Effects of a 12-Week Moderate-to-High Intensity Strength Training Program on the Gait Parameters and Their Variability of Stroke Survivors
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention
- Warm-up phase (5–10 min): involved breathing, posture, and mobility exercises;
- Main program (35–50 min): comprised of personalized strength exercises targeting the upper and lower body. The duration varied based on individual tolerance and progression;
- Cool-down (5-min): included breathing exercises and stretching.
2.3. Experimental Protocol
2.4. Data Analysis
3. Results
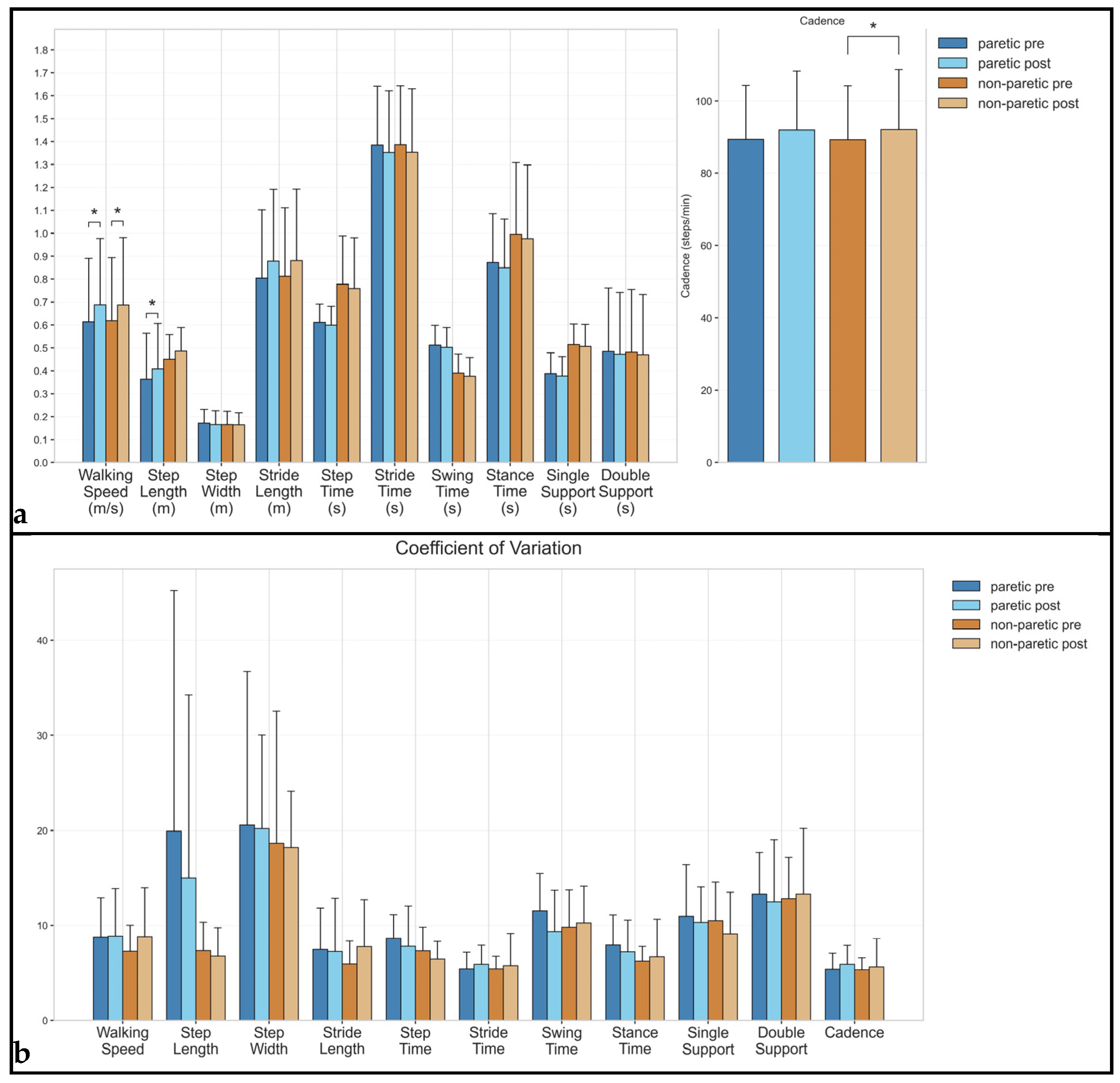
3.1. Spatiotemporal Parameters
3.2. Spatial Parameters
3.3. Temporal Parameters
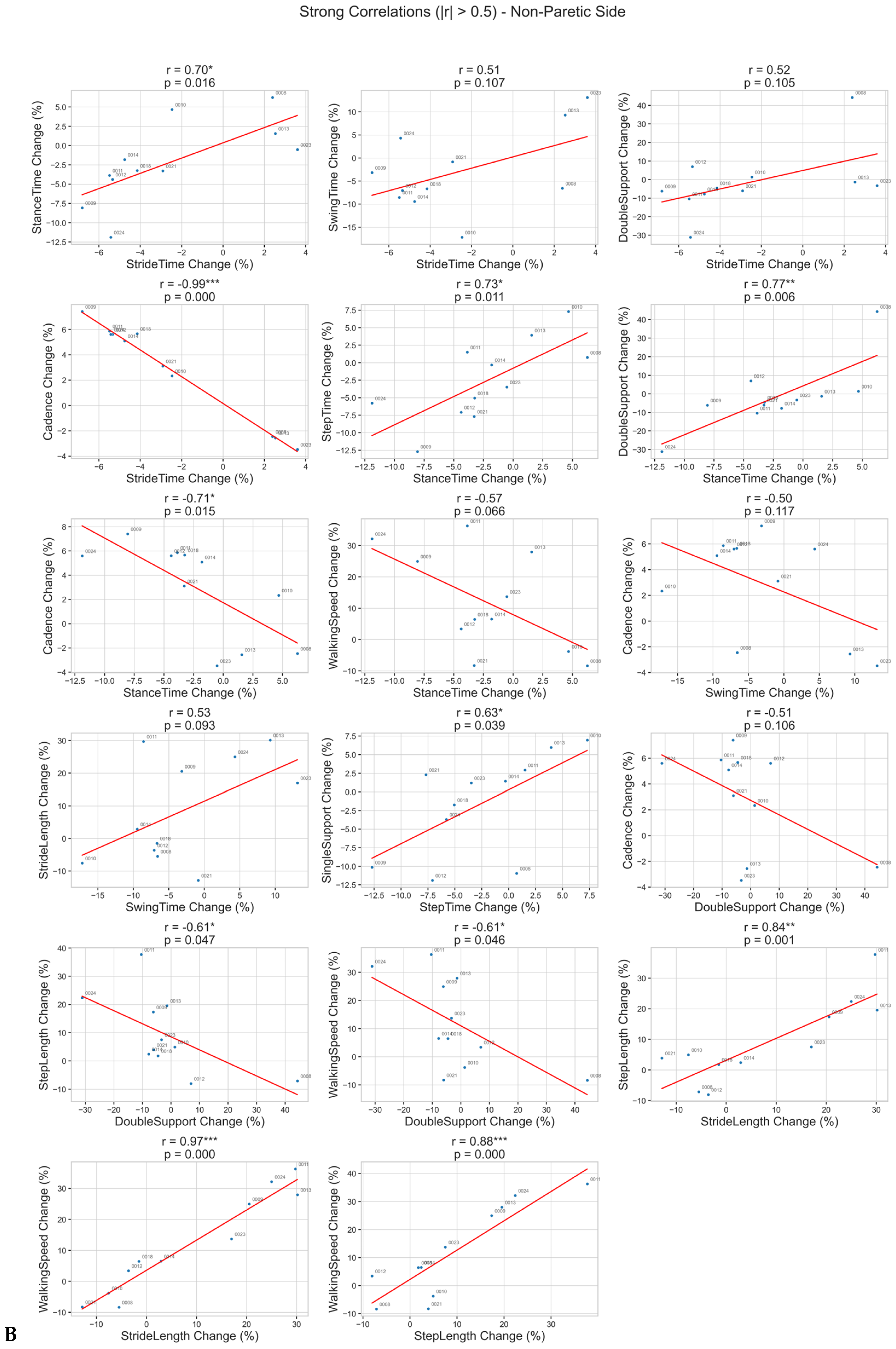
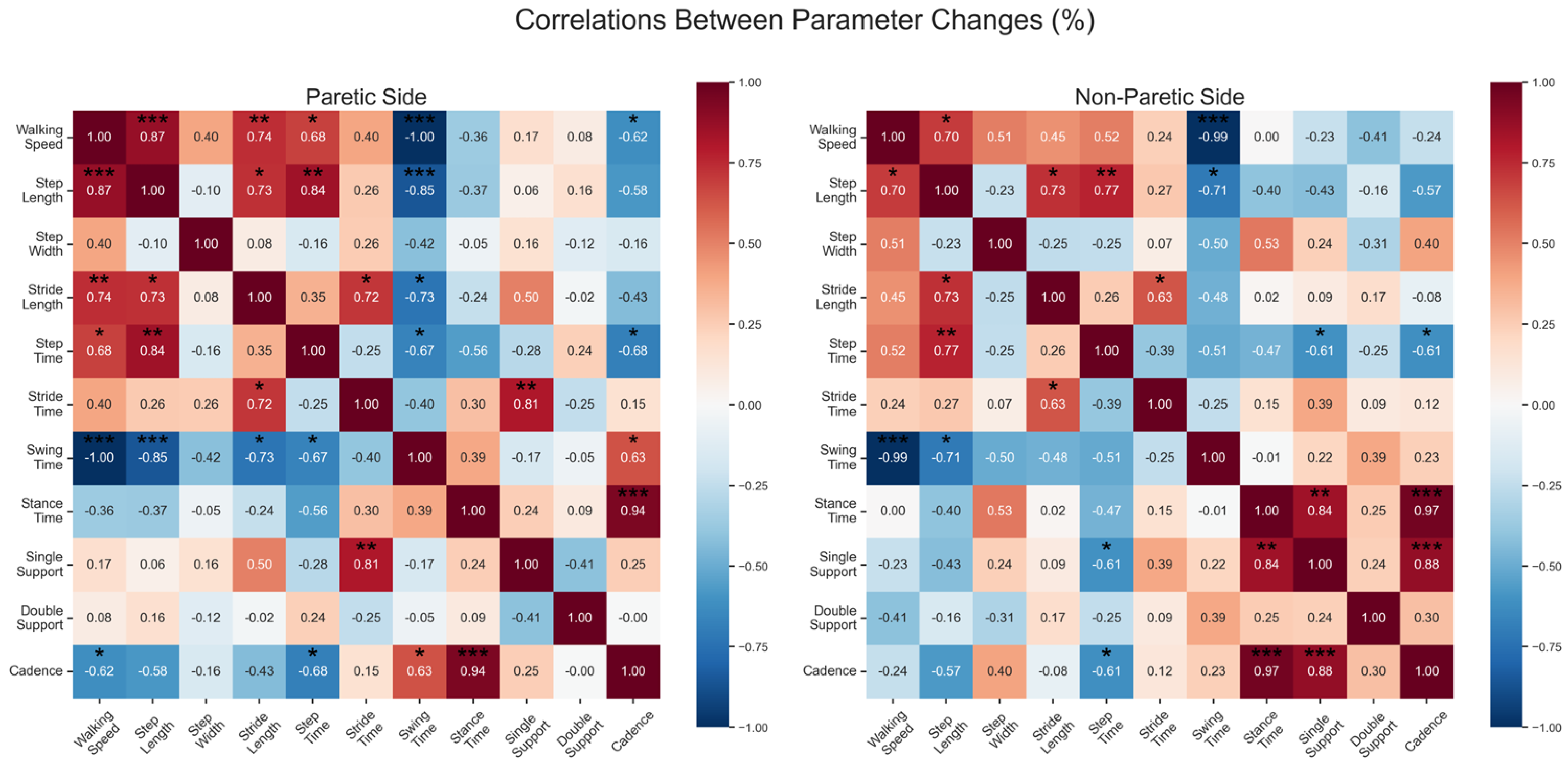
Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Luengo-Fernandez, R.; Violato, M.; Candio, P.; Leal, J. Economic burden of stroke across Europe: A population-based cost analysis. Eur. Stroke J. 2020, 5, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, O.H.; Stenager, E.; Dalgas, U. Muscle Strength and Poststroke Hemiplegia: A Systematic Review of Muscle Strength Assessment and Muscle Strength Impairment. Arch. Phys. Med. Rehabil. 2017, 98, 368–380. [Google Scholar] [CrossRef]
- Slater, L.; Gilbertson, N.M.; Hyngstrom, A.S. Improving gait efficiency to increase movement and physical activity—The impact of abnormal gait patterns and strategies to correct. Prog. Cardiovasc. Dis. 2021, 64, 83–87. [Google Scholar] [CrossRef]
- Kramer, S.; Johnson, L.; Bernhardt, J.; Cumming, T. Energy Expenditure and Cost During Walking After Stroke: A Systematic Review. Arch. Phys. Med. Rehabil. 2016, 97, 619–632.e1. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhang, W.; Kang, L.; Ma, Y.; Fu, L.; Jia, L.; Yu, H.; Chen, X.; Hou, L.; Wang, L.; et al. Clinical evidence of exercise benefits for stroke. In Exercise for Cardiovascular Disease Prevention and Treatment; Xiao, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2017; Volume 1000, pp. 131–151. [Google Scholar]
