Semaglutide and High-Intensity Interval Exercise Attenuate Cognitive Impairment in Type 2 Diabetic Mice via BDNF Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Semaglutide Treatment
2.3. HIIE Intervention
2.4. Behavioral Tests
2.4.1. Open Field Test (OFT)
2.4.2. Tail Suspension Test (TST)
2.4.3. Morris Water Maze (MWM) Test
2.5. Cell Culture and Treatment
2.6. Transmission Electron Microscopy (TEM)
2.7. Hematoxylin–Eosin (HE) Staining
2.8. Lactate Levels
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. RNA Sequencing
2.11. Western Blotting (WB)
2.12. Statistical Analysis
3. Results
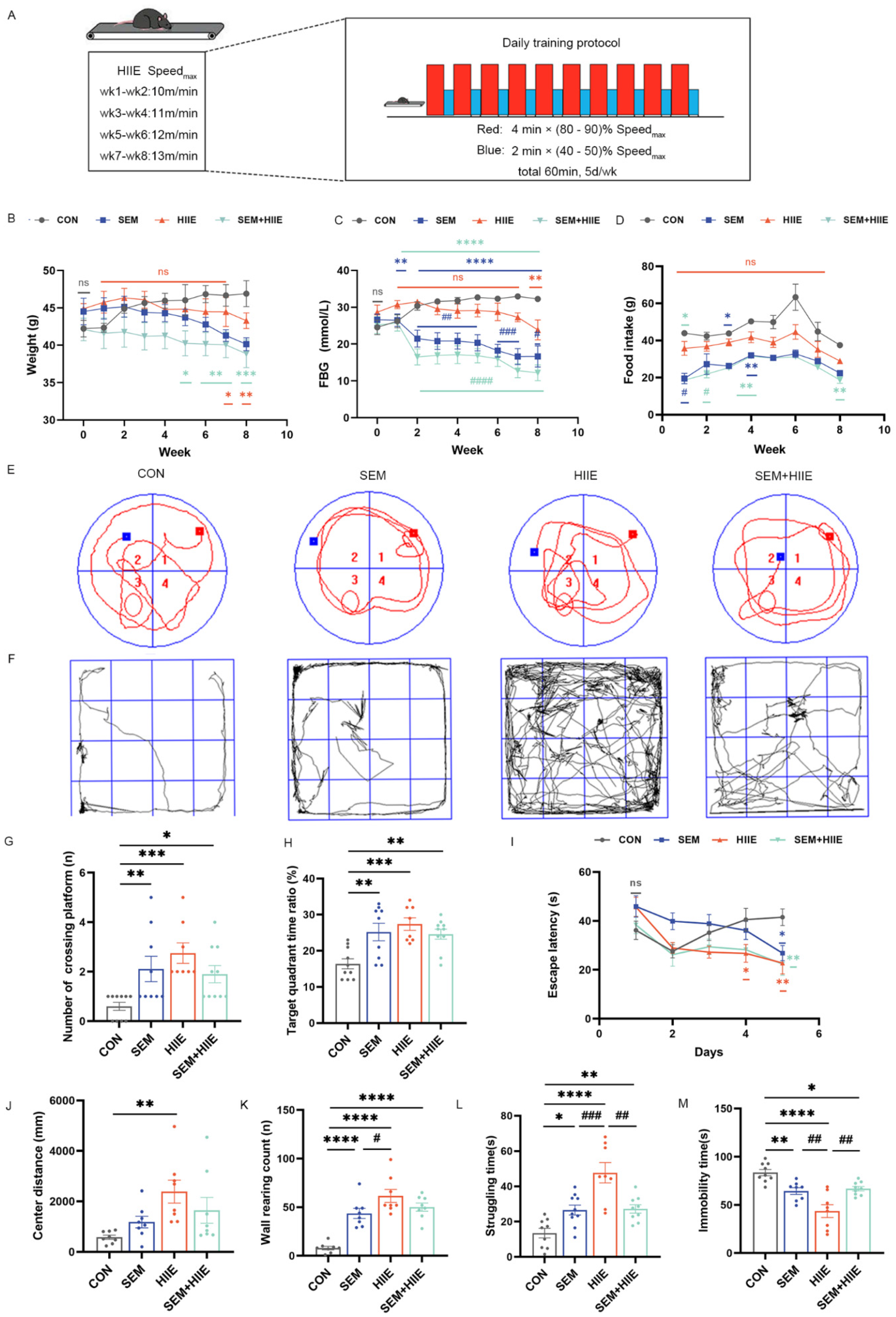
3.1. HIIE and Semaglutide Induce Weight Loss and Improve Glycemic Control in db/db Mice
3.2. HIIE and Semaglutide Improve Cognition and Alleviate Depression-like Behavior in db/db Mice
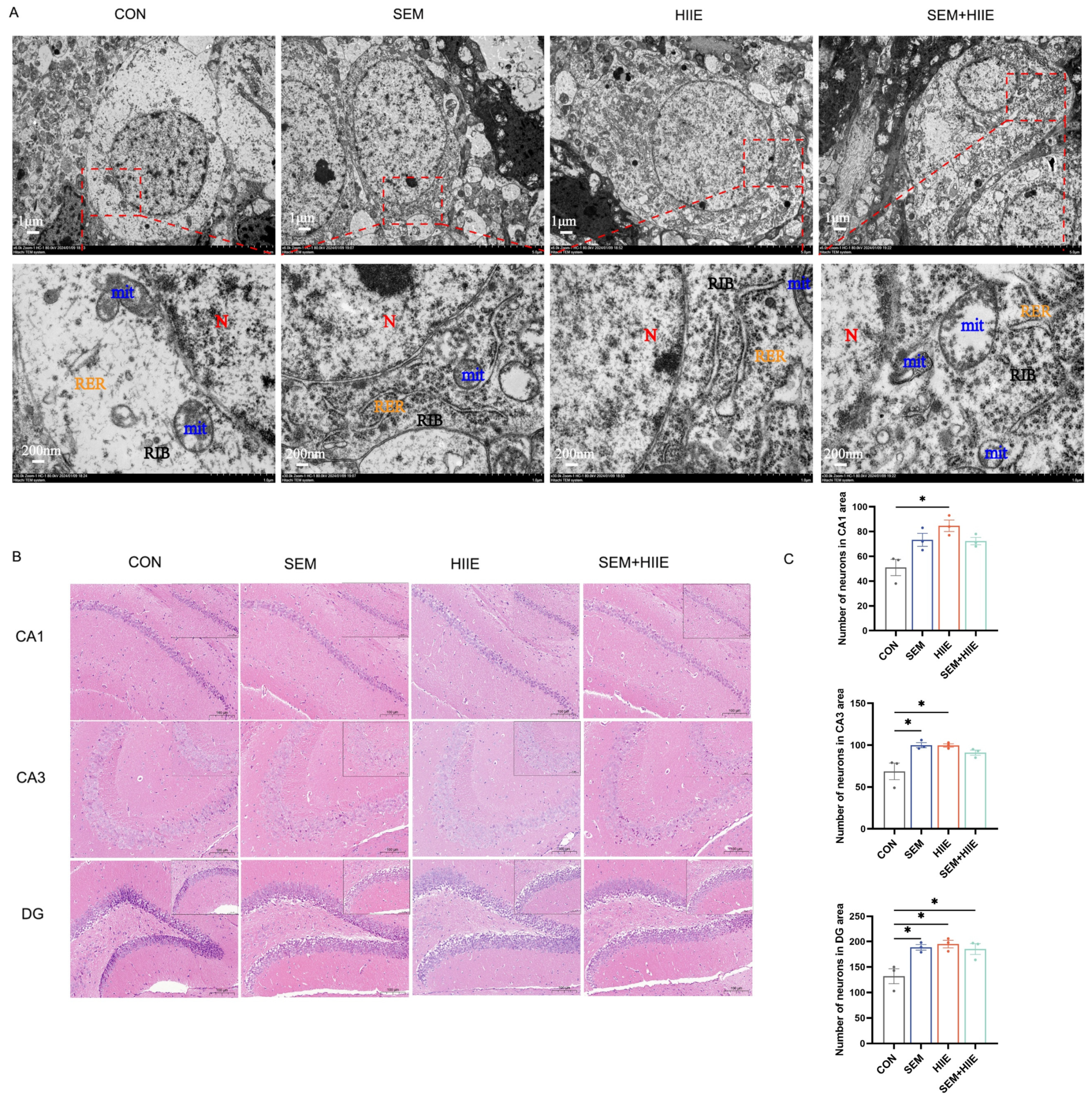
3.3. Semaglutide and HIIE Improve Morphology of Hippocampal Neurons in db/db Mice
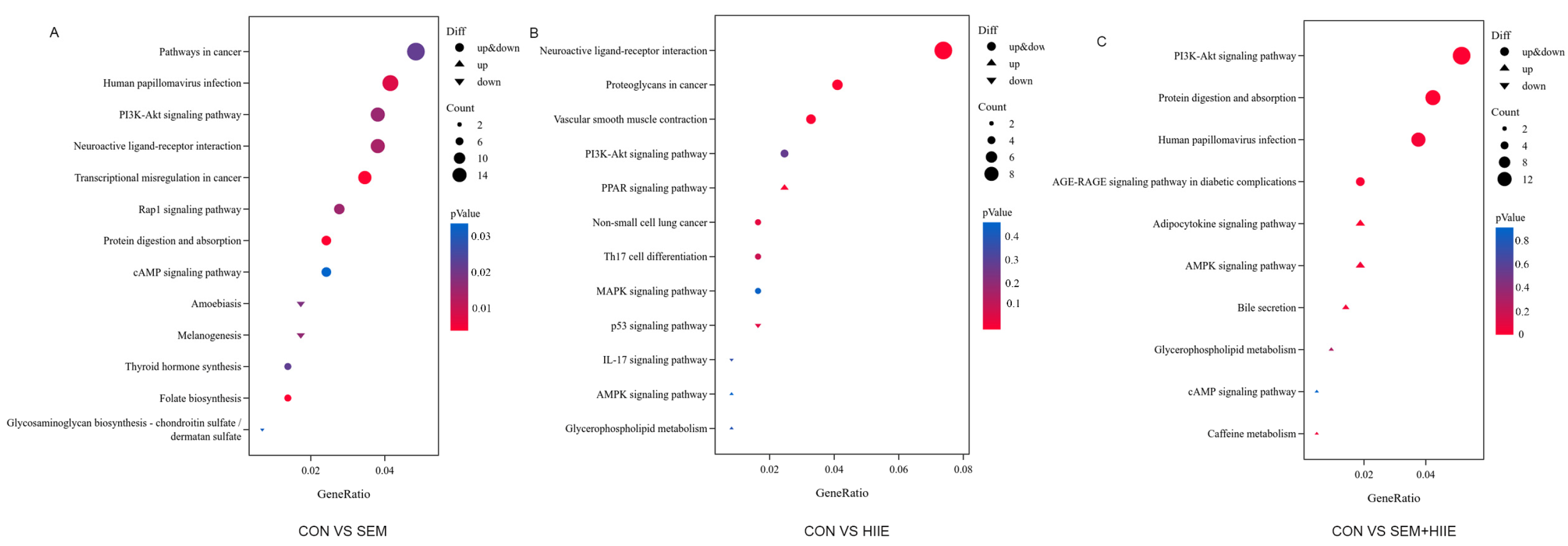
3.4. Transcriptome Analysis Reveals Distinct Mechanisms of Semaglutide and HIIE in Improving Diabetic Cognitive Impairment
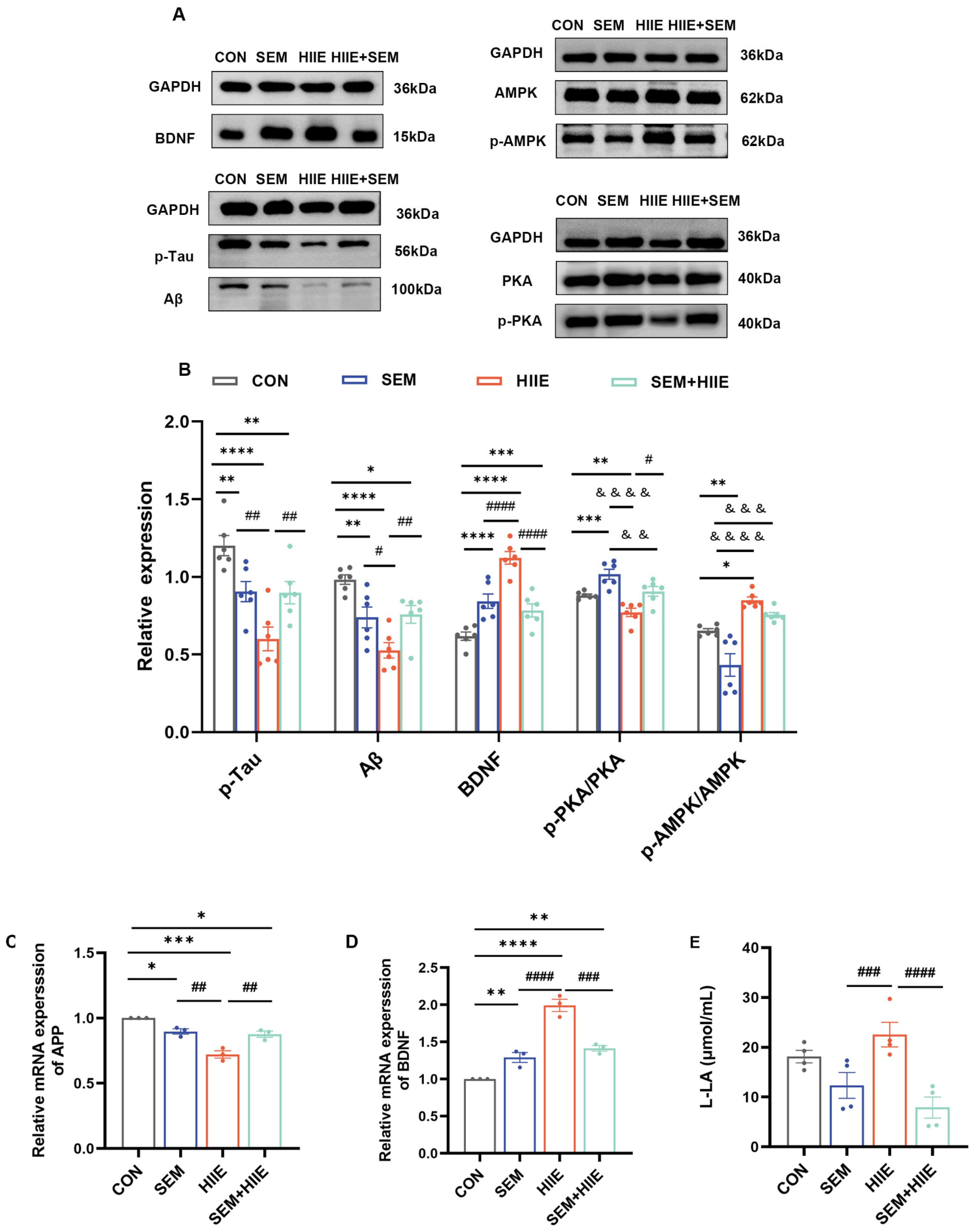
3.5. HIIE and Semaglutide Increase BDNF and Decrease Aβ, p-Tau Expressions in the Hippocampus of db/db Mice
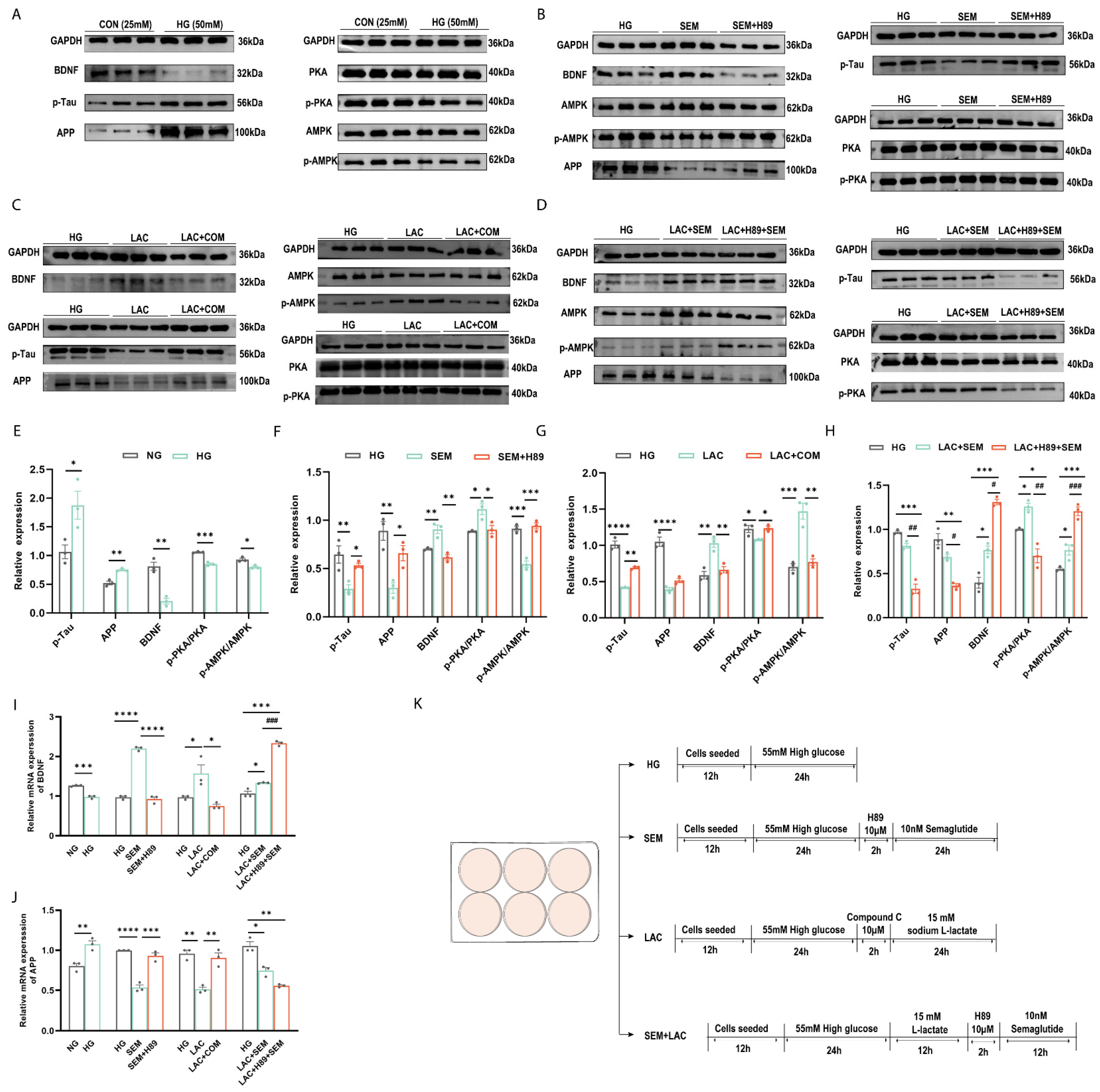
3.6. HIIE and Semaglutide Regulate L-Lactate, p-PKA/PKA, and p-AMPK/AMPK Expressions in the Hippocampus of db/db Mice
3.7. Lactate and Semaglutide Regulate PKA, BDNF, and AMPK Expression in HT22 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edgerton-Fulton, M.; Ergul, A. Vascular contributions to cognitive impairment/dementia in diabetes: Role of endothelial cells and pericytes. Am. J. Physiol. Cell Physiol. 2022, 323, C1177–C1189. [Google Scholar] [CrossRef] [PubMed]
- Lardaro, A.; Quarta, L.; Pagnotta, S.; Sodero, G.; Mariani, S.; Del Ben, M.; Desideri, G.; Ettorre, E.; Baratta, F. Impact of Sodium Glucose Cotransporter 2 Inhibitors (SGLT2i) Therapy on Dementia and Cognitive Decline. Biomedicines 2024, 12, 1750. [Google Scholar] [CrossRef] [PubMed]
- Mantik, K.E.K.; Kim, S.; Gu, B.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Repositioning of Anti-Diabetic Drugs against Dementia: Insight from Molecular Perspectives to Clinical Trials. Int. J. Mol. Sci. 2023, 24, 11450. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Caracciolo, B.; Wang, H.X.; Winblad, B.; Backman, L.; Qiu, C.; Fratiglioni, L. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes 2010, 59, 2928–2935. [Google Scholar] [CrossRef]
