Comparative Effects of Temporal Interference and High-Definition Transcranial Direct Current Stimulation on Spontaneous Neuronal Activity in the Primary Motor Cortex: A Randomized Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
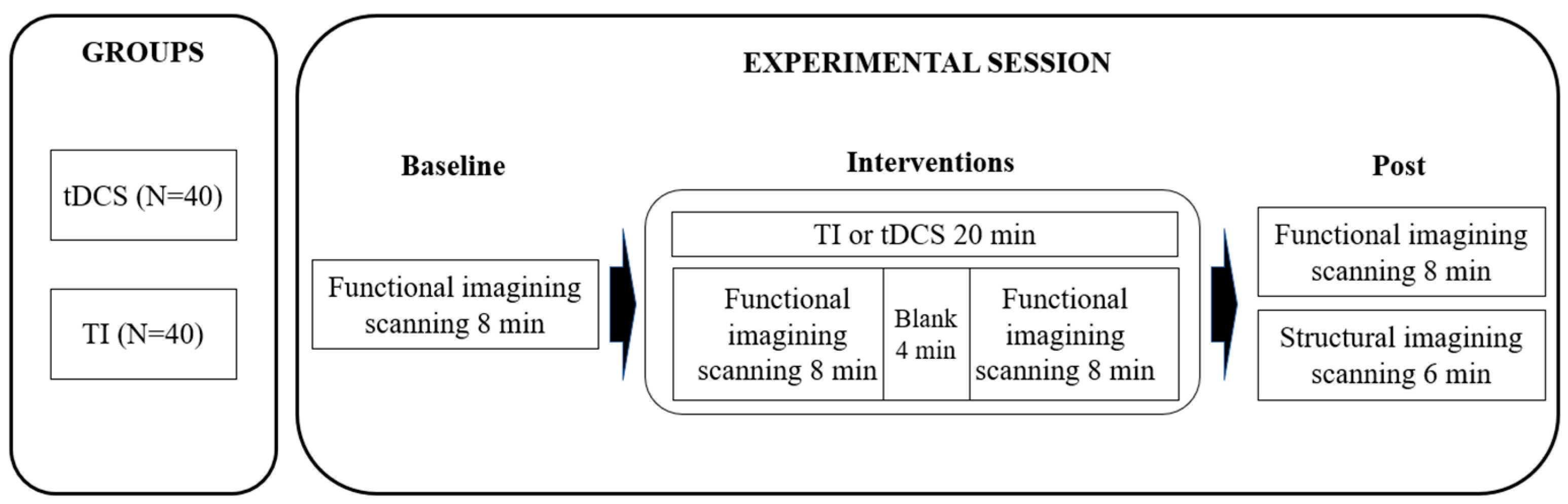
2.2. Experimental Design
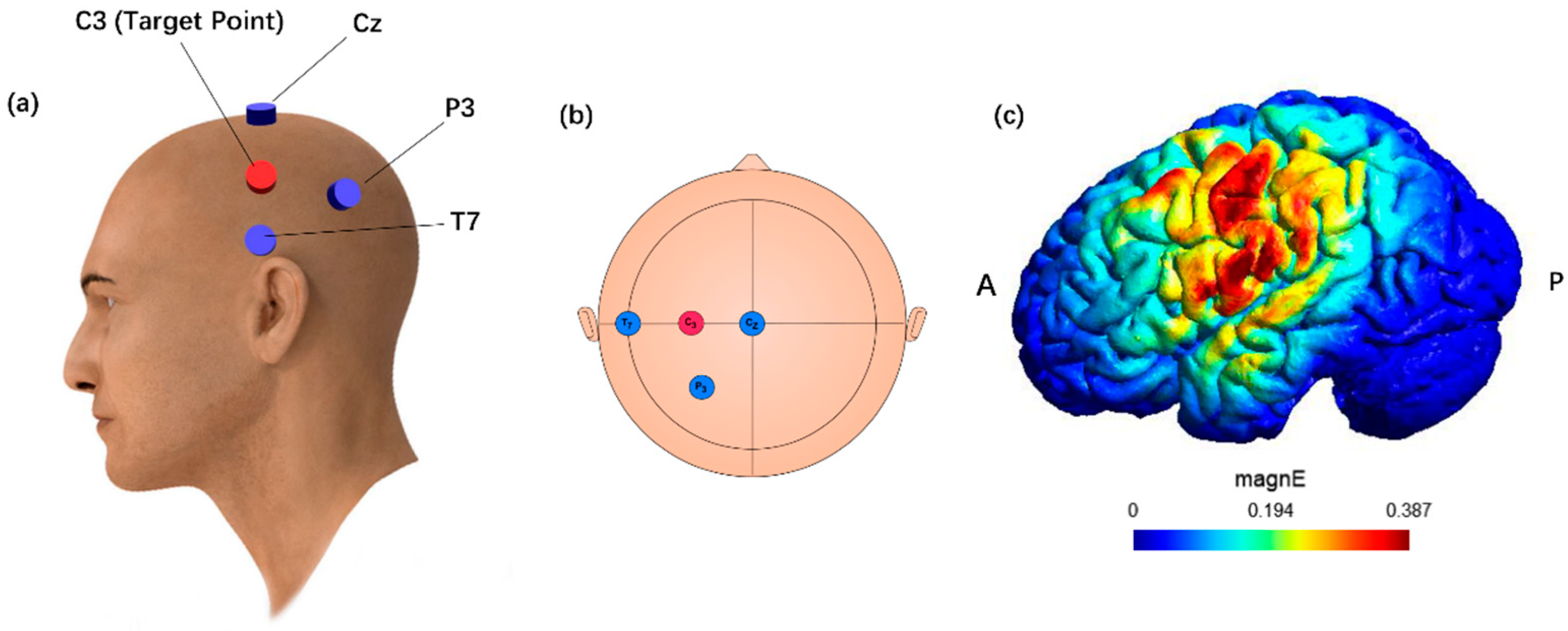
2.3. Brain Stimulation Parameters and Session Procedures
2.3.1. TI
2.3.2. HD-tDCS
2.4. Image Acquisition and Preprocessing
2.5. Calculation of dReHo and dfALFFs of Resting-State fMRI
2.6. Statistical Analysis
3. Results
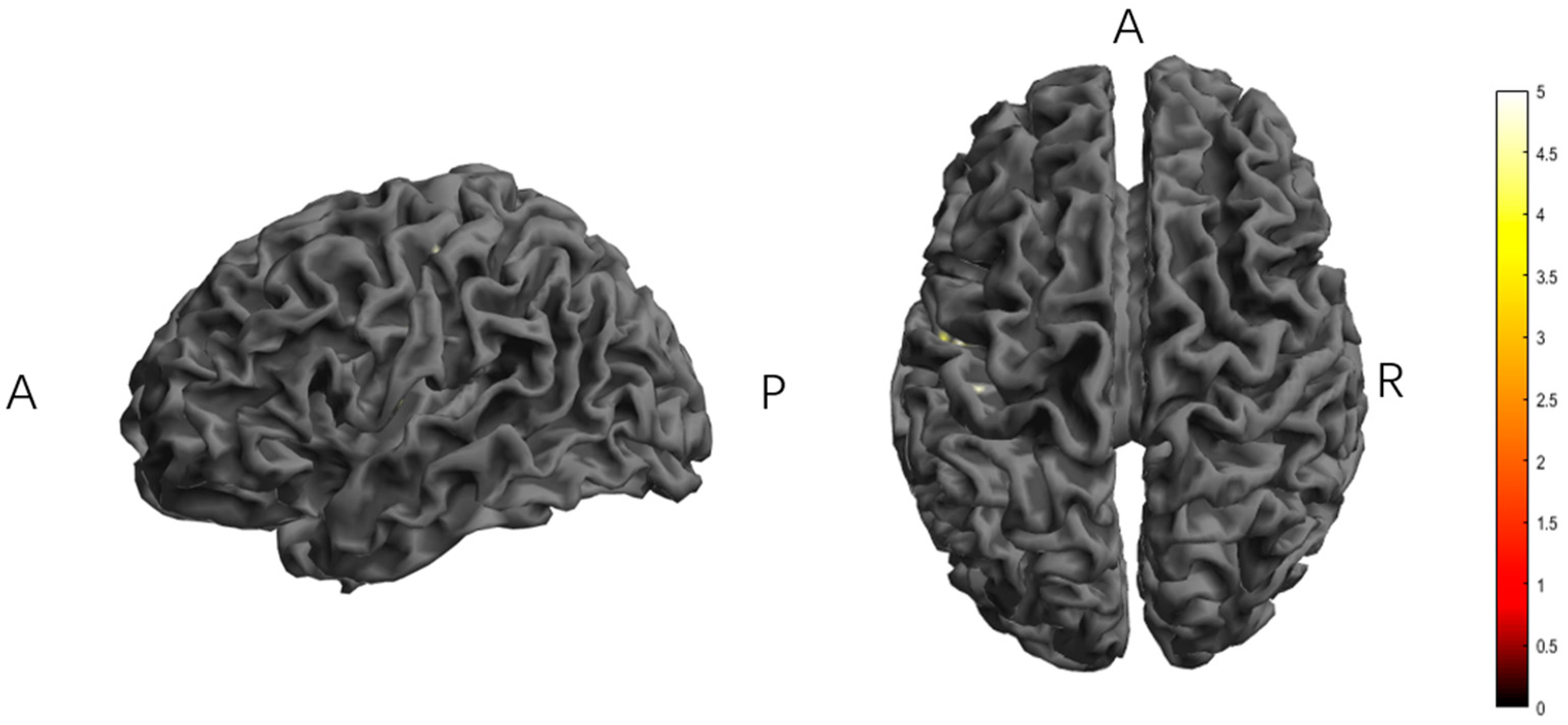
3.1. Effect of Different Transcranial Electrical Stimulation on ReHo
3.2. Effect of Different Transcranial Electrical Stimulation on dReHo
3.3. Effect of Different Transcranial Electrical Stimulation on fALFFs
3.4. Effect of Different Transcranial Electrical Stimulation on dfALFFs
4. Discussion
4.1. ReHo and dReHo
4.2. fALFFs and dfALFFs
4.3. Mechanisms
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Kucyi, A.; Davis, K.D. Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage 2014, 100, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Chin, J.; Mucke, L. A network dysfunction perspective on neurodegenerative diseases. Nature 2006, 443, 768–773. [Google Scholar] [CrossRef]
- Northoff, G.; Duncan, N.W. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Prog. Neurobiol. 2016, 145–146, 26–45. [Google Scholar] [CrossRef]
- Soekadar, S.R.; Birbaumer, N.; Slutzky, M.W.; Cohen, L.G. Brain-machine interfaces in neurorehabilitation of stroke. Neurobiol. Dis. 2015, 83, 172–179. [Google Scholar] [CrossRef]
- Yavari, F.; Jamil, A.; Mosayebi Samani, M.; Vidor, L.P.; Nitsche, M.A. Basic and functional effects of transcranial Electrical Stimulation (tES)-An introduction. Neurosci. Biobehav. Rev. 2018, 85, 81–92. [Google Scholar] [CrossRef]
- Alam, M.; Truong, D.Q.; Khadka, N.; Bikson, M. Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Phys. Med. Biol. 2016, 61, 4506–4521. [Google Scholar] [CrossRef]
- Caulfield, K.A.; George, M.S. Treating the mental health effects of COVID-19: The need for at-home neurotherapeutics is now. Brain Stimul. 2020, 13, 939–940. [Google Scholar] [CrossRef]