- Saunders, D.H.; Greig, C.A.; Mead, G.E. Physical activity and exercise after stroke: Review of multiple meaningful benefits. Stroke 2014, 45, 3742–3747. [Google Scholar] [CrossRef]
- Wang, Y.; Mukaino, M.; Ohtsuka, K.; Otaka, Y.; Tanikawa, H.; Matsuda, F.; Tsuchiyama, K.; Yamada, J.; Saitoh, E. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. Int. J. Rehabil. Res. 2020, 43, 69–75. [Google Scholar] [CrossRef]
- Balasubramanian, C.K.; Neptune, R.R.; Kautz, S.A. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture 2009, 29, 408–414. [Google Scholar] [CrossRef]
- Jang, S.H. The recovery of walking in stroke patients: A review. Int. J. Rehabil. Res. 2010, 33, 285–289. [Google Scholar] [CrossRef]
- Moe-Nilssen, R.; Aaslund, M.K.; Hodt-Billington, C.; Helbostad, J.L. Gait variability measures may represent different constructs. Gait Posture 2010, 32, 98–101. [Google Scholar] [CrossRef]
- Takakusaki, K. Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov. Disord. 2013, 28, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Couto, A.G.B.; Vaz, M.A.P.; Pinho, L.; Félix, J.; Moreira, J.; Pinho, F.; Mesquita, I.A.; Montes, A.M.; Crasto, C.; Sousa, A.S.P. Repeatability and Temporal Consistency of Lower Limb Biomechanical Variables Expressing Interlimb Coordination during the Double-Support Phase in People with and without Stroke Sequelae. Sensors 2023, 23, 2526. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Robbins, M.; Holtzer, R.; Zimmerman, M.; Wang, C.; Xue, X.; Lipton, R.B. Gait Dysfunction in Mild Cognitive Impairment Syndromes. J. Am. Geriatr. Soc. 2008, 56, 1244–1251. [Google Scholar] [CrossRef]
- Brach, J.S.; Studenski, S.A.; Perera, S.; VanSwearingen, J.M.; Newman, A.B. Gait Variability and the Risk of Incident Mobility Disability in Community-Dwelling Older Adults. J. Gerontol. Ser. A 2007, 62, 983–988. [Google Scholar] [CrossRef]