- Tang, X.; Cardoso, M.A.; Yang, J.; Zhou, J.B.; Simo, R. Impact of Intensive Glucose Control on Brain Health: Meta-Analysis of Cumulative Data from 16,584 Patients with Type 2 Diabetes Mellitus. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2021, 12, 765–779. [Google Scholar] [CrossRef]
- Drucker, D.J. Efficacy and Safety of GLP-1 Medicines for Type 2 Diabetes and Obesity. Diabetes Care 2024, 47, 1873–1888. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Karuppasamy, M.; Sahoo, B.M. Therapeutic Potential of Semaglutide, a Newer GLP-1 Receptor Agonist, in Abating Obesity, Non-Alcoholic Steatohepatitis and Neurodegenerative diseases: A Narrative Review. Pharm. Res. 2022, 39, 1233–1248. [Google Scholar] [CrossRef]
- Nowell, J.; Blunt, E.; Edison, P. Incretin and insulin signaling as novel therapeutic targets for Alzheimer’s and Parkinson’s disease. Mol. Psychiatry 2023, 28, 217–229. [Google Scholar] [CrossRef]
- Holscher, C. Brain insulin resistance: Role in neurodegenerative disease and potential for targeting. Expert Opin. Investig. Drugs 2020, 29, 333–348. [Google Scholar] [CrossRef]
- Iwai, S.; Kaji, K.; Nishimura, N.; Kubo, T.; Tomooka, F.; Shibamoto, A.; Suzuki, J.; Tsuji, Y.; Fujinaga, Y.; Kitagawa, K.; et al. Glucagon-like peptide-1 receptor agonist, semaglutide attenuates chronic liver disease-induced skeletal muscle atrophy in diabetic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166770. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Yuan, P.; Cheng, L.; Sun, H.; Gui, J.; Pan, Y.; Huang, D.; Chen, H.; Jiang, L. Role of PKA/CREB/BDNF signaling in PM2.5-induced neurodevelopmental damage to the hippocampal neurons of rats. Ecotoxicol. Environ. Saf. 2021, 214, 112005. [Google Scholar] [CrossRef] [PubMed]
- Ghadernezhad, N.; Khalaj, L.; Pazoki-Toroudi, H.; Mirmasoumi, M.; Ashabi, G. Metformin pretreatment enhanced learning and memory in cerebral forebrain ischaemia: The role of the AMPK/BDNF/P70SK signalling pathway. Pharm. Biol. 2016, 54, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Ma, L.; Jiao, X.; Dong, W.; Lin, J.; Qiu, Y.; Wu, W.; Liu, Q.; Chen, C.; Huang, H.; et al. Impaired synaptic plasticity and decreased excitability of hippocampal glutamatergic neurons mediated by BDNF downregulation contribute to cognitive dysfunction in mice induced by repeated neonatal exposure to ketamine. CNS Neurosci. Ther. 2024, 30, e14604. [Google Scholar] [CrossRef]
