Chronic Fluoxetine Treatment Desensitizes Serotoninergic Inhibition of GABAergic Inputs and Intrinsic Excitability of Dorsal Raphe Serotonin Neurons
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Brain Slice Preparation
2.3. Electrophysiological Recording
2.4. Statistics
2.5. Drugs
2.6. DRN 5-HT Neuron Mapping with Tryptophan Hydroxylase (TPH) Immunostaining
3. Results
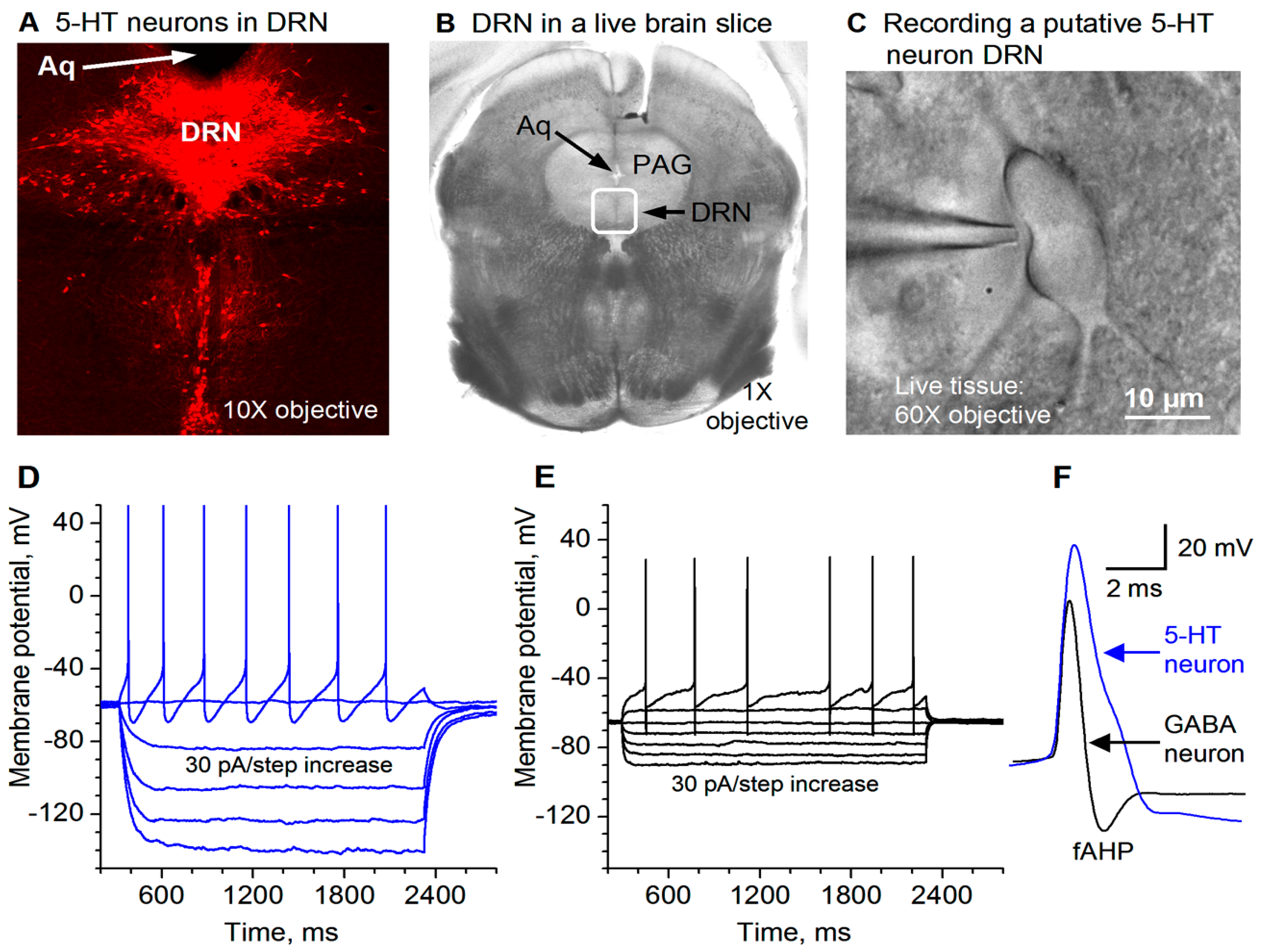
3.1. Electrophysiological Identification of Dorsal Raphe 5-HT Neurons
3.2. GABAergic Inhibition of DR 5-HT Cell Firing
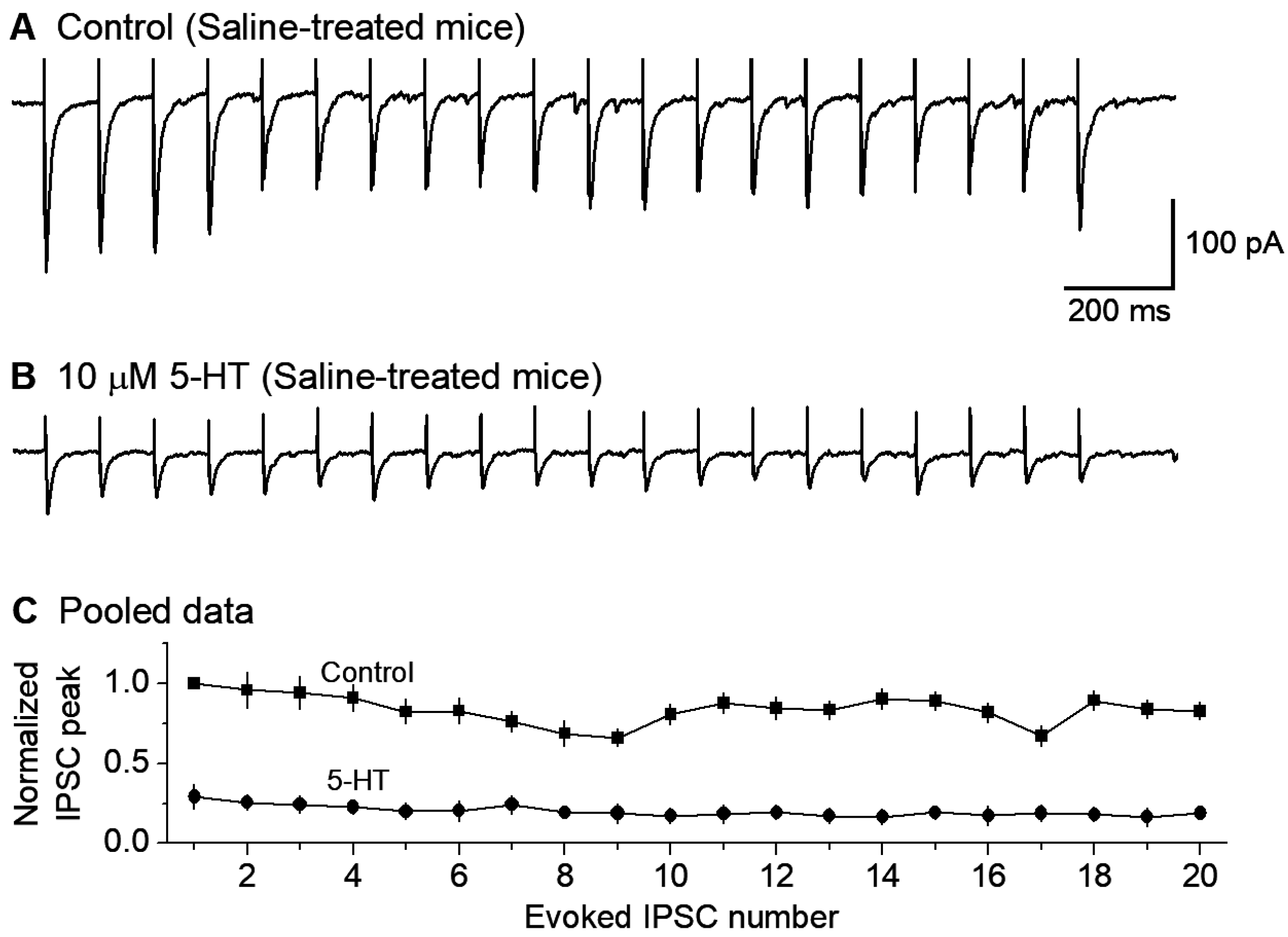
3.3. 5-HT Inhibition of GABAergic Inputs to DR 5-HT Neurons
3.4. GirK Channel Blocker Tertiapin-Q Prevents 5-HT Inhibition of GABAergic Inputs to 5-HT Neurons
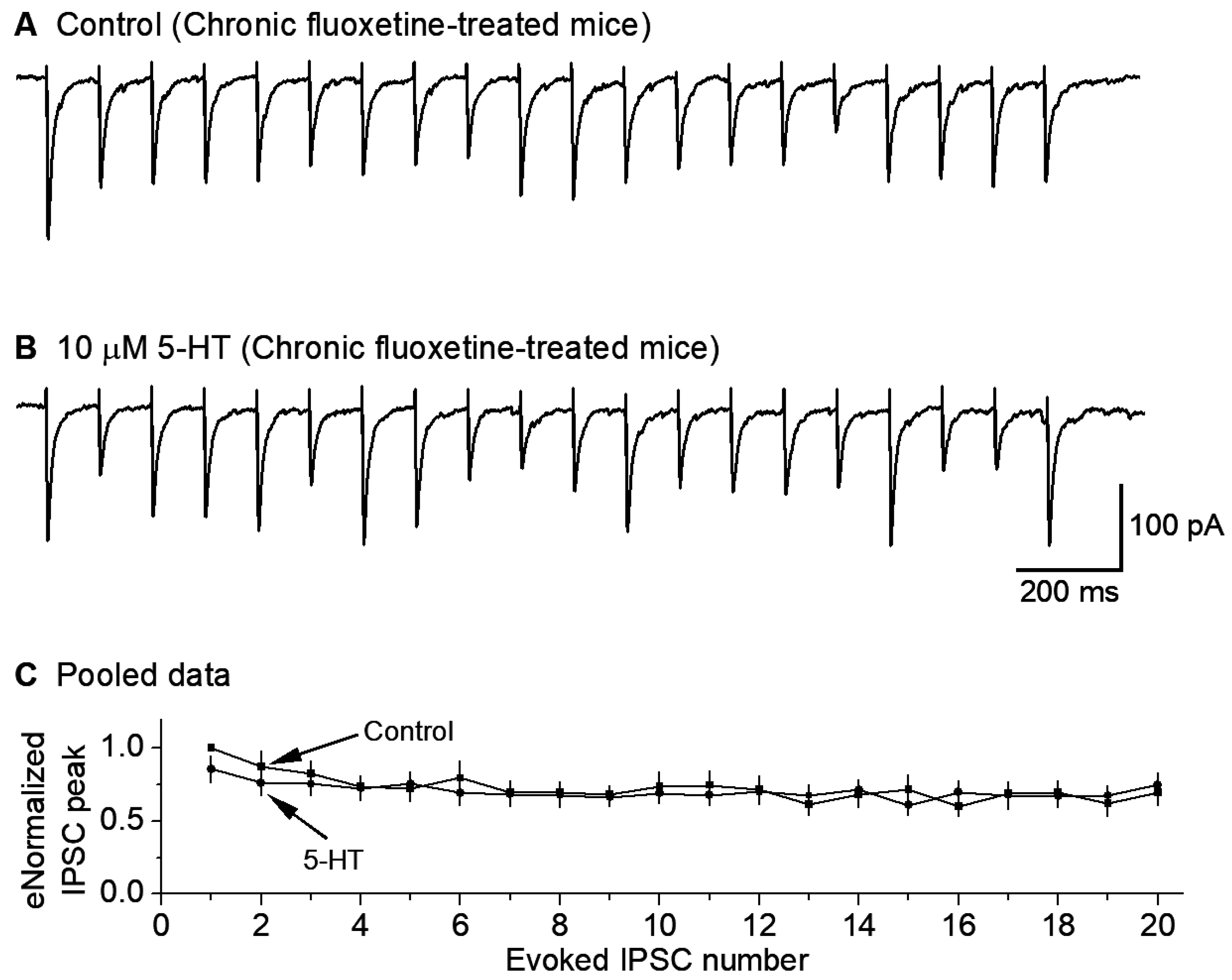
3.5. Chronic Fluoxetine Treatment Downregulates 5-HT Inhibition of GABAergic Inputs to 5-HT Neurons
3.6. Chronic Fluoxetine Treatment Downregulates 5-HT Neuron Autoinhibition

4. Discussion
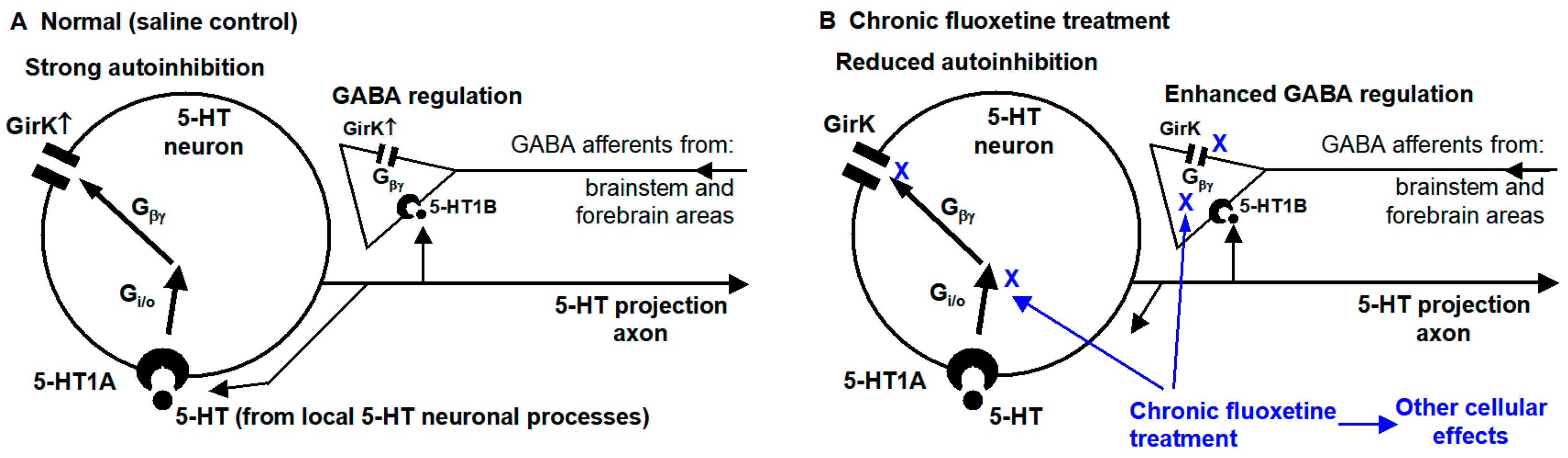
4.1. Chronic Fluoxetine Treatment Enhances GABAergic Inhibitory Influence on Dorsal Raphe 5-HT Neurons by Downregulating Presynaptic 5-HT Inhibition
4.2. Chronic Antidepressant Treatment Renders DRN 5-HT Neurons Resistant to 5-HT Autoinhibition by Downregulating 5-HT Inhibition of the Intrinsic Excitability
4.3. Limitations and Alternative Interpretation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, K.G.; Halliday, G.M.; Törk, I. Cytoarchitecture of the human dorsal raphe nucleus. J. Comp. Neurol. 1990, 301, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Charara, A.; Parent, A. Chemoarchitecture of the primate dorsal raphe nucleus. J. Chem. Neuroanat. 1998, 15, 111–127. [Google Scholar] [PubMed]
- Commons, K.G. Ascending serotonin neuron diversity under two umbrellas. Brain Struct. Funct. 2016, 221, 3347–3360. [Google Scholar] [CrossRef] [PubMed]
- Hornung, J.P. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003, 26, 331–343. [Google Scholar] [PubMed]
- Parent, M.; Wallman, M.J.; Gagnon, D.; Parent, A. Serotonin innervation of basal ganglia in monkeys and humans. J. Chem. Neuroanat. 2011, 41, 256–265. [Google Scholar] [PubMed]
- Steinbusch, H.W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat—Cell bodies and terminals. Neuroscience 1981, 6, 557–618. [Google Scholar] [PubMed]
- Smiley, J.F.; Goldman-Rakic, P.S. Serotonergic axons in monkey prefrontal cerebral cortex synapse predominantly on interneurons as demonstrated by serial section electron microscopy. J. Comp. Neurol. 1996, 367, 431–443. [Google Scholar] [CrossRef] [PubMed]
- De Stasi, A.M.; Zorrilla de San Martin, J.; Soto, N.; Aguirre, A.; Olusakin, J.; Lourenço, J.; Gaspar, P.; Bacci, A. Alterations of Adult Prefrontal Circuits Induced by Early Postnatal Fluoxetine Treatment Mediated by 5-HT7 Receptors. J. Neurosci. 2025, 45, e2393232024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mosienko, V.; Beis, D.; Pasqualetti, M.; Waider, J.; Matthes, S.; Qadri, F.; Bader, M.; Alenina, N. Life without brain serotonin: Reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behav. Brain Res. 2015, 277, 78–88. [Google Scholar] [PubMed]