- Patel, P.; Tiwari, A.; Lodha, N. Gait variability predicts real-life falls in high-functioning stroke survivors. Clin. Biomech. 2025, 121, 106393. [Google Scholar] [CrossRef]
- Stein, J.; Katz, D.I.; Black Schaffer, R.M.; Cramer, S.C.; Deutsch, A.F.; Harvey, R.L.; Lang, C.E.; Ottenbacher, K.J.; Prvu-Bettger, J.; Roth, E.J.; et al. Clinical Performance Measures for Stroke Rehabilitation: Performance Measures from the American Heart Association/American Stroke Association. Stroke 2021, 52, E675–E700. [Google Scholar] [CrossRef] [PubMed]
- Wist, S.; Clivaz, J.; Sattelmayer, M. Muscle strengthening for hemiparesis after stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2016, 59, 114–124. [Google Scholar] [CrossRef]
- Teasell, R.W.; Bhogal, S.K.; Foley, N.C.; Speechley, M.R. Gait Retraining Post Stroke. Top. Stroke Rehabil. 2003, 10, 34–65. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Elsner, B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2017, 8, CD002840. [Google Scholar] [CrossRef]
- Bohannon, R.W. Muscle strength and muscle training after stroke. J. Rehabil. Med. 2007, 39, 14–20. [Google Scholar] [CrossRef]
- Hollands, K.L.; Pelton, T.A.; Tyson, S.F.; Hollands, M.A.; van Vliet, P.M. Interventions for coordination of walking following stroke: Systematic review. Gait Posture 2012, 35, 349–359. [Google Scholar] [CrossRef]
- Manning, C.D.; Pomeroy, V.M. Effectiveness of Treadmill Retraining on Gait of Hemiparetic Stroke Patients: Systematic review of current evidence. Physiotherapy 2003, 89, 337–349. [Google Scholar] [CrossRef]
- Ouellette, M.M.; LeBrasseur, N.K.; Bean, J.F.; Phillips, E.; Stein, J.; Frontera, W.R.; Fielding, R.A. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke 2004, 35, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Van Abbema, R.; De Greef, M.; Crajé, C.; Krijnen, W.; Hobbelen, H.; Van Der Schans, C. What type, or combination of exercise can improve preferred gait speed in older adults? A meta-analysis. BMC Geriatr. 2015, 15, 72. [Google Scholar] [CrossRef]