- Yu, X.; He, H.; Wen, J.; Xu, X.; Ruan, Z.; Hu, R.; Wang, F.; Ju, H. Diabetes-related cognitive impairment: Mechanisms, symptoms, and treatments. Open Med. 2025, 20, 20241091. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Sanabria, L.C.; Cavero-Redondo, I.; Martinez-Vizcaino, V.; Cano-Gutierrez, C.A.; Alvarez-Bueno, C. Effect of multicomponent exercise in cognitive impairment: A systematic review and meta-analysis. BMC Geriatr. 2022, 22, 617. [Google Scholar] [CrossRef]
- Setayesh, S.; Mohammad Rahimi, G.R. The impact of resistance training on brain-derived neurotrophic factor and depression among older adults aged 60 years or older: A systematic review and meta-analysis of randomized controlled trials. Geriatr. Nurs. 2023, 54, 23–31. [Google Scholar] [CrossRef]
- Yu, P.; Zhu, Z.; He, J.; Gao, B.; Chen, Q.; Wu, Y.; Zhou, J.; Cheng, Y.; Ling, J.; Zhang, J.; et al. Effects of high-intensity interval training, moderate-intensity continuous training, and guideline-based physical activity on cardiovascular metabolic markers, cognitive and motor function in elderly sedentary patients with type 2 diabetes (HIIT-DM): A protocol for a randomized controlled trial. Front. Aging Neurosci. 2023, 15, 1211990. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pan, C.Y.; Tseng, Y.T.; Chen, F.C.; Chang, Y.C.; Wang, T.C. Acute effects of high-intensity interval training and moderate-intensity continuous exercise on BDNF and irisin levels and neurocognitive performance in late middle-aged and older adults. Behav. Brain Res. 2021, 413, 113472. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Khoramipour, K.; Bejeshk, M.A.; Rajizadeh, M.A.; Najafipour, H.; Dehghan, P.; Farahmand, F. High-Intensity Interval Training Ameliorates Molecular Changes in the Hippocampus of Male Rats with the Diabetic Brain: The Role of Adiponectin. Mol. Neurobiol. 2023, 60, 3486–3495. [Google Scholar] [CrossRef]
- Kim, D.M.; Leem, Y.H. Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction. Neuroscience 2016, 324, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, M.; Hu, B.; Wang, L. Exercise Combined with a Chinese Medicine Herbal Tea for Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. J. Integr. Complement. Med. 2022, 28, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, F.; Wu, Y.; Li, F. The influence of hypoglycemic drugs on exercise-mediated hypoglycemic effects in elderly type 2 diabetic patients. Int. J. Clin. Exp. Med. 2015, 8, 14054–14059. [Google Scholar]
- Guo, W.; Xu, Z.; Zou, H.; Li, F.; Li, Y.; Feng, J.; Zhu, Z.; Zheng, Q.; Zhu, R.; Wang, B.; et al. Discovery of ecnoglutide—A novel, long-acting, cAMP-biased glucagon-like peptide-1 (GLP-1) analog. Mol. Metab. 2023, 75, 101762. [Google Scholar] [CrossRef]
