Differences in Anatomical Structures and Resting-State Brain Networks Between Elite Wrestlers and Handball Athletes
Abstract
1. Introduction
- (i)
- Anatomical structures of the brain tissue represented by the segmented T1 images differ between wrestlers and handball athletes.
- (ii)
- Resting-state networks obtained through the analysis of Blood Oxygenation Level-Dependent (BOLD) signal differ between wrestlers and handball athletes.
2. Materials and Methods
2.1. Subjects
2.2. MRI Protocol and Methods
2.3. Statistical Analysis
2.3.1. Whole-Brain Metric Statistics
2.3.2. Voxel-Based Morphometry Statistics
2.3.3. Resting-State Networks Comparison Statistics
3. Results
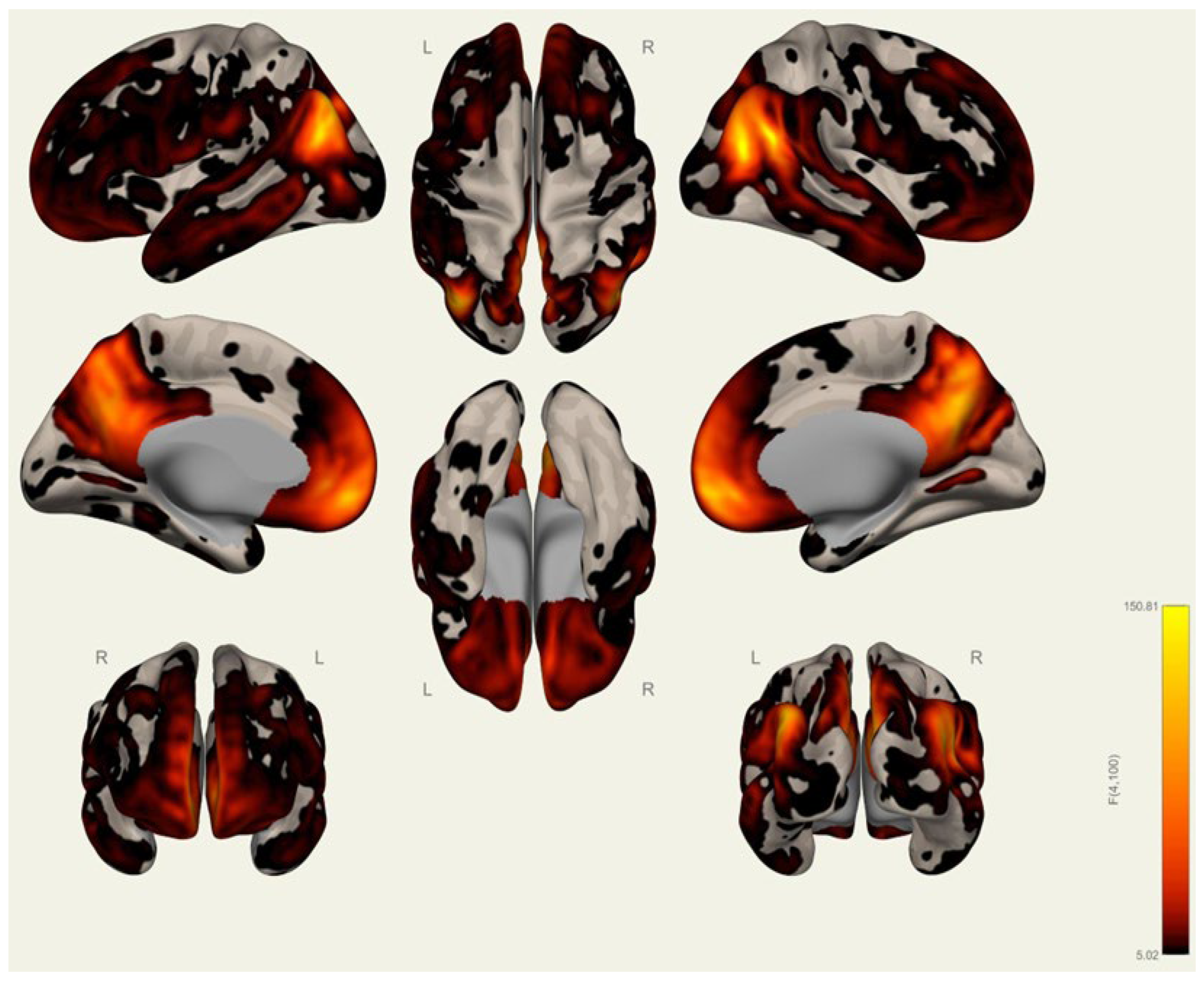
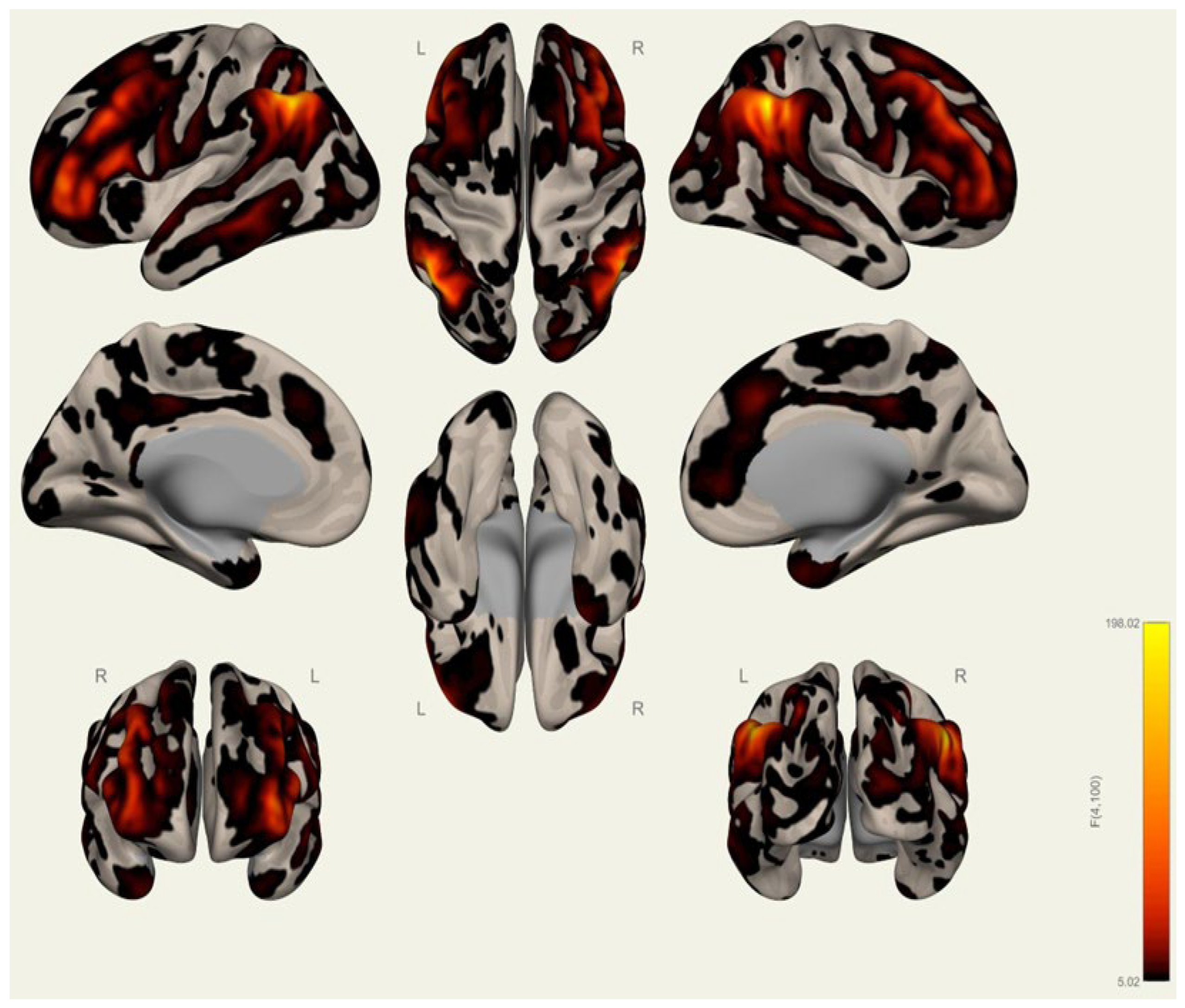
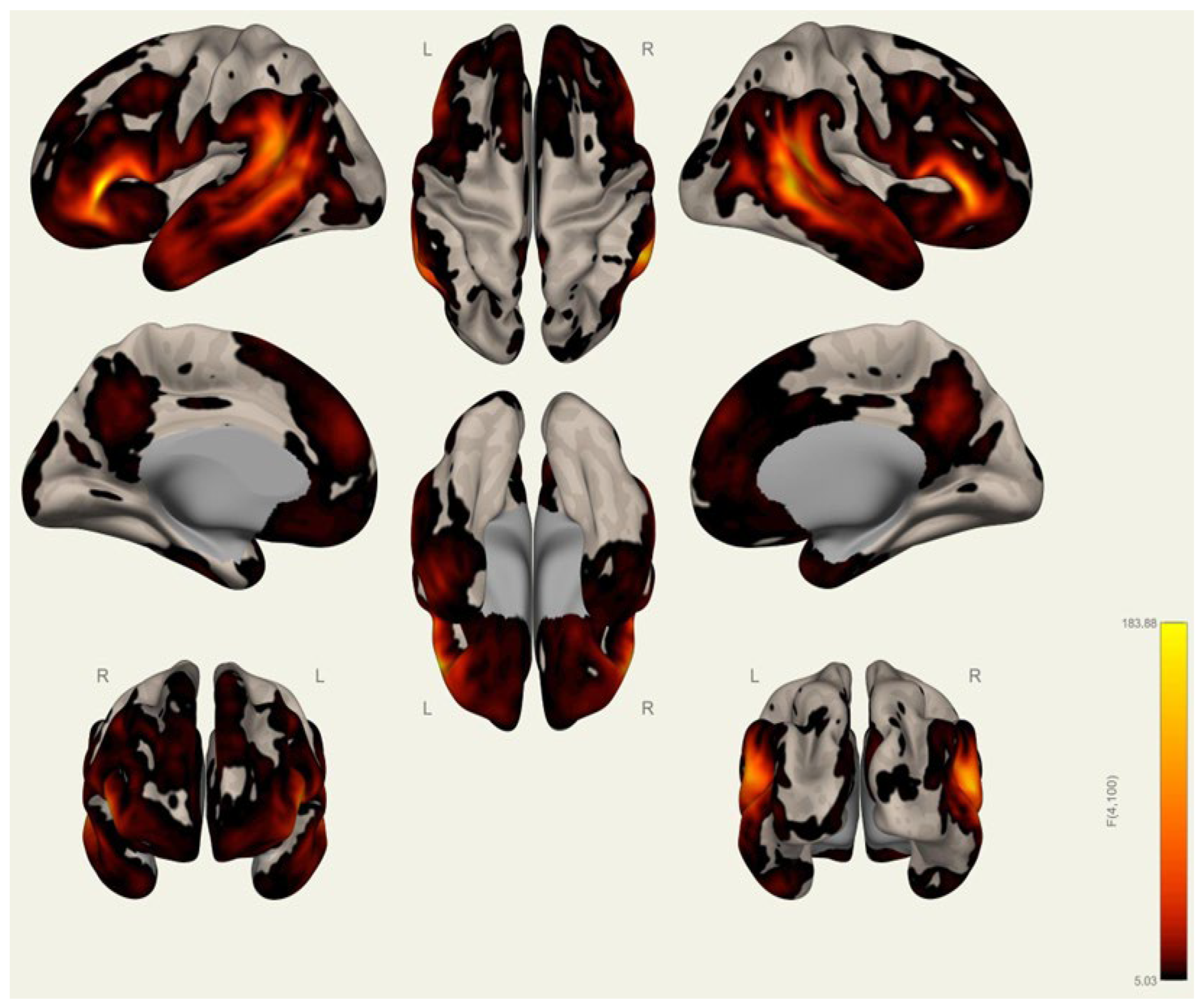
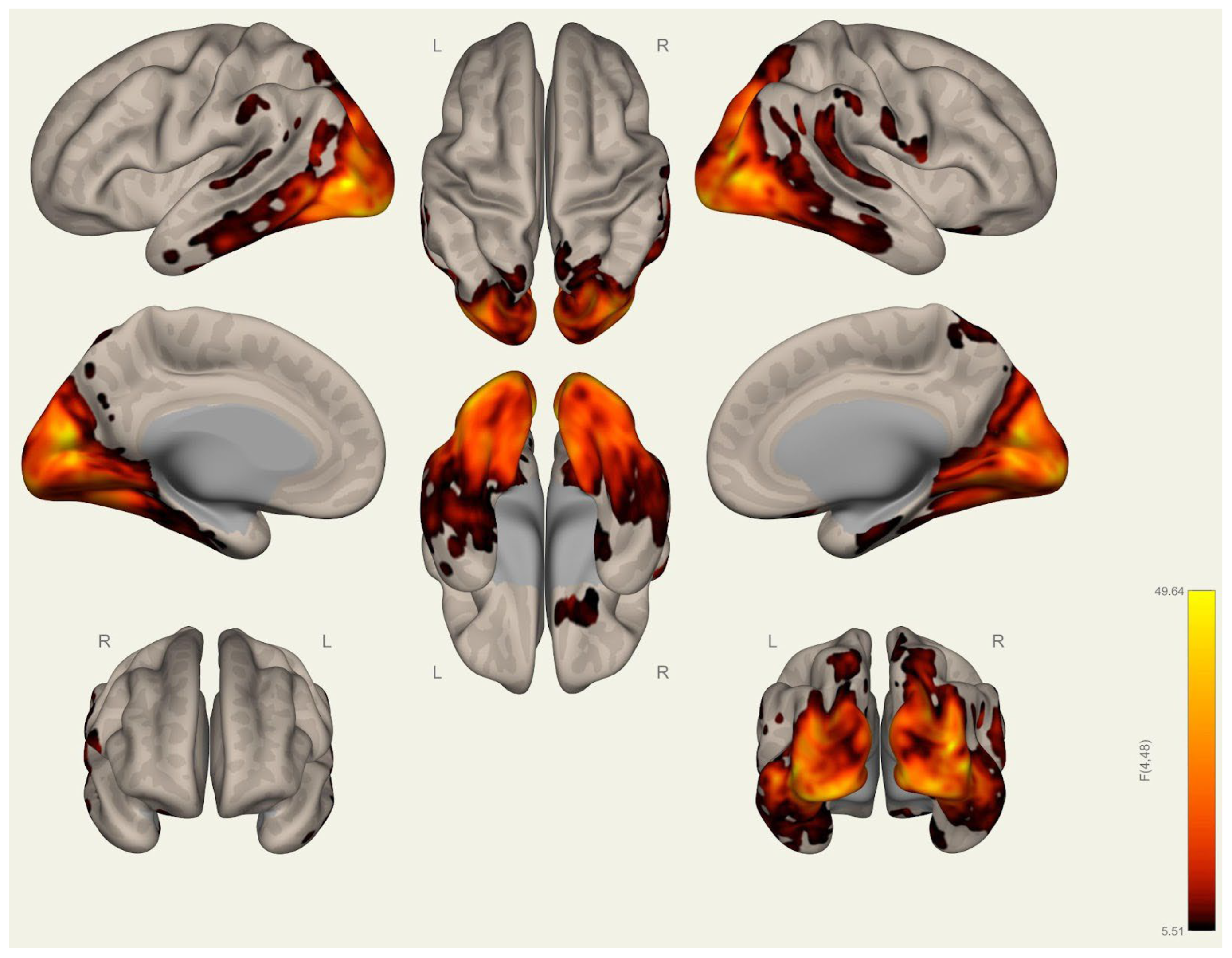
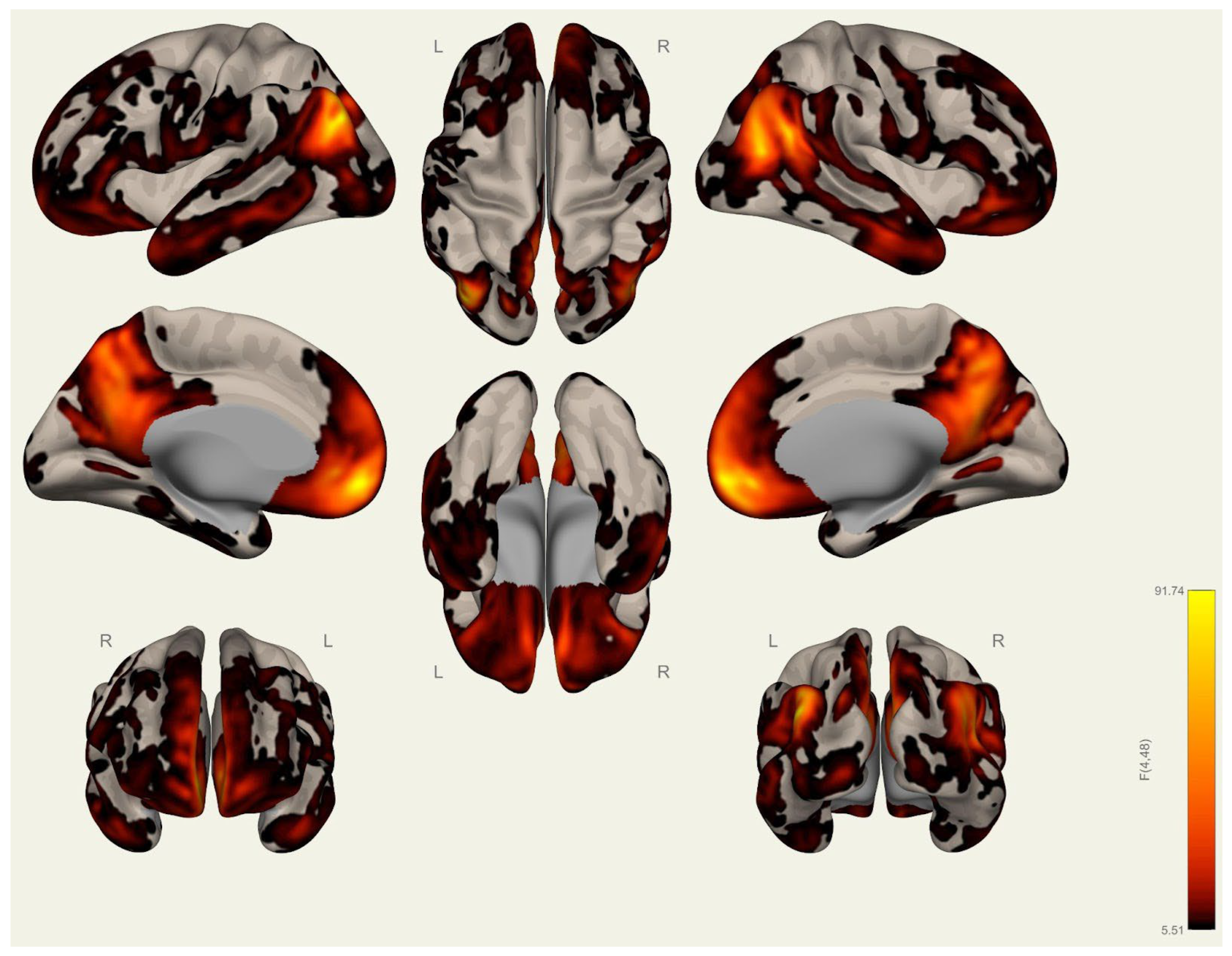
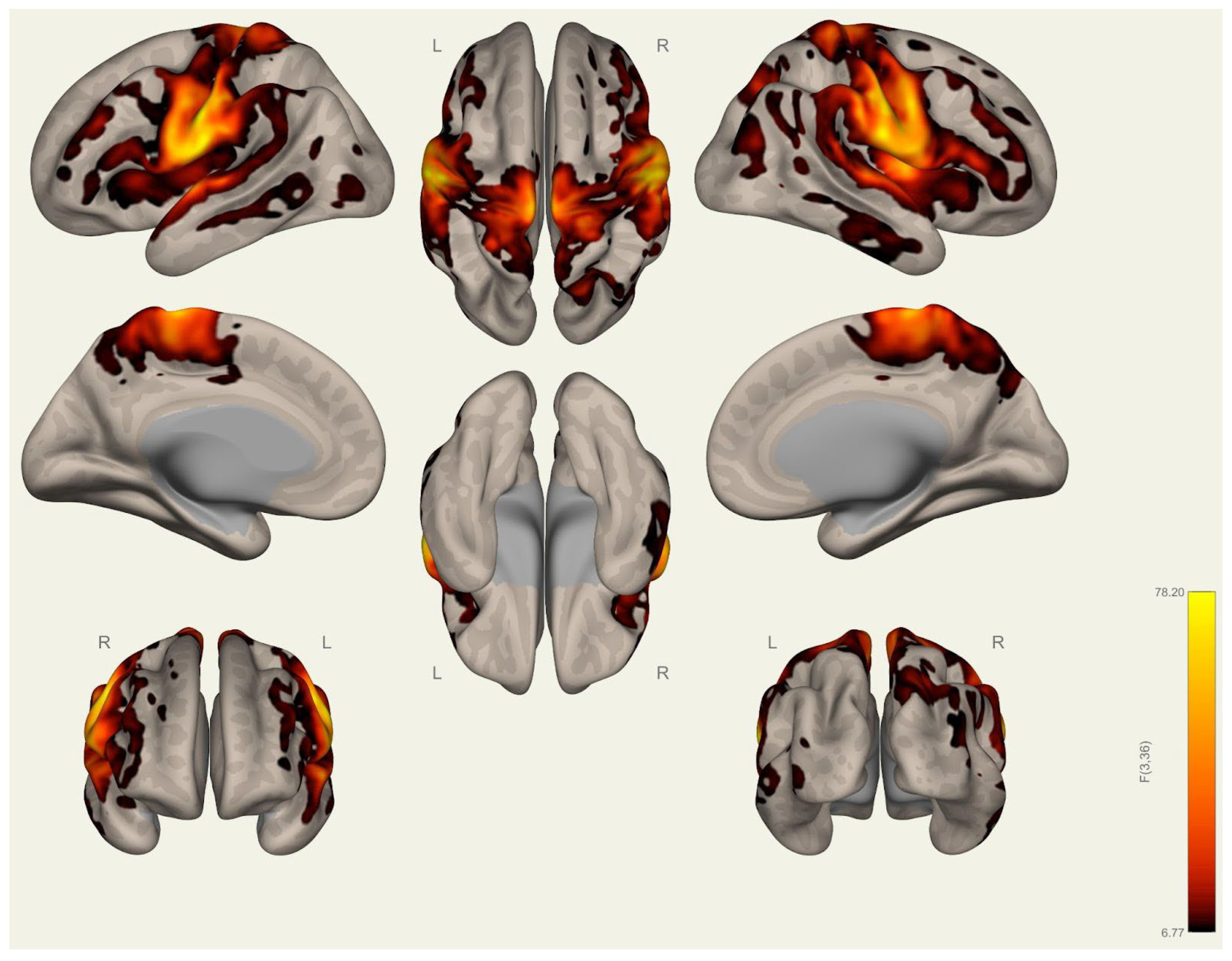
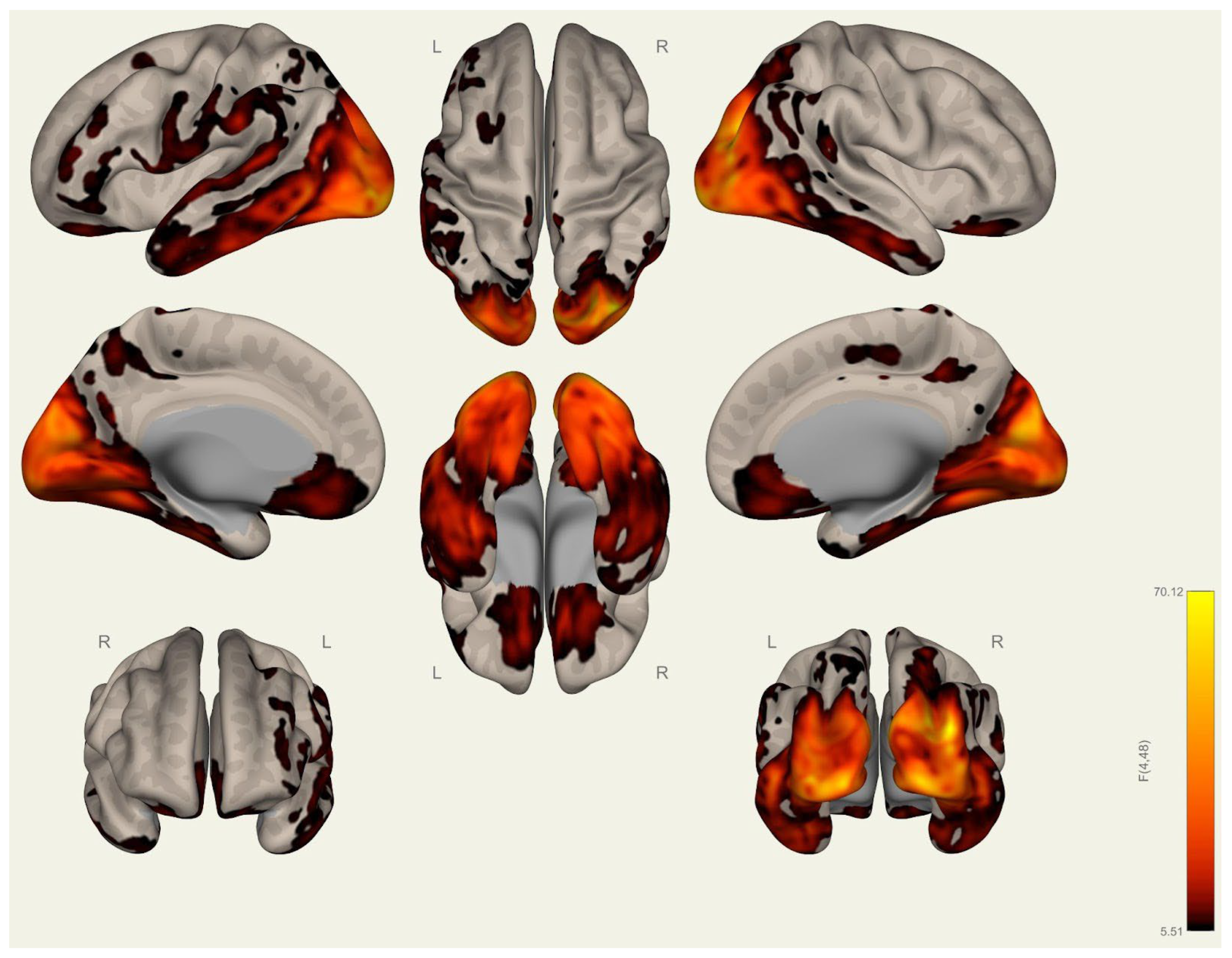
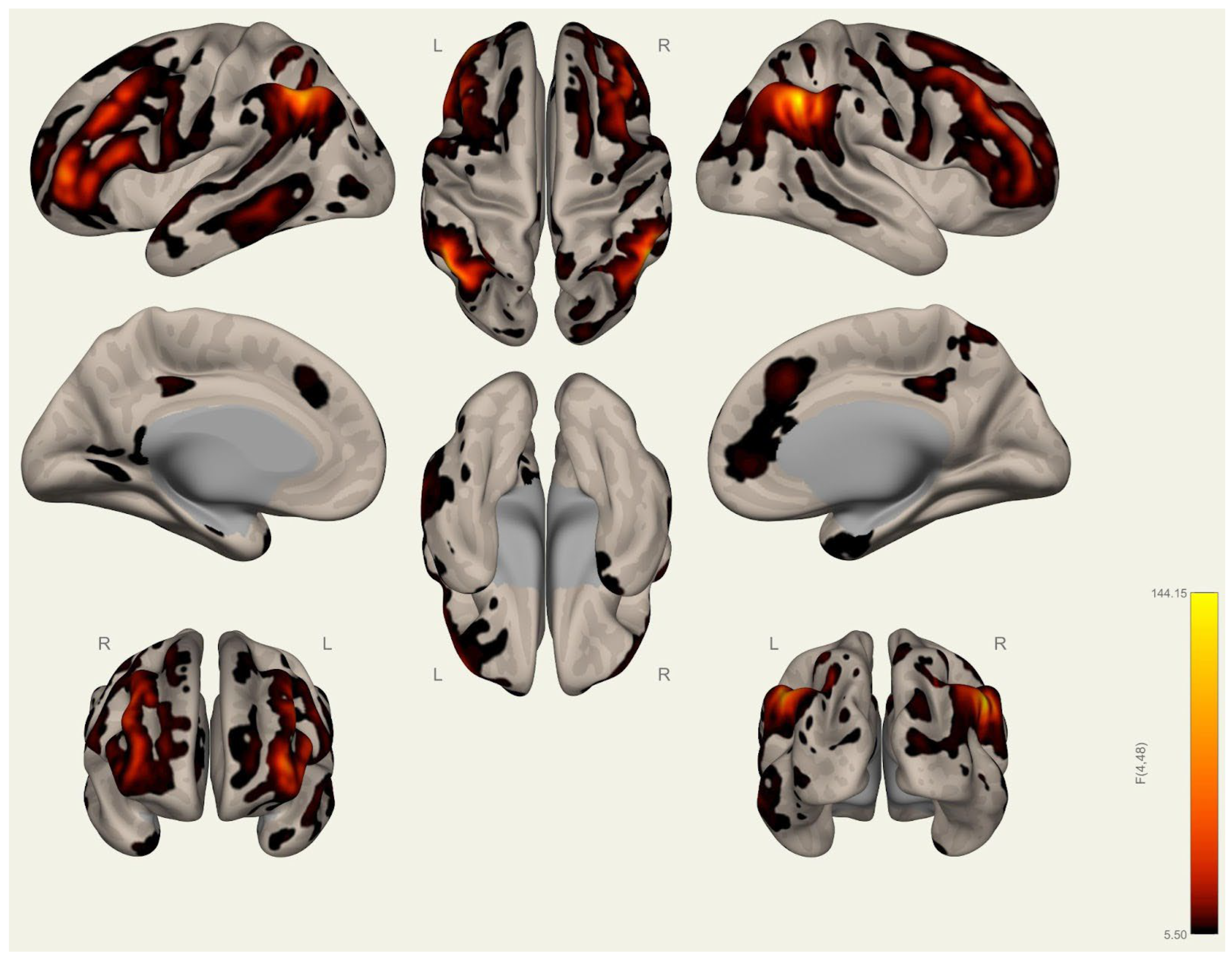