- Vila-Chã, C.; Falla, D. Strength training, but not endurance training, reduces motor unit discharge rate variability. J. Electromyogr. Kinesiol. 2016, 26, 88–93. [Google Scholar] [CrossRef]
- Patel, P.; Casamento-Moran, A.; Christou, E.A.; Lodha, N. Force-Control vs. Strength Training: The Effect on Gait Variability in Stroke Survivors. Front. Neurol. 2021, 12, 667340. [Google Scholar] [CrossRef]
- Steele, J.; Bruce-Low, S.; Smith, D.; Jessop, D.; Osborne, N. A Randomized Controlled Trial of the Effects of Isolated Lumbar Extension Exercise on Lumbar Kinematic Pattern Variability During Gait in Chronic Low Back Pain. PM&R 2016, 8, 105–114. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Wang, Y.-L.; Cheng, Y.; Chao, Y.-H.; Chen, C.-L.; Yang, Y.-R. Effects of combined exercise on gait variability in community-dwelling older adults. Age 2015, 37, 9780. [Google Scholar] [CrossRef]
- Han, S.; Park, D. Effects of Reformer-based Trunk Stability Exercise on Trunk Control, Balance, and Gait Parameters in Patients with Chronic Stroke. NeuroRehabilitation 2025. [Google Scholar] [CrossRef]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef]
- Sage, M.; Middleton, L.; Tang, A.; Sibley, K.; Brooks, D.; McIlroy, W. Validity of rating of perceived exertion ranges in individuals in the subacute stage of stroke recovery. Top. Stroke Rehabil. 2013, 20, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, F.; Baker, R.; Barré, A.; Reay, J.; Jones, R.; Sangeux, M. The conventional gait model, an open-source implementation that reproduces the past but prepares for the future. Gait Posture 2019, 69, 235–241. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Whittle, M.W. Normal gait. In Gait Analysis, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2007; pp. 47–100. [Google Scholar] [CrossRef]