- Hu, J.; Cai, M.; Shang, Q.; Li, Z.; Feng, Y.; Liu, B.; Xue, X.; Lou, S. Elevated Lactate by High-Intensity Interval Training Regulates the Hippocampal BDNF Expression and the Mitochondrial Quality Control System. Front. Physiol. 2021, 12, 629914. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S59–S85. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S181–S206. [Google Scholar] [CrossRef]
- Liu, T.; Hettish, L.; Lo, W.J.; Gray, M.; Li, C. FEASibility testing a randomized controlled trial of an exercise program to improve cognition for T2DM patients (the FEAST trial): A study protocol. Res. Nurs. Health 2021, 44, 746–757. [Google Scholar] [CrossRef]
- Espeland, M.A.; Lipska, K.; Miller, M.E.; Rushing, J.; Cohen, R.A.; Verghese, J.; McDermott, M.M.; King, A.C.; Strotmeyer, E.S.; Blair, S.N.; et al. Effects of Physical Activity Intervention on Physical and Cognitive Function in Sedentary Adults With and Without Diabetes. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 861–866. [Google Scholar] [CrossRef]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Cholerton, B.A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 22, 569–579. [Google Scholar] [CrossRef]
- de Lima, N.S.; De Sousa, R.A.L.; Amorim, F.T.; Gripp, F.; Diniz, E.M.C.O.; Henrique Pinto, S.; Peixoto, M.F.D.; Monteiro-Junior, R.S.; Bourbeau, K.; Cassilhas, R.C. Moderate-intensity continuous training and high-intensity interval training improve cognition, and BDNF levels of middle-aged overweight men. Metab. Brain Dis. 2022, 37, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Q.; Qi, X.; Gurney, M.; Perry, G.; Volkow, N.D.; Davis, P.B.; Kaelber, D.C.; Xu, R. Associations of semaglutide with first-time diagnosis of Alzheimer’s disease in patients with type 2 diabetes: Target trial emulation using nationwide real-world data in the US. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2024, 20, 8661–8672. [Google Scholar] [CrossRef]
- Estato, V.; Obadia, N.; Chateaubriand, P.H.; Figueiredo, V.; Curty, M.; Costa Silva, M.; Ferreira, R.G.L.; Santa-Ritta, J.; Campos Baroni, M.; Aragao, A.; et al. Semaglutide restores astrocyte-vascular interactions and blood-brain barrier integrity in a model of diet-induced metabolic syndrome. Diabetol. Metab. Syndr. 2025, 17, 2. [Google Scholar] [CrossRef]
