Dance Training and the Neuroplasticity of the Vestibular-Ocular Reflex: Preliminary Findings †
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Dancers Profile
2.3. Procedure
2.4. Statistical Analysis
3. Results
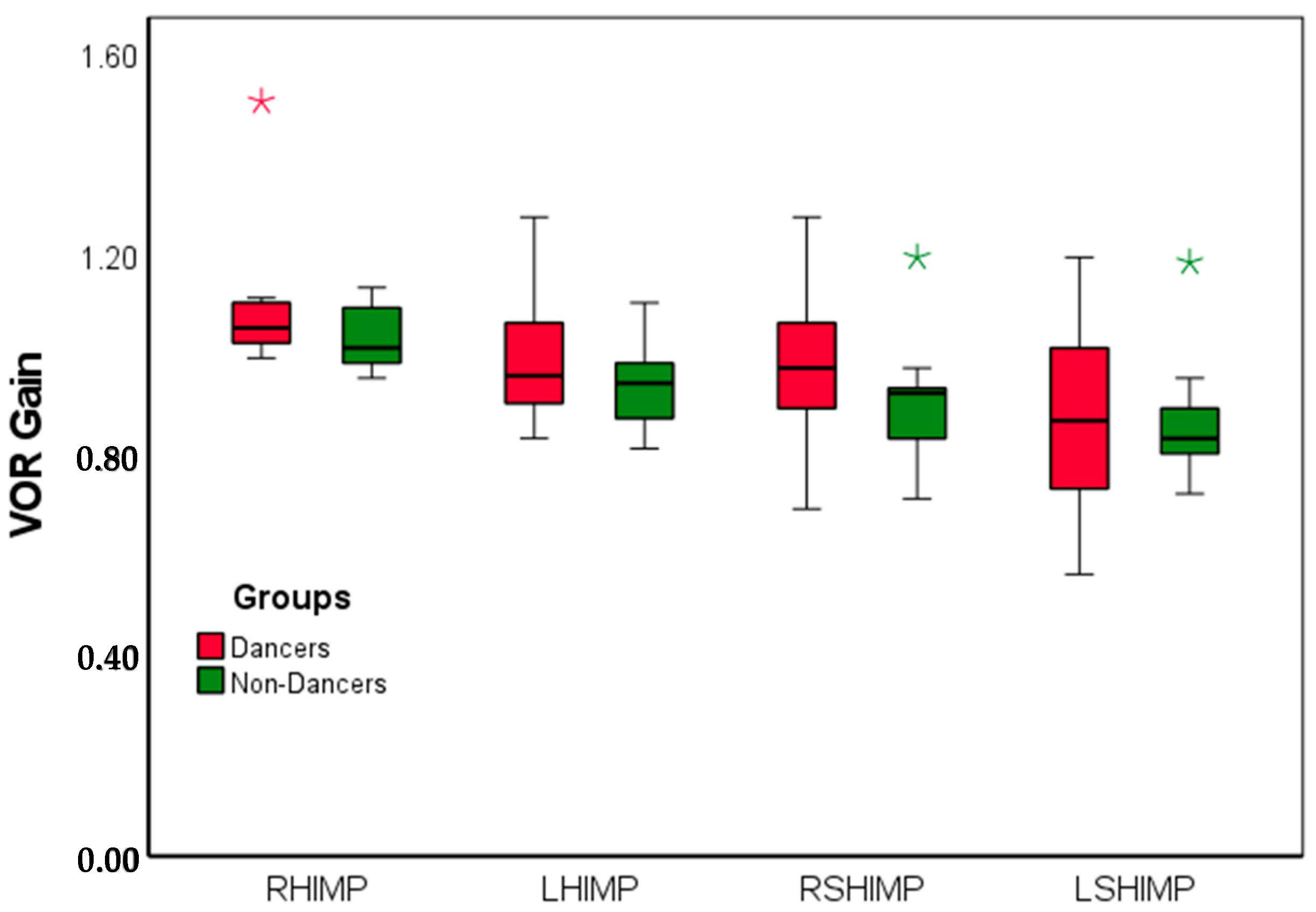
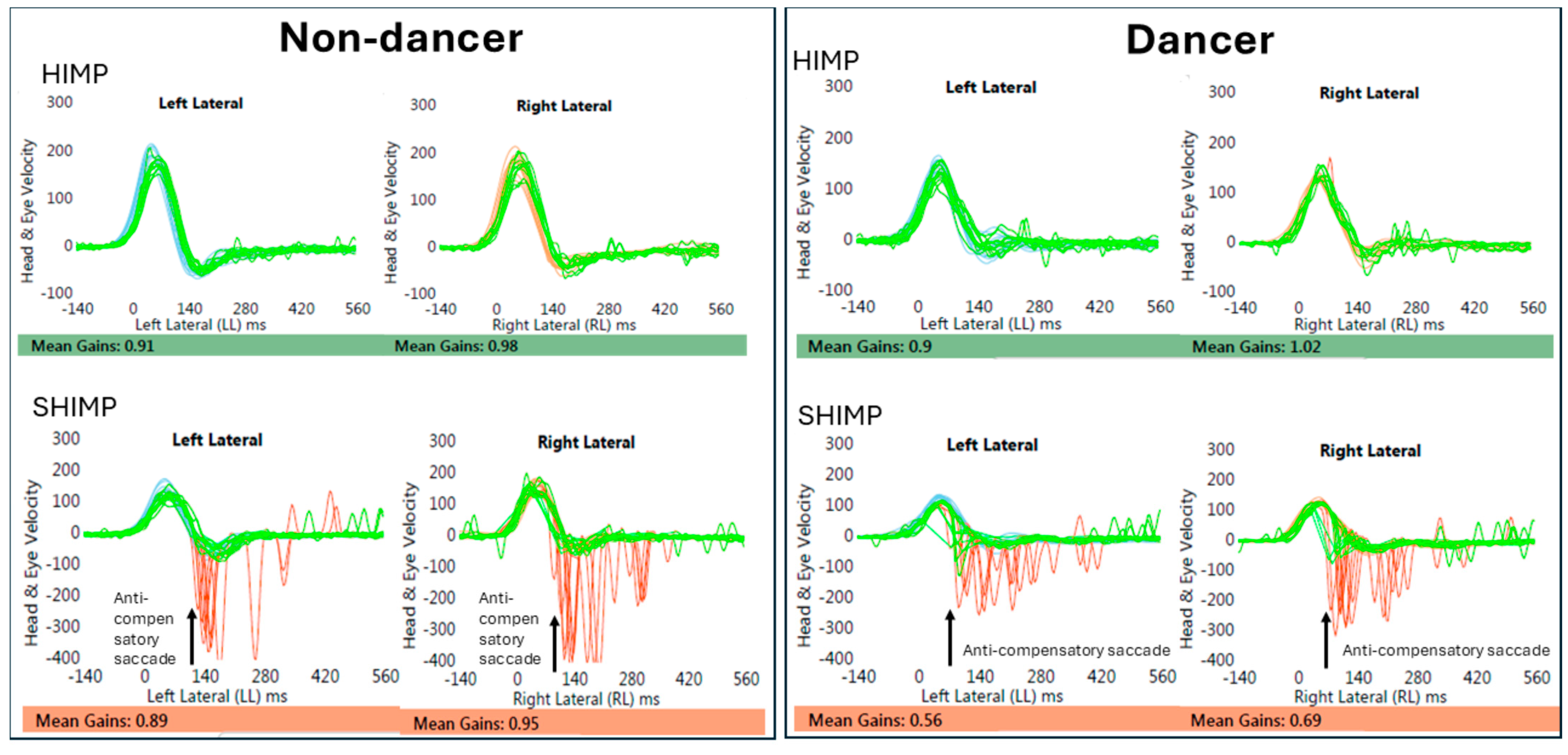
3.1. VOR Gain Using HIMP and SHIMP
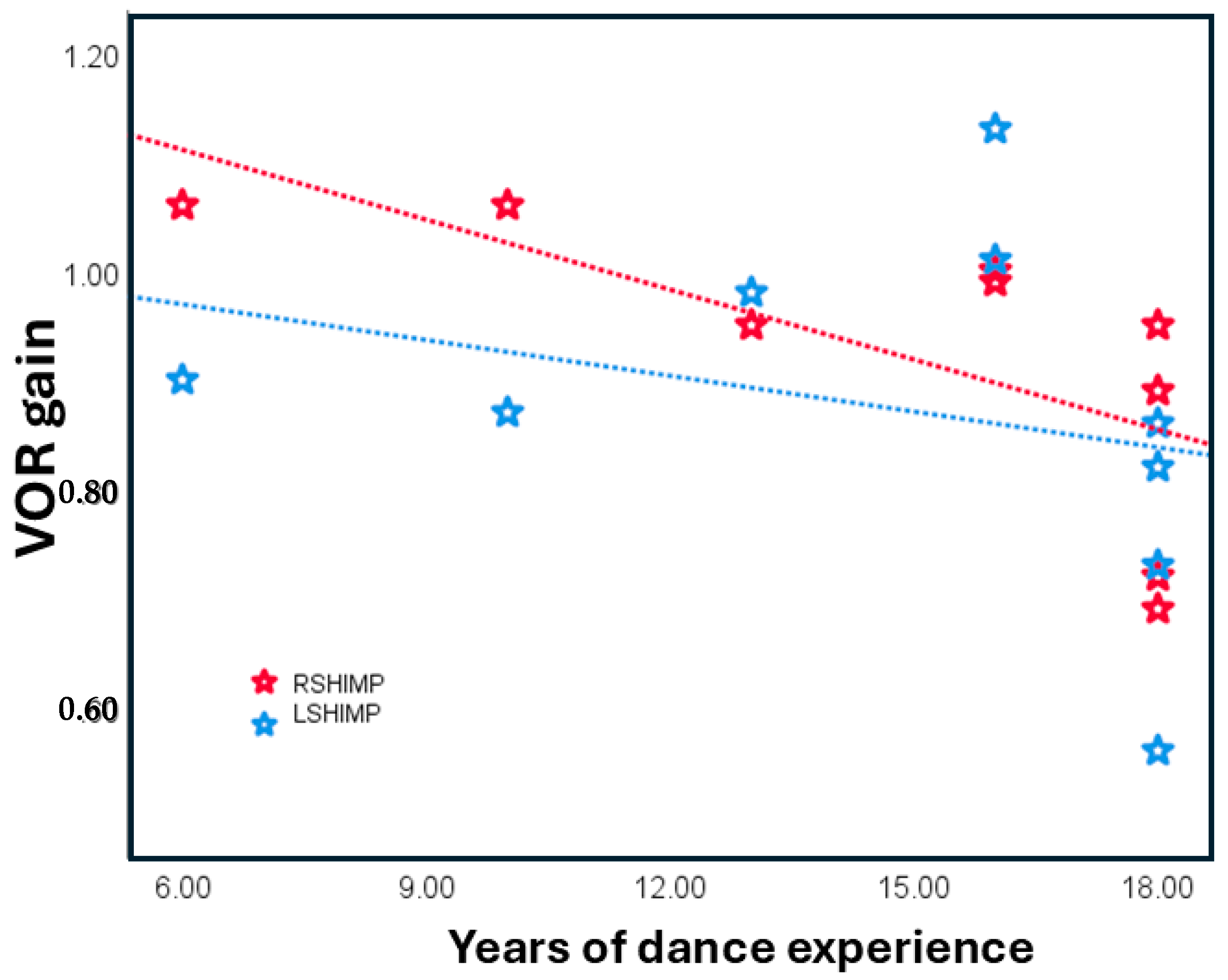
3.2. Dance Experience as a Continuous Variable
3.3. Anti-Compensatory Saccade Latency
4. Discussion
4.1. The Effects of Dance Training on the HIMP and the SHIMP Gain
4.2. Anti-Compensatory Catch up Saccade
4.3. Asymmetry Between the Right and the Left VOR Gain
5. Implications
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitch, W.T. The biology and evolution of rhythm: Unraveling a paradox. In Lang Music as Cogn Syst; Oxford University Press: Oxford, UK, 2012; pp. 73–95. [Google Scholar]
- Iversen, J.R. In the beginning was the beat. In Cambridge Companion to Percuss; Cambridge University Press: Cambridge, UK, 2016; pp. 281–295. [Google Scholar] [CrossRef]
- Hanna, J.L. To Dance Is Human: A Theory of Nonverbal Communication; University of Chicago Press: Chicago, IL, USA, 1987. [Google Scholar]
- Karpati, F.J.; Giacosa, C.; Foster, N.E.V.; Penhune, V.B.; Hyde, K.L. Dance and the brain: A review. Ann. N. Y. Acad. Sci. 2015, 1337, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Muiños, M.; Ballesteros, S. Does dance counteract age-related cognitive and brain declines in middle-aged