QEVO®-Assisted Anatomical Inspection of Adjacent Perforators in Microsurgical Clipping—Technical Note
Abstract
1. Introduction
2. Materials and Methods
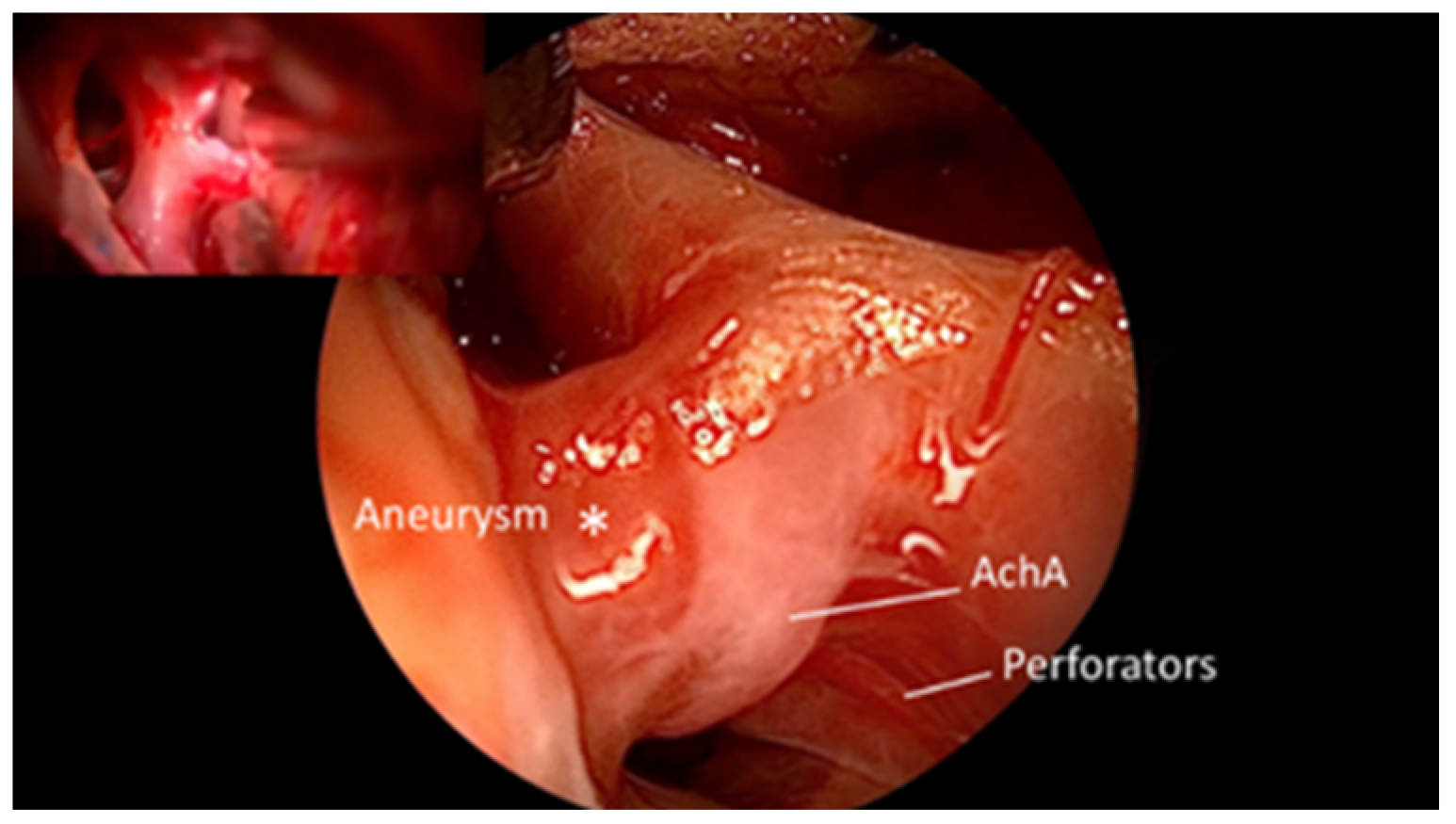
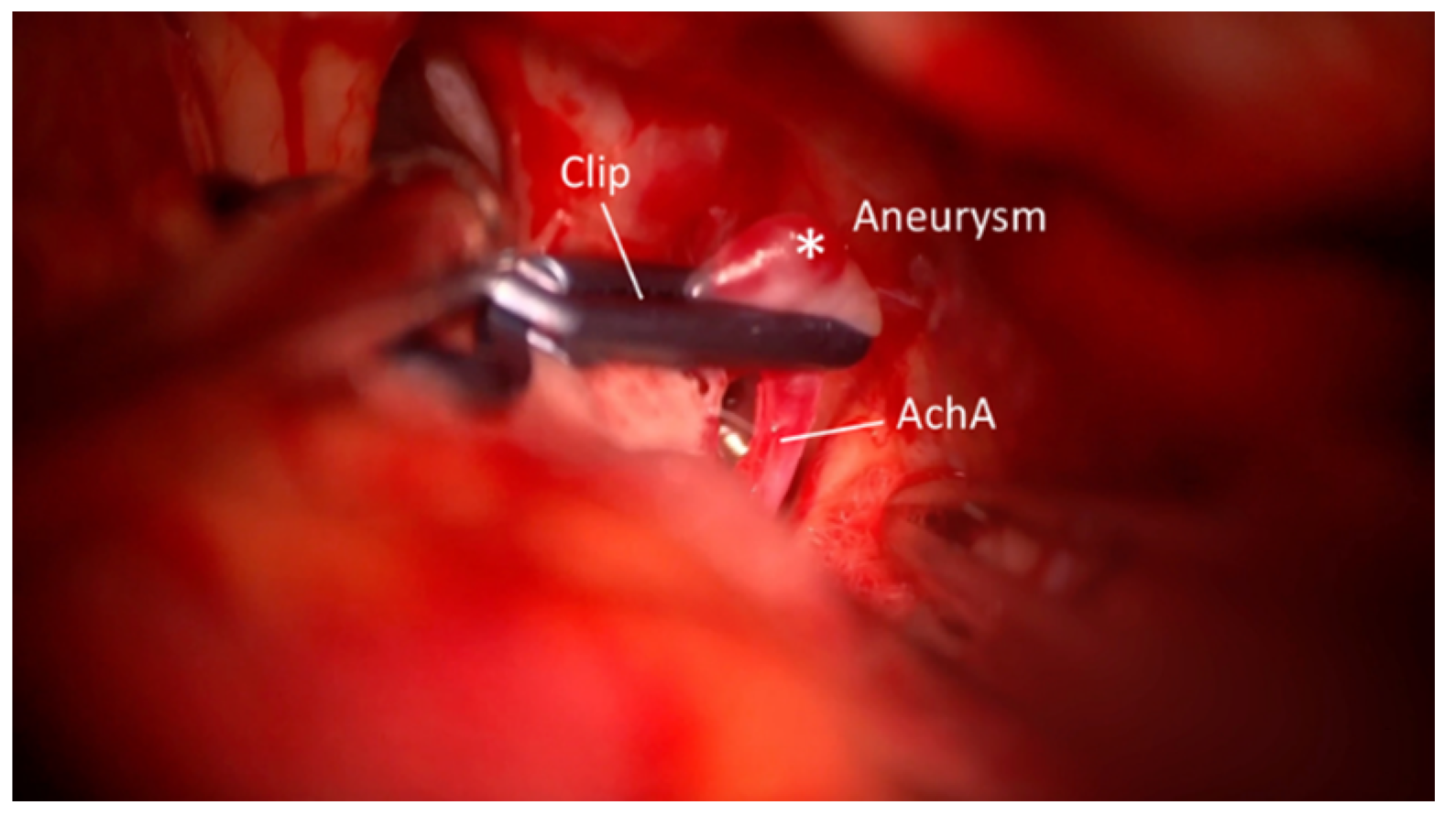
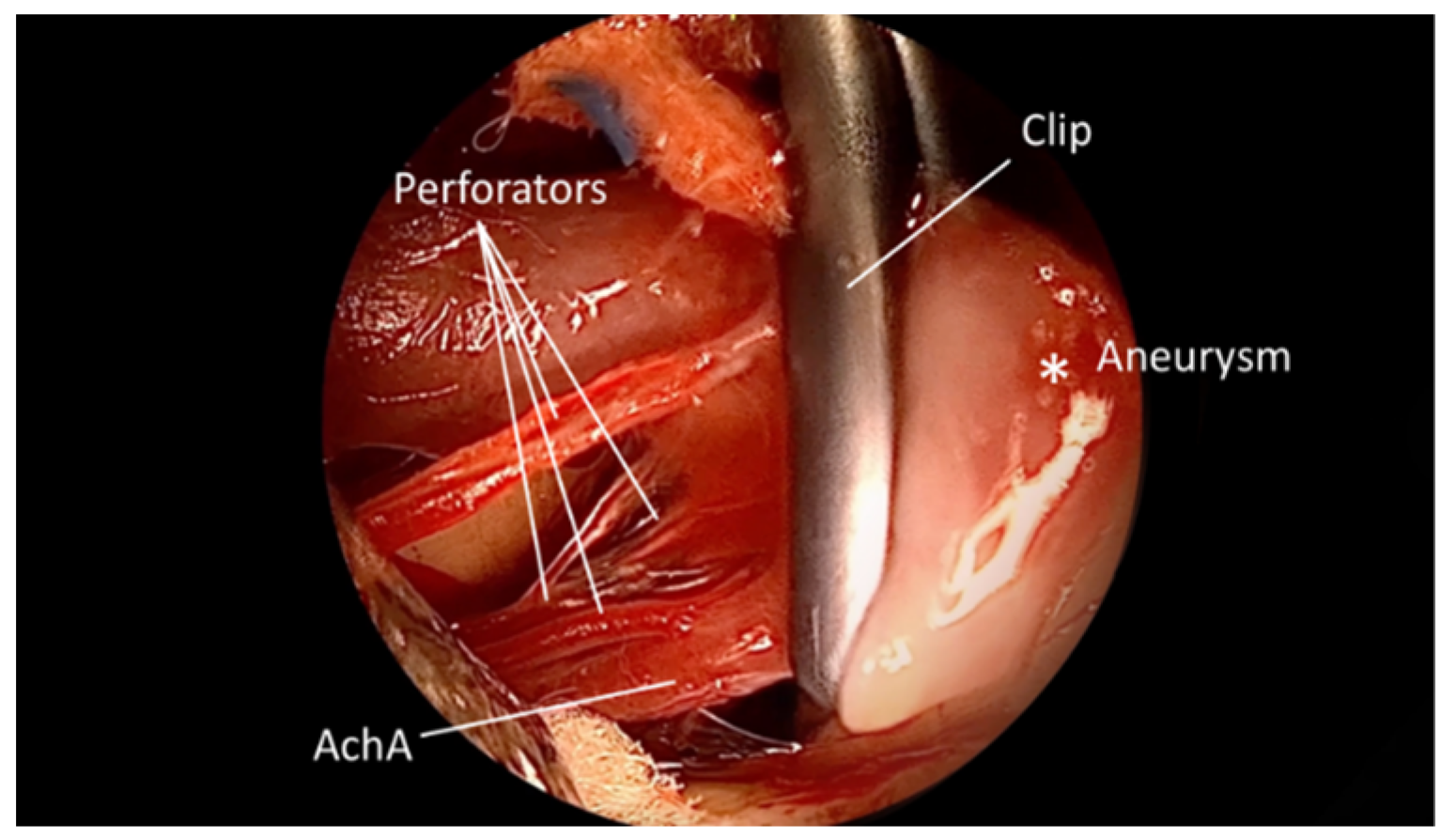
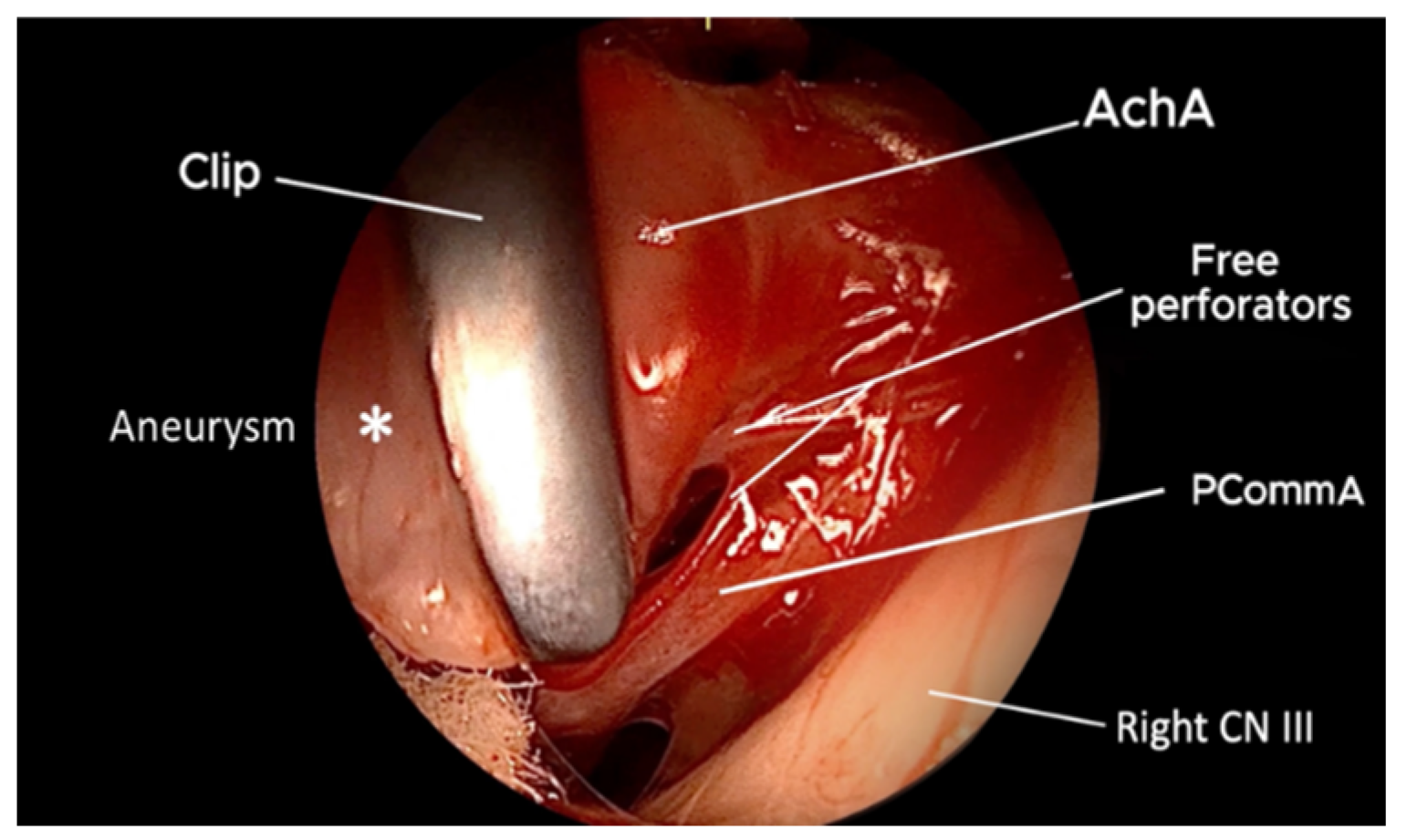
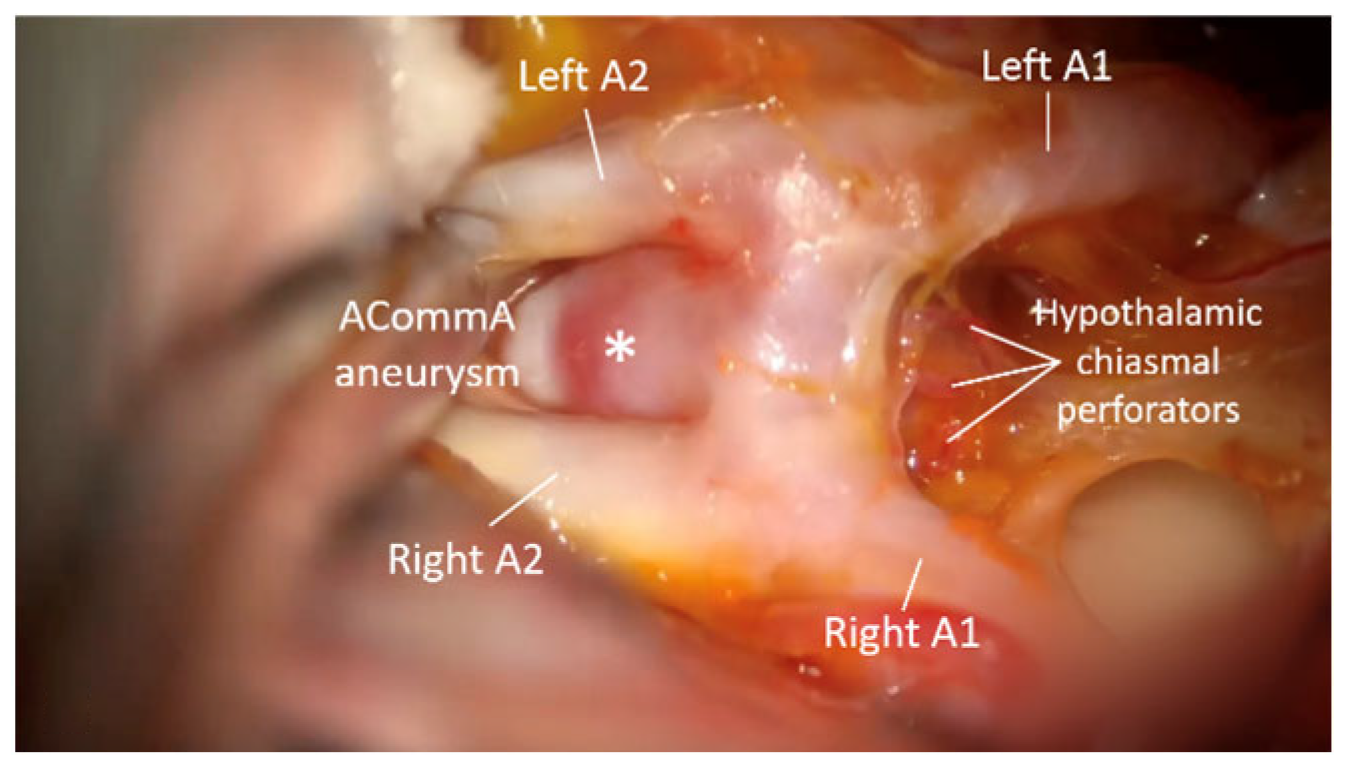
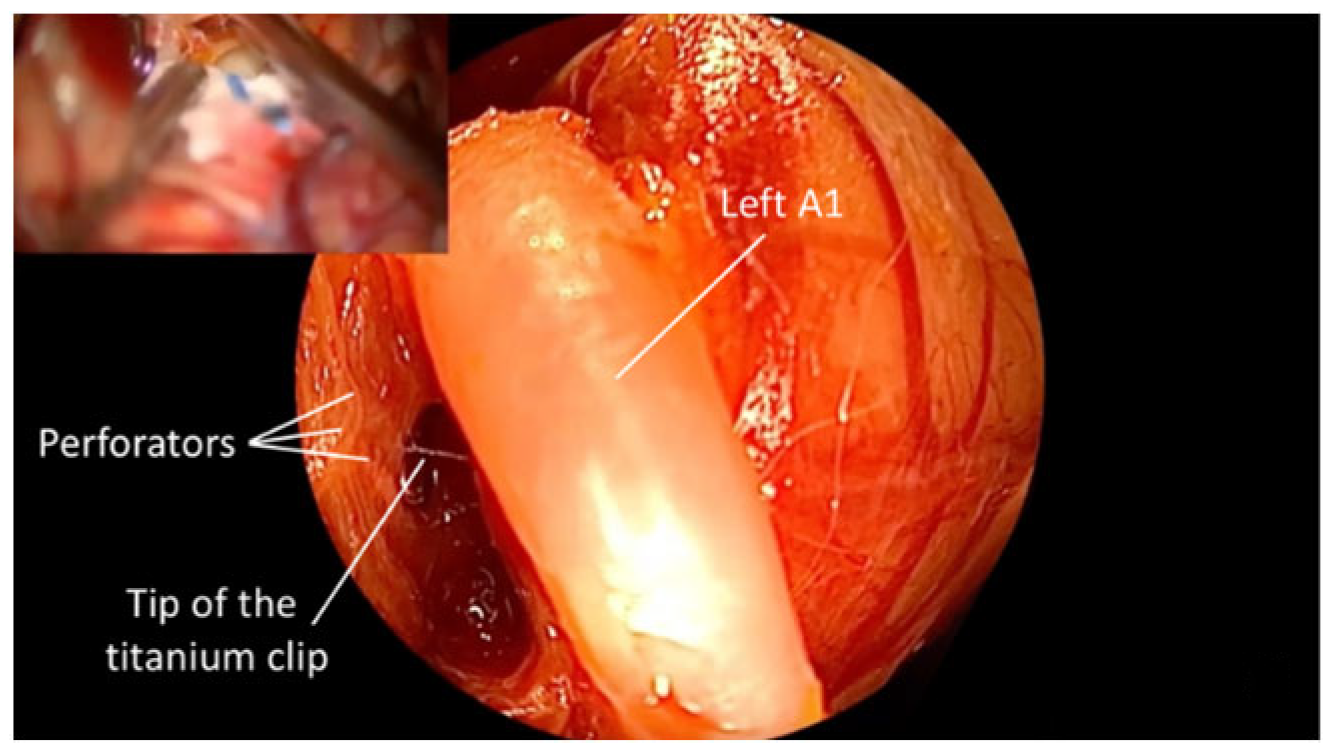
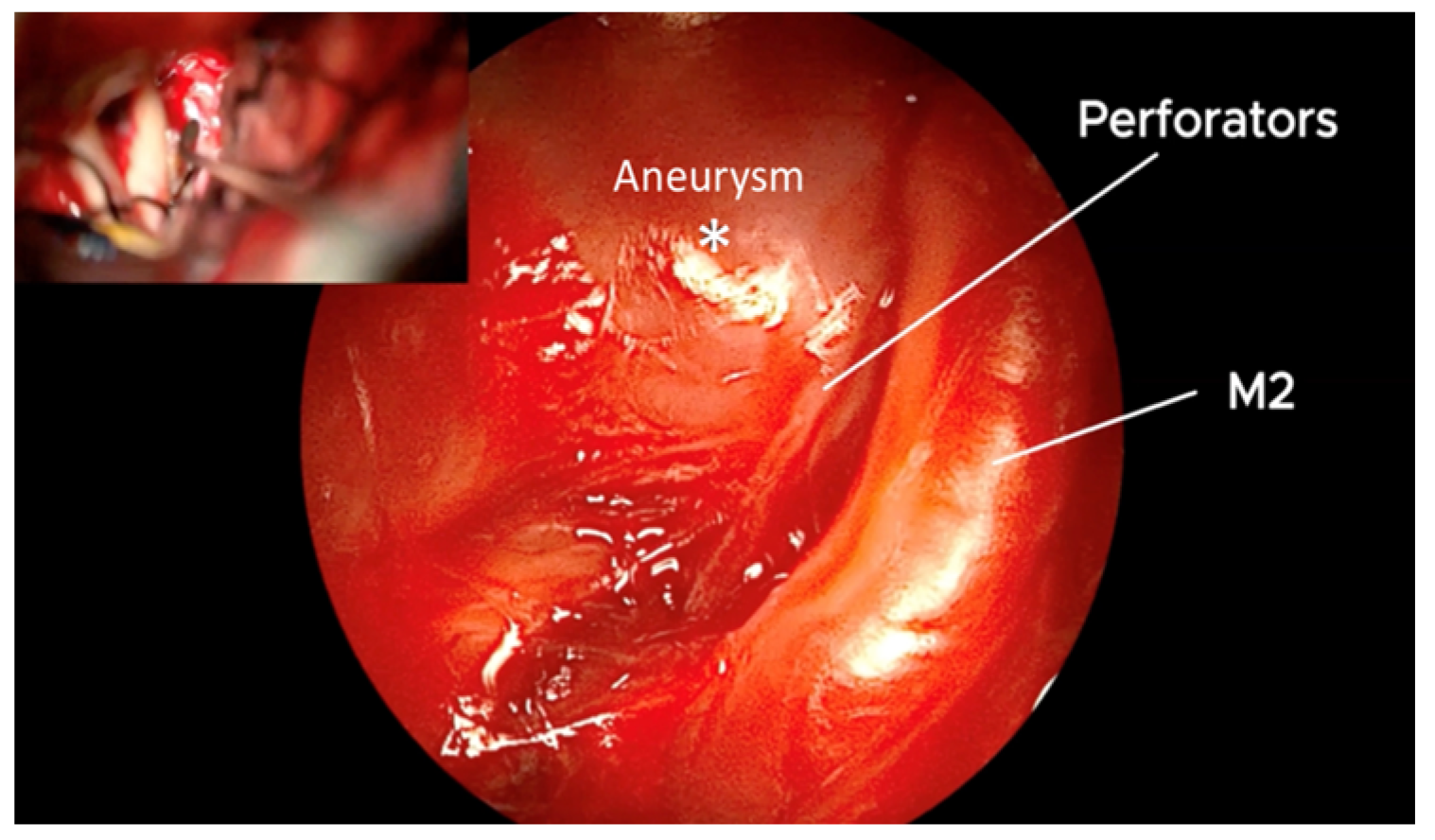
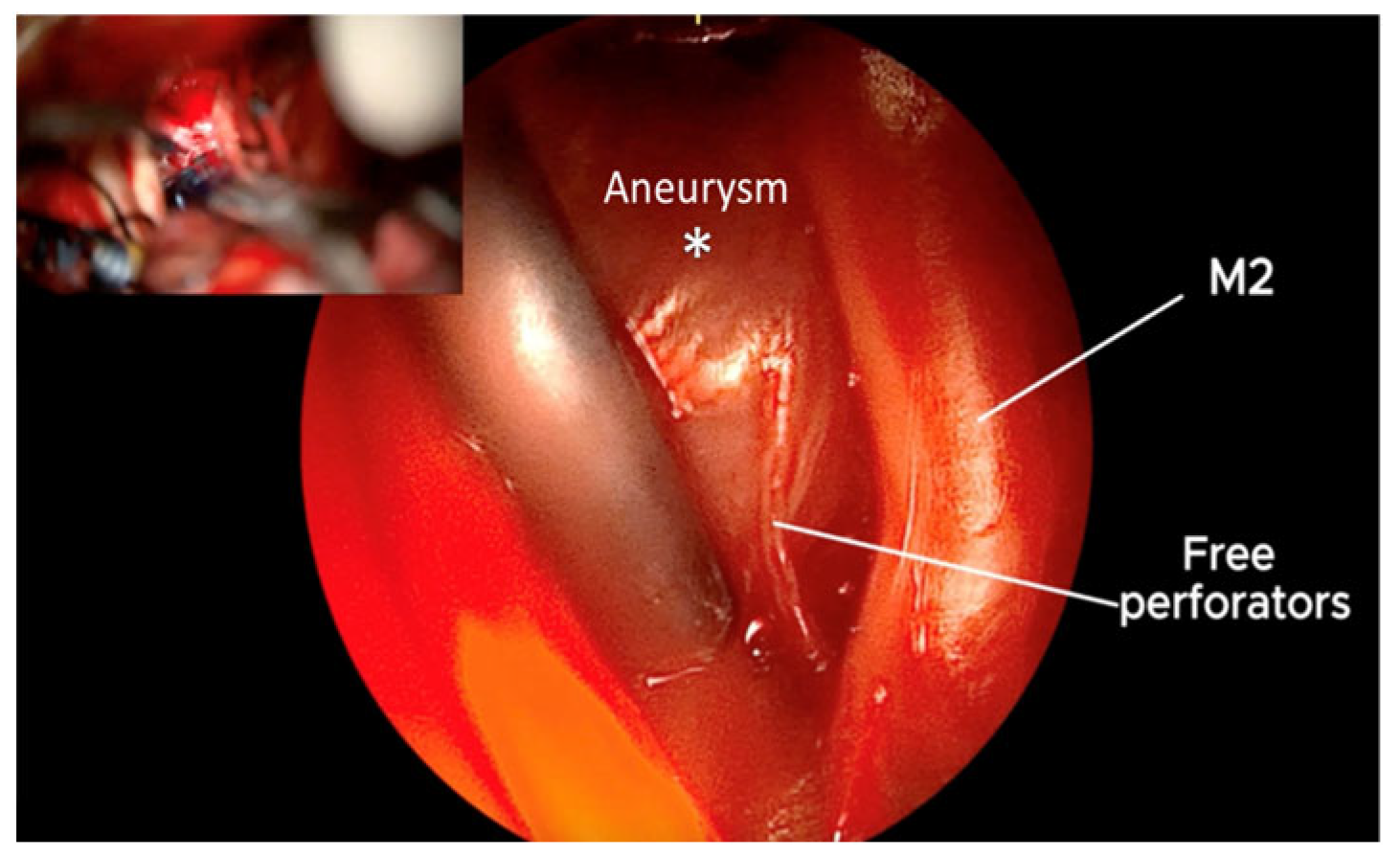
3. Results
4. Discussion
Limitations vs. Advantages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AchA | Anterior choroidal artery |
| ACommA | Anterior communicating artery |
| ICA | Internal carotid artery |
| MCA | Middle cerebral artery |
| PCommA | Posterior communicating artery |
References