- Yang, X.; Feng, P.; Zhang, X.; Li, D.; Wang, R.; Ji, C.; Li, G.; Holscher, C. The diabetes drug semaglutide reduces infarct size, inflammation, and apoptosis, and normalizes neurogenesis in a rat model of stroke. Neuropharmacology 2019, 158, 107748. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, X.R.; Chai, S.F.; Li, W.R.; Li, S.; Hou, M.; Li, J.L.; Ye, Y.C.; Cai, H.Y.; Holscher, C.; et al. Semaglutide ameliorates cognition and glucose metabolism dysfunction in the 3xTg mouse model of Alzheimer’s disease via the GLP-1R/SIRT1/GLUT4 pathway. Neuropharmacology 2023, 240, 109716. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, S.J.; Willing, L.B.; Goto, S.; Alkayed, N.J.; Brucklacher, R.M.; Wood, T.L.; Towfighi, J.; Hurn, P.D.; Simpson, I.A. Experimental stroke in the female diabetic, db/db, mouse. J. Cereb. Blood Flow Metab. 2001, 21, 52–60. [Google Scholar] [CrossRef]
- Shi, J.-J.; Liu, H.-F.; Hu, T.; Gao, X.; Zhang, Y.-B.; Li, W.-R.; Wang, Q.; Zhang, S.-J.; Tang, D.; Chen, Y.-B. Danggui-Shaoyao-San improves cognitive impairment through inhibiting O-GlcNAc-modification of estrogen α receptor in female db/db mice. J. Ethnopharmacol. 2021, 281, 114562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, Z.; Pan, J.; Yuan, M.; Lang, Y.; Wei, X.; Zhang, C. The interaction of BDNF with estrogen in the development of hypertension and obesity, particularly during menopause. Front. Endocrinol. 2024, 15, 1384159. [Google Scholar] [CrossRef]
- Chen, X.; Chen, A.; Wei, J.; Huang, Y.; Deng, J.; Chen, P.; Yan, Y.; Lin, M.; Chen, L.; Zhang, J.; et al. Dexmedetomidine alleviates cognitive impairment by promoting hippocampal neurogenesis via BDNF/TrkB/CREB signaling pathway in hypoxic-ischemic neonatal rats. CNS Neurosci. Ther. 2024, 30, e14486. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.; Penninx, B.W.; Elzinga, B.M. Serum BDNF concentrations as peripheral manifestations of depression: Evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 2014, 19, 791–800. [Google Scholar] [CrossRef]
- Li, G.; Peskind, E.R.; Millard, S.P.; Chi, P.; Sokal, I.; Yu, C.E.; Bekris, L.M.; Raskind, M.A.; Galasko, D.R.; Montine, T.J. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS ONE 2009, 4, e5424. [Google Scholar] [CrossRef]
- Alwhaibi, M. Depression, Anxiety, and Health-Related Quality of Life in Adults with Type 2 Diabetes. J. Clin. Med. 2024, 13, 6028. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.; Handelsman, Y.; Heile, M.; Shannon, M. Burden of Illness in Type 2 Diabetes Mellitus. J. Manag. Care Spec. Pharm. 2018, 24, S5–S13. [Google Scholar] [CrossRef]
