Neural Oscillatory Mechanisms Underlying Step Accuracy: Integrating Microstate Segmentation with eLORETA-Independent Component Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Methodology
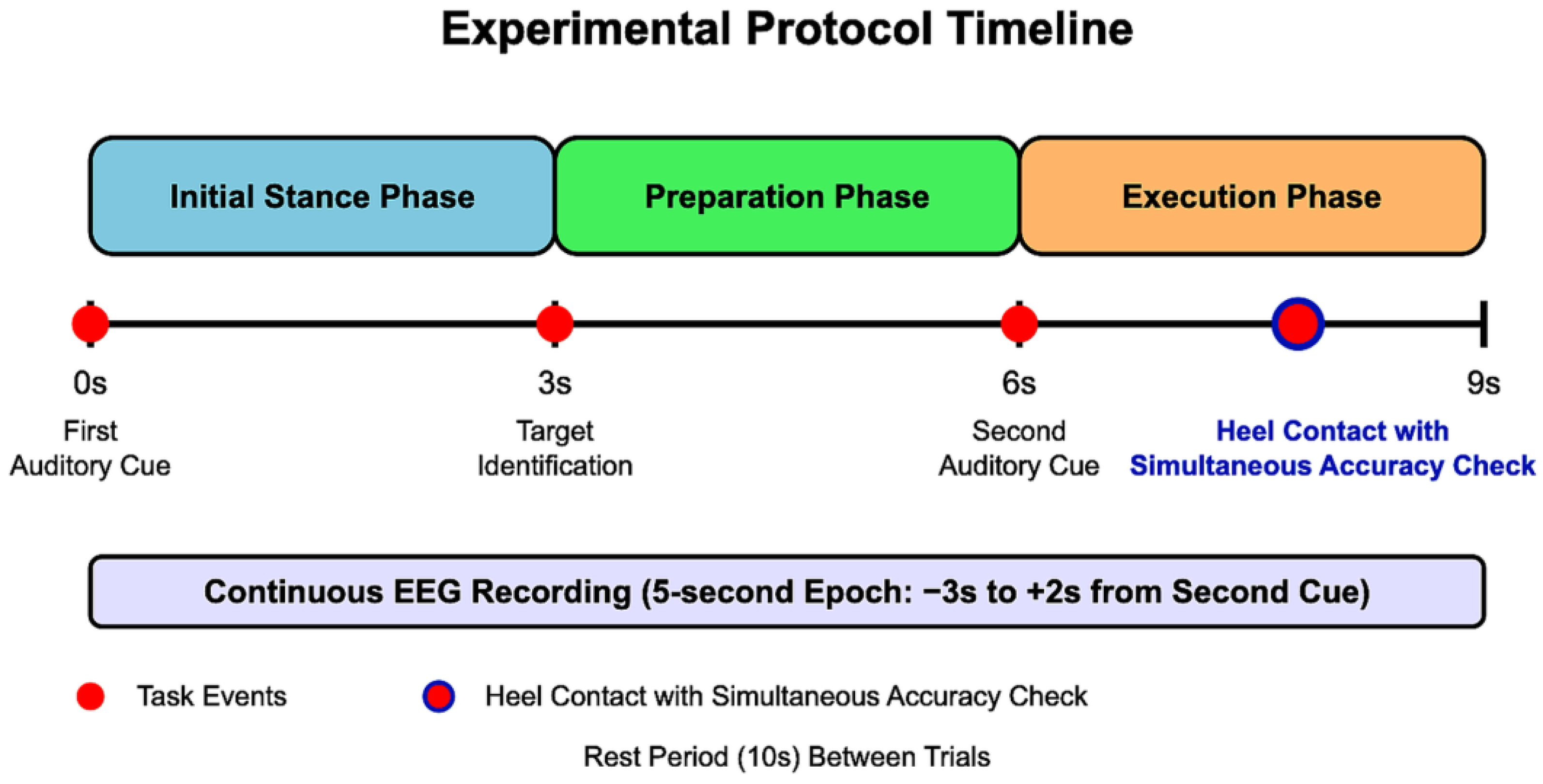
2.2.1. Stepping Task Protocol
- Initial Stance Phase (0–3 s): Participants stood in a stable, comfortable, and natural posture with their weight evenly distributed between both feet. An auditory cue signaled the beginning of the trial.
- Preparation Phase (3–6 s): Participants visually identified the target point (marked with an 8 mm circular marker) and prepared their stepping. They were instructed to maintain their initial stance during this period.
- Execution Phase (6–9 s): A second auditory cue prompted participants to take a single step forward with their dominant leg, aiming to align the calcaneal tuberosity with the designated target point. No specific instructions regarding step speed were given to ensure natural movement patterns. After heel contact, participants simultaneously performed a visual accuracy check by comparing their heel position to the target marker before returning to the starting position for the next trial.