- Soiza-Reilly, M.; Meye, F.J.; Olusakin, J.; Telley, L.; Petit, E.; Chen, X.; Mameli, M.; Jabaudon, D.; Sze, J.Y.; Gaspar, P. SSRIs target pre-frontal to raphe circuits during development modulating synaptic connectivity and emotional behavior. Mol. Psychiatry 2019, 24, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Teissier, A.; Soiza-Reilly, M.; Gaspar, P. Refining the Role of 5-HT in Postnatal Development of Brain Circuits. Front. Cell Neurosci. 2017, 11, 139. [Google Scholar] [PubMed]
- Van Kleef, E.S.; Gaspar, P.; Bonnin, A. Insights into the complex influence of 5-HT signaling on thalamocortical axonal system development. Eur. J. Neurosci. 2012, 35, 1563–1572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holze, F.; Vizeli, P.; Ley, L.; Müller, F.; Dolder, P.; Stocker, M.; Duthaler, U.; Varghese, N.; Eckert, A.; Borgwardt, S.; et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology 2021, 46, 537–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraehenmann, R.; Pokorny, D.; Vollenweider, L.; Preller, K.H.; Pokorny, T.; Seifritz, E.; Vollenweider, F.X. Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology 2017, 234, 2031–2046. [Google Scholar] [PubMed]
- Preller, K.H.; Vollenweider, F.X. Phenomenology, Structure, and Dynamic of Psychedelic States. Curr. Top. Behav. Neurosci. 2018, 36, 221–256. [Google Scholar] [PubMed]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. Case history: The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Collins, H. Mechanisms of SSRI Therapy and Discontinuation. Curr. Top. Behav. Neurosci. 2024, 66, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Dougalis, A.G.; Matthews, G.A.C.; Liss, B.; Ungless, M.A. Ionic currents influencing spontaneous firing and pacemaker frequency in dopamine neurons of the ventrolateral periaqueductal gray and dorsal raphe nucleus (vlPAG/DRN): A voltage-clamp and computational modelling study. J. Comput. Neurosci. 2017, 42, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar]
- Jacobs, B.L.; Martín-Cora, F.J.; Fornal, C.A. Activity of medullary serotonergic neurons in freely moving animals. Brain Res. Brain Res. Rev. 2002, 40, 45–52. [Google Scholar] [CrossRef]
- Sakai, K. Sleep-waking discharge profiles of dorsal raphe nucleus neurons in mice. Neuroscience 2011, 197, 200–224. [Google Scholar] [PubMed]
- Tuckwell, H.C.; Penington, N.J. Computational modeling of spike generation in serotonergic neurons of the dorsal raphe nucleus. Prog. Neurobiol. 2014, 118, 59–101. [Google Scholar] [PubMed]
- Levine, E.S.; Jacobs, B.L. Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: Microiontophoretic studies in the awake cat. J. Neurosci. 1992, 12, 4037–4044. [Google Scholar] [PubMed]