- Ruffini, G.; Wendling, F.; Sanchez-Todo, R.; Santarnecchi, E. Targeting brain networks with multichannel transcranial current stimulation (tCS). Curr. Opin. Biomed. Eng. 2018, 8, 70–77. [Google Scholar] [CrossRef]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e1016. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tanaka, S.; Gomez-Tames, J.; Okabe, T.; Cho, K.; Iso, N.; Hirata, A. Nonequivalent After-Effects of Alternating Current Stimulation on Motor Cortex Oscillation and Inhibition: Simulation and Experimental Study. Brain Sci. 2022, 12, 195. [Google Scholar] [CrossRef]
- Wang, L.; Nitsche, M.A.; Zschorlich, V.R.; Liu, H.; Kong, Z.; Qi, F. 20 Hz Transcranial Alternating Current Stimulation Inhibits Observation-Execution-Related Motor Cortex Excitability. J. Pers. Med. 2021, 11, 979. [Google Scholar] [CrossRef]
- Zeng, Y.; Cheng, R.; Zhang, L.; Fang, S.; Zhang, S.; Wang, M.; Lv, Q.; Dai, Y.; Gong, X.; Liang, F. Clinical Comparison between HD-tDCS and tDCS for Improving Upper Limb Motor Function: A Randomized, Double-Blinded, Sham-Controlled Trial. Neural Plast. 2024, 2024, 2512796. [Google Scholar] [CrossRef] [PubMed]
- Aberra, A.S.; Wang, R.; Grill, W.M.; Peterchev, A.V. Multi-scale model of axonal and dendritic polarization by transcranial direct current stimulation in realistic head geometry. Brain Stimul. 2023, 16, 1776–1791. [Google Scholar] [CrossRef]
- Zhu, Z.; Xiong, Y.; Chen, Y.; Jiang, Y.; Qian, Z.; Lu, J.; Liu, Y.; Zhuang, J. Temporal Interference (TI) Stimulation Boosts Functional Connectivity in Human Motor Cortex: A Comparison Study with Transcranial Direct Current Stimulation (tDCS). Neural Plast. 2022, 2022, 7605046. [Google Scholar] [CrossRef]
- Zheng, J.; Stevenson, R.F.; Mander, B.A.; Mnatsakanyan, L.; Hsu, F.P.K.; Vadera, S.; Knight, R.T.; Yassa, M.A.; Lin, J.J. Multiplexing of Theta and Alpha Rhythms in the Amygdala-Hippocampal Circuit Supports Pattern Separation of Emotional Information. Neuron 2019, 102, 887–898.e885. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Conforto, A.B.; Z’Graggen, W.J.; Kohl, A.S.; Rösler, K.M.; Kaelin-Lang, A. Impact of coil position and electrophysiological monitoring on determination of motor thresholds to transcranial magnetic stimulation. Clin. Neurophysiol. 2004, 115, 812–819. [Google Scholar] [CrossRef]
