Ethanol Administration in Mice Leads to Sex-Specific Changes in the Acetylation of α-Tubulin in the Cerebellum
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Brain Tissue Extraction and Protein Estimation
2.3. Western Blot
2.4. Perchloric Acid Extraction
2.5. High-Performance Liquid Chromatography (HPLC) Analysis
2.6. Statistical Analysis
3. Results
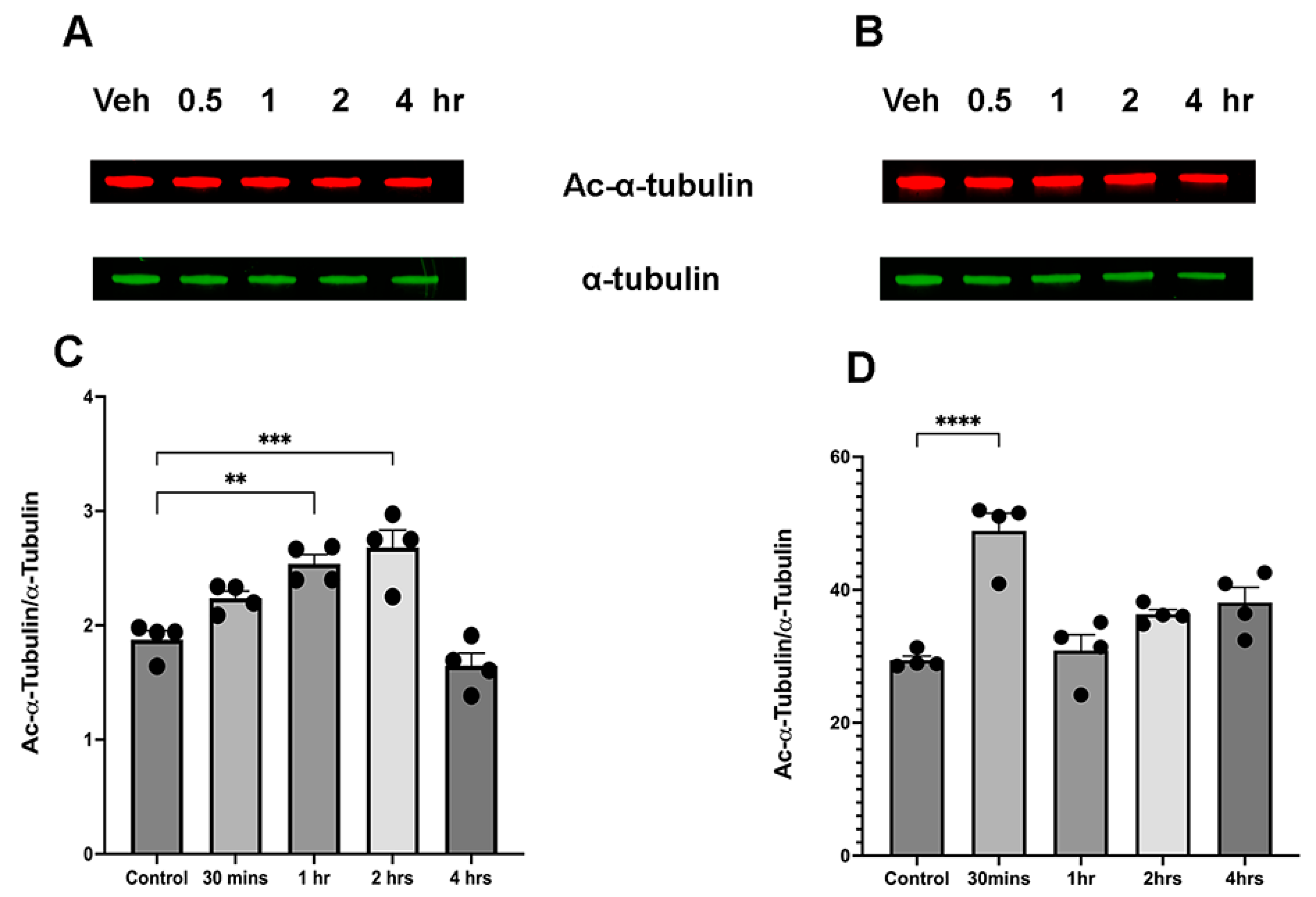
3.1. Ethanol Induces Sex-Dependent Temporal Alterations in α-Tubulin Acetylation
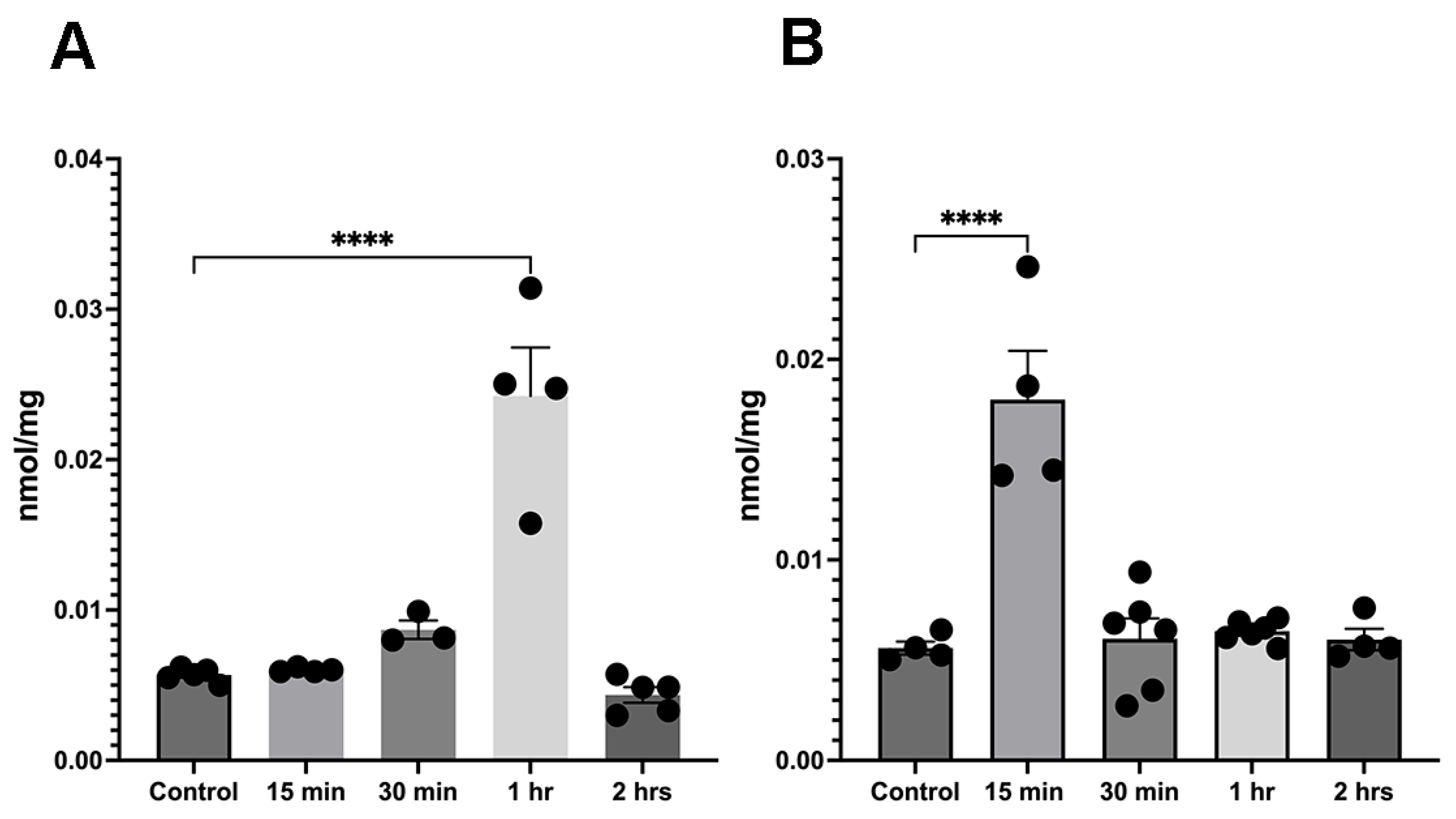
3.2. α-Tubulin Acetylation Changes Coincide with an Ethanol-Induced Increase in Acetyl-CoA
3.3. Ethanol Induced Transient Changes in Acetyl-CoA Synthetase Levels Are Sex Dependent
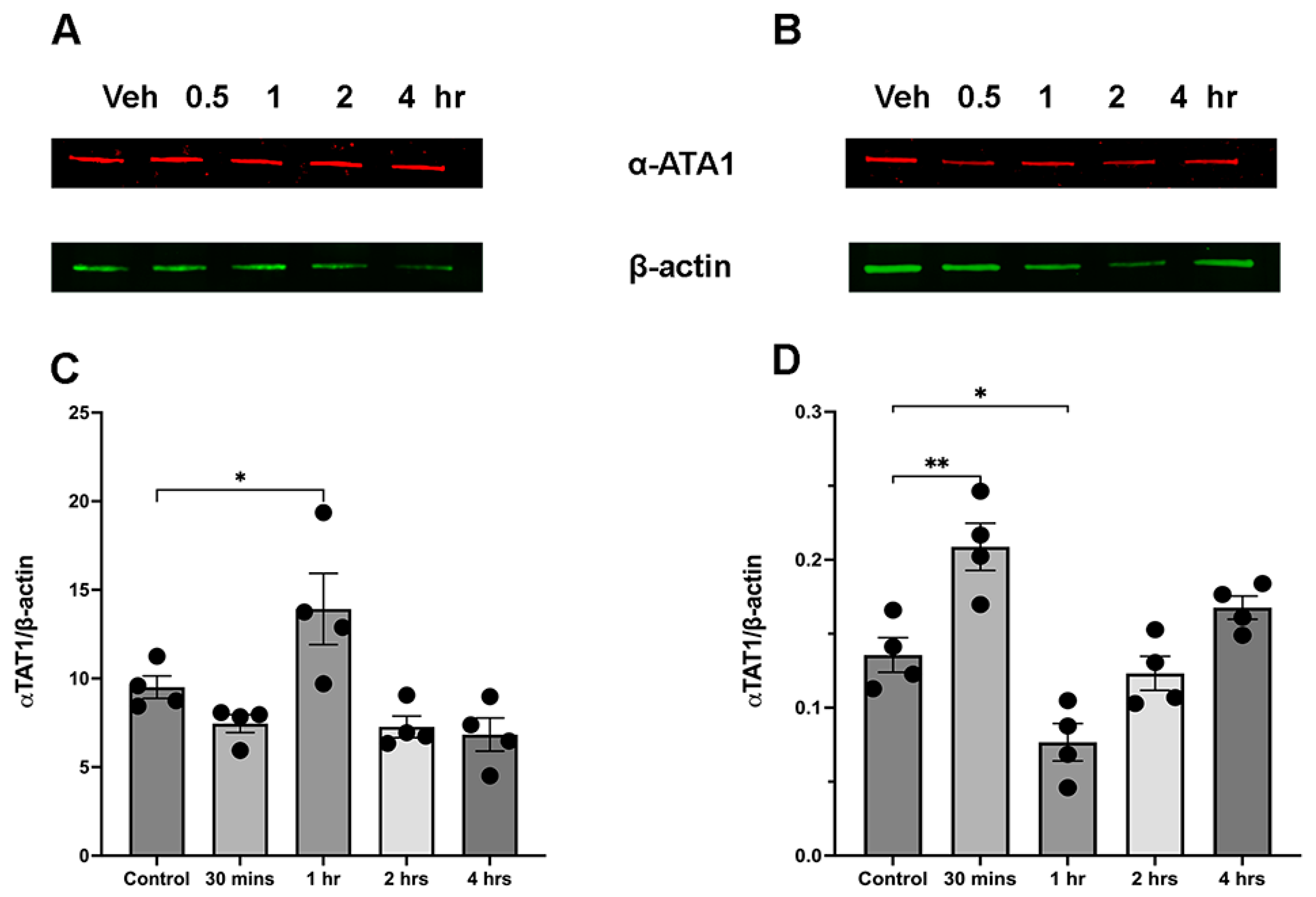
3.4. Ethanol Administration Leads to Sex-Dependent Transient Increase in aTAT1 Expression
3.5. Ethanol Intake Does Not Affect the HDAC6 Expression Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glozak, M.A.; Sengupta, N.; Zhang, X.; Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 2005, 363, 15–23. [Google Scholar] [CrossRef]
- Bar, J.; Popp, Y.; Bucher, M.; Mikhaylova, M. Direct and indirect effects of tubulin post-translational modifications on microtubule stability: Insights and regulations. Biochim. Biophys. Acta Mol. Cell. Res. 2022, 1869, 119241. [Google Scholar] [CrossRef]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury, M.V.; Toso, D.B.; Lohr, T.; Vendruscolo, M.; Bonomi, M.; Fraser, J.S.; Nogales, E. Effects of alpha-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef]
- Macedo, G.C.; Kreifeldt, M.; Goulding, S.P.; Okhuarobo, A.; Sidhu, H.; Contet, C. Chronic MAP4343 reverses escalated alcohol drinking in a mouse model of alcohol use disorder. Neuropsychopharmacology 2023, 48, 821–830. [Google Scholar] [CrossRef]