- Milot, M.-H.; Nadeau, S.; Gravel, D.; Bourbonnais, D. Gait Performance and Lower-Limb Muscle Strength Improved in Both Upper-Limb and Lower-Limb Isokinetic Training Programs in Individuals with Chronic Stroke. Int. Sch. Res. Not. 2013, 2013, 929758. [Google Scholar] [CrossRef]
- Flansbjer, U.B.; Miller, M.; Downham, D.; Lexell, J. Progressive resistance training after stroke: Effects on muscle strength, muscle tone, gait performance and perceived participation. J. Rehabil. Med. 2008, 40, 42–48. [Google Scholar] [CrossRef]
- Lexell, J.; Flansbjer, U. Muscle strength training, gait performance and physiotherapy after stroke. Minerva Med. 2008, 99, 353–368. Available online: https://api.semanticscholar.org/CorpusID:22226117 (accessed on 24 March 2025).
- Teixeira-Salmela, L.F.; Nadeau, S.; McBride, I.; Olney, S.J. Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors. J. Rehabil. Med. 2001, 33, 53–60. [Google Scholar] [CrossRef][Green Version]
- Nardelli, V.M.F.; Okazaki, V.H.A.; Guimarães, A.N.; Nascimento, V.B.; Dascal, J.B. Effects on older adult Women’s precision, strength and flexibility from resistance training and handicrafts practice. J. Bodyw. Mov. Ther. 2024, 40, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Keen, D.A.; Yue, G.H.; Enoka, R.M. Training-related enhancement in the control of motor output in elderly humans. J. Appl. Physiol. 1994, 77, 2648–2658. [Google Scholar] [CrossRef]
- Skiadopoulos, A.; Moore, E.E.; Sayles, H.R.; Schmid, K.K.; Stergiou, N. Step width variability as a discriminator of age-related gait changes. J. Neuroeng. Rehabil. 2020, 17, 41. [Google Scholar] [CrossRef]
- Bauby, C.E.; Kuo, A.D. Active control of lateral balance in human walking. J. Biomech. 2000, 33, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Orr, R.; Raymond, J.; Singh, M.F. Efficacy of progressive resistance training on balance performance in older adults: A systematic review of randomized controlled trials. Sport. Med. 2008, 38, 317–343. [Google Scholar] [CrossRef]