- Ansari, A.; Kalyan, S.; Sae-Ngow, T.; Yamada, Y.; Tanaka, R.; Kawase, T.; Kato, Y. Review of Avoidance of Complications in Cerebral Aneurysm Surgery: The Fujita Experience. Asian J. Neurosurg. 2019, 14, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Schebesch, K.M.; Doenitz, C.; Haj, A.; Höhne, J.; Schmidt, N.O. Application of the Endoscopic Micro-Inspection Tool QEVO® in the Surgical Treatment of Anterior Circulation Aneurysms-A Technical Note and Case Series. Front. Surg. 2020, 7, 602080. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Li, Y.; Zhang, B.; Wu, J.; Xie, S.; Zheng, X.; Jiang, Z. Endoscope-assisted microneurosurgery for intracranial aneurysms: A systematic review and meta-analysis. J. Clin. Neurosci. 2022, 103, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Gallieni, M.; Del Maestro, M.; Luzzi, S.; Trovarelli, D.; Ricci, A.; Galzio, R. Endoscope-Assisted Microneurosurgery for Intracranial Aneurysms: Operative Technique, Reliability, and Feasibility Based on 14 Years of Personal Experience. Acta Neurochir. Suppl. 2018, 129, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, S.B.; Hendricks, B.K.; Cohen-Gadol, A. Single-Surgeon in Vivo Experience with the Zeiss QEVO Microinspection Tool: An Analysis of Its Use for Extending the Reach of Operative Visualization. World Neurosurg. 2021, 147, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Schebesch, K.M.; Brawanski, A.; Tamm, E.R.; Kühnel, T.S.; Höhne, J. QEVO®—A new digital endoscopic microinspection tool—A cadaveric study and first clinical experiences (case series). Surg. Neurol. Int. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Preul, M.; Belykh, E.; George, L.; Zhao, X.; Carotenuto, A.; Moreira, L.; Yağmurlu, K.; Bozkurt, B.; Byvaltsev, V.; Nakaji, P. Microvascular anastomosis under 3D exoscope or endoscope magnification: A proof-of-concept study. Surg. Neurol. Int. 