- Wang, R.Y.; Gallager, D.W.; Aghajanian, G.K. Stimulation of pontine reticular formation suppresses firing of serotonergic neuronses in the dorsal raphe. Nature 1976, 264, 365–368. [Google Scholar] [PubMed]
- Jouvet, M. Sleep and serotonin: An unfinished story. Neuropsychopharmacology 1999, 21 (Suppl. S2), 24S–27S. [Google Scholar] [PubMed]
- Iwasaki, K.; Komiya, H.; Kakizaki, M.; Miyoshi, C.; Abe, M.; Sakimura, K.; Funato, H.; Yanagisawa, M. Ablation of Central Serotonergic Neurons Decreased REM Sleep and Attenuated Arousal Response. Front. Neurosci. 2018, 12, 535. [Google Scholar] [PubMed]
- Nitz, D.; Siegel, J. GABA release in the dorsal raphe nucleus: Role in the control of REM sleep. Am. J. Physiol. 1997, 273, R451–R455. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Crochet, S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neuroscience 2001, 104, 1141–1155. [Google Scholar] [PubMed]
- Siegel, J.M. The neurotransmitters of sleep. J. Clin. Psychiatry 2004, 65 (Suppl. S16), 4–7. [Google Scholar] [PubMed]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep state switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef] [PubMed]
- Bernard, R.; Veh, R.W. Individual neurons in the rat lateral habenular complex project mostly to the dopaminergic ventral tegmental area or to the serotonergic raphe nuclei. J. Comp. Neurol. 2012, 520, 2545–2558. [Google Scholar] [PubMed]
- Gervasoni, D.; Peyron, C.; Rampon, C.; Barbagli, B.; Chouvet, G.; Urbain, N.; Fort, P.; Luppi, P.H. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J. Neurosci. 2000, 20, 4217–4225. [Google Scholar] [PubMed]
- Kirouac, G.J.; Li, S.; Mabrouk, G. GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. J. Comp. Neurol. 2004, 469, 170–184. [Google Scholar]
- Lavezzi, H.N.; Parsley, K.P.; Zahm, D.S. Mesopontine rostromedial tegmental nucleus neurons projecting to the dorsal raphe and pedunculopontine tegmental nucleus: Psychostimulant-elicited Fos expression and collateralization. Brain Struct. Funct. 2012, 217, 719–734. [Google Scholar]
- Pollak Dorocic, I.; Fürth, D.; Xuan, Y.; Johansson, Y.; Pozzi, L.; Silberberg, G.; Carlén, M.; Meletis, K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 2014, 83, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Reisine, T.D.; Soubrié, P.; Artaud, F.; Glowinski, J. Involvement of lateral habenula-dorsal raphe neurons in the differential regulation of striatal and nigral serotonergic transmission in cats. J. Neurosci. 1982, 2, 1062–1071. [Google Scholar] [PubMed]
- Sego, C.; Gonçalves, L.; Lima, L.; Furigo, I.C.; Donato, J.; Metzger, M. Lateral habenula and the rostromedial tegmental nucleus innervate neurochemically distinct subdivisions of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 2014, 522, 1454–1484. [Google Scholar] [CrossRef] [PubMed]