- Topp, R. Effect of resistance training on strength, postural control, and gait velocity among older adults. Clin. Nurs. Res. 1996, 5, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Baricich, A.; Borg, M.B.; Battaglia, M.; Facciorusso, S.; Spina, S.; Invernizzi, M.; Scotti, L.; Cosenza, L.; Picelli, A.; Santamato, A. High-Intensity Exercise Training Impact on Cardiorespiratory Fitness, Gait Ability, and Balance in Stroke Survivors: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 5498. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Glenney, S.S. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2014, 20, 295–300. [Google Scholar] [CrossRef]
- Allen, J.L.; Kautz, S.A.; Neptune, R.R. Forward propulsion asymmetry is indicative of changes in plantarflexor coordination during walking in individuals with post-stroke hemiparesis. Clin. Biomech. 2014, 29, 780–786. [Google Scholar] [CrossRef]
| Parameter | Leg | Mean Pre (Range) | Mean Post (Range) | Hedges’ g | Mean p-Value | CV Pre (Range) | CV Post (Range) | CV Hedges’ g | CV p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Spatiotemporal Parameters | |||||||||
| Walking Speed | paretic | 0.61 (0.20–1.13) | 0.69 (0.20–1.20) | 0.25 | 0.032 | 8.75 (4.09–18.47) | 8.84 (3.05–17.63) | 0.02 | 0.765 |
| non-paretic | 0.62 (0.20–1.14) | 0.69 (0.19–1.21) | 0.23 | 0.024 | 7.26 (3.12–12.15) | 8.78 (3.83–20.59) | 0.35 | 0.465 | |
| Cadence | paretic | 89.35 (60.65–112.91) | 91.92 (60.27–119.23) | 0.16 | 0.147 | 5.37 (3.59–8.40) | 5.91 (3.60–9.98) | 0.28 | 0.365 |
| non-paretic | 89.24 (60.79–112.62) | 92.01 (59.24–118.93) | 0.17 | 0.024 | 5.31 (3.33–7.51) | 5.61 (2.92–13.22) | 0.12 | 1.000 | |
| Spatial Parameters | |||||||||
| Step Length | paretic | 0.36 (0.04–0.71) | 0.41 (0.07–0.74) | 0.21 | 0.042 | 19.91 (3.53–90.83) | 14.99 (3.11–71.11) | −0.21 | 0.102 |
