Transcranial Direct Current Stimulation Enhances Motor Performance by Modulating Beta-Phase Synchronization in the Sensorimotor Network: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Participants
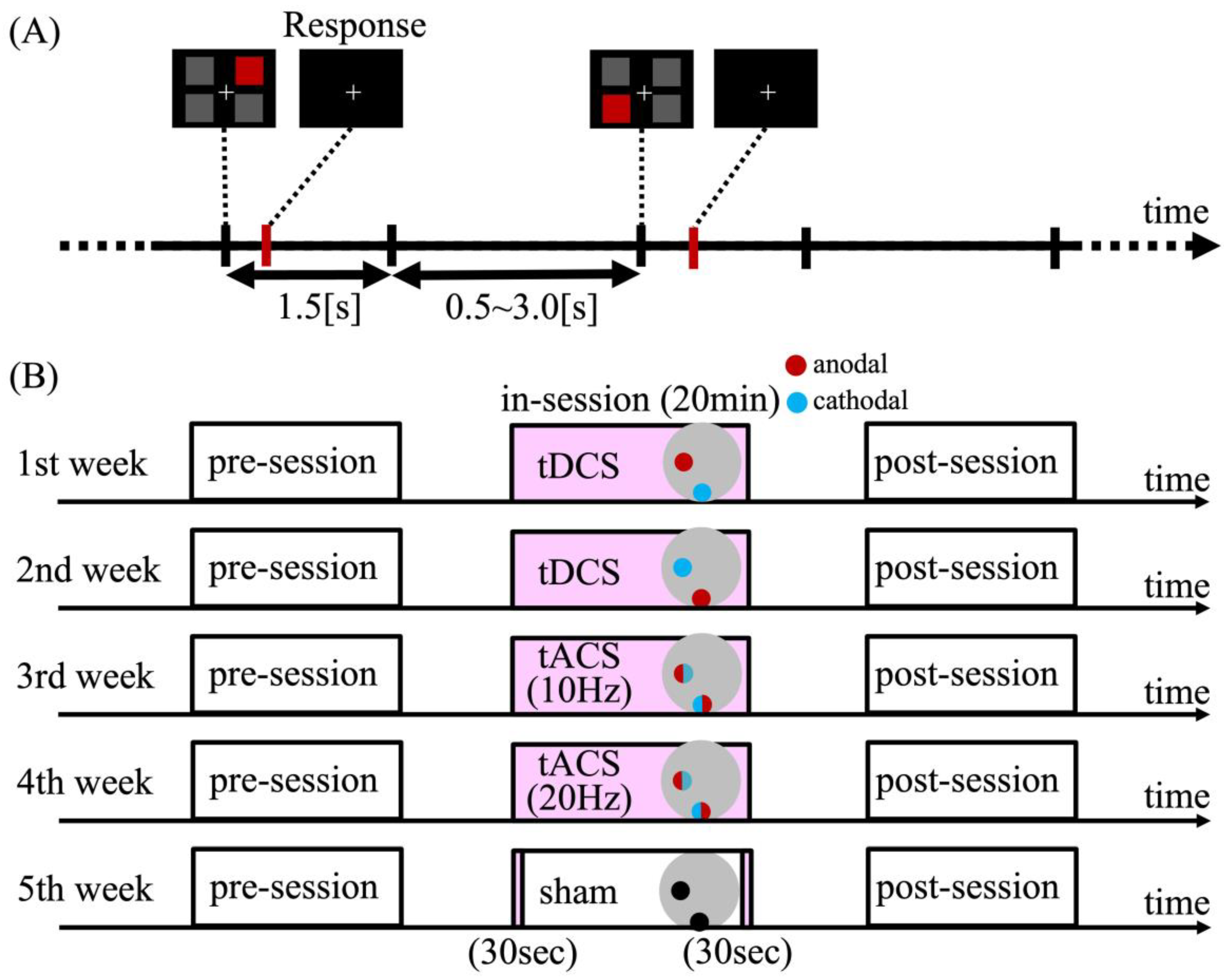
2.1.2. Task Procedure
2.1.3. Experimental Procedure
2.1.4. Transcranial Electrical Stimulation
2.1.5. Electroencephalography Recording
2.2. Analyses
2.2.1. Behavioral Analysis
2.2.2. Amplitude Analysis
2.2.3. Phase Synchronization Analysis
3. Results
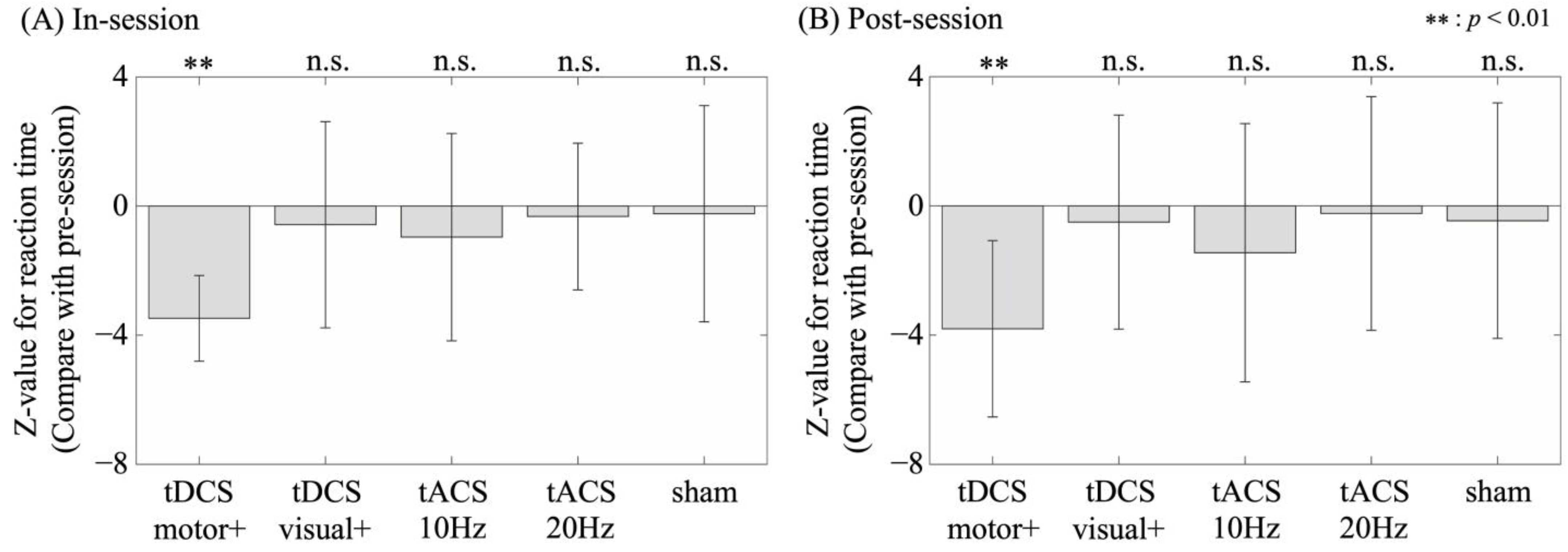
3.1. Motor Performance
3.2. Oscillatory Activity
3.2.1. Alpha and Beta Amplitudes
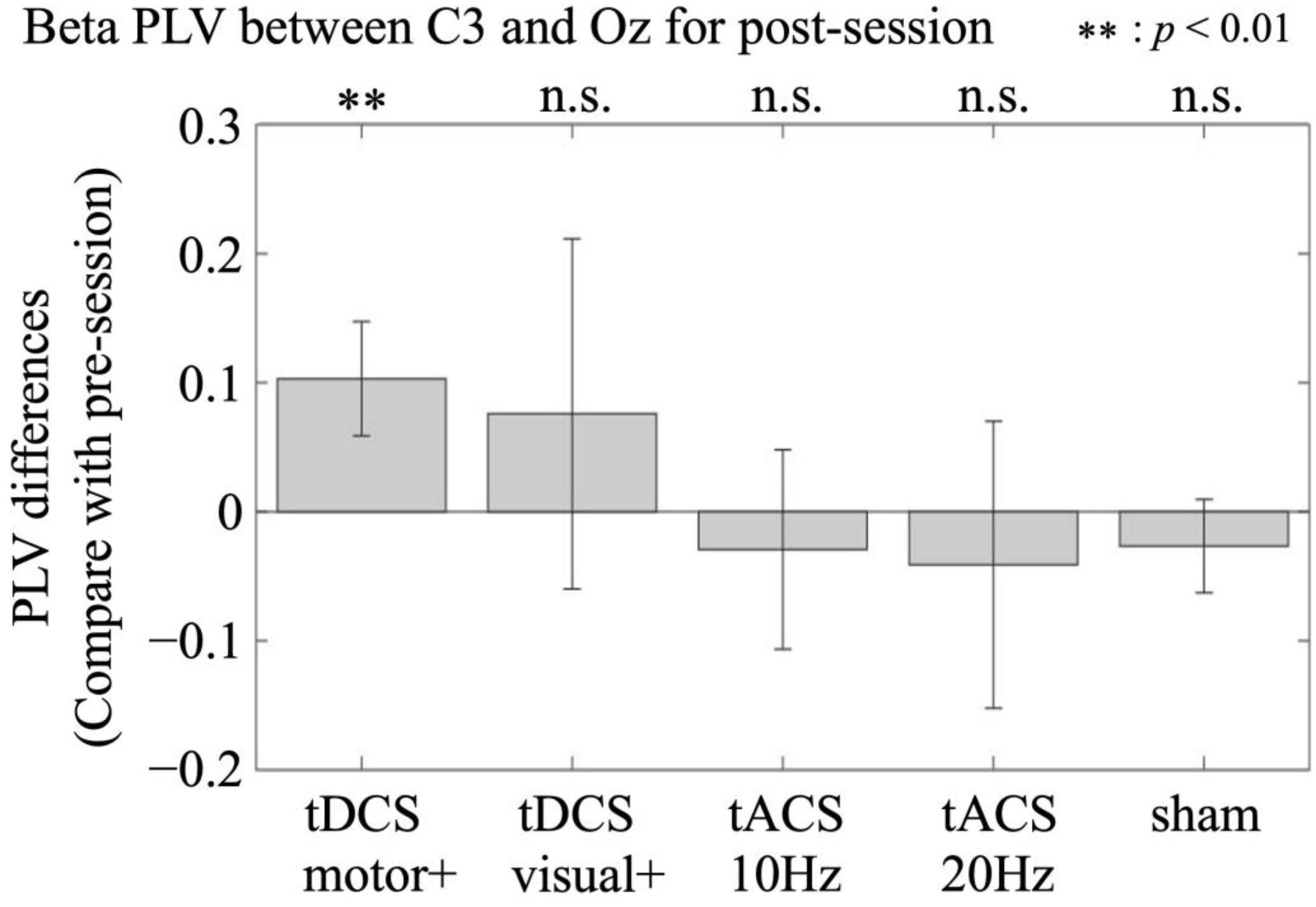
3.2.2. Phase Synchrony Between the Motor and Visual Areas
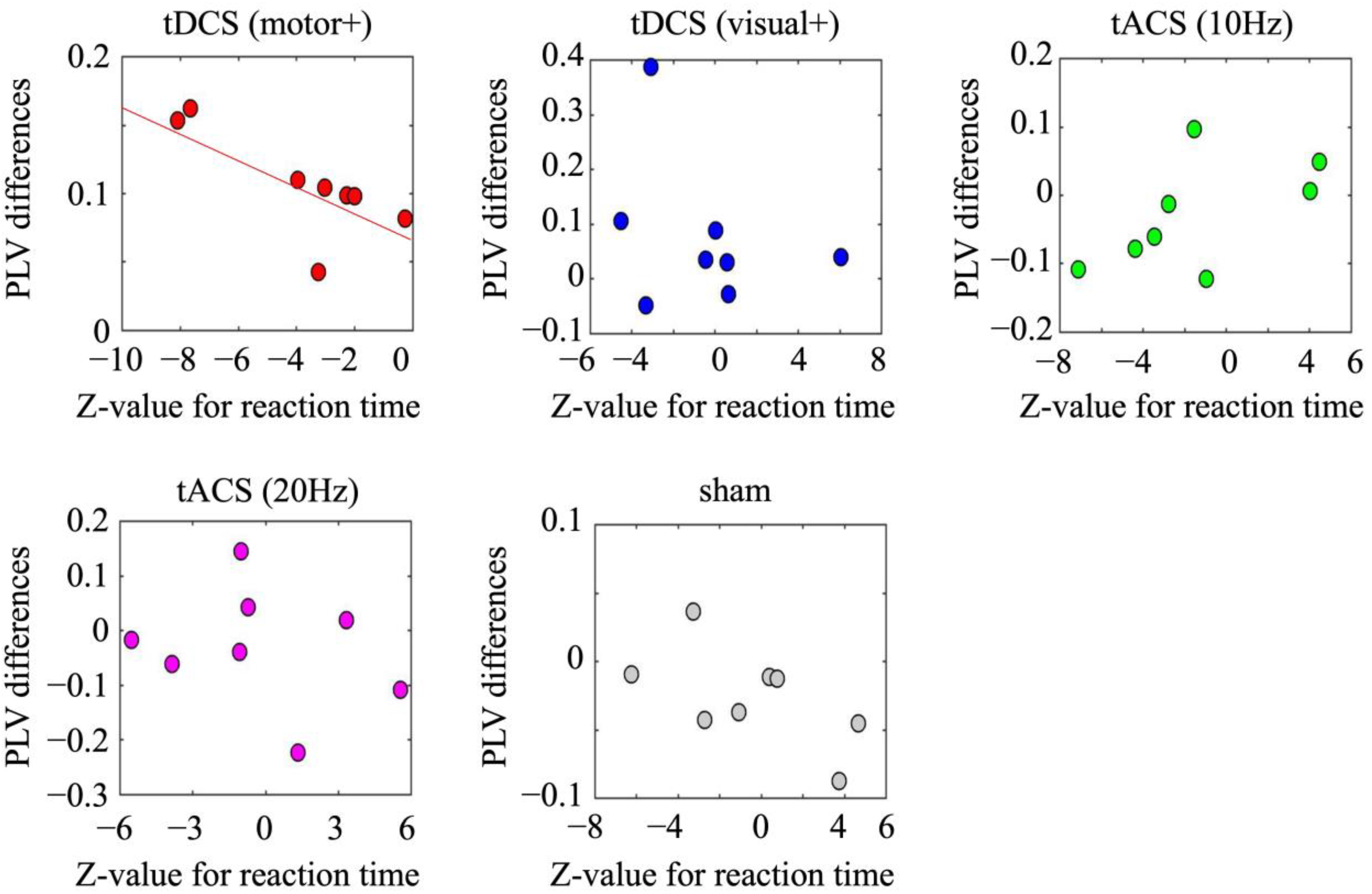
3.2.3. Correlation Between Pre- and Post-Differences in Motor Performance and EEG Oscillatory Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| fMRI | Functional magnetic resonance imaging |
| EEG | Electroencephalography |
| TMS | Transcranial magnetic stimulation |
| tES | Transcranial electric stimulation |
| tDCS | Transcranial direct current stimulation |
| tACS | Transcranial alternating current stimulation |
| RT | Response time |
| PLV | Phase-locking value |
References