- Violante, I.R.; Alania, K.; Cassarà, A.M.; Neufeld, E.; Acerbo, E.; Carron, R.; Williamson, A.; Kurtin, D.L.; Rhodes, E.; Hampshire, A.; et al. Non-invasive temporal interference electrical stimulation of the human hippocampus. Nat. Neurosci. 2023, 26, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Windhoff, M.; Opitz, A.; Thielscher, A. Electric field calculations in brain stimulation based on finite elements: An optimized processing pipeline for the generation and usage of accurate individual head models. Human. Brain Mapp. 2013, 34, 923–935. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Shereen, A.D.; Ghobadi-Azbari, P.; Datta, A.; Woods, A.J.; Ironside, M.; O’Shea, J.; Kirk, U.; Bikson, M.; Ekhtiari, H. Methodology for tDCS integration with fMRI. Hum. Brain Mapp. 2020, 41, 1950–1967. [Google Scholar] [CrossRef] [PubMed]
- Chao-Gan, Y.; Yu-Feng, Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Yan, C.G.; Yang, Z.; Colcombe, S.J.; Zuo, X.N.; Milham, M.P. Concordance among indices of intrinsic brain function: Insights from inter-individual variation and temporal dynamics. Sci. Bull. 2017, 62, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Zalesky, A.; Breakspear, M. Towards a statistical test for functional connectivity dynamics. Neuroimage 2015, 114, 466–470. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Li, M.; Wang, W.; Li, R.; Zhang, Z.; Lu, G.; Chen, H. Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum. Brain Mapp. 2017, 38, 957–973. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, A.A.; Lafon, B.; Friedman, D.; Dayan, M.; Wang, X.; Bikson, M.; Doyle, W.K.; Devinsky, O.; Parra, L.C. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife 2017, 6, e18834. [Google Scholar] [CrossRef]
- Turi, Z.; Mittner, M.; Paulus, W.; Antal, A. Placebo Intervention Enhances Reward Learning in Healthy Individuals. Sci. Rep. 2017, 7, 41028. [Google Scholar] [CrossRef]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.J.; Reiser, M.; Padberg, F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef] [PubMed]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Sun, W.; Liu, X.; Guo, Z.; Mao, D.; Yu, E.; Chen, Y. Altered dynamic intrinsic brain activity of the default mode network in Alzheimer’s disease: A resting-state fMRI study. Front. Hum. Neurosci. 2022, 16, 951114. [Google Scholar] [CrossRef]
- Zou, Q.H.; Zhu, C.Z.; Yang, Y.; Zuo, X.N.; Long, X.Y.; Cao, Q.J.; Wang, Y.F.; Zang, Y.F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef]
- Cabral-Calderin, Y.; Williams, K.A.; Opitz, A.; Dechent, P.; Wilke, M. Transcranial alternating current stimulation modulates spontaneous low frequency fluctuations as measured with fMRI. Neuroimage 2016, 141, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, C.; Cao, X.; Yuan, Q.; Qi, W.; Xu, W.; Zhang, S.; Huang, Q. Altered Patterns of Amplitude of Low-Frequency Fluctuations and Fractional Amplitude of Low-Frequency Fluctuations Between Amnestic and Vascular Mild Cognitive Impairment: An ALE-Based Comparative Meta-Analysis. Front. Aging Neurosci. 2021, 13, 711023. [Google Scholar] [CrossRef]
- Gong, J.; Wang, J.; Luo, X.; Chen, G.; Huang, H.; Huang, R.; Huang, L.; Wang, Y. Abnormalities of intrinsic regional brain activity in first-episode and chronic schizophrenia: A meta-analysis of resting-state functional MRI. J. Psychiatry Neurosci. 2020, 45, 55–68. [Google Scholar] [CrossRef]
- Vieira, P.G.; Krause, M.R.; Pack, C.C. Temporal interference stimulation disrupts spike timing in the primate brain. Nat. Commun. 2024, 15, 4558. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef]
- Polanía, R.; Nitsche, M.A.; Paulus, W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum. Brain Mapp. 2011, 32, 1236–1249. [Google Scholar] [CrossRef]
- Zheng, X.; Alsop, D.C.; Schlaug, G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage 2011, 58, 26–33. [Google Scholar] [CrossRef]
- Meinzer, M.; Lindenberg, R.; Antonenko, D.; Flaisch, T.; Flöel, A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J. Neurosci. 2013, 33, 12470–12478. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.J.; Cicchetti, F. Cellular and molecular mechanisms of action of transcranial direct current stimulation: Evidence from in vitro and in vivo models. Int. J. Neuropsychopharmacol. 2014, 18, pyu047. [Google Scholar] [CrossRef]
- Ma, R.; Xia, X.; Zhang, W.; Lu, Z.; Wu, Q.; Cui, J.; Song, H.; Fan, C.; Chen, X.; Zha, R.; et al. High Gamma and Beta Temporal Interference Stimulation in the Human Motor Cortex Improves Motor Functions. Front. Neurosci. 2021, 15, 800436. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, L. A mini-review: Recent advancements in temporal interference stimulation in modulating brain function and behavior. Front. Hum. Neurosci. 2023, 17, 1266753. [Google Scholar] [CrossRef]