| non-paretic | 0.45 (0.33–0.71) | 0.49 (0.35–0.72) | 0.33 | 0.083 | 7.33 (3.16–11.64) | 6.79 (3.73–13.10) | −0.18 | 0.898 | |
| Step Width | paretic | 0.17 (0.07–0.24) | 0.17 (0.07–0.25) | −0.10 | 0.765 | 20.55 (5.95–52.27) | 20.18 (6.57–32.90) | −0.03 | 0.700 |
| non-paretic | 0.17 (0.07–0.24) | 0.16 (0.07–0.25) | −0.02 | 0.966 | 18.64 (7.58–53.76) | 18.19 (6.67–27.16) | −0.04 | 0.898 | |
| Stride Length | paretic | 0.80 (0.32–1.40) | 0.88 (0.28–1.46) | 0.23 | 0.102 | 7.47 (2.45–18.94) | 7.25 (2.15–22.71) | −0.04 | 0.966 |
| non-paretic | 0.81 (0.32–1.42) | 0.88 (0.28–1.47) | 0.21 | 0.175 | 5.94 (2.16–9.11) | 7.77 (3.39–20.33) | 0.45 | 0.206 | |
| Temporal Parameters | |||||||||
| Step Time | paretic | 0.61 (0.49–0.72) | 0.60 (0.42–0.70) | −0.15 | 0.413 | 8.63 (5.19–11.71) | 7.80 (2.92–16.77) | −0.23 | 0.320 |
| non-paretic | 0.78 (0.55–1.30) | 0.76 (0.51–1.35) | −0.08 | 0.365 | 7.32 (4.13–13.65) | 6.46 (3.21–9.26) | −0.37 | 0.175 | |
| Stride Time | paretic | 1.38 (1.06–1.98) | 1.35 (1.01–2.00) | −0.12 | 0.206 | 5.41 (3.54–8.75) | 5.91 (3.62–9.53) | 0.25 | 0.365 |
| non-paretic | 1.39 (1.07–1.98) | 1.35 (1.01–2.03) | −0.12 | 0.054 | 5.41 (3.31–7.97) | 5.76 (2.89–14.66) | 0.13 | 0.898 | |
| Stance Time | paretic | 0.87 (0.66–1.30) | 0.85 (0.61–1.31) | −0.10 | 0.175 | 7.94 (4.23–14.58) | 7.21 (4.09–14.72) | −0.21 | 0.278 |
| non-paretic | 1.00 (0.68–1.73) | 0.98 (0.61–1.76) | −0.06 | 0.240 | 6.24 (3.95–9.21) | 6.72 (3.14–17.23) | 0.15 | 0.700 | |
| Swing Time | paretic | 0.51 (0.40–0.68) | 0.50 (0.38–0.69) | −0.11 | 0.320 | 11.53 (4.84–17.03) | 9.34 (4.42–18.30) | −0.51 | 0.365 |
| non-paretic | 0.39 (0.24–0.49) | 0.38 (0.23–0.48) | −0.16 | 0.175 | 9.80 (5.03–16.76) | 10.23 (4.97–18.08) | 0.11 | 0.577 | |
| Single Support | paretic | 0.39 (0.22–0.50) | 0.38 (0.24–0.48) | −0.11 | 0.365 | 10.94 (4.77–22.40) | 10.28 (5.01–16.46) | −0.14 | 0.966 |