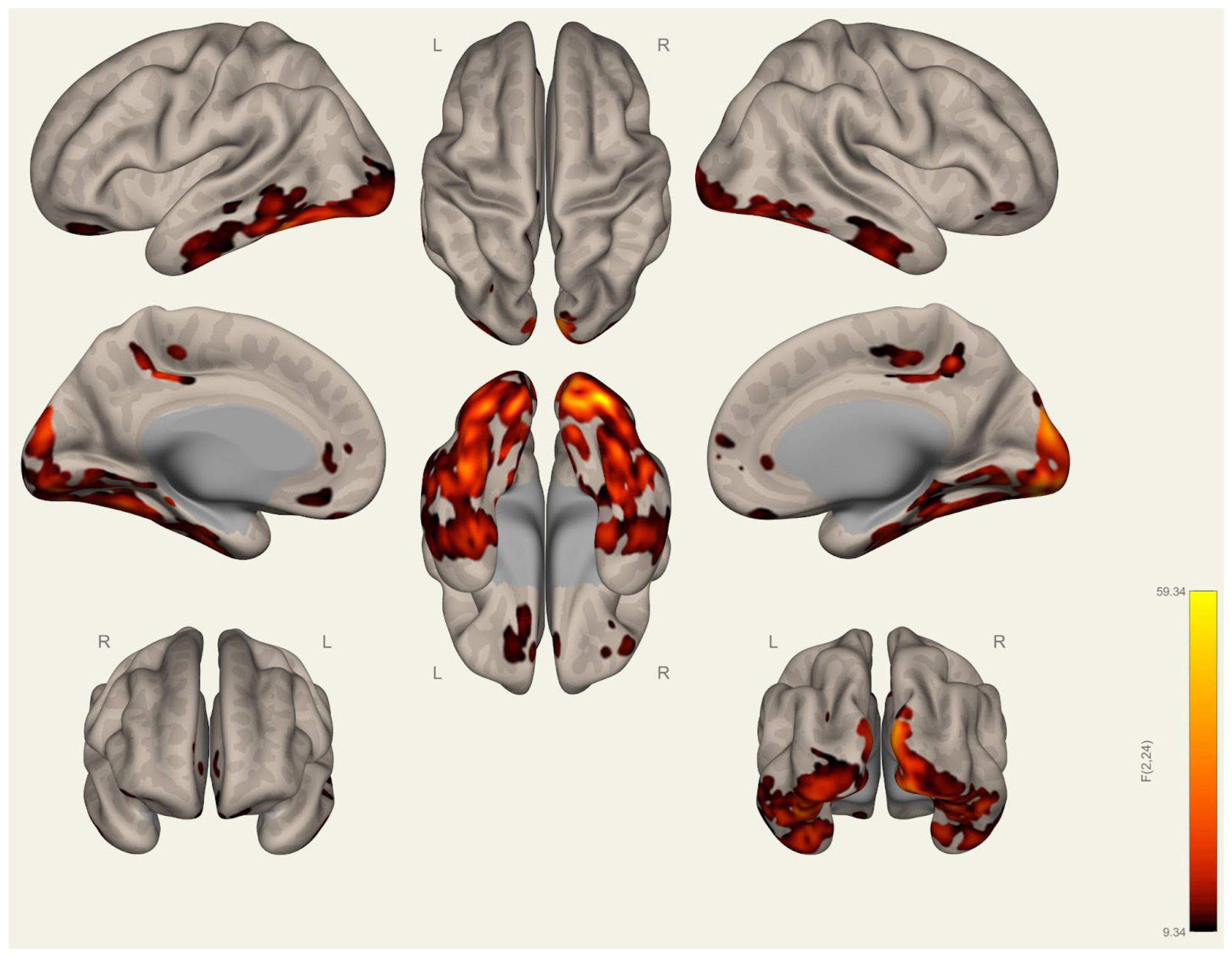
3.1. Anatomical Differences in Wrestlers and Handball Athletes
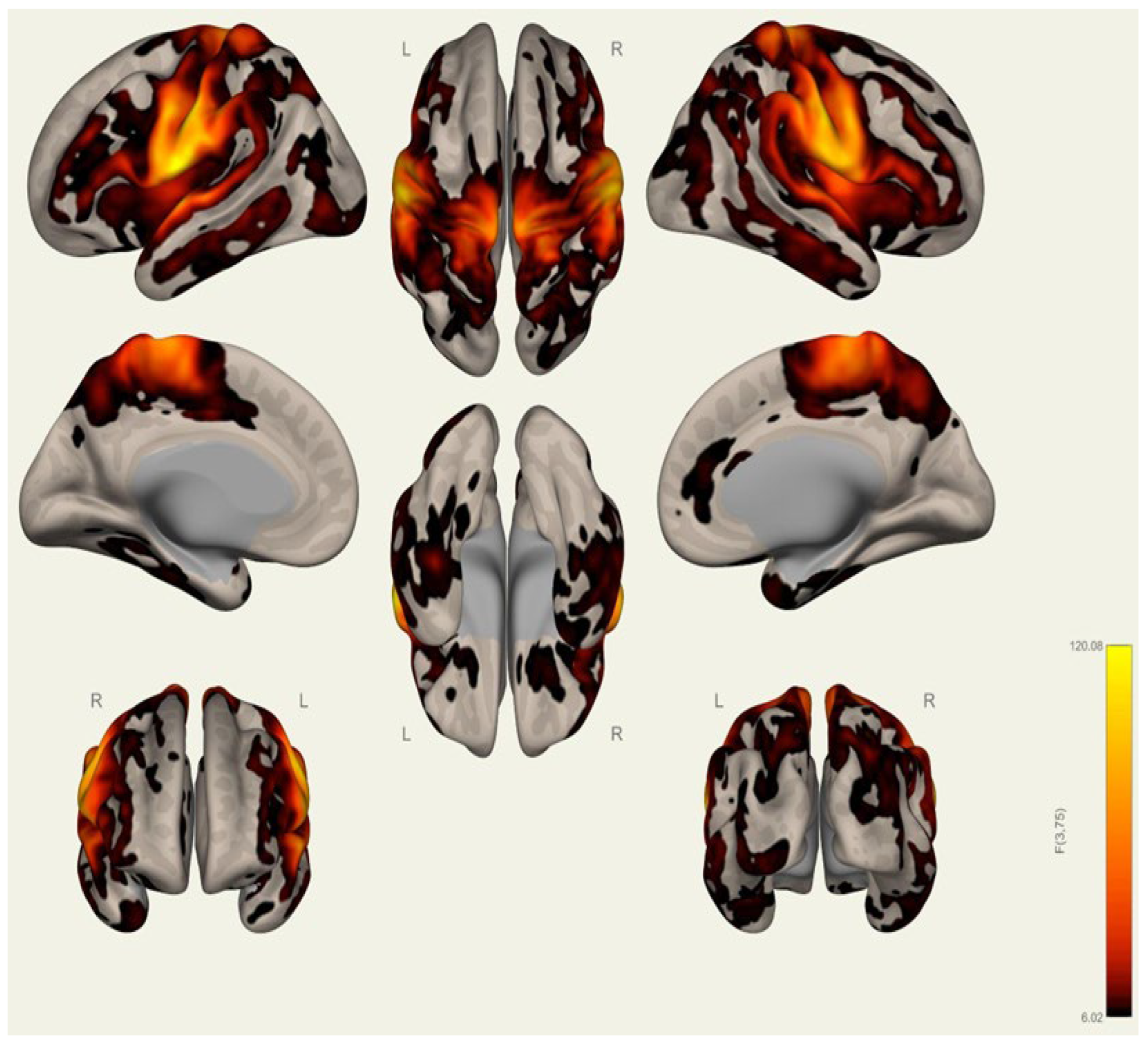
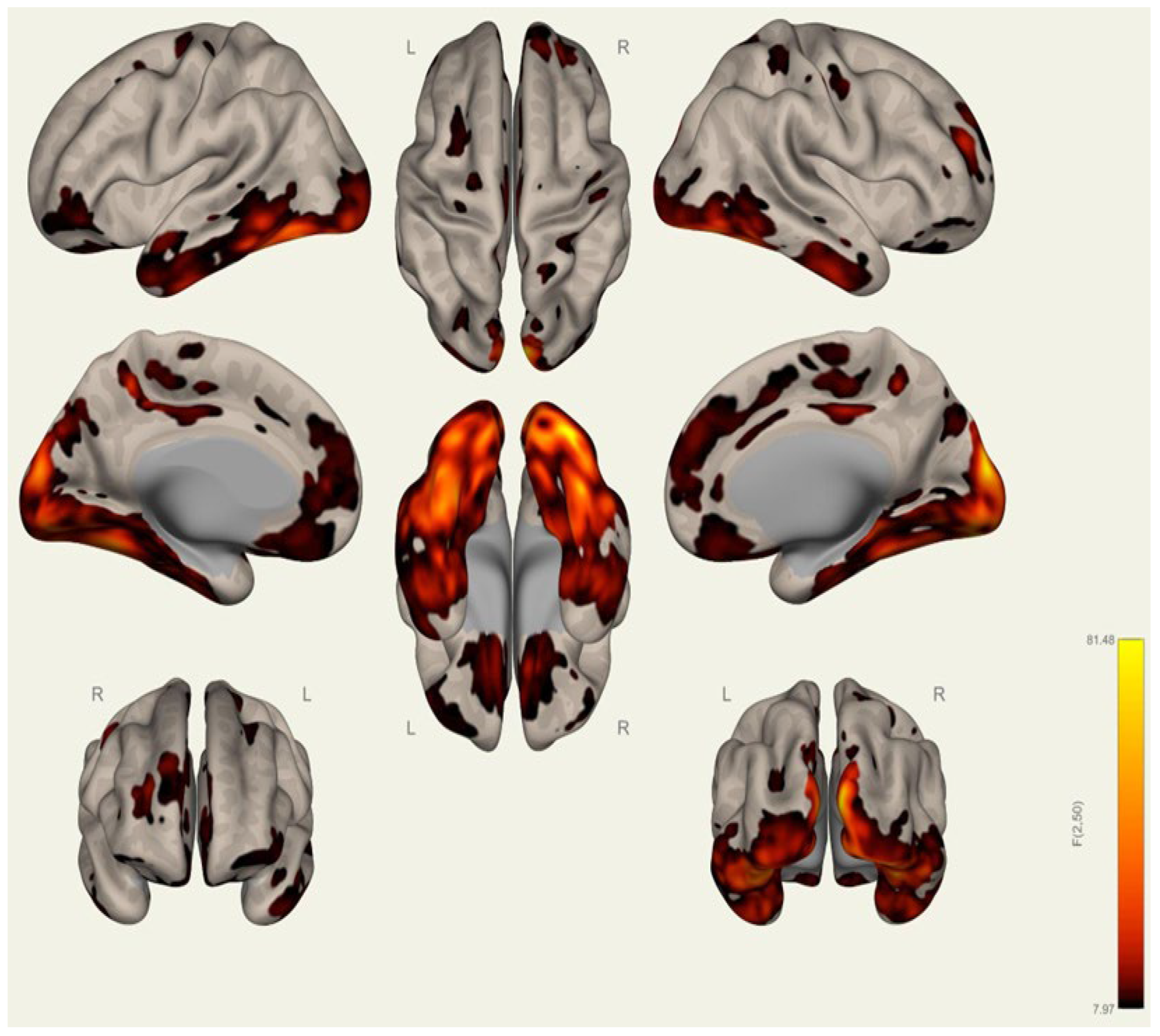
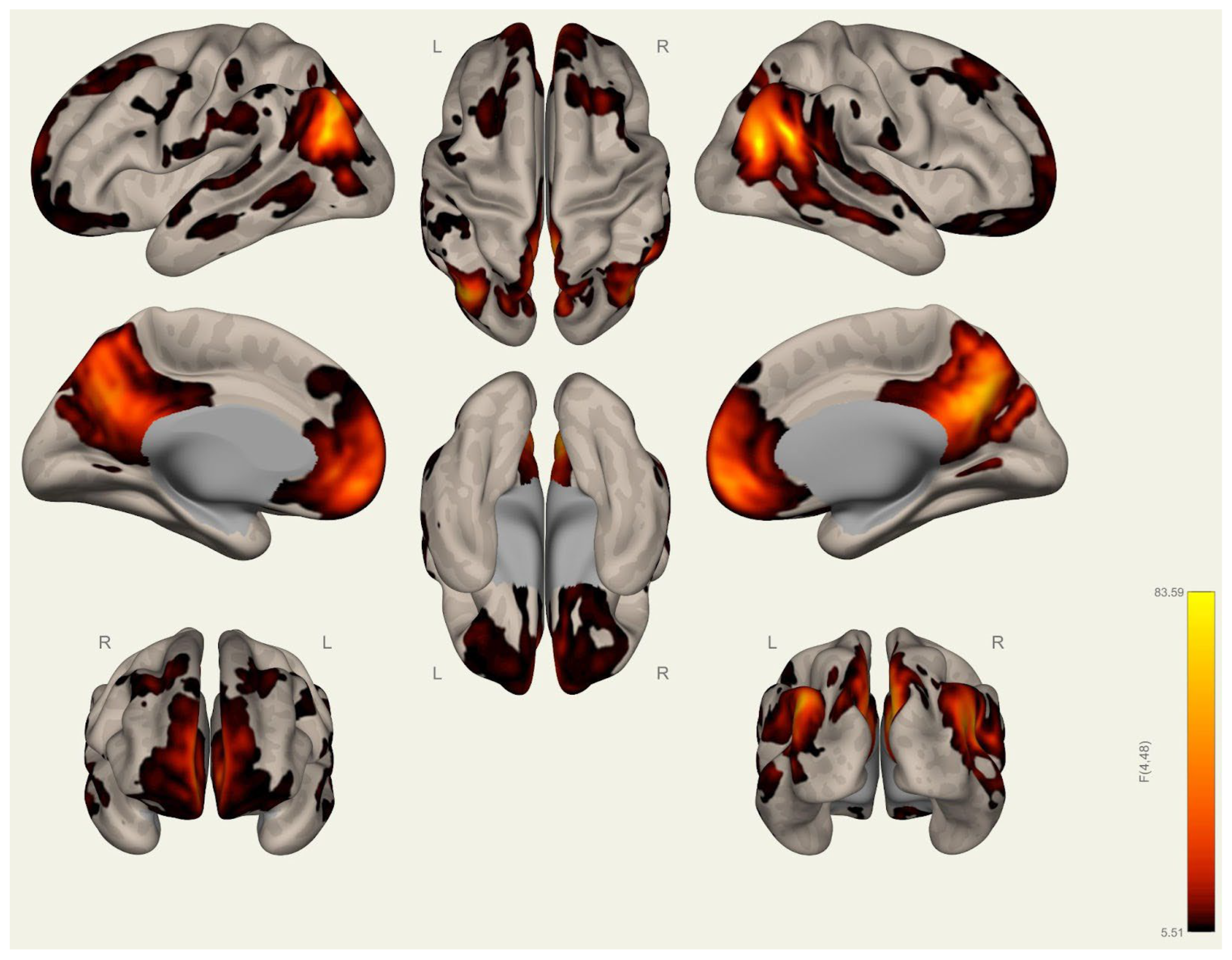
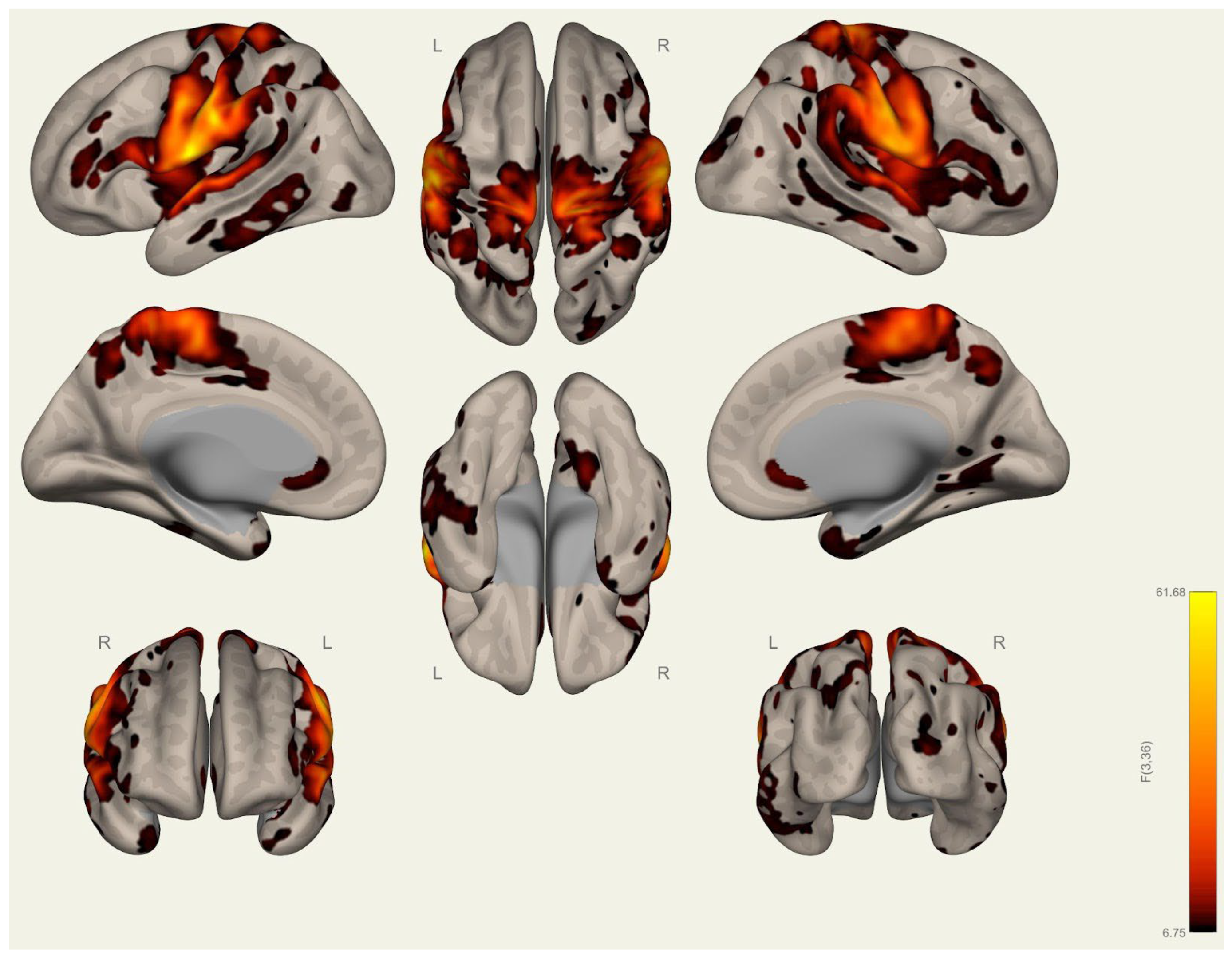
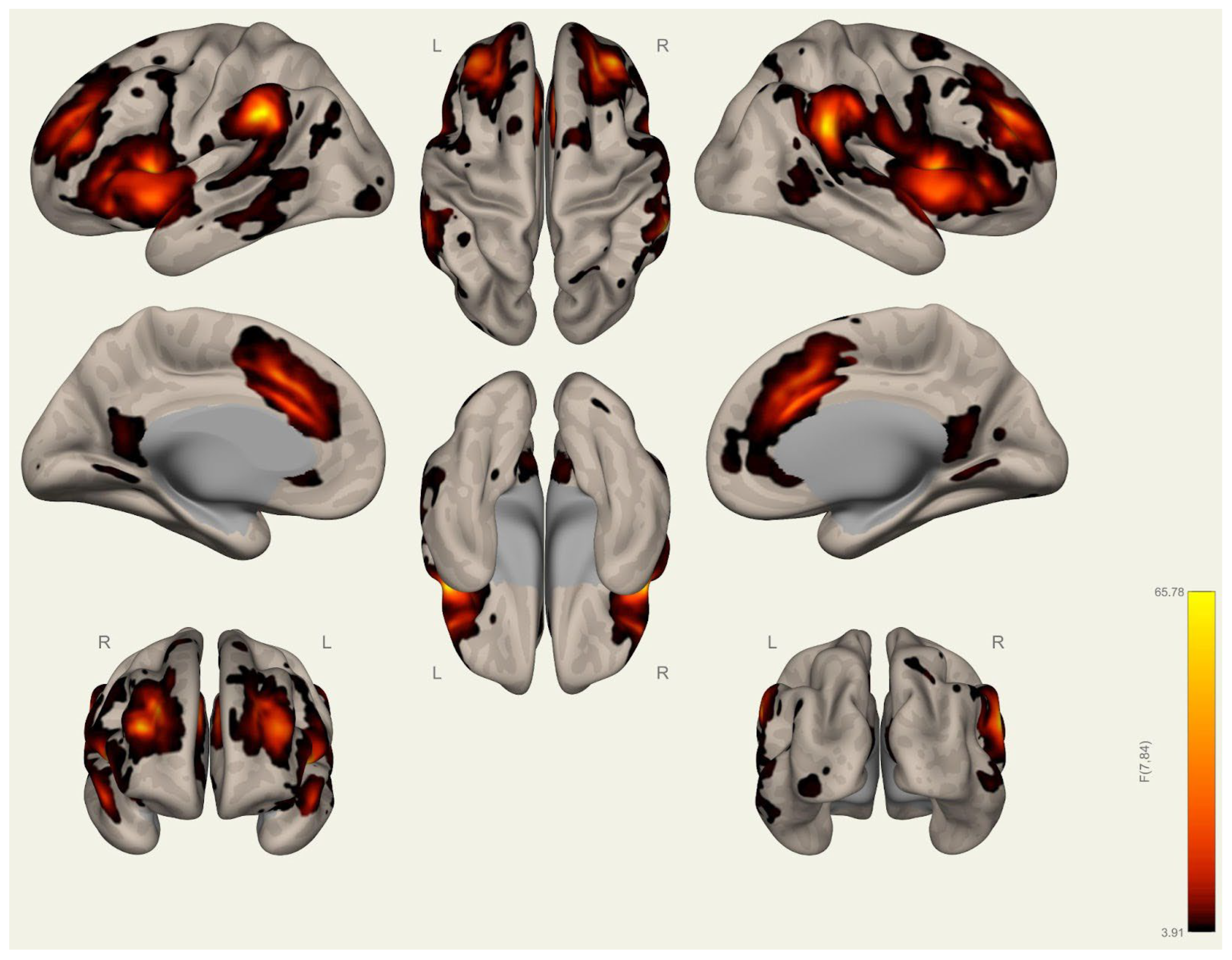