- Dayan, E.; Cohen, L.G. Neuroplasticity subserving motor skill learning. Neuron 2011, 72, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Rottschy, C.; Miall, R.C.; Eickhoff, S.B. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 2013, 67, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Solis-Escalante, T. Could the beta rebound in the EEG be suitable to realize a “brain switch”? Clin. Neurophysiol. 2009, 120, 24–29. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Aslan, A.; Staudigl, T.; Klimesch, W.; Herrmann, C.S.; Bäuml, K.H. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage 2007, 37, 1465–1473. [Google Scholar] [CrossRef]
- Wróbel, A. Beta activity: A carrier for visual attention. Acta Neurobiol. Exp. (Wars) 2000, 60, 247–260. [Google Scholar] [CrossRef]
- Basile, L.F.; Anghinah, R.; Ribeiro, P.; Ramos, R.T.; Piedade, R.; Ballester, G.; Brunetti, E.P. Interindividual variability in EEG correlates of attention and limits of functional mapping. Int. J. Psychophysiol. 2007, 65, 238–251. [Google Scholar] [CrossRef]
- Buchholz, V.N.; Jensen, O.; Medendorp, W.P. Different roles of alpha and beta band oscillations in anticipatory sensorimotor gating. Front. Hum. Neurosci. 2014, 8, 446. [Google Scholar] [CrossRef]
- Roelfsema, P.R.; Engel, A.K.; König, P.; Singer, W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature 1997, 385, 157–161. [Google Scholar] [CrossRef]
- Edagawa, K.; Kawasaki, M. Beta phase synchronization in the frontal-temporal-cerebellar network during auditory-to-motor rhythm learning. Sci. Rep. 2017, 7, 42721. [Google Scholar] [CrossRef]