- Soiza-Reilly, M.; Commons, K.G. Unraveling the architecture of the dorsal raphe synaptic neuropil using high-resolution neuroanatomy. Front. Neural. Circuits 2014, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Badurek, S.; Dileone, R.J.; Nashmi, R.; Minichiello, L.; Picciotto, M.R. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J. Comp. Neurol. 2014, 522, 3308–3334. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Aghajanian, G.K. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science 1977, 197, 89–91. [Google Scholar] [PubMed]
- Zhou, L.; Liu, M.Z.; Li, Q.; Deng, J.; Mu, D.; Sun, Y.G. Organization of Functional Long-Range Circuits Controlling the Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus. Cell Rep. 2017, 18, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.C.; Pan, Y.Z.; Ma, X.; Lamy, C.; Akanwa, A.C.; Beck, S.G. Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur. J. Neurosci. 2006, 24, 3415–3430. [Google Scholar] [PubMed]
- Adell, A.; Celada, P.; Artigas, F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J. Neurochem. 2001, 79, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Adell, A.; Celada, P.; Abellán, M.T.; Artigas, F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res. Brain Res. Rev. 2002, 39, 154–180. [Google Scholar] [CrossRef] [PubMed]
- Descarries, L.; Riad, M. Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2416–2425. [Google Scholar] [PubMed]
- Riad, M.; Garcia, S.; Watkins, K.C.; Jodoin, N.; Doucet, E.; Langlois, X.; el Mestikawy, S.; Hamon, M.; Descarries, L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000, 417, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Stamford, J.A.; Davidson, C.; McLaughlin, D.P.; Hopwood, S.E. Control of dorsal raphe 5-HT function by multiple 5-HT(1) autoreceptors: Parallel purposes or pointless plurality? Trends Neurosci. 2000, 23, 459–465. [Google Scholar] [PubMed]
- Bayliss, D.A.; Li, Y.W.; Talley, E.M. Effects of serotonin on caudal raphe neurons: Activation of an inwardly rectifying potassium conductance. J. Neurophysiol. 1997, 77, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Jones, M.B.; Talley, E.M.; Schrier, A.D.; McIntire, W.E.; Garrison, J.C.; Bayliss, D.A. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein beta gamma subunits. Proc. Natl. Acad. Sci. USA 2000, 97, 9771–9776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Penington, N.J.; Kelly, J.S.; Fox, A.P. Unitary properties of potassium channels activated by 5-HT in acutely isolated rat dorsal raphe neurones. J. Physiol. 1993, 469, 407–426. [Google Scholar] [PubMed]
- Penington, N.J.; Kelly, J.S.; Fox, A.P. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J. Physiol. 1993, 469, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.; Corradetti, R.; Mlinar, B. Pharmacological Characterization of 5-HT1A Autoreceptor-Coupled GIRK Channels in Rat Dorsal Raphe 5-HT Neurons. PLoS ONE 2015, 10, e0140369. [Google Scholar] [CrossRef] [PubMed]