2018, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Peris-Celda, M.; Da Roz, L.; Monroy-Sosa, A.; Morishita, T.; Rhoton, A.L. Surgical anatomy of endoscope-assisted approaches to common aneurysm sites. Neurosurgery 2014, 10 (Suppl. S1), 121–144; discussion 144. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sano, H.; Nagahisa, S.; Iwata, S.; Yoshida, K.; Yamamoto, K.; Kanno, T. Endoscope-assisted microsurgery for cerebral aneurysms. Minim. Invasive Neurosurg. 2000, 43, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ebner, F.H.; Marquardt, J.S.; Hirt, B.; Tatagiba, M.; Schuhmann, M.U. Visualization of the anterior cerebral artery complex with a continuously variable-view rigid endoscope: New options in aneurysm surgery. Neurosurgery 2010, 67 (Suppl. S2), 321–324. [Google Scholar] [CrossRef] [PubMed]
- Bohnstedt, B.N.; Kemp, W.J.; Li, Y.; Payner, T.D.; Horner, T.G.; Leipzig, T.J.; Cohen-Gadol, A.A. Surgical treatment of 127 anterior choroidal artery aneurysms: A cohort study of resultant ischemic complications. Neurosurgery 2013, 73, 933–939; discussion 939–940. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Lu, A.; Burkhardt, J.K.; Rutledge, W.C.; Yue, J.K.; Birk, H.S.; Alotaibi, N.; Choudhri, O.; Lawton, M.T. Microsurgical Clipping of Anterior Choroidal Artery Aneurysms: A Systematic Approach to Reducing Ischemic Complications in an Experience with 146 Patients. Oper. Neurosurg. 2019, 17, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Andaluz, N.; Van Loveren, H.R.; Keller, J.T.; Zuccarello, M. Anatomic and clinical study of the orbitopterional approach to anterior communicating artery aneurysms. Neurosurgery 2003, 52, 1140–1148; discussion 1148–1149. [Google Scholar] [PubMed]