- Priori, A. Brain polarization in humans: A reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin. Neurophysiol. 2003, 114, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W. Transcranial electrical stimulation (tES-tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 2011, 21, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003, 15, 619–626. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef]
- Galea, J.M.; Celnik, P. Brain polarization enhances the formation and retention of motor memories. J. Neurophysiol. 2009, 102, 294–301. [Google Scholar] [CrossRef]
- Bergmann, T.O.; Groppa, S.; Seeger, M.; Mölle, M.; Marshall, L.; Siebner, H.R. Acute changes in motor cortical excitability during slow oscillatory and constant anodal transcranial direct current stimulation. J. Neurophysiol. 2009, 102, 2303–2311. [Google Scholar] [CrossRef]
- Reis, J.; Fritsch, B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr. Opin. Neurol. 2011, 24, 590–596. [Google Scholar] [CrossRef]
- Antal, A.; Boros, K.; Poreisz, C.; Chaieb, L.; Terney, D.; Paulus, W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008, 1, 97–105. [Google Scholar] [CrossRef]
- Feurra, M.; Bianco, G.; Santarnecchi, E.; Del Testa, M.; Rossi, A.; Rossi, S. Frequency-dependent tuning of the human motor system induced by transcranial oscillatory potentials. J. Neurosci. 