2.2.2. Data Acquisition Systems
EEG Recording
Supplementary Physiological Recordings
- Electrooculography (EOG): Vertical EOG was recorded by placing electrodes above and below the dominant eye to detect eye movement and blink artifacts.
- Electromyography (EMG): Surface EMG was recorded bilaterally from the tibialis anterior muscles. Electrode placement followed SENIAM’s recommendations [40]. A bipolar derivation was used with an inter-electrode distance of 2 cm. The EMG signals were sampled at 1 kHz.
Pressure-Sensitive Mat System
- Sensor Configuration: Three pressure-sensitive mats were positioned as follows:
- Two mats at the initial standing position (one for the right foot and one for the left foot);
- One mat at the target position (around the heel contact area).
- Data Acquisition and Synchronization: Signals from each mat sensor were directly synchronized with the EEG recording system. Trigger signals were collected at the same sampling rate as the EEG (1 kHz), with a temporal precision of ±5 ms.
- Trigger Functions: The mat sensors generated trigger signals to accurately detect the following two key events:
- Moment of unloading of the dominant foot (decrease in pressure on the initial position mat at step initiation);
- Moment of heel contact (increase in pressure on the target position mat).
Video Recording System
2.2.3. Step Performance Assessment
- Video Processing: Videos of the stepping trials were processed using the Fiji software (Fiji Is Just ImageJ, Version 1.54f) [38] to extract individual frames corresponding to heel contact moments.
- Calibration: A 10 mm reference marker was placed in each video frame for accurate spatial calibration and distance measurement.
- Reference Points: The heel contact position was defined using the following anatomical landmarks:
- X-axis reference point: Dorsal (posterior) side of the calcaneal tuberosity;
- Y-axis reference point: Lateral side of the calcaneal tuberosity.
- Measurement: These reference points were marked with small (8 mm diameter) high-contrast circular markers to enhance visibility. ED was calculated as the Euclidean distance between the target point (xt, yt) and the heel contact point (xh, yh) using the following formula.
2.3. Data Analysis
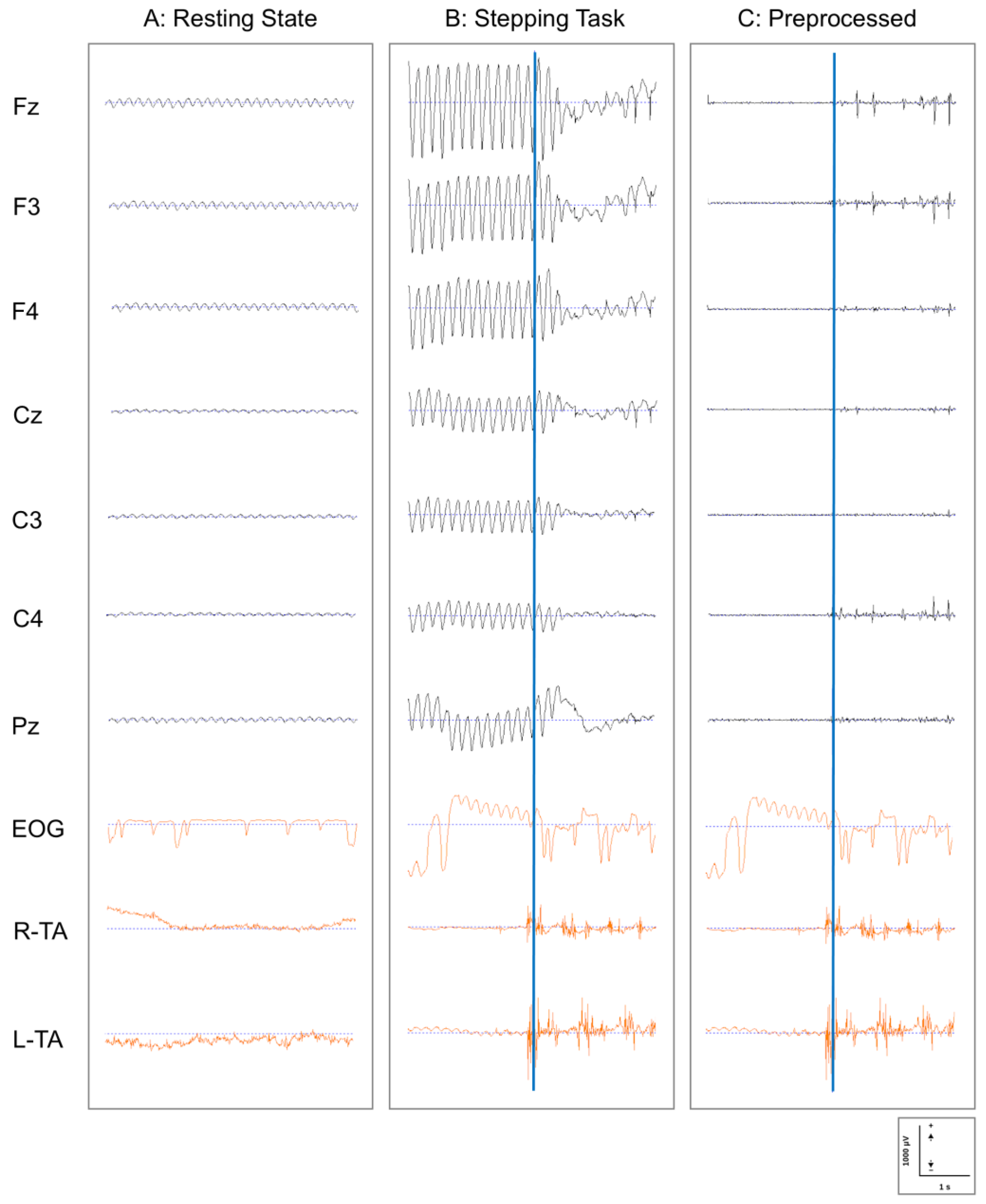
2.3.1. EEG Preprocessing
- Filtering: Initially, continuous EEG data were filtered using the following parameters:
- High-pass filter: 1.5 Hz (to remove low-frequency drift);
- Low-pass filter: 30 Hz (to remove high-frequency noise);
- Zero-phase digital filtering was implemented using the filter pipeline in EMSE to minimize phase distortion.
- Power Line Noise Removal: A band-stop filter (notch filter) was applied to selectively remove power line noise:
- Since the experiment was conducted in western Japan (Kyoto), where the commercial power frequency is 60 Hz, a band-stop filter centered at 60 Hz with a bandwidth of 2 Hz was applied.
- EOG Artifact Removal: Eye movement and blink artifacts were removed using the template-based EOG artifact removal function implemented in EMSE. Using the simultaneously recorded EOG signal as a reference, typical eye movement and blink patterns were defined as templates, and these patterns were detected and removed from each EEG channel.
- Segmentation: The preprocessed continuous data were segmented according to each stepping trial as follows:
- From 3 s before to 2 s after the presentation of the second auditory cue (stepping initiation instruction);
- Each segment covered a 5 s time window, encompassing the preparation phase through post-movement completion.
- Quality Control: The segmented data underwent quality verification through the following procedures:
- Automatic rejection of epochs containing values exceeding ±100 μV;
- Visual inspection by experienced researchers (K.O. and T.K.) to identify any remaining artifacts;
- Re-referencing to average reference to minimize the influence of the reference electrode location.
2.3.2. eLORETA-ICA Analysis
- Source Estimation: Preprocessed scalp EEG data were transformed into current density distributions in the brain using the eLORETA algorithm. eLORETA features minimal localization bias for source estimation at 6239 cortical gray matter voxels with 5 mm spatial resolution within a realistic head model [42].
- Frequency-Domain ICA Application: The frequency-domain ICA (transposed fICA networks) module integrated into the eLORETA was directly applied to the preprocessed EEG data. This approach decomposes cortical electrical activity across multiple frequency bands into independent components (ICs), enabling simultaneous analysis of multiple frequency bands that would be difficult with conventional structural source analysis [29,31]. This module was configured to analyze the following four frequency bands:
- Delta band (1.5–3.5 Hz);
- Theta band (4–7.5 Hz);
- Alpha/mu band (8–13 Hz, with the mu rhythm specifically referring to activity recorded over the sensorimotor areas) [43];
- Beta band (13.5–30 Hz).
- 3.
- Component Evaluation: Three experienced researchers (K.O., K.M., and T.K.) evaluated the resulting components and collaboratively classified them into neural activity and artifact components based on their spatial and spectral characteristics.
2.3.3. Microstate Segmentation Analysis
- Clustering Algorithm: The k-means clustering algorithm was applied to the source-space data to identify prototypical microstate maps.