| non-paretic | 0.51 (0.41–0.69) | 0.51 (0.36–0.73) | −0.08 | 0.638 | 10.47 (5.64–15.52) | 9.10 (3.56–18.30) | −0.31 | 0.365 | |
| Double Support | paretic | 0.48 (0.22–1.06) | 0.47 (0.14–1.04) | −0.04 | 0.240 | 13.31 (7.85–23.29) | 12.47 (3.55–25.41) | −0.15 | 0.520 |
| non-paretic | 0.48 (0.21–1.04) | 0.47 (0.14–1.03) | −0.04 | 0.147 | 12.82 (9.28–24.06) | 13.30 (3.31–25.93) | 0.08 | 0.898 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giarmatzis, G.; Giannakou, E.; Karagiannakidou, I.; Makri, E.; Tsiakiri, A.; Christidi, F.; Malliou, P.; Vadikolias, K.; Aggelousis, N. Effects of a 12-Week Moderate-to-High Intensity Strength Training Program on the Gait Parameters and Their Variability of Stroke Survivors. Brain Sci. 2025, 15, 354. https://doi.org/10.3390/brainsci15040354
Giarmatzis G, Giannakou E, Karagiannakidou I, Makri E, Tsiakiri A, Christidi F, Malliou P, Vadikolias K, Aggelousis N. Effects of a 12-Week Moderate-to-High Intensity Strength Training Program on the Gait Parameters and Their Variability of Stroke Survivors. Brain Sciences. 2025; 15(4):354. https://doi.org/10.3390/brainsci15040354
Chicago/Turabian StyleGiarmatzis, Georgios, Erasmia Giannakou, Ioanna Karagiannakidou, Evangelia Makri, Anna Tsiakiri, Foteini Christidi, Paraskevi Malliou, Konstantinos Vadikolias, and Nikolaos Aggelousis. 2025. "Effects of a 12-Week Moderate-to-High Intensity Strength Training Program on the Gait Parameters and Their Variability of Stroke Survivors" Brain Sciences 15, no. 4: 354. https://doi.org/10.3390/brainsci15040354
APA StyleGiarmatzis, G., Giannakou, E., Karagiannakidou, I., Makri, E., Tsiakiri, A., Christidi, F., Malliou, P., Vadikolias, K., & Aggelousis, N. (2025). Effects of a 12-Week Moderate-to-High Intensity Strength Training Program on the Gait Parameters and Their Variability of Stroke Survivors. Brain Sciences, 15(4), 354. https://doi.org/10.3390/brainsci15040354