- Shepard, B.D.; Joseph, R.A.; Kannarkat, G.T.; Rutledge, T.M.; Tuma, D.J.; Tuma, P.L. Alcohol-induced alterations in hepatic microtubule dynamics can be explained by impaired histone deacetylase 6 function. Hepatology 2008, 48, 1671–1679. [Google Scholar] [CrossRef]
- Kelliher, M.T.; Saunders, H.A.; Wildonger, J. Microtubule control of functional architecture in neurons. Curr. Opin. Neurobiol. 2019, 57, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.M.; Menko, A.S. Microtubules: Evolving roles and critical cellular interactions. Exp. Biol. Med. 2019, 244, 1240–1254. [Google Scholar] [CrossRef] [PubMed]
- Waddell, J.; Banerjee, A.; Kristian, T. Acetylation in Mitochondria Dynamics and Neurodegeneration. Cells 2021, 10, 3031. [Google Scholar] [CrossRef] [PubMed]
- Labisso, W.L.; Raulin, A.C.; Nwidu, L.L.; Kocon, A.; Wayne, D.; Erdozain, A.M.; Morentin, B.; Schwendener, D.; Allen, G.; Enticott, J.; et al. The Loss of alpha- and beta-Tubulin Proteins Are a Pathological Hallmark of Chronic Alcohol Consumption and Natural Brain Ageing. Brain Sci. 2018, 8, 175. [Google Scholar] [CrossRef]
- Ryu, N.M.; Kim, J.M. The role of the alpha-tubulin acetyltransferase alphaTAT1 in the DNA damage response. J. Cell Sci. 2020, 133, jcs246702. [Google Scholar] [CrossRef]
- Carmona, B.; Marinho, H.S.; Matos, C.L.; Nolasco, S.; Soares, H. Tubulin Post-Translational Modifications: The Elusive Roles of Acetylation. Biology 2023, 12, 561. [Google Scholar] [CrossRef]
- Khatoon, R.; Fick, J.; Elesinnla, A.; Waddell, J.; Kristian, T. Sexual Dimorphism of Ethanol-Induced Mitochondrial Dynamics in Purkinje Cells. Int. J. Mol. Sci. 2024, 25, 13714. [Google Scholar] [CrossRef]
- Jin, S.; Cao, Q.; Yang, F.; Zhu, H.; Xu, S.; Chen, Q.; Wang, Z.; Lin, Y.; Cinar, R.; Pawlosky, R.J.; et al. Brain ethanol metabolism by astrocytic ALDH2 drives the behavioural effects of ethanol intoxication. Nat. Metab. 2021, 3, 337–351. [Google Scholar] [CrossRef]
