Personal Quality of Life as an Outcome Measure of Antipsychotic Drug Management of Problem Behaviours in Adults with Intellectual Developmental Disorders with or Without Autism Spectrum Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Procedure and Assessments
2.4. Instruments
2.4.1. Wing’s Handicap, Behaviours, and Skills Schedule (HBS)
2.4.2. Dosage Record and Treatment Emergent Symptoms Scale (DOTES)
2.4.3. BASIQ (BAtteria di Strumenti per l’Indagine Della Qualità di Vita) QoL Assessment Tool
2.5. Data Elaboration and Statistical Analysis
2.6. Ethics
3. Results
3.1. Background Characteristics
3.2. Psychopathological Co-Occurrences
3.3. Compounds and Dosages
3.4. AP Discontinuation
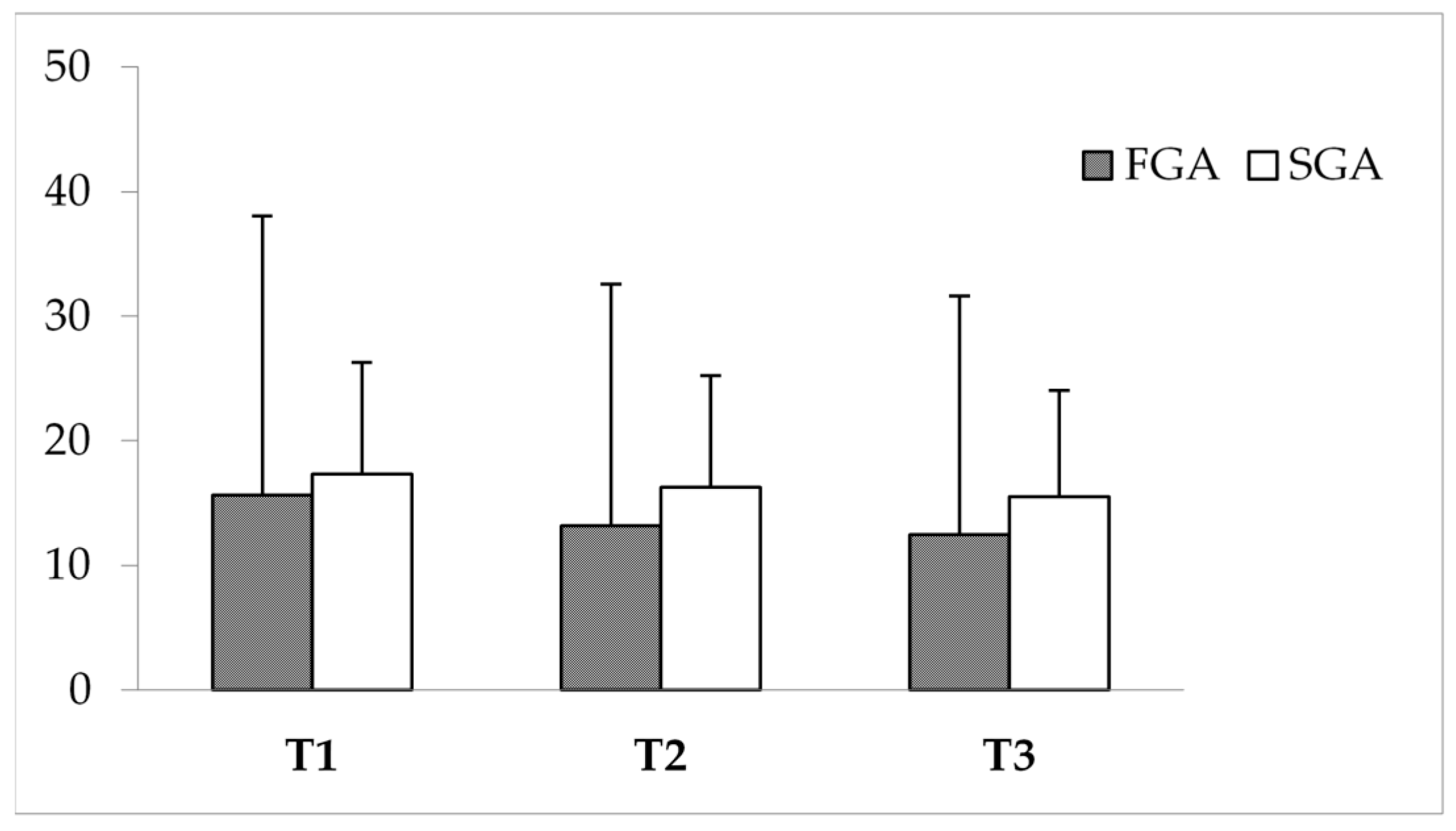
3.5. Impact of APs on PBs
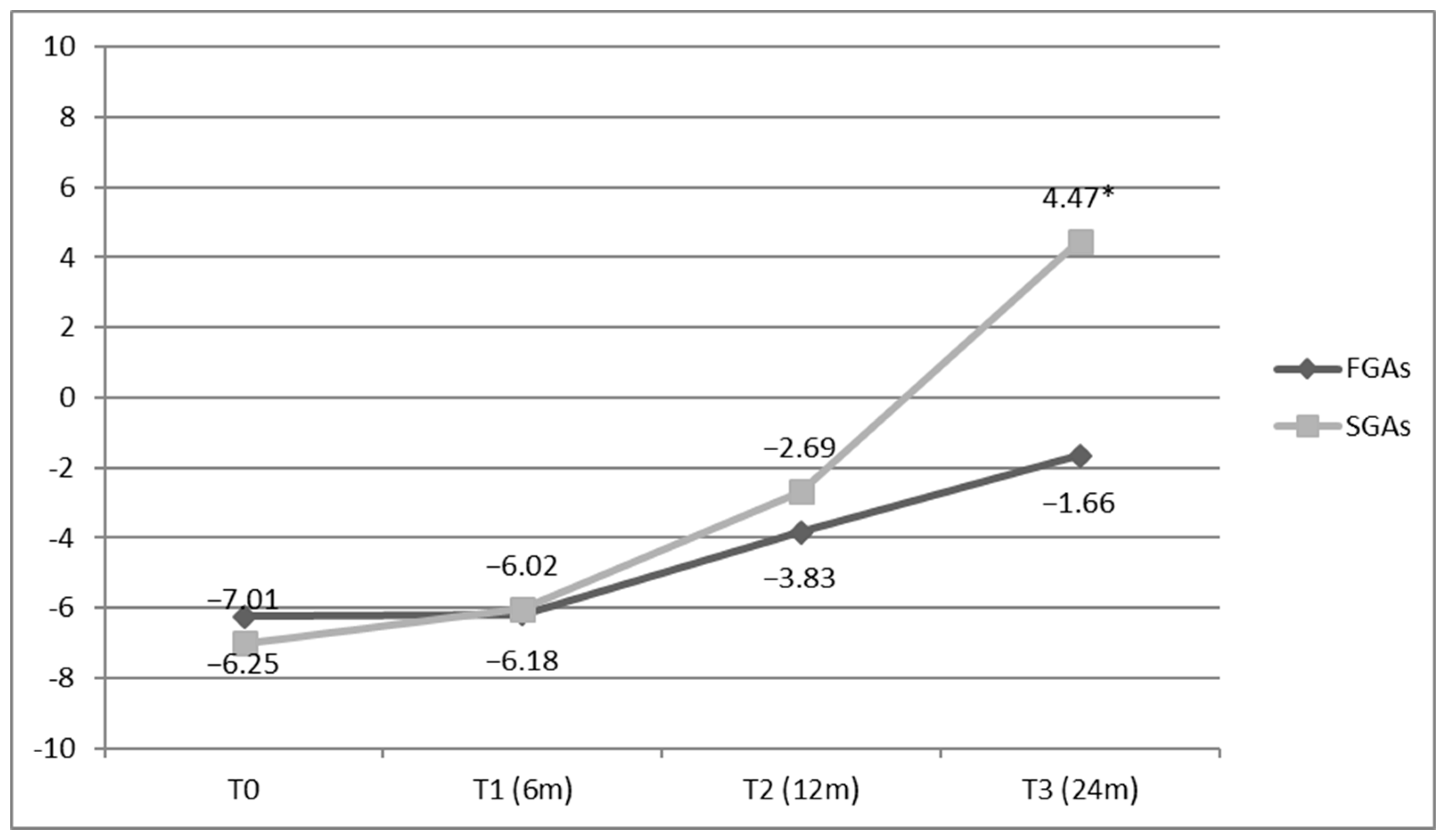
3.6. Impact of APs on QoL
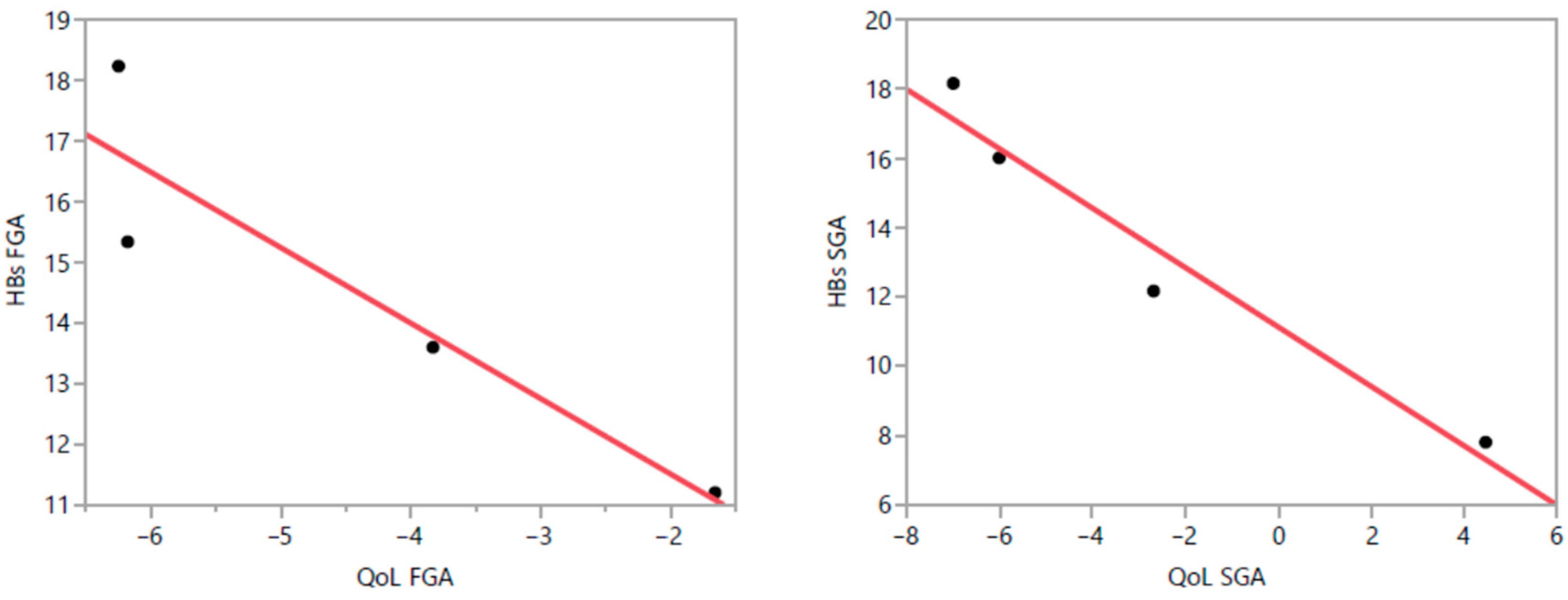