- Joo, S.P.; Kim, T.S. The Clinical Importance of Perforator Preservation in Intracranial Aneurysm Surgery: An Overview with a Review of the Literature. Chonnam Med. J. 2017, 53, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Acik, V.; Cavus, G.; Bilgin, E.; Arslan, A.; Gezercan, Y.; Okten, A.I. Surgical Treatment of Mirror Middle Cerebral Artery Aneurysms: Bilateral and Unilateral Approach. World Neurosurg. 2017, 108, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.; Gabarrós, A.; Lawton, M.T. Contralateral clipping of middle cerebral artery aneurysms: Rationale, indications, and surgical technique. Neurosurgery 2012, 71 (Suppl. S1), 116–123; discussion 123–124. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, A.A.; Filho, M.A.D.; Faglioni, W.; Carvalho, G.T.C. Unilateral pterional approach to bilateral aneurysms of the middle cerebral artery. Surg. Neurol. 2005, 63 (Suppl. S1), S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Ahmetspahić, A.; Janković, D.; Burazerovic, E.; Rovčanin, B.; Šahbaz, A.; Hasanagić, E.; Džurlić, A.; Granov, N.; Feletti, A. Clinical Characteristics of Poor-Grade Aneurysmal Subarachnoid Hemorrhage Treatment. Asian J. Neurosurg. 2023, 18, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Feletti, A.; Marton, E.; Fiorindi, A.; Longatti, P. Neuroendoscopic aspiration of tumors in the posterior third ventricle and aqueduct lumen: A technical update. Acta Neurochir. 2013, 155, 1467–1473. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmetspahic, A.; Burazerovic, E.; Rizvanovic, H.; Selimovic, E.; Kujaca, E.; Pojskic, M.; Feletti, A.; Arnautovic, K. QEVO®-Assisted Anatomical Inspection of Adjacent Perforators in Microsurgical Clipping—Technical Note. Brain Sci. 2025, 15, 300. https://doi.org/10.3390/brainsci15030300
Ahmetspahic A, Burazerovic E, Rizvanovic H, Selimovic E, Kujaca E, Pojskic M, Feletti A, Arnautovic K. QEVO®-Assisted Anatomical Inspection of Adjacent Perforators in Microsurgical Clipping—Technical Note. Brain Sciences. 2025; 15(3):300. https://doi.org/10.3390/brainsci15030300
Chicago/Turabian StyleAhmetspahic, Adi, Eldin Burazerovic, Hana Rizvanovic, Ema Selimovic, Eleonora Kujaca, Mirza Pojskic, Alberto Feletti, and Kenan Arnautovic. 2025. "QEVO®-Assisted Anatomical Inspection of Adjacent Perforators in Microsurgical Clipping—Technical Note" Brain Sciences 15, no. 3: 300. https://doi.org/10.3390/brainsci15030300
APA StyleAhmetspahic, A., Burazerovic, E., Rizvanovic, H., Selimovic, E., Kujaca, E., Pojskic, M., Feletti, A., & Arnautovic, K. (2025). QEVO®-Assisted Anatomical Inspection of Adjacent Perforators in Microsurgical Clipping—Technical Note. Brain Sciences, 15(3), 300. https://doi.org/10.3390/brainsci15030300