- Mansouri, A.; Demeilliers, C.; Amsellem, S.; Pessayre, D.; Fromenty, B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: Protective effects of antioxidants. J. Pharmacol. Exp. Ther. 2001, 298, 737–743. [Google Scholar]
- Escarabajal, M.D.; Aragon, C.M. The effect of cyanamide and 4-methylpyrazole on the ethanol-induced locomotor activity in mice. Pharmacol. Biochem. Behav. 2002, 72, 389–395. [Google Scholar] [CrossRef]
- Hasegawa, H.; Kondo, M. Astrocytic Responses to Binge Alcohol Intake in the Mouse Hindbrain. Biol. Pharm. Bull. 2023, 46, 1194–1202. [Google Scholar] [CrossRef]
- Rewal, M.; Wen, Y.; Simpkins, J.W.; Jung, M.E. Ethanol withdrawal reduces cerebellar parvalbumin expression in a manner reversed by estrogens. Neurosci. Lett. 2005, 377, 44–48. [Google Scholar] [CrossRef]
- Acquaah-Mensah, G.K.; Misra, V.; Biswal, S. Ethanol sensitivity: A central role for CREB transcription regulation in the cerebellum. BMC Genom. 2006, 7, 308. [Google Scholar] [CrossRef]
- Southwood, C.M.; Peppi, M.; Dryden, S.; Tainsky, M.A.; Gow, A. Microtubule deacetylases, SirT2 and HDAC6, in the nervous system. Neurochem. Res. 2007, 32, 187–195. [Google Scholar] [CrossRef]
- Waddell, J.; McKenna, M.C.; Kristian, T. Brain ethanol metabolism and mitochondria. Curr. Top. Biochem. Res. 2022, 23, 1–13. [Google Scholar]
- Wilson, D.F.; Matschinsky, F.M. Ethanol metabolism: The good, the bad, and the ugly. Med. Hypotheses 2020, 140, 109638. [Google Scholar] [CrossRef]
- Egervari, G.; Siciliano, C.A.; Whiteley, E.L.; Ron, D. Alcohol and the brain: From genes to circuits. Trends Neurosci. 2021, 44, 1004–1015. [Google Scholar] [CrossRef]