3.7. Correlation Between PBs and QoL
3.8. Treatment Interruption and Side Effects
4. Discussion
4.1. Over-Prescription and Concerns of AP Use for PBs in IDD
4.2. AP Side Effects
4.3. PBs and Psychopathology
4.4. AP Discontinuation
4.5. Impact of APs on QoL
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FGAs | First-generation antipsychotics |
| SGAs | Second-generation antipsychotics |
| PBs | Problem behaviours |
| IDDs | Intellectual developmental disorders |
| ASD | Autism spectrum disorders |
| QoL | Quality of life |
| HBS | Handicaps, behaviours, and skills schedule |
| PwIDDs | People with intellectual developmental disorders |
| PDs | Psychiatric disorders |
| BEs | Behavioural equivalents |
| APs | Antipsychotics |
| HR-QoL | Health-related quality of life |
| G-QoL | Generic quality of life |
| SPAIDD-G | Systematic psychopathological assessment for persons with intellectual and developmental disabilities |
| ECG | Electrocardiogram |
| DOTES | Dosage record and treatment of emergent symptoms |
| BASIQ | Batteria di strumenti per l’indagine della qualità di vita (QoL assessment tool) |
| DDD | Defined daily dose |
References
- Hemmings, C.; Deb, S.; Chaplin, E.; Hardy, S.; Mukherjee, R. Research for people with intellectual disabilities and mental health problems: A view from the UK. J. Ment. Health Res. Intellect. Disabil. 2013, 6, 127–158. [Google Scholar] [CrossRef]
- American Psychiatric Association. DSM-5 Task Force. In Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 11th ed.; WHO: Geneva, Switzerland, 2022; Available online: https://icd.who.int/browse11/l-m/en#http%3a%2f%2fid.who.int%2ficd%2fentity%2f605267007 (accessed on 1 February 2024).