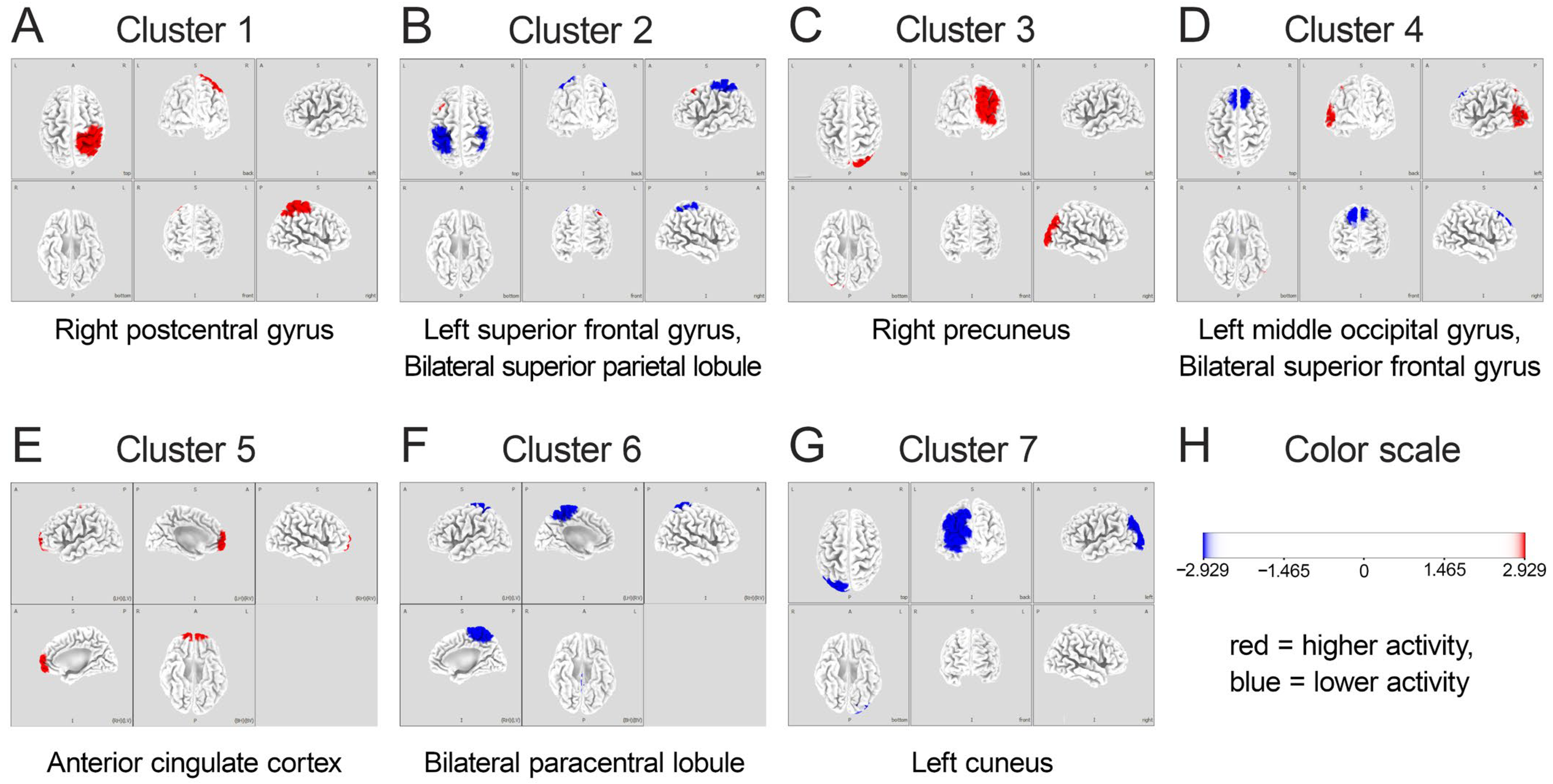
- Number of Clusters: Based on previous literature on gait-related brain activity [10], the number of clusters was set to seven, corresponding to brain regions previously identified as associated with gait and stepping motions. The optimal number of clusters was verified using the cross-validation criterion, which confirmed that seven clusters provided the best balance between model complexity and explanatory power.
- Microstate Parameter: For each identified microstate, its probability (the likelihood of each microstate occurring during the task) was calculated and used for subsequent analysis.
2.3.4. Statistical Analysis
- High-Performing (HiP) Group: Participants with smaller ED values (below the median value), indicating more accurate stepping (n = 8).
- Low-Performing (LoP) Group: Participants with larger ED values (above the median value), indicating less accurate stepping (n = 8).
2.4. Ethical Considerations
3. Results
3.1. Quality of EEG Data and Preprocessing Efficacy
3.2. IC and Microstate Analysis
3.3. Comparison Between HiP and LoP Groups
4. Discussion
4.1. Amplified ACC Theta-Band Activity: Role in Performance Monitoring
4.2. Suppression of Mu- and Beta-Band Activity in Sensorimotor Regions: Enhanced Motor Preparation and Control
4.3. Visual Processing in the Occipital Region: Enhanced Visuospatial Integration
4.4. A Proposed Neural Network Framework for Stepping Accuracy
- Error Monitoring System: Centered on the ACC, this system generates theta oscillations that monitor performance in real-time, detecting discrepancies between intended and actual foot placement. The enhanced theta activity in the HiP group suggests more efficient error monitoring processes facilitating precise stepping, aligning with findings from Luu et al. [27] and Jacobsen and Ferris [23] on ACC theta activity in error monitoring and locomotor adaptation.