- Even, A.; Morelli, G.; Turchetto, S.; Shilian, M.; Bail, R.L.; Laguesse, S.; Krusy, N.; Brisker, A.; Brandis, A.; Inbar, S.; et al. ATP-citrate lyase promotes axonal transport across species. Nat. Commun. 2021, 12, 5878. [Google Scholar] [CrossRef]
- Erdozain, A.M.; Morentin, B.; Bedford, L.; King, E.; Tooth, D.; Brewer, C.; Wayne, D.; Johnson, L.; Gerdes, H.K.; Wigmore, P.; et al. Alcohol-related brain damage in humans. PLoS ONE 2014, 9, e93586. [Google Scholar] [CrossRef] [PubMed]
- Groebner, J.L.; Giron-Bravo, M.T.; Rothberg, M.L.; Adhikari, R.; Tuma, D.J.; Tuma, P.L. Alcohol-induced microtubule acetylation leads to the accumulation of large, immobile lipid droplets. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G373–G386. [Google Scholar] [CrossRef]
- Kannarkat, G.T.; Tuma, D.J.; Tuma, P.L. Microtubules are more stable and more highly acetylated in ethanol-treated hepatic cells. J. Hepatol. 2006, 44, 963–970. [Google Scholar] [CrossRef]
- Bobrowska, A.; Donmez, G.; Weiss, A.; Guarente, L.; Bates, G. SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington's disease phenotypes in vivo. PLoS ONE 2012, 7, e34805. [Google Scholar] [CrossRef]
- Kalebic, N.; Sorrentino, S.; Perlas, E.; Bolasco, G.; Martinez, C.; Heppenstall, P.A. alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nat. Commun. 2013, 4, 1962. [Google Scholar] [CrossRef]
- Shida, T.; Cueva, J.G.; Xu, Z.; Goodman, M.B.; Nachury, M.V. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA 2010, 107, 21517–21522. [Google Scholar] [CrossRef]
- Pascual, M.; Do Couto, B.R.; Alfonso-Loeches, S.; Aguilar, M.A.; Rodriguez-Arias, M.; Guerri, C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology 2012, 62, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Zhang, Y.; Wang, Q.; Miao, Q.; Xu, Y.; Soares, J.C.; Zhang, X.; Zhang, R. Correlation between the epigenetic