their chronological age. Children with ASD + DD showed low scores across all subscales and the lowest score for B (Language and Communication) and D (Personal–Social–Emotional) subscales. The DD group exhibited a consistent delay across all subscales. Gender differences were not computed. | |
| Case report study | ||||||||
| 4 | Jansen et al., [1] | Not specified | Cross-sectional study | N = 1 child with ASD (male). Age: 6 years and 4 months. | Child’s developmental profile: Griffith Scales of Child Development (Griffiths III [11]). Autistic symptoms: Childhood Autism Rating Scale (CARS [88]). Child’s hyperactivity and attention deficits: Conners 3-Parent and Teacher Surveys, Long Form [89]. Child’s cognitive profile: Goodenough–Harris Draw-a-Person Intellectual Ability Test (DAP: IQ [90]). | Use Griffiths III to clarify a diagnosis of Autism Spectrum Disorder and possible Attention Deficit/Hyperactivity Disorder in a 6-year-old male child. | The child exhibited a low developmental age (DA) across all subscales, with extremely low scores in subscales A (Learning Foundation), B (Language and Communication), and D (Personal–Social–Emotional). For subscales C (Eye and Hand Coordination) and E (Gross Motor), the child fell within the borderline range. | |
| Intervention studies | ||||||||
| References [Study Authors (Year)], | Country | Study Design (Cross-Sectional vs. Longitudinal) | Description of the Intervention | Sample Characteristics: Sample Size, Mean Age and Standard Deviation, Age Range | Measures | Study Purpose(s) | Relevant Results | |
| 5 | Colombi et al., [80] | Italy | Pre- and post- longitudinal study | Intervention duration: 6-months intervention Intervention wave: 6 h/week Child’s evaluation: baseline, after 3 months, and post-test (after 6 months). | Experimental group (ESDM intervention group) N = 22 children with ASD (gender distribution: n.s.) M(SD) = 31.1 (8.0) months. Control group (TAU intervention group) N = 70 children with ASD (gender distribution: n.s.) M(SD) = 35.2 (7.6) months. Total sample age range: 18–48 months. | Child’s developmental profile: Griffith Scales of Child Development (Griffiths III [11]). Autistic symptoms: Autism Diagnostic Observation Schedule, Second Edition (ADOS-2 [85]). Child’s adaptive behaviours: Vineland Adaptive Behaviour Scales-2 (VABS-II [91]). | Focusing on the children’s DP and their adaptive behaviours, evaluating the effectiveness of the Early Start Denver Model (ESDM) intervention by comparing a group of children who received ESDM to a group of children who received treatment as usual. | Children in both groups improved in cognitive, adaptive, and social skills after 3 and 6 months of treatment. However, the ESDM group achieved greater improvements in cognitive and social skills after 3 and 6 months of treatment than the control group. The ESDM group achieved greater improvements in adaptive skills than the control group after 3 months of treatment. Results on children’s developmental profiles indicated that, after 3 and 6 months of intervention, the ESDM group exhibited increased scores in subscales B (Language and Communication) and D (Personal–Social–Emotional) compared to the TAU group. Furthermore, after 6 months, the ESDM group also showed improved scores in subscales C (Hand and Eye Coordination) and E (Gross Motor) compared to the TAU group. Gender differences were not computed. |
| 6 | Colombi et al., [79] | Italy | Single-case report longitudinal study | Intervention duration: Child aged 6–8 months: 2 h/week (P-ESDM intervention). Child aged 9 months: 30 min twice a day (ESDM intervention). Child aged 11–20 months: 1 h/week (P-ESDM intervention) and 2 h/week (ESDM intervention). Child aged 23–32 months: 1 h/week (ESDM intervention). Child’s evaluation: 6–8 months, 14, 19, and 32 months. | N = 1 child at risk of ASD (male). Age: 6 months. | Child’s developmental profile: Griffith Scales of Child Development (Griffiths III [11]). Autistic symptoms: Autism Diagnostic Observation Schedule, Second Edition (ADOS-2 [85]). Child’s adaptive behaviours: Vineland Adaptive Behaviour Scales-2 (VABS-II [91]). Child’s risky behaviours for autism: Social Attention and Communication Surveillance-Revised (SACS-R [92,93]). Child’s neurological evaluation: EEG/MRI. | To report the case of a child showing early signs of ASD in the first few months of life. The child received parent-mediated preventive intervention based on the Infant Start model, an adaptation of the Early Start Denver (ESDM) model. The progression of the child’s developmental profile was investigated. | Repetitive evaluations showed progressive improvements in developmental level and ASD symptoms. The child showed improvement over time in all developmental domains evaluated by Griffiths III. Specifically, B (Language and Communication) and D (Personal–Social–Emotional) subscales were the most increased. The child’s age equivalent was close to the chronological one at the end of the intervention. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecciso, F.; Martis, C.; Levante, A. The Use of Griffiths III in the Appraisal of the Developmental Profile in Autism: A Systematic Search and Review. Brain Sci. 2025, 15, 506. https://doi.org/10.3390/brainsci15050506
Lecciso F, Martis C, Levante A. The Use of Griffiths III in the Appraisal of the Developmental Profile in Autism: A Systematic Search and Review. Brain Sciences. 2025; 15(5):506. https://doi.org/10.3390/brainsci15050506
Chicago/Turabian StyleLecciso, Flavia, Chiara Martis, and Annalisa Levante. 2025. "The Use of Griffiths III in the Appraisal of the Developmental Profile in Autism: A Systematic Search and Review" Brain Sciences 15, no. 5: 506. https://doi.org/10.3390/brainsci15050506
APA StyleLecciso, F., Martis, C., & Levante, A. (2025). The Use of Griffiths III in the Appraisal of the Developmental Profile in Autism: A Systematic Search and Review. Brain Sciences, 15(5), 506. https://doi.org/10.3390/brainsci15050506