- Sensorimotor Integration System: Encompassing the paracentral lobules and postcentral gyrus, this system features task-specific mu and beta desynchronization patterns supporting flexible motor execution. The greater suppression of these rhythms in the HiP group may enable more precise motor adjustments, corresponding with findings from Wagner et al. [25] and Nordin et al. [55] on active locomotion and adaptation.
- Visuospatial Processing System: Including the precuneus and occipital regions, this system processes visual information and integrates it with body-centered coordinates to create a spatial map for guiding foot placement. Enhanced activity in these regions among high performers suggests more effective visuospatial processing, consistent with studies by Wagner et al. [51] on parietal regions in locomotion.
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Anterior cingulate cortex |
| BCI | Brain–computer interface |
| ED | Error distance |
| EEG | Electroencephalography |
| eLORETA | Exact low-resolution electromagnetic tomography |
| EMG | Electromyography |
| EOG | Electrooculography |
| MMSE | Mini-mental state examination |
| ERD | Event-related desynchronization |
| fMRI | Functional magnetic resonance imaging |
| HiP group | High-performing group |
| ICA | Independent component analysis |
| ICs | Independent components |
| LoP group | Low-performing group |
| MoBI | Mobile brain/body imaging |
| MSA | Microstate segmentation analysis |
| PPC | Posterior parietal cortex |
Appendix A. Images of Independent Components from eLORETA-ICA Analysis


References
- Bruijn, S.M.; van Dieën, J.H. Control of human gait stability through foot placement. J. R. Soc. Interface 2018, 15, 20170816. [Google Scholar] [CrossRef]
- Robinovitch, S.N.; Feldman, F.; Yang, Y.; Schonnop, R.; Leung, P.M.; Sarraf, T.; Sims-Gould, J.; Loughin, M. Video capture of the circumstances of falls in elderly people residing in long-term care: An observational study. Lancet 2013, 381, 47–54. [Google Scholar] [CrossRef]
- Tisserand, R.; Robert, T.; Chabaud, P.; Bonnefoy, M.; Chèze, L. Elderly fallers enhance dynamic stability through anticipatory postural adjustments during a choice stepping reaction time. Front. Hum. Neurosci. 2016, 10, 613. [Google Scholar] [CrossRef]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. 2004, 19, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Bloem, B.R.; Giladi, N.; Hallett, M.; Horak, F.B.; Nieuwboer, A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011, 10, 734–744. [Google Scholar] [CrossRef]
- Chapman, G.J.; Hollands, M.A. Evidence that older adult fallers prioritise the planning of future stepping actions over the accurate execution of ongoing steps during complex locomotor tasks. Gait Posture 2007, 26, 59–67. [Google Scholar] [CrossRef]
- Young, W.R.; Wing, A.M.; Hollands, M.A. Influences of state anxiety on gaze behavior and stepping accuracy in older adults during adaptive locomotion. J. Gerontol. B Psychol. Sci. Soc. Sci. 2012, 67, 43–51. [Google Scholar] [CrossRef]
- Caetano, M.J.D.; Lord, S.R.; Brodie, M.A.; Schoene, D.; Pelicioni, P.H.S.; Sturnieks, D.L.; Menant, J.C. Executive functioning, concern about falling and quadriceps strength mediate the relationship between impaired gait adaptability and fall risk in older people. Gait Posture 2018, 59, 188–192. [Google Scholar] [CrossRef]
- Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Hamacher, D.; Herold, F.; Wiegel, P.; Hamacher, D.; Schega, L. Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 2015, 57, 310–327. [Google Scholar] [CrossRef]
- Yiou, E.; Teyssèdre, C.; Artico, R.; Fourcade, P. Comparison of base of support size during gait initiation using force-plate and motion-capture system: A bland and Altman analysis. J. Biomech. 2016, 49, 4168–4172. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, R.; Talebian, S.; Hadian, M.R.; Olyaei, G.; Bagheri, H. Time and frequency domain analysis of gait initiation in younger and older adults. J. Aging Phys. Act. 2017, 25, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.-T.; Cho, H.-Y. A novel method for gait analysis on center of pressure excursion based on a pressure-sensitive mat. Int. J. Environ. Res. Public Health 2020, 17, 7845. [Google Scholar] [CrossRef]