and older adults? A systematic review. Neurosci. Biobehav. Rev. 2021, 121, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Stawicki, P.; Wareńczak, A.; Lisiński, P. Does regular dancing improve static balance? Int. J. Environ. Res. Public Health 2021, 18, 5056. [Google Scholar] [CrossRef]
- Buizza, A.; Schmid, R. Velocity characteristics of smooth pursuit eye movements to different patterns of target motion. Exp. Brain Res. 1986, 63, 395–401. [Google Scholar] [CrossRef]
- Cullen, K.E.; Belton, T.; McCrea, R.A. A non-visual mechanism for voluntary cancellation of the vestibulo-ocular reflex. Exp. Brain Res. 1991, 83, 237–252. [Google Scholar] [CrossRef]
- MacDougall, H.G.; McGarvie, L.A.; Halmagyi, G.M.; Rogers, S.J.; Manzari, L.; Burgess, A.M.; Curthoys, I.S.; Weber, K.P. A new saccadic indicator of peripheral vestibular function based on the video head impulse test. Neurology 2016, 87, 410–418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, Q.; Magnani, C.; Sterkers, O.; Lamas, G.; Vidal, P.-P.; Sadoun, J.; Curthoys, I.S.; de Waele, C. Saccadic velocity in the new suppression head impulse test: A new indicator of horizontal vestibular canal paresis and of vestibular compensation. Front. Neurol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Nigmatullina, Y.; Hellyer, P.J.; Nachev, P.; Sharp, D.J.; Seemungal, B.M. The neuroanatomical correlates of training-related perceptuo-reflex uncoupling in dancers. Cereb. Cortex 2015, 25, 554–562. [Google Scholar] [CrossRef]