- Royal College of Psychiatrists. Diagnostic Criteria for Psychiatric Disorders for Use with Adults with Learning Disabilities/Mental Retardation (DCLD); Gaskell Press: London, UK, 2001. [Google Scholar]
- Holden, B.; Gitlesen, J.P. A total population study of challenging behaviour in the county of Hedmark, Norway: Prevalence and risk markers. Res. Dev. Disabil. 2006, 27, 456–465. [Google Scholar] [CrossRef]
- Bertelli, M.; Hassiotis, A.; Deb, S.; Salvador-Carulla, L. New Contributions of Psychiatric Research in the Field of Intellectual Disability. In Advances in Psychiatry; Christodoulou, G.N., Jorge, M., Mezzich, J.E., Eds.; Beta Medical Publishers: Athens, Greece, 2009; Volume 3, pp. 37–43. [Google Scholar]
- Felce, D.; Kerr, M.; Hastings, R.P. A general practice-based study of the relationship between indicators of mental illness and challenging behaviour among adults with intellectual disabilities. J. Intellect. Disabil. Res. 2009, 53, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Kwok, H.; Bertelli, M.; Salvador-Carulla, L.; Bradley, E.; Torr, J.; Barnhill, J. International guide to prescribing psychotropic medication for the management of problem behaviours in adults with intellectual disabilities. World Psychiatry 2009, 8, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Shankar, R.; Wilcock, M.; Deb, S.; Goodey, R.; Corson, E.; Pretorius, C.; Praed, G.; Pell, A.; Vujkovic, D.; Wilkinson, E.; et al. A structured programme to withdraw antipsychotics among adults with intellectual disabilities: The Cornwall experience. J. Appl. Res. Intellect. Disabil. 2019, 32, 1389–1400. [Google Scholar] [CrossRef]
- Ramerman, L.; Hoekstra, P.J.; de Kuijper, G. Exploring barriers and facilitators in the implementation and use of guideline recommendations on antipsychotic drug prescriptions for people with intellectual disability. J. Appl. Res. Intellect. Disabil. 2018, 31, 1062–1070. [Google Scholar] [CrossRef]
- Bowring, D.L.; Totsika, V.; Hastings, R.P.; Toogood, S.; Griffith, G.M. Challenging behaviours in adults with an intellectual disability: A total population study and exploration of risk indices. Br. J. Clin. Psychol. 2017, 56, 16–32. [Google Scholar] [CrossRef]
- Perry, B.I.; Cooray, S.E.; Mendis, J.; Purandare, K.; Wijeratne, A.; Manjubhashini, S.; Dasari, M.; Esan, F.; Gunaratna, I.; Naseem, R.A.; et al. Problem behaviours and psychotropic medication use in intellectual disability: A multinational cross-sectional survey. J. Intellect. Disabil. Res. 2018, 62, 140–149. [Google Scholar] [CrossRef]
- Iasevoli, F.; Barone, A.; Buonaguro, E.F.; Vellucci, L.; de Bartolomeis, A. Safety and tolerability of antipsychotic agents in neurodevelopmental disorders: A systematic review. Expert Opin. Drug Saf. 2020, 19, 1419–1444. [Google Scholar] [CrossRef]
- De Leon, J.; Greenlee, B.; Barber, J.; Sabaawi, M.; Singh, N.N. Practical guidelines for the use of new generation antipsychotic drugs (except clozapine) in adult individuals with intellectual disabilities. Res. Dev. Disabil. 2009, 30, 613–669. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Tomasetti, C.; Iasevoli, F. Update on the mechanism of action of aripiprazole: Translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs 2015, 29, 773–799. [Google Scholar] [CrossRef]