- Lee, M.; Kim, J.; Son, J.; Kim, Y. Kinematic and kinetic analysis during forward and backward walking. Gait Posture 2013, 38, 674–678. [Google Scholar] [CrossRef]
- Ogata, T.; Hashiguchi, H.; Hori, K.; Hirobe, Y.; Ono, Y.; Sawada, H.; Inaba, A.; Orimo, S.; Miyake, Y. Foot trajectory features in gait of Parkinson’s disease patients. Front. Physiol. 2022, 13, 726677. [Google Scholar] [CrossRef]
- Bishnoi, A.; Holtzer, R.; Hernandez, M.E. Brain activation changes while walking in adults with and without neurological disease: Systematic review and meta-analysis of functional near-infrared spectroscopy studies. Brain Sci. 2021, 11, 291. [Google Scholar] [CrossRef]
- Makeig, S.; Gramann, K.; Jung, T.-P.; Sejnowski, T.J.; Poizner, H. Linking brain, mind and behavior. Int. J. Psychophysiol. 2009, 73, 95–100. [Google Scholar] [CrossRef]
- Song, S.; Nordin, A.D. Mobile electroencephalography for studying neural control of human locomotion. Front. Hum. Neurosci. 2021, 15, 749017. [Google Scholar] [CrossRef]
- Gramann, K.; Ferris, D.P.; Gwin, J.; Makeig, S. Imaging natural cognition in action. Int. J. Psychophysiol. 2014, 91, 22–29. [Google Scholar] [CrossRef]
- Ladouce, S.; Donaldson, D.I.; Dudchenko, P.A.; Ietswaart, M. Mobile EEG identifies the re-allocation of attention during real-world activity. Sci. Rep. 2019, 9, 15851. [Google Scholar] [CrossRef]
- Kim, H.; Miyakoshi, M.; Iversen, J.R. Approaches for hybrid coregistration of marker-based and markerless coordinates describing complex body/object interactions. Sensors 2023, 23, 6542. [Google Scholar] [CrossRef] [PubMed]
- Callan, D.E.; Torre-Tresols, J.J.; Laguerta, J.; Ishii, S. Shredding artifacts: Extracting brain activity in EEG from extreme artifacts during skateboarding using ASR and ICA. Front. Neuroergonomics 2024, 5, 1358660. [Google Scholar] [CrossRef]
- Jacobsen, N.A.; Ferris, D.P. Electrocortical activity correlated with locomotor adaptation during split-belt treadmill walking. J. Physiol. 2023, 601, 3921–3944. [Google Scholar] [CrossRef]
- Peterson, S.M.; Ferris, D.P. Group-level cortical and muscular connectivity during perturbations to walking and standing balance. Neuroimage 2019, 198, 93–103. [Google Scholar] [CrossRef]
- Wagner, J.; Makeig, S.; Gola, M.; Neuper, C.; Müller-Putz, G. Distinct β band oscillatory networks subserving motor and cognitive control during gait adaptation. J. Neurosci. 2016, 36, 2212–2226. [Google Scholar] [CrossRef]
- Luu, T.P.; Brantley, J.A.; Nakagome, S.; Zhu, F.; Contreras-Vidal, J.L. Electrocortical correlates of human level-ground, slope, and stair walking. PLoS ONE 2017, 12, e0188500. [Google Scholar] [CrossRef]
- Luu, T.P.; Nakagome, S.; He, Y.; Contreras-Vidal, J.L. Real-time EEG-based brain-computer interface to a virtual avatar enhances cortical involvement in human treadmill walking. Sci. Rep. 2017, 7, 8895. [Google Scholar] [CrossRef]
- Li, J.; Dimitrakopoulos, G.N.; Thangavel, P.; Chen, G.; Sun, Y.; Guo, Z.; Yu, H.; Thakor, N.; Bezerianos, A. What are spectral and spatial distributions of EEG-EMG correlations in overground walking? An exploratory study. IEEE Access 2019, 7, 143935–143946. [Google Scholar] [CrossRef]
- Aoki, Y.; Ishii, R.; Pascual-Marqui, R.D.; Canuet, L.; Ikeda, S.; Hata, M.; Imajo, K.; Matsuzaki, H.; Musha, T.; Asada, T.; et al. Detection of EEG-resting state independent networks by ELORETA-ICA method. Front. Hum. Neurosci. 2015, 9, 31. [Google Scholar] [CrossRef]
- Aoki, Y.; Takahashi, R.; Suzuki, Y.; Pascual-Marqui, R.D.; Kito, Y.; Hikida, S.; Maruyama, K.; Hata, M.; Ishii, R.; Iwase, M.; et al. EEG resting-state networks in Alzheimer’s disease associated with clinical symptoms. Sci. Rep. 2023, 13, 3964. [Google Scholar] [CrossRef]
- Katayama, O.; Stern, Y.; Habeck, C.; Coors, A.; Lee, S.; Harada, K.; Makino, K.; Tomida, K.; Morikawa, M.; Yamaguchi, R.; et al. Detection of neurophysiological markers of cognitive reserve: An EEG study. Front. Aging Neurosci. 2024, 16, 1401818. [Google Scholar] [CrossRef] [PubMed]