- Sanaeifar, F.; Pourranjbar, S.; Pourranjbar, M.; Ramezani, S.; Mehr, S.R.; Wadan, A.S.; Khazeifard, F. Beneficial effects of physical exercise on cognitive-behavioral impairments and brain-derived neurotrophic factor alteration in the limbic system induced by neurodegeneration. Exp. Gerontol. 2024, 195, 112539. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wei, R.; Lu, Y. Effect of a Nurse-Led Exercise Program on Depression in Elderly Patients with Diabetes: A Retrospective Study. Actas Esp. Psiquiatr. 2024, 52, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Gabe, M.B.N.; Breitschaft, A.; Knop, F.K.; Hansen, M.R.; Kirkeby, K.; Rathor, N.; Adrian, C.L. Effect of oral semaglutide on energy intake, appetite, control of eating and gastric emptying in adults living with obesity: A randomized controlled trial. Diabetes Obes. Metab. 2024, 26, 4480–4489. [Google Scholar] [CrossRef]
- Manoharan, S.; Madan, R. GLP-1 Agonists Can Affect Mood: A Case of Worsened Depression on Ozempic (Semaglutide). Innov. Clin. Neurosci. 2024, 21, 25–26. [Google Scholar]
- McCarthy, S.F.; Tucker, J.A.L.; Hazell, T.J. Exercise-induced appetite suppression: An update on potential mechanisms. Physiol. Rep. 2024, 12, e70022. [Google Scholar] [CrossRef]
- Khodabandeh, S.; Rahmani-Nia, F.; Mirzaei, B.; Fairchild, T.J.; Hazell, T.J. The effects of acute aerobic exercise on appetite-regulating parameters and energy intake in males with obesity. Health Sci. Rep. 2024, 7, e70067. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L. Targeting cAMP/PKA pathway for glycemic control and type 2 diabetes therapy. J. Mol. Endocrinol. 2016, 57, R93–R108. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, Y.; Cao, L.X.; Li, Y.Z.; Wan, P.; Qiu, D.L. Glucagon-like peptide-1 facilitates cerebellar parallel fiber glutamate release through PKA signaling in mice in vitro. Sci. Rep. 2023, 13, 7948. [Google Scholar] [CrossRef]
- Marzook, A.; Tomas, A.; Jones, B. The Interplay of Glucagon-Like Peptide-1 Receptor Trafficking and Signalling in Pancreatic Beta Cells. Front. Endocrinol. 2021, 12, 678055. [Google Scholar] [CrossRef] [PubMed]
- Tramutola, A.; Arena, A.; Cini, C.; Butterfield, D.A.; Barone, E. Modulation of GLP-1 signaling as a novel therapeutic approach in the treatment of Alzheimer’s disease pathology. Expert Rev. Neurother. 2017, 17, 59–75. [Google Scholar] [CrossRef]
- Rajizadeh, M.A.; Moslemizadeh, A.; Hosseini, M.S.; Rafiei, F.; Soltani, Z.; Khoramipour, K. Adiponectin receptor 1 could explain the sex differences in molecular basis of cognitive improvements induced by exercise training in type 2 diabetic rats. Sci. Rep. 2023, 13, 16267. [Google Scholar] [CrossRef] [PubMed]
- Kakoti, B.B.; Alom, S.; Deka, K.; Halder, R.K. AMPK pathway: An emerging target to control diabetes mellitus and its related complications. J. Diabetes Metab. Disord. 2024, 23, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Przygrodzka, E.; Hou, X.; Zhang, P.; Plewes, M.R.; Franco, R.; Davis, J.S. PKA and AMPK Signaling Pathways Differentially Regulate Luteal Steroidogenesis. Endocrinology 2021, 162, bqab015. [Google Scholar] [CrossRef]
- Zhao, M.; Cao, N.; Gu, H.; Xu, J.; Xu, W.; Zhang, D.; Wei, T.W.; Wang, K.; Guo, R.; Cui, H.; et al. AMPK Attenuation of beta-Adrenergic Receptor-Induced Cardiac Injury via Phosphorylation of beta-Arrestin-1-ser330. Circ. Res. 2024, 135, 651–667. [Google Scholar] [CrossRef]
- Djouder, N.; Tuerk, R.D.; Suter, M.; Salvioni, P.; Thali, R.F.; Scholz, R.; Vaahtomeri, K.; Auchli, Y.; Rechsteiner, H.; Brunisholz, R.A.; et al. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010, 29, 469–481. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Liu, X.; Dagda, R.K.; Zhang, Y. How AMPK and PKA Interplay to Regulate Mitochondrial Function and Survival in Models of Ischemia and Diabetes. Oxid. Med. Cell. Longev. 2017, 2017, 4353510. [Google Scholar] [CrossRef]
- He, L.; Chang, E.; Peng, J.; An, H.; McMillin, S.M.; Radovick, S.; Stratakis, C.A.; Wondisford, F.E. Activation of the cAMP-PKA pathway Antagonizes Metformin Suppression of Hepatic Glucose Production. J. Biol. Chem. 2016, 291, 10562–10570. [Google Scholar] [CrossRef]
- Aw, D.K.; Sinha, R.A.; Xie, S.Y.; Yen, P.M. Differential AMPK phosphorylation by glucagon and metformin regulates insulin signaling in human hepatic cells. Biochem. Biophys. Res. Commun. 2014, 447, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ladilov, Y. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, L.H. Lactate transport and signaling in the brain: Potential therapeutic targets and roles in body-brain interaction. J. Cereb. Blood Flow Metab. 2015, 35, 176–185. [Google Scholar] [CrossRef]
- Colucci, A.C.M.; Tassinari, I.D.; Loss, E.D.S.; de Fraga, L.S. History and Function of the Lactate Receptor GPR81/HCAR1 in the Brain: A Putative Therapeutic Target for the Treatment of Cerebral Ischemia. Neuroscience 2023, 526, 144–163. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.R.; Leichner, T.M.; Zhao, S.; Lee, G.S.; Chowansky, A.; Zimmer, D.; De Jonghe, B.C.; Kanoski, S.E.; Grill, H.J.; Bence, K.K. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011, 13, 320–330. [Google Scholar] [CrossRef]