- Buonaguro, E.F.; Iasevoli, F.; Marmo, F.; Eramo, A.; Latte, G.; Avagliano, C.; Tomasetti, C.; de Bartolomeis, A. Re-arrangements of gene transcripts at glutamatergic synapses after prolonged treatments with antipsychotics: A putative link with synaptic remodeling. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 76, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.D.; Vogel, A.; Becker, T.; Salize, H.-J.; Voss, E.; Werner, A.; Arnold, K.; Schützwohl, M. Proxy and self-reported Quality of Life in adults with intellectual disabilities: Impact of psychiatric symptoms, problem behaviour, psychotropic medication and unmet needs. Res. Dev. Disabil. 2015, 45–46, 136–146. [Google Scholar] [CrossRef]
- Rand, S.; Malley, J. The factors associated with care-related quality of life of adults with intellectual disabilities in England: Implications for policy and practice. Health Soc. Care Community 2017, 25, 1607–1619. [Google Scholar] [CrossRef]
- Brown, I.; Brown, R.I.; Edwards, M.; Bertelli, M.O.; Schalock, R.L. Quality of Life as an Outcome Measure. In Textbook of Psychiatry for Intellectual Disability and Autism Spectrum Disorder; Bertelli, M.O., Deb, S., Munir, K., Hassiotis, A., Salvador-Carulla, L., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Brown, I.; Brown, R.I. Quality of Life and Disability: An Approach for Community Practitioners; Jessica Kingsley Publishers: London, UK, 2003. [Google Scholar]
- Ramerman, L.; Hoekstra, P.J.; de Kuijper, G. Changes in Health-Related Quality of Life in People with Intellectual Disabilities Who Discontinue Long-Term Used Antipsychotic Drugs for Challenging Behaviors. J. Clin. Pharmacol. 2019, 59, 280–287. [Google Scholar] [CrossRef]
- Fletcher, R.; Loschen, E.; Stavrakaki, C.; First, M. (Eds.) . Diagnostic Manual-Intellectual Disability: A Clinical Guide for Diagnosis of Mental Disorders in Persons with Intellectual Disability; NADD Press: Kingston, NY, USA, 2007. [Google Scholar]
- Bertelli, M.; Scuticchio, D.; Ferrandi, A.; Lassi, S.; Mango, F.; Ciavatta, C.; Porcelli, C.; Bianco, A.; Monchieri, S. Reliability and validity of the SPAID-G checklist for detecting psychiatric disorders in adults with intellectual disability. Res. Dev. Disabil. 2012, 33, 382–390. [Google Scholar] [CrossRef]
- Bertelli, M.O.; Bianco, A.; Piva Merli, M.; Scuticchio, D.; Lassi, S.; Lorenzoni, L.; Carbone Viviani, D.; Brown, I. Psychometric Properties of the Italian Adaptation of a Quality of Life Instrument as Applied to Adults With Intellectual and Developmental Disabilities. J. Policy Pract. Intellect. Disabil. 2016, 13, 227–235. [Google Scholar] [CrossRef]
- Wing, L.; Gould, J. Systematic recording of behaviors and skills of retarded and psychotic children. J. Autism. Child. Schizophr. 1978, 8, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Guy, W. Dosage Record and Treatment Emergent Symptoms Scale. In ECDEU Assessment Manual for Psychopharmacology (Revised), US Department of Health, Education and Human Welfare Publication (ADM) 76-338; US Department of Health, Education, and Welfare: Rockville, MD, USA, 1976; pp. 223–244. [Google Scholar]
- Brown, I.; Renwick, R.; Raphael, D. Quality of Life Instrument Package for Adults with Developmental Disabilities; Centre for Health Promotion, University of Toronto: Toronto, ON, Canada, 1997. [Google Scholar]
- Leucht, S.; Chaimani, A.; Cipriani, A.S.; Davis, J.M.; Furukawa, T.A.; Salanti, G. Network meta-analyses should be the highest level of evidence in treatment guidelines. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, R.; Hassiotis, A.; Walters, K.; Osborn, D.; Strydom, A.; Horsfall, L. Mental illness, challenging behaviour, and psychotropic drug prescribing in people with intellectual disability: UK population-based cohort study. BMJ 2015, 351, h4326. [Google Scholar] [CrossRef]