- Saenz del Burgo, L.; Cortes, R.; Mengod, G.; Zarate, J.; Echevarria, E.; Salles, J. Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J. Comp. Neurol. 2008, 510, 581–606. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; El Mansari, M. Serotonin and beyond: Therapeutics for major depression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120536. [Google Scholar] [CrossRef] [PubMed]
- Hensler, J.G. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology 2002, 26, 565–573. [Google Scholar] [CrossRef]
- Rainer, Q.; Nguyen, H.T.; Quesseveur, G.; Gardier, A.M.; David, D.J.; Guiard, B.P. Functional status of somatodendritic serotonin 1A autoreceptor after long-term treatment with fluoxetine in a mouse model of anxiety/depression based on repeated corticosterone administration. Mol. Pharmacol. 2012, 81, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; De Montigny, C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. J. Neurosci. 1983, 3, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; Chaput, Y.; de Montigny, C. Long-term 5-HT reuptake blockade, but not monoamine oxidase inhibition, decreases the function of terminal 5-HT autoreceptors: An electrophysiological study in the rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 1988, 337, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Chaput, Y.; Blier, P.; de Montigny, C. In vivo electrophysiological evidence for the regulatory role of autoreceptors on serotonergic terminals. J. Neurosci. 1986, 6, 2796–2801. [Google Scholar] [CrossRef] [PubMed]
- Chaput, Y.; de Montigny, C.; Blier, P. Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. An in vivo electrophysiologic study in the rat. Neuropsychopharmacology 1991, 5, 219–229. [Google Scholar] [PubMed]
- Czachura, J.F.; Rasmussen, K. Effects of acute and chronic administration of fluoxetine on the activity of serotonergic neurons in the dorsal raphe nucleus of the rat. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 266–275. [Google Scholar] [PubMed]
- Guiard, B.P.; Mansari, M.E.; Murphy, D.L.; Blier, P. Altered response to the selective serotonin reuptake inhibitor escitalopram in mice heterozygous for the serotonin transporter: An electrophysiological and neurochemical study. Int. J. Neuropsychopharmacol. 2012, 15, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Boothman, L.; Raley, J.; Quérée, P. Important messages in the ’post’: Recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 2007, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Jones, J.W.; Craige, C.P.; Guiard, B.P.; Stephen, A.; Metzger, K.L.; Kung, H.F.; Gardier, A.M.; Dranovsky, A.; David, D.J.; Beck, S.G.; et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 2010, 65, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Ferrés-Coy, A.; Santana, N.; Castañé, A.; Cortés, R.; Carmona, M.C.; Toth, M.; Montefeltro, A.; Artigas, F.; Bortolozzi, A. Acute 5-HT1A autoreceptor knockdown increases antidepressant responses and serotonin release in stressful conditions. Psychopharmacology 2013, 225, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, M.S.; Morelli, E.; Gingrich, J.A. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J. Neurosci. 2008, 28, 199–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawyer, E.K.; Howell, L.L. Pharmacokinetics of fluoxetine in rhesus macaques following multiple routes of administration. Pharmacology 2011, 88, 44–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2019, 73 (Suppl. S1), 1–19. [Google Scholar] [CrossRef]
- Zhou, F.W.; Jin, Y.; Matta, S.G.; Xu, M.; Zhou, F.M. An ultra-short dopamine pathway regulates basal ganglia output. J. Neurosci. 2009, 29, 10424–10435. [Google Scholar] [PubMed]
- Ding, S.; Li, L.; Zhou, F.M. Robust presynaptic serotonin 5-HT(1B) receptor inhibition of the striatonigral output and its sensitization by chronic fluoxetine treatment. J. Neurophysiol. 2015, 113, 3397–3409. [Google Scholar] [PubMed]
- Liu, R.J.; Van den Pol, A.N.; Aghajanian, G.K. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J. Neurosci. 2002, 22, 9453–9464. [Google Scholar] [PubMed]
- Allers, K.A.; Sharp, T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience 2003, 122, 193–204. [Google Scholar] [PubMed]
- Haj-Dahmane, S. D2-like dopamine receptor activation excites rat dorsal raphe 5-HT neurons in vitro. Eur. J. Neurosci. 2001, 14, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.J.; Lambe, E.K.; Aghajanian, G.K. Somatodendritic autoreceptor regulation of serotonergic neurons: Dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur. J. Neurosci. 2005, 21, 945–958. [Google Scholar] [PubMed]
- Mlinar, B.; Montalbano, A.; Piszczek, L.; Gross, C.; Corradetti, R. Firing Properties of Genetically Identified Dorsal Raphe Serotonergic Neurons in Brain Slices. Front. Cell Neurosci. 2016, 10, 195. [Google Scholar] [PubMed]
- Fernández-Alacid, L.; Aguado, C.; Ciruela, F.; Martín, R.; Colón, J.; Cabañero, M.J.; Gassmann, M.; Watanabe, M.; Shigemoto, R.; Wickman, K.; et al. Subcellular compartment-specific molecular diversity of pre- and post-synaptic GABA-activated GIRK channels in Purkinje cells. J. Neurochem. 2009, 110, 1363–1376. [Google Scholar] [PubMed]
- Fernández-Alacid, L.; Watanabe, M.; Molnár, E.; Wickman, K.; Luján, R. Developmental regulation of G protein-gated inwardly-rectifying K+ (GIRK/Kir3) channel subunits in the brain. Eur. J. Neurosci. 2011, 34, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Ladera, C.; del Carmen Godino, M.; José Cabañero, M.; Torres, M.; Watanabe, M.; Luján, R.; Sánchez-Prieto, J. Pre-synaptic GABA receptors inhibit glutamate release through GIRK channels in rat cerebral cortex. J. Neurochem. 2008, 107, 1506–1517. [Google Scholar] [PubMed]