- Khosravi, P.; Shahidi, F.; Eskandari, A.; Khoramipour, K. High-intensity interval training reduces Tau and beta-amyloid accumulation by improving lactate-dependent mitophagy in rats with type 2 diabetes. Iran. J. Basic Med. Sci. 2024, 27, 1430–1439. [Google Scholar] [CrossRef]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Huang, C.; Lin, D. Lactate Activates AMPK Remodeling of the Cellular Metabolic Profile and Promotes the Proliferation and Differentiation of C2C12 Myoblasts. Int. J. Mol. Sci. 2022, 23, 13996. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tsukamoto, H.; Takenaka, S.; Olesen, N.D.; Petersen, L.G.; Sorensen, H.; Nielsen, H.B.; Secher, N.H.; Ogoh, S. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 1417–1427. [Google Scholar] [CrossRef]
- Lin, J.Y.; Kuo, W.W.; Baskaran, R.; Kuo, C.H.; Chen, Y.A.; Chen, W.S.; Ho, T.J.; Day, C.H.; Mahalakshmi, B.; Huang, C.Y. Swimming exercise stimulates IGF1/PI3K/Akt and AMPK/SIRT1/PGC1alpha survival signaling to suppress apoptosis and inflammation in aging hippocampus. Aging 2020, 12, 6852–6864. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Gu, J.; Li, T.; Chen, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X. Inhibition of aerobic glycolysis alleviates sepsis-induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK-regulated autophagy. Int. J. Mol. Med. 2021, 47, 19. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.; Asrih, M.; Repond, C.; Sempoux, C.; Stehle, J.C.; Leloup, C.; Jornayvaz, F.R.; Pellerin, L. AMPK activation caused by reduced liver lactate metabolism protects against hepatic steatosis in MCT1 haploinsufficient mice. Mol. Metab. 2017, 6, 1625–1633. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, S.; Kang, Z.; Sun, J.; Wang, Z.; Xu, Y.; Xing, S.; Feng, M.; Wang, Y.; Liu, H. Semaglutide and High-Intensity Interval Exercise Attenuate Cognitive Impairment in Type 2 Diabetic Mice via BDNF Modulation. Brain Sci. 2025, 15, 480. https://doi.org/10.3390/brainsci15050480
Lai S, Kang Z, Sun J, Wang Z, Xu Y, Xing S, Feng M, Wang Y, Liu H. Semaglutide and High-Intensity Interval Exercise Attenuate Cognitive Impairment in Type 2 Diabetic Mice via BDNF Modulation. Brain Sciences. 2025; 15(5):480. https://doi.org/10.3390/brainsci15050480
Chicago/Turabian StyleLai, Sijie, Zhenghong Kang, Jianting Sun, Ziyu Wang, Yanzi Xu, Sisi Xing, Mengying Feng, Yiyi Wang, and Hua Liu. 2025. "Semaglutide and High-Intensity Interval Exercise Attenuate Cognitive Impairment in Type 2 Diabetic Mice via BDNF Modulation" Brain Sciences 15, no. 5: 480. https://doi.org/10.3390/brainsci15050480
APA StyleLai, S., Kang, Z., Sun, J., Wang, Z., Xu, Y., Xing, S., Feng, M., Wang, Y., & Liu, H. (2025). Semaglutide and High-Intensity Interval Exercise Attenuate Cognitive Impairment in Type 2 Diabetic Mice via BDNF Modulation. Brain Sciences, 15(5), 480. https://doi.org/10.3390/brainsci15050480