- Deb, S.; Nancarrow, T.; Limbu, B.; Sheehan, R.; Wilcock, M.; Branford, D.; Courtenay, K.; Perera, B.; Shankar, R. UK psychiatrists’ experience of withdrawal of antipsychotics prescribed for challenging behaviours in adults with intellectual disabilities and/or autism. BJPsych Open 2020, 17, e112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Branford, D.; Gerrard, D.; Saleem, N.; Shaw, C.; Webster, A. Stopping over-medication of people with intellectual disability, autism or both (STOMP) in England part 1—History and background of STOMP. Adv. Ment. Health Intellect. Disabil. 2019, 13, 31–40. [Google Scholar] [CrossRef]
- Fung, L.K.; Mahajan, R.; Nozzolillo, A.; Bernal, P.; Krasner, A.; Jo, B.; Coury, D.; Whitaker, A.; Veenstra-Vanderweele, J.; Hardan, A.Y. Pharmacologic Treatment of Severe Irritability and Problem Behaviors in Autism: A Systematic Review and Meta-analysis. Pediatrics 2016, 137 (Suppl. 2), S124–S135. [Google Scholar] [CrossRef] [PubMed]
- Amore, M.; Bertelli, M.; Villani, D.; Tamborini, S.; Rossi, M. Olanzapine vs. risperidone in treating aggressive behaviours in adults with intellectual disability: A single blind study. J. Intellect. Disabil. Res. 2011, 55, 210–218. [Google Scholar] [CrossRef]
- Park, S.Y.; Cervesi, C.; Galling, B.; Molteni, S.; Walyzada, F.; Ameis, S.H.; Gerhard, T.; Olfson, M.; Correll, C.U. Antipsychotic Use Trends in Youth with Autism Spectrum Disorder and/or Intellectual Disability: A Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 456–468.e4. [Google Scholar] [CrossRef] [PubMed]
- Rothärmel, M.; Szymoniak, F.; Pollet, C.; Beherec, L.; Quesada, P.; Leclerc, S.; Belhaine, A.; Rosier, A.; Guillin, O. Eleven Years of Clozapine Experience in Autism Spectrum Disorder: Efficacy and Tolerance. J. Clin. Psychopharmacol. 2018, 38, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Unwin, G.; Deb, T. Characteristics and the trajectory of psychotropic medication use in general and antipsychotics in particular among adults with an intellectual disability who exhibit aggressive behaviour. J. Intellect. Disabil. Res. 2015, 59, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Pigato, G.; Kane, J.M.; Correll, C.U. Clinical risk factors for the development of tardive dyskinesia. J. Neurol. Sci. 2018, 389, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.O. Psychotropic medication for problem behaviours in intellectual disability and autism spectrum disorder: The need for caution. BJPsych Adv. 2023, 29, 334–336. [Google Scholar] [CrossRef]
- Branford, D. Factors associated with successful or unsuccessful withdrawal of antipsychotic drug therapy prescribed for people with learning disabilities. J. Intellect. Disabil. Res. 1996, 40, 322–329. [Google Scholar] [CrossRef]
- Ahmed, Z.; Fraser, W.; Kerr, M.P.; Kiernan, C.; Emerson, E.; Robertson, J.; Felce, D.; Allen, D.; Baxter, H.; Thomas, J. Reducing antipsychotic medication in people with a learning disability. Br. J. Psychiatry 2000, 176, 42–46. [Google Scholar] [CrossRef]
- de Kuijper, G.M.; Hoekstra, P.J. An open-label discontinuation trial of long-term, off-label antipsychotic medication in people with intellectual disability: Determinants of success and failure. J. Clin. Pharmacol. 2018, 58, 1418–1426. [Google Scholar] [CrossRef]
- de Kuijper, G.; Evenhuis, H.; Mindera, R.B.; Hoekstra, P.J. Effects of controlled discontinuation of long-term used antipsychotics for behavioural symptoms in individuals with intellectual disability. J. Intellect. Disabil. Res. 2014, 58, 71–83. [Google Scholar] [CrossRef]