2011, 31, 12165–12170. [Google Scholar] [CrossRef] [PubMed]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr. Biol. 2009, 19, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Wach, C.; Krause, V.; Moliadze, V.; Paulus, W.; Schnitzler, A.; Pollok, B. Effects of 10 Hz and 20 Hz transcranial alternating current stimulation (tACS) on motor functions and motor cortical excitability. Behav. Brain Res. 2013, 241, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Jamil, A.; Mosayebi Samani, M.M.; Vidor, L.P.; Nitsche, M.A. Basic and functional effects of transcranial electrical stimulation (tES)—An introduction. Neurosci. Biobehav. Rev. 2018, 85, 81–92. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O.; Delpuech, C.; Pernier, J. Stimulus specificity of phase-locked and non-phase-locked 40Hz visual responses in human. J. Neurosci. 1996, 16, 4240–4249. [Google Scholar] [CrossRef]
- Vecchio, F.; Di Iorio, R.; Miraglia, F.; Granata, G.; Romanello, R.; Bramanti, P.; Rossini, P.M. Transcranial direct current stimulation generates a transient increase of small-world in brain connectivity: An EEG graph theoretical analysis. Exp. Brain Res. 2018, 236, 1117–1127. [Google Scholar] [CrossRef]
- Miyauchi, E.; Ide, M.; Tachikawa, H.; Nemoto, K.; Arai, T.; Kawasaki, M. A novel approach for assessing neuromodulation using phase-locked information measured with TMS-EEG. Sci. Rep. 2019, 9, 428. [Google Scholar] [CrossRef]
- Zaepffel, M.; Trachel, R.; Kilavik, B.E.; Brochier, T. Modulations of EEG beta power during planning and execution of grasping movements. PLoS ONE 2013, 8, e60060. [Google Scholar] [CrossRef]