- Férat, V.; Seeber, M.; Michel, C.M.; Ros, T. Beyond broadband: Towards a spectral decomposition of EEG microstates. Hum. Brain Mapp. 2022, 43, 3047–3061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, W.; Wang, C.; Li, Y.; Liu, Z.; Liu, T.; Li, J.; Yan, X.; Wang, Q.; Cao, Z.; et al. Reliability of EEG microstate analysis at different electrode densities during propofol-induced transitions of brain states. Neuroimage 2021, 231, 117861. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Creutzfeldt, O.D.; Schwibbe, M.; Wuttke, W. Changes in physiological, EEG and psychological parameters in women during the spontaneous menstrual cycle and following oral contraceptives. Psychoneuroendocrinology 1982, 7, 75–90. [Google Scholar] [CrossRef]
- de Souza, R.F.L.; Mendes, T.M.A.S.; de Araujo Lima, L.A.B.; Brandão, D.S.; Laplagne, D.A.; de Sousa, M.B.C. Effect of the menstrual cycle on electroencephalogram alpha and beta bands during motor imagery and action observation. Front. Hum. Neurosci. 2022, 16, 878887. [Google Scholar] [CrossRef]
- Gibbings, A.; Ray, L.B.; Berberian, N.; Nguyen, T.; Shahidi Zandi, A.; Owen, A.M.; Comeau, F.J.E.; Fogel, S.M. EEG and behavioural correlates of mild sleep deprivation and vigilance. Clin. Neurophysiol. 2021, 132, 45–55. [Google Scholar] [CrossRef]
- Goda, A.; Izumi, M.; Kido, H.; Yamashita, H.; Murata, S. Differences in foot morphology and center of gravity sway between dominant and nondominant legs in young healthy adults. Jpn. J. Health Promot. Phys. Ther. 2024, 11, 79–83. [Google Scholar] [CrossRef]
- Chatrian, G.E.; Lettich, E.; Nelson, P.L. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activities. Am. J. EEG Technol. 1985, 25, 83–92. [Google Scholar] [CrossRef]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C. Motor imagery activates primary sensorimotor area in humans. Neurosci. Lett. 1997, 239, 65–68. [Google Scholar] [CrossRef]
- Iacobucci, D.; Posavac, S.S.; Kardes, F.R.; Schneider, M.J.; Popovich, D.L. The median split: Robust, refined, and revived. J. Consum. Psychol. 2015, 25, 690–704. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Cohen, J.D.; Carter, C.S. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn. Sci. 2004, 8, 539–546. [Google Scholar] [CrossRef]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Suzuki, M.; Miyai, I.; Ono, T.; Oda, I.; Konishi, I.; Kochiyama, T.; Kubota, K. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: An optical imaging study. Neuroimage 2004, 23, 1020–1026. [Google Scholar] [CrossRef]
- Koenraadt, K.L.M.; Roelofsen, E.G.J.; Duysens, J.; Keijsers, N.L.W. Cortical control of normal gait and precision stepping: An FNIRS Study. Neuroimage 2014, 85 Pt 1, 415–422. [Google Scholar] [CrossRef]
- Haefeli, J.; Vögeli, S.; Michel, J.; Dietz, V. Preparation and Performance of Obstacle Steps: Interaction between Brain and Spinal neuronal activity. Eur. J. Neurosci. 2011, 33, 338–348. [Google Scholar] [CrossRef]
- Wagner, J.; Solis-Escalante, T.; Scherer, R.; Neuper, C.; Müller-Putz, G. It’s how you get there: Walking down a virtual alley activates premotor and parietal areas. Front. Hum. Neurosci. 2014, 8, 93. [Google Scholar] [CrossRef]
- Wagner, J.; Martinez-Cancino, R.; Delorme, A.; Makeig, S.; Solis-Escalante, T.; Neuper, C.; Mueller-Putz, G. High-density EEG mobile brain/body imaging data recorded during a challenging auditory gait pacing task. Sci. Data 2019, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Seeber, M.; Scherer, R.; Wagner, J.; Solis-Escalante, T.; Müller-Putz, G.R. EEG beta suppression and low gamma modulation are different elements of human upright walking. Front. Hum. Neurosci. 2014, 8, 485. [Google Scholar] [CrossRef]
- Seeber, M.; Scherer, R.; Wagner, J.; Solis-Escalante, T.; Müller-Putz, G.R. High and low gamma EEG oscillations in central sensorimotor areas are conversely modulated during the human gait cycle. Neuroimage 2015, 112, 318–326. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Faster gait speeds reduce alpha and beta EEG spectral power from human sensorimotor cortex. IEEE Trans. Biomed. Eng. 2020, 67, 842–853. [Google Scholar] [CrossRef]