- Schärli, A.; Hecht, H.; Mast, F.W.; Hossner, E.J. How spotting technique affects dizziness and postural stability after full-body rotations in dancers. Hum. Mov. Sci. 2024, 95, 103211. [Google Scholar] [CrossRef]
- Barrett, H. Quantifying Ballet Technique Through Turn Kinematics for Injury Assessment. Bachelor’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2015. [Google Scholar]
- Land, W.M.; Volchenkov, D.; Bläsing, B.E.; Schack, T. From action representation to action execution: Exploring the links between cognitive and biomechanical levels of motor control. Front. Comput. Neurosci. 2013, 7, 127. [Google Scholar] [CrossRef]
- Osterhammel, P.; Terkildsen, K.; Zilstorff, K. Vestibular habituation in ballet dancers. Acta Otolaryngol. 1968, 66, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, K.; Sakata, E.; Ohtsu, K. Use of the visual suppression test using post-rotatory nystagmus to determine skill in ballet dancers. Eur. Arch. Oto-Rhino-Laryngol. 1994, 251, 218–223. [Google Scholar] [CrossRef]
- Tanguy, S.; Quarck, G.; Etard, O.; Gauthier, A.; Denise, P. Vestibulo-ocular reflex and motion sickness in figure skaters. Eur. J. Appl. Physiol. 2008, 104, 1031–1037. [Google Scholar] [CrossRef]
- Maheu, M.; Bethani, L.; Nooristani, M.; Jemel, B.; Delcenserie, A.; Champoux, F. Influence of dance training on challenging postural control task. Gait Posture 2019, 69, 31–35. [Google Scholar] [CrossRef]