- Krause, V.; Meier, A.; Dinkelbach, L.; Pollok, B. Beta band transcranial alternating (tACS) and direct current stimulation (tDCS) applied after initial learning facilitate retrieval of a motor sequence. Front. Behav. Neurosci. 2016, 10, 4. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyauchi, E.; Henmi, Y.; Kawasaki, M. Transcranial Direct Current Stimulation Enhances Motor Performance by Modulating Beta-Phase Synchronization in the Sensorimotor Network: A Preliminary Study. Brain Sci. 2025, 15, 286. https://doi.org/10.3390/brainsci15030286
Miyauchi E, Henmi Y, Kawasaki M. Transcranial Direct Current Stimulation Enhances Motor Performance by Modulating Beta-Phase Synchronization in the Sensorimotor Network: A Preliminary Study. Brain Sciences. 2025; 15(3):286. https://doi.org/10.3390/brainsci15030286
Chicago/Turabian StyleMiyauchi, Eri, Yoshiki Henmi, and Masahiro Kawasaki. 2025. "Transcranial Direct Current Stimulation Enhances Motor Performance by Modulating Beta-Phase Synchronization in the Sensorimotor Network: A Preliminary Study" Brain Sciences 15, no. 3: 286. https://doi.org/10.3390/brainsci15030286
APA StyleMiyauchi, E., Henmi, Y., & Kawasaki, M. (2025). Transcranial Direct Current Stimulation Enhances Motor Performance by Modulating Beta-Phase Synchronization in the Sensorimotor Network: A Preliminary Study. Brain Sciences, 15(3), 286. https://doi.org/10.3390/brainsci15030286