- Llamosas, N.; Ugedo, L.; Torrecilla, M. Inactivation of GIRK channels weakens the pre- and postsynaptic inhibitory activity in dorsal raphe neurons. Physiol. Rep. 2017, 5, e13141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Johnston, D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J. Neurosci. 2005, 25, 3787–3792. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, H.; Manzoni, O.J.; Crabbe, J.C.; Williams, J.T. Regulation of central synaptic transmission by 5-HT1B auto- and heteroreceptors. Mol. Pharmacol. 2000, 58, 1271–1278. [Google Scholar] [PubMed]
- Pan, Z.Z.; Williams, J.T. GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J. Neurophysiol. 1989, 61, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.T.; Colmers, W.F.; Pan, Z.Z. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J. Neurosci. 1988, 8, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Boschert, U.; Amara, D.A.; Segu, L.; Hen, R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience 1994, 58, 167–182. [Google Scholar] [CrossRef]
- Ding, S.; Li, L.; Zhou, F.M. Presynaptic serotonergic gating of the subthalamonigral glutamatergic projection. J. Neurosci. 2013, 33, 4875–4885. [Google Scholar] [PubMed]
- Li, Y.W.; Bayliss, D.A. Presynaptic inhibition by 5-HT1B receptors of glutamatergic synaptic inputs onto serotonergic caudal raphe neurones in rat. J. Physiol. 1998, 510, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y. Serotonin1B receptors: From protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004, 28, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Argañaraz, C.V.; Adjimann, T.S.; Perissinotti, P.P.; Soiza-Reilly, M. Selective refinement of glutamate and GABA synapses on dorsal raphe 5-HT neurons during postnatal life. Development 2022, 149, dev201121. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.; Bueno, E.; Kentros, C.; Vega-Saenz de Miera, E.; Chow, A.; Hillman, D.; Chen, S.; Zhu, L.; Wu, M.B.; Wu, X.; et al. G-protein-gated inward rectifier K+ channel proteins (GIRK1) are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. J. Neurosci. 1996, 16, 1990–2001. [Google Scholar] [PubMed]
- Michaeli, A.; Yaka, R. Dopamine inhibits GABA(A) currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience 2010, 165, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.P.; Sexton, T.J.; Neumaier, J.F. Antidepressant-induced regulation of 5-HT(1b) mRNA in rat dorsal raphe nucleus reverses rapidly after drug discontinuation. J. Neurosci. Res. 2000, 61, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, J.F.; Root, D.C.; Hamblin, M.W. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology 1996, 15, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.E.; Shalom, G.; Ran, A.; Gur, E.; Van de Kar, L.D. Chronic fluoxetine-induced desensitization of 5-HT1A and 5-HT1B autoreceptors: Regional differences and effects of WAY-100635. Eur. J. Pharmacol. 