- Reinhart, R.M.G.; Nguyen, J.A. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat. Neurosci. 2019, 22, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Baxter, B.; He, B. High-definition transcranial direct current stimulation induces both acute and persistent changes in broadband cortical synchronization: A simultaneous tDCS-EEG study. IEEE Trans. Biomed. Eng. 2014, 61, 1967–1978. [Google Scholar] [CrossRef]
- Wu, C.W.; Lin, S.N.; Hsu, L.M.; Yeh, S.C.; Guu, S.F.; Lee, S.H.; Chen, C.C. Synchrony Between Default-Mode and Sensorimotor Networks Facilitates Motor Function in Stroke Rehabilitation: A Pilot fMRI Study. Front. Neurosci. 2020, 14, 548. [Google Scholar] [CrossRef]
- Morya, E.; Monte-Silva, K.; Bikson, M.; Esmaeilpour, Z.; Biazoli, C.E., Jr.; Fonseca, A.; Bocci, T.; Farzan, F.; Chatterjee, R.; Hausdorff, J.M.; et al. Beyond the target area: An integrative view of tDCS-induced motor cortex modulation in patients and athletes. J. Neuroeng. Rehabil. 2019, 16, 141. [Google Scholar] [CrossRef]
- Alonzo, A.; Brassil, J.; Taylor, J.L.; Martin, D.; Loo, C.K. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul. 2012, 5, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, S.; Boessenkool, S.; Pflanz, C.P.; Basting, R.; Betts, J.F.; Jenkinson, M.; Clare, S.; Muhammed, K.; LeHeron, C.; Armstrong, R.; et al. Subthalamic nucleus shows opposite functional connectivity pattern in Huntington’s and Parkinson’s disease. Brain Commun. 2023, 5, fcad282. [Google Scholar] [CrossRef] [PubMed]
- Fabri, A.; Giezeman, G.J.; Kettner, L.; Schirra, S.; Schönherr, S. On the design of CGAL a computational geometry algorithms library. Softw. Pract. Exp. 2000, 30, 1167–1202. [Google Scholar] [CrossRef]
- Dular, P.; Geuzaine, C.; Henrotte, F.; Legros, W. A general environment for the treatment of discrete problems and its application to the finite element method. IEEE Trans. Magn. 1998, 34, 3395–3398. [Google Scholar] [CrossRef]




| Comparisons | Brain Regions/BA | Peak MNI Coordinates | Cluster Voxels | Peak t Values | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Interaction effect group × time | Temporal_Sup_R | 54 | −12 | 3 | 46 | 4.57 |
| Insula_R | 36 | −15 | 15 | 20 | 4.21 | |