- Scheifes, A.; Walraven, S.; Stolker, J.J.; Nijman, H.L.; Egberts, T.C.; Heerdink, E.R. Adverse events and the relation with quality of life in adults with intellectual disability and challenging behaviour using psychotropic drugs. Res. Dev. Disabil. 2016, 49–50, 13–21. [Google Scholar] [CrossRef]


| Diagnosis | Baseline (T0) N (%) | FGA Group (n = 22) N (%) | SGA Group (n = 23) N (%) |
|---|---|---|---|
| Only IDD | 2 (4.5) | 1 (4.6) | 1 (4.4) |
| Schizophrenia | 9 (20.0) | 4 (18.1) | 5 (21.7) |
| ICD | 10 (22.2) | 5 (22.7) | 5 (21.7) |
| OCD | 2 (4.5) | 1 (4.6) | 1 (4.4) |
| BD I | 9 (20.0) | 4 (18.1) | 5 (21.7) |
| Schizotypical PD | 1 (2.2) | 1 (4.6) | - |
| Narcissistic PD | 1 (2.2) | - | 1 (4.4) |
| Antisocial PD | 1 (2.2) | 1 (4.6) | - |
| Comorbidity | 10 (22.2) | 5 (22.7) | 5 (21.7) |
| Schizophrenia + SRD | 3 (30.0) | 2 (40.0) | 1 (20.0) |
| BD I + SRD | 1 (10.0) | - | 1 (20.0) |
| BD I + Cluster A PD | 2 (20.0) | 2 (40.0) | - |
| BD I + Cluster B PD | 3 (30.0) | 1 (20.0) | 2 (40.0) |
| BD I + Cluster C PD | 1 (10.0) | - | 1 (20.0) |
| Compound | N (%) | Mean Dosage mg/day | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| FGAs | ||||
| Haloperidol | 11 (24.4) | 44.5 | 38.2 | 37.3 |
| Chlorpromazine | 4 (8.9) | 200.0 | 162.5 | 137.5 |
| Levomepromazine | 3 (6.7) | 91.7 | 91.7 | 83.3 |
| Promazine | 2 (4.4) | 210.0 | 195.0 | 180.0 |
| Perphenazine | 2 (4.4) | 16.0 | 8.0 | 7.0 |
| SGAs | ||||
| Risperidone | 2 (4.4) | 6.5 | 5.5 | 5.0 |
| Clozapine | 10 (22.2) | 235.0 | 220.5 | 212.5 |
| Olanzapine | 6 (13.3) | 20.0 | 19.17 | 19.17 |
| Quetiapine | 5 (11.1) | 1140.0 | 1100.0 | 1030.0 |
| FGAs (n = 22) | SGAs (n = 23) | |
|---|---|---|
| Drop-outs for adverse events | N (%) | N (%) |
| Extrapyramidal side effects | 5 (22.7) | - |
| Electrocardiogram abnomalities | 3 (13.6) | - |
| Abnormal laboratory test | 2 (9) | 1 (4.3) |
| Weight gain | - | 1 (4.3) |
| Side effects | N (%) | N (%) |
| Extrapyramidal side effects | 6 (27.7) | 1 (4.3) |
| Hyperglicemia | 1 (4.5) | 3 (13) |
| Abnormalities lipid profile | 1 (4.5) | 5 (21.7) |
| Hyperprolactinemia | 7 (31.8) | - |
| Weight gain | 4 (18.1) | 5 (21.7) |
| Sedation | 4 (18.1) | 3 (13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertelli, M.O.; Bianco, A.; Piva Merli, M.; Vescio, M.S.; Rossi, M.; Buonaguro, E.F. Personal Quality of Life as an Outcome Measure of Antipsychotic Drug Management of Problem Behaviours in Adults with Intellectual Developmental Disorders with or Without Autism Spectrum Disorder. Brain Sci. 2025, 15, 316. https://doi.org/10.3390/brainsci15030316
Bertelli MO, Bianco A, Piva Merli M, Vescio MS, Rossi M, Buonaguro EF. Personal Quality of Life as an Outcome Measure of Antipsychotic Drug Management of Problem Behaviours in Adults with Intellectual Developmental Disorders with or Without Autism Spectrum Disorder. Brain Sciences. 2025; 15(3):316. https://doi.org/10.3390/brainsci15030316
Chicago/Turabian StyleBertelli, Marco O., Annamaria Bianco, Micaela Piva Merli, Maria Stella Vescio, Michele Rossi, and Elisabetta F. Buonaguro. 2025. "Personal Quality of Life as an Outcome Measure of Antipsychotic Drug Management of Problem Behaviours in Adults with Intellectual Developmental Disorders with or Without Autism Spectrum Disorder" Brain Sciences 15, no. 3: 316. https://doi.org/10.3390/brainsci15030316
APA StyleBertelli, M. O., Bianco, A., Piva Merli, M., Vescio, M. S., Rossi, M., & Buonaguro, E. F. (2025). Personal Quality of Life as an Outcome Measure of Antipsychotic Drug Management of Problem Behaviours in Adults with Intellectual Developmental Disorders with or Without Autism Spectrum Disorder. Brain Sciences, 15(3), 316. https://doi.org/10.3390/brainsci15030316