- Luu, T.P.; He, Y.; Brown, S.; Nakagame, S.; Contreras-Vidal, J.L. Gait adaptation to visual kinematic perturbations using a real-time closed-loop brain-computer interface to a virtual reality avatar. J. Neural Eng. 2016, 13, 036006. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, T.; Szarkowski, R.; Rios, C.; Ashe, J.; He, B. Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: An EEG and FMRI study of motor imagery and movements. Neuroimage 2010, 49, 2596–2606. [Google Scholar] [CrossRef]
- Vanni, S.; Tanskanen, T.; Seppä, M.; Uutela, K.; Hari, R. Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc. Natl. Acad. Sci. USA 2001, 98, 2776–2780. [Google Scholar] [CrossRef]
- Neuner, I.; Arrubla, J.; Werner, C.J.; Hitz, K.; Boers, F.; Kawohl, W.; Shah, N.J. The default mode network and EEG regional spectral power: A simultaneous FMRI-EEG study. PLoS ONE 2014, 9, e88214. [Google Scholar] [CrossRef]
- Bradford, J.C.; Lukos, J.R.; Ferris, D.P. Electrocortical activity distinguishes between uphill and level walking in humans. J. Neurophysiol. 2016, 115, 958–966. [Google Scholar] [CrossRef]

| Characteristic | Mean ± SD or n (%) |
|---|---|
| Age (years) | 23.2 ± 2.7 |
| Height (cm) | 169.9 ± 4.5 |
| Weight (kg) | 62.4 ± 6.9 |
| Dominant foot (Right/Left) | 13 (81.3)/3 (18.7) |
| Sleep duration, previous night (hours) | 6.2 ± 1.4 |
| MMSE (points) | 30 (100) |
| MMSE: Mini-Mental State Examination; SD: standard deviation. | |
| All participants scored 30 points on the MMSE, indicating no cognitive impairment. | |
| HiP, n = 8 | LoP, n = 8 | |||||
|---|---|---|---|---|---|---|
| Cluster | Brain Lobe | Region | BA | Brain Lobe | Region | BA |
| 1 | Right parietal lobe | Postcentral gyrus | 3 | No EEG activity | ||
| 2 | Left frontal lobe | Superior frontal gyrus | 8 | Bilateral parietal lobes | Superior parietal lobule | 7 |
| 3 | Right parietal lobe | Precuneus | 7 | No EEG activity | ||
| 4 | Left occipital lobe | Middle occipital gyrus | 19 | Bilateral frontal lobes | Superior frontal gyrus | 8 |
| 5 | Limbic lobe | Anterior cingulate cortex | 32 | No EEG activity | ||
| 6 | No EEG activity | Bilateral parietal lobes | Paracentral lobule | 4 | ||
| 7 | No EEG activity | Left occipital lobe | Cuneus | 19 | ||
| HiP: high-performing group characterized by a small error distance between the target point and the heel contact point; LoP: low-performing group characterized by a large error distance between the target point and the heel contact point; BA: Brodmann area; EEG: electroencephalography. | ||||||
| Cluster | Total | δ | θ | α (μ) | β |
|---|---|---|---|---|---|
| 1 | 7.69 | 7.69 | 7.69 | 7.69 | 7.69 |
| 2 | 15.39 | 15.39 | 15.39 | 15.39 | 15.39 |
| 3 | 17.31 | 23.08 | 15.39 | 15.39 | 15.39 |
| 4 | 5.77 | 0.00 | 7.69 | 7.69 | 7.69 |
| 5 | 21.15 | 23.08 | 38.46 | 15.39 | 7.69 |
| 6 | 17.31 | 15.39 | 7.69 | 23.08 | 23.08 |
| 7 | 15.39 | 15.39 | 7.69 | 15.39 | 23.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyama, K.; Maeda, K.; Yamauchi, R.; Harada, D.; Kodama, T. Neural Oscillatory Mechanisms Underlying Step Accuracy: Integrating Microstate Segmentation with eLORETA-Independent Component Analysis. Brain Sci. 2025, 15, 356. https://doi.org/10.3390/brainsci15040356
Okuyama K, Maeda K, Yamauchi R, Harada D, Kodama T. Neural Oscillatory Mechanisms Underlying Step Accuracy: Integrating Microstate Segmentation with eLORETA-Independent Component Analysis. Brain Sciences. 2025; 15(4):356. https://doi.org/10.3390/brainsci15040356
Chicago/Turabian StyleOkuyama, Kohei, Kota Maeda, Ryosuke Yamauchi, Daichi Harada, and Takayuki Kodama. 2025. "Neural Oscillatory Mechanisms Underlying Step Accuracy: Integrating Microstate Segmentation with eLORETA-Independent Component Analysis" Brain Sciences 15, no. 4: 356. https://doi.org/10.3390/brainsci15040356
APA StyleOkuyama, K., Maeda, K., Yamauchi, R., Harada, D., & Kodama, T. (2025). Neural Oscillatory Mechanisms Underlying Step Accuracy: Integrating Microstate Segmentation with eLORETA-Independent Component Analysis. Brain Sciences, 15(4), 356. https://doi.org/10.3390/brainsci15040356