modification of histone H3K9 acetylation of NR2B gene promoter in rat hippocampus and ethanol withdrawal syndrome. Mol. Biol. Rep. 2019, 46, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Mews, P.; Egervari, G.; Nativio, R.; Sidoli, S.; Donahue, G.; Lombroso, S.I.; Alexander, D.C.; Riesche, S.L.; Heller, E.A.; Nestler, E.J.; et al. Alcohol metabolism contributes to brain histone acetylation. Nature 2019, 574, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Shibasaki, M.; Mizuno, K.; Kurokawa, K.; Ohkuma, S. Enhancement of histone acetylation in midbrain of mice with ethanol physical dependence and its withdrawal. Synapse 2011, 65, 1244–1250. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elesinnla, A.; Khatoon, R.; Kleinert, N.; Wu, J.; Waddell, J.; Kristian, T. Ethanol Administration in Mice Leads to Sex-Specific Changes in the Acetylation of α-Tubulin in the Cerebellum. Brain Sci. 2025, 15, 326. https://doi.org/10.3390/brainsci15040326
Elesinnla A, Khatoon R, Kleinert N, Wu J, Waddell J, Kristian T. Ethanol Administration in Mice Leads to Sex-Specific Changes in the Acetylation of α-Tubulin in the Cerebellum. Brain Sciences. 2025; 15(4):326. https://doi.org/10.3390/brainsci15040326
Chicago/Turabian StyleElesinnla, Abosede, Rehana Khatoon, Nicholas Kleinert, Junfang Wu, Jaylyn Waddell, and Tibor Kristian. 2025. "Ethanol Administration in Mice Leads to Sex-Specific Changes in the Acetylation of α-Tubulin in the Cerebellum" Brain Sciences 15, no. 4: 326. https://doi.org/10.3390/brainsci15040326
APA StyleElesinnla, A., Khatoon, R., Kleinert, N., Wu, J., Waddell, J., & Kristian, T. (2025). Ethanol Administration in Mice Leads to Sex-Specific Changes in the Acetylation of α-Tubulin in the Cerebellum. Brain Sciences, 15(4), 326. https://doi.org/10.3390/brainsci15040326