| Postcentral_R | 36 | −33 | 63 | 18 | 4.09 | |
| TI S3–tDCS S3 | Temporal_Sup_L | −48 | −12 | −3 | 26 | 4.53 |
| Postcentral_L | −45 | −18 | 57 | 17 | 3.66 | |
| tDCS S3–tDCS S1 | Precentral_R | 39 | −27 | 60 | 51 | 4.32 |
| Temporal_Sup_R | 54 | −9 | 0 | 24 | 4.04 | |
| Postcentral_L | −27 | −39 | 66 | 34 | 3.86 | |
| tDCS S3–tDCS S4 | Postcentral_R | 45 | −27 | 60 | 20 | 4.74 |
| Temporal_Sup_R | 54 | −12 | 3 | 20 | 4.17 | |
| TI S3–TI S1 | Heschl_R | 48 | −21 | 6 | 204 | 5.48 |
| Postcentral_R | 30 | −33 | 63 | 158 | 5.38 | |
| Postcentral_R | −48 | −24 | 9 | 155 | 5.09 | |
| Postcentral_L | −45 | −18 | 54 | 91 | 4.63 | |
| Postcentral_R | 24 | −45 | 69 | 25 | 4.51 | |
| Postcentral_L | −27 | −39 | 60 | 92 | 4.23 | |
| TI S3–TI S2 | Temporal_Sup_L | −48 | −15 | −3 | 57 | 5.40 |
| Heschl_R | 48 | −21 | 6 | 36 | 5.05 | |
| Postcentral_R | 30 | −33 | 60 | 26 | 4.49 | |
| TI S4–TI S1 | Precentral_R | 51 | −18 | 45 | 68 | 4.73 |
| TI S3–TI S4 | Temporal_Sup_R | 60 | −33 | 9 | 20 | 4.86 |
| Heschl_L | −45 | −15 | 6 | 69 | 4.33 | |
| Postcentral_R | 33 | −33 | 63 | 24 | 4.32 | |
| Comparisons | Brain Regions/BA | Peak MNI Coordinates | Cluster Voxels | Peak t Values | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Interaction effect group × time | Heschl_L | −45 | −15 | 6 | 20 | 4.76 |
| Postcentral_R | 33 | −30 | 66 | 63 | 4.58 | |
| Heschl_R | 45 | −21 | 6 | 31 | 4.37 | |
| tDCS S1–tDCS S3 | Temporal_Sup_R | 54 | −18 | 6 | 13 | 4.59 |
| Postcentral_R | 33 | −33 | 66 | 26 | 4.46 | |
| Cingulate_Mid_R | 9 | −15 | 45 | 27 | 4.43 | |
| Heschl_L | −45 | −15 | 6 | 15 | 4.29 | |
| Precentral_R | 48 | −15 | 39 | 15 | 3.72 | |
| tDCS S4–tDCS S3 | Heschl_L | −45 | −15 | 6 | 18 | 4.92 |
| Precentral_R | 30 | −30 | 66 | 91 | 4.74 | |
| Postcentral_R | 45 | −12 | 33 | 18 | 4.60 | |
| Temporal_Sup_R | 57 | −9 | 3 | 19 | 3.84 | |
| TI S1–TI S3 | Heschl_R | 45 | −21 | 6 | 81 | 5.34 |
| Precuneus_L | −9 | −45 | 63 | 44 | 5.16 | |
| Postcentral_L | −45 | −18 | 51 | 56 | 4.92 | |
| Postcentral_R | 12 | −39 | 72 | 10 | 4.76 | |
| Heschl_R | −42 | −27 | 9 | 31 | 4.70 | |
| Temporal_Sup_L | −54 | −9 | 3 | 24 | 4.54 | |
| Postcentral_R | 27 | −35 | 60 | 16 | 4.44 | |
| Paracentral_Lobule_L | −6 | −36 | 72 | 18 | 4.37 | |
| Precentral_R | 33 | −21 | 57 | 12 | 4.17 | |
| TI S4–TI S3 | Supp_Motor_Area_R | 6 | −3 | 45 | 44 | 4.96 |