2004, 486, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tiger, M.; Varnäs, K.; Okubo, Y.; Lundberg, J. The 5-HT(1B) receptor—A potential target for antidepressant treatment. Psychopharmacology 2018, 235, 1317–1334. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, R.A.; Hiroi, R.; Mackenzie, S.M.; Robin, N.C.; Cohn, A.; Kim, J.J.; Neumaier, J.F. Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol. Psychiatry 2011, 69, 780–787. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, R.A.; Neumaier, J.F. Regulation of dorsal raphe nucleus function by serotonin autoreceptors: A behavioral perspective. J. Chem. Neuroanat. 2011, 41, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Diaz, A.; del Olmo, E.; Pazos, A. Chronic fluoxetine induces opposite changes in G protein coupling at pre and postsynaptic 5-HT1A receptors in rat brain. Neuropharmacology 2003, 44, 93–101. [Google Scholar] [CrossRef]
- Cornelisse, L.N.; Van der Harst, J.E.; Lodder, J.C.; Baarendse, P.J.; Timmerman, A.J.; Mansvelder, H.D.; Spruijt, B.M.; Brussaard, A.B. Reduced 5-HT1A- and GABAB receptor function in dorsal raphe neurons upon chronic fluoxetine treatment of socially stressed rats. J. Neurophysiol. 2007, 98, 196–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Q.; Muma, N.A.; Van de Kar, L.D. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: Reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J. Pharmacol. Exp. Ther. 1996, 279, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Muma, N.A.; Battaglia, G.; Van de Kar, L.D. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: Reduction in the levels of G(i) and G(o) proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J. Pharmacol. Exp. Ther. 1997, 282, 1581–1590. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Jin, Y.; Zhou, F.-M. Chronic Fluoxetine Treatment Desensitizes Serotoninergic Inhibition of GABAergic Inputs and Intrinsic Excitability of Dorsal Raphe Serotonin Neurons. Brain Sci. 2025, 15, 384. https://doi.org/10.3390/brainsci15040384
Zhang W, Jin Y, Zhou F-M. Chronic Fluoxetine Treatment Desensitizes Serotoninergic Inhibition of GABAergic Inputs and Intrinsic Excitability of Dorsal Raphe Serotonin Neurons. Brain Sciences. 2025; 15(4):384. https://doi.org/10.3390/brainsci15040384
Chicago/Turabian StyleZhang, Wei, Ying Jin, and Fu-Ming Zhou. 2025. "Chronic Fluoxetine Treatment Desensitizes Serotoninergic Inhibition of GABAergic Inputs and Intrinsic Excitability of Dorsal Raphe Serotonin Neurons" Brain Sciences 15, no. 4: 384. https://doi.org/10.3390/brainsci15040384
APA StyleZhang, W., Jin, Y., & Zhou, F.-M. (2025). Chronic Fluoxetine Treatment Desensitizes Serotoninergic Inhibition of GABAergic Inputs and Intrinsic Excitability of Dorsal Raphe Serotonin Neurons. Brain Sciences, 15(4), 384. https://doi.org/10.3390/brainsci15040384