- Moin-Darbari, K.; Nooristani, M.; Bacon, B.A.; Champoux, F.; Maheu, M. Long-term dance training modifies eye-head coordination in response to passive head impulse. J. Neurophysiol. 2023, 130, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, M.; Piker, E.G.; Jha, R.H.; Trinidad, D. Effects of Dance training on VOR. In Proceedings of the American Balance Society Conference, Scottsdale, AZ, USA, 28 February–1 March 2023. [Google Scholar]
- Crane, B.T.; Demer, J.L. Latency of voluntary cancellation of the human vestibule-ocular reflex during transient yaw rotation. Exp. Brain Res. 1999, 127, 67–74. [Google Scholar] [CrossRef]
- McGarvie, L.A.; MacDougall, H.G.; Halmagyi, G.M.; Burgess, A.M.; Weber, K.P.; Curthoys, I.S. The video head impulse test (vHIT) of semicircular canal function—Age-dependent normative values of VOR gain in healthy subjects. Front. Neurol. 2015, 6, 154. [Google Scholar] [CrossRef]
- Weber, K.P.; Aw, S.T.; Todd, M.J.; McGarvie, L.A.; Curthoys, I.S.; Halmagyi, G.M. Head impulse test in unilateral vestibular loss: Vestibulo-ocular reflex and catch-up saccades. Neurology 2008, 70, 454–463. [Google Scholar] [PubMed]
- Janky, K.L.; Patterson, J.; Shepard, N.; Thomas, M.; Barin, K.; Creutz, T.; Schmid, K.; Honaker, J.A. Video Head Impulse Test (vHIT): The Role of Corrective Saccades in Identifying Patients with Vestibular Loss. Otol. Neurotol. 2018, 39, 467–473. [Google Scholar] [CrossRef]
- Jha, R.H.; Singh, N.K.; Kumar, P. Video Head Impulse Test in Persons with Blindness: Feasibility and Outcomes. J. Am. Acad. Audiol. 2022, 33, 116–124. [Google Scholar] [CrossRef]
- van der Veen, S.M.; Stamenkovic, A.; Thomas, J.S.; Pidcoe, P.E. Skill-Related Adaptive Modifications of Gaze Stabilization in Elite and Non-Elite Athletes. Front. Sport Act. Living 2022, 4, 824990. [Google Scholar] [CrossRef] [PubMed]
- Bläsing, B.; Calvo-Merino, B.; Cross, E.S.; Jola, C.; Honisch, J.; Stevens, C.J. Neurocognitive control in dance perception and performance. Acta Psychol. 2012, 139, 300–308. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, R.H.; Piker, E.G.; Scalzo, M.; Trinidad, D. Dance Training and the Neuroplasticity of the Vestibular-Ocular Reflex: Preliminary Findings. Brain Sci. 2025, 15, 355. https://doi.org/10.3390/brainsci15040355
Jha RH, Piker EG, Scalzo M, Trinidad D. Dance Training and the Neuroplasticity of the Vestibular-Ocular Reflex: Preliminary Findings. Brain Sciences. 2025; 15(4):355. https://doi.org/10.3390/brainsci15040355
Chicago/Turabian StyleJha, Raghav H., Erin G. Piker, Miranda Scalzo, and Diana Trinidad. 2025. "Dance Training and the Neuroplasticity of the Vestibular-Ocular Reflex: Preliminary Findings" Brain Sciences 15, no. 4: 355. https://doi.org/10.3390/brainsci15040355
APA StyleJha, R. H., Piker, E. G., Scalzo, M., & Trinidad, D. (2025). Dance Training and the Neuroplasticity of the Vestibular-Ocular Reflex: Preliminary Findings. Brain Sciences, 15(4), 355. https://doi.org/10.3390/brainsci15040355