| Temporal_Sup_R | 60 | −36 | 9 | 31 | 4.90 | |
| Temporal_Sup_R | −33 | −27 | 12 | 57 | 4.54 | |
| Insula_R | 36 | −15 | 6 | 11 | 4.52 | |
| Heschl_R | 48 | −18 | 9 | 31 | 4.42 | |
| Precentral_L | −33 | −27 | 54 | 12 | 3.43 | |
| Comparisons | Brain Regions/BA | Peak MNI Coordinates | Cluster Voxels | Peak t Values | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Interaction effect group × time | Temporal_Sup_R | 51 | −12 | 0 | 10 | 3.89 |
| TI S3–TI S1 | Temporal_Sup_L | −51 | −12 | 0 | 9 | 4.70 |
| Postcentral_R | 30 | −36 | 63 | 45 | 4.37 | |
| Postcentral_R | 24 | −42 | 72 | 8 | 4.36 | |
| Heschl_R | 54 | −6 | 3 | 16 | 4.27 | |
| TI S4–TI S1 | Postcentral_R | 48 | −21 | 48 | 6 | 4.53 |
| Precentral_R | 42 | −18 | 45 | 15 | 4.06 | |
| Postcentral_L | −48 | −24 | 51 | 7 | 4.06 | |
| Precentral_L | −27 | −27 | 72 | 8 | 3.89 | |
| Postcentral_L | −33 | −45 | 66 | 7 | 3.71 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Qin, L.; Tang, D.; Qian, Z.; Zhuang, J.; Liu, Y. Comparative Effects of Temporal Interference and High-Definition Transcranial Direct Current Stimulation on Spontaneous Neuronal Activity in the Primary Motor Cortex: A Randomized Crossover Study. Brain Sci. 2025, 15, 317. https://doi.org/10.3390/brainsci15030317
Zhu Z, Qin L, Tang D, Qian Z, Zhuang J, Liu Y. Comparative Effects of Temporal Interference and High-Definition Transcranial Direct Current Stimulation on Spontaneous Neuronal Activity in the Primary Motor Cortex: A Randomized Crossover Study. Brain Sciences. 2025; 15(3):317. https://doi.org/10.3390/brainsci15030317
Chicago/Turabian StyleZhu, Zhiqiang, Lang Qin, Dongsheng Tang, Zhenyu Qian, Jie Zhuang, and Yu Liu. 2025. "Comparative Effects of Temporal Interference and High-Definition Transcranial Direct Current Stimulation on Spontaneous Neuronal Activity in the Primary Motor Cortex: A Randomized Crossover Study" Brain Sciences 15, no. 3: 317. https://doi.org/10.3390/brainsci15030317
APA StyleZhu, Z., Qin, L., Tang, D., Qian, Z., Zhuang, J., & Liu, Y. (2025). Comparative Effects of Temporal Interference and High-Definition Transcranial Direct Current Stimulation on Spontaneous Neuronal Activity in the Primary Motor Cortex: A Randomized Crossover Study. Brain Sciences, 15(3), 317. https://doi.org/10.3390/brainsci15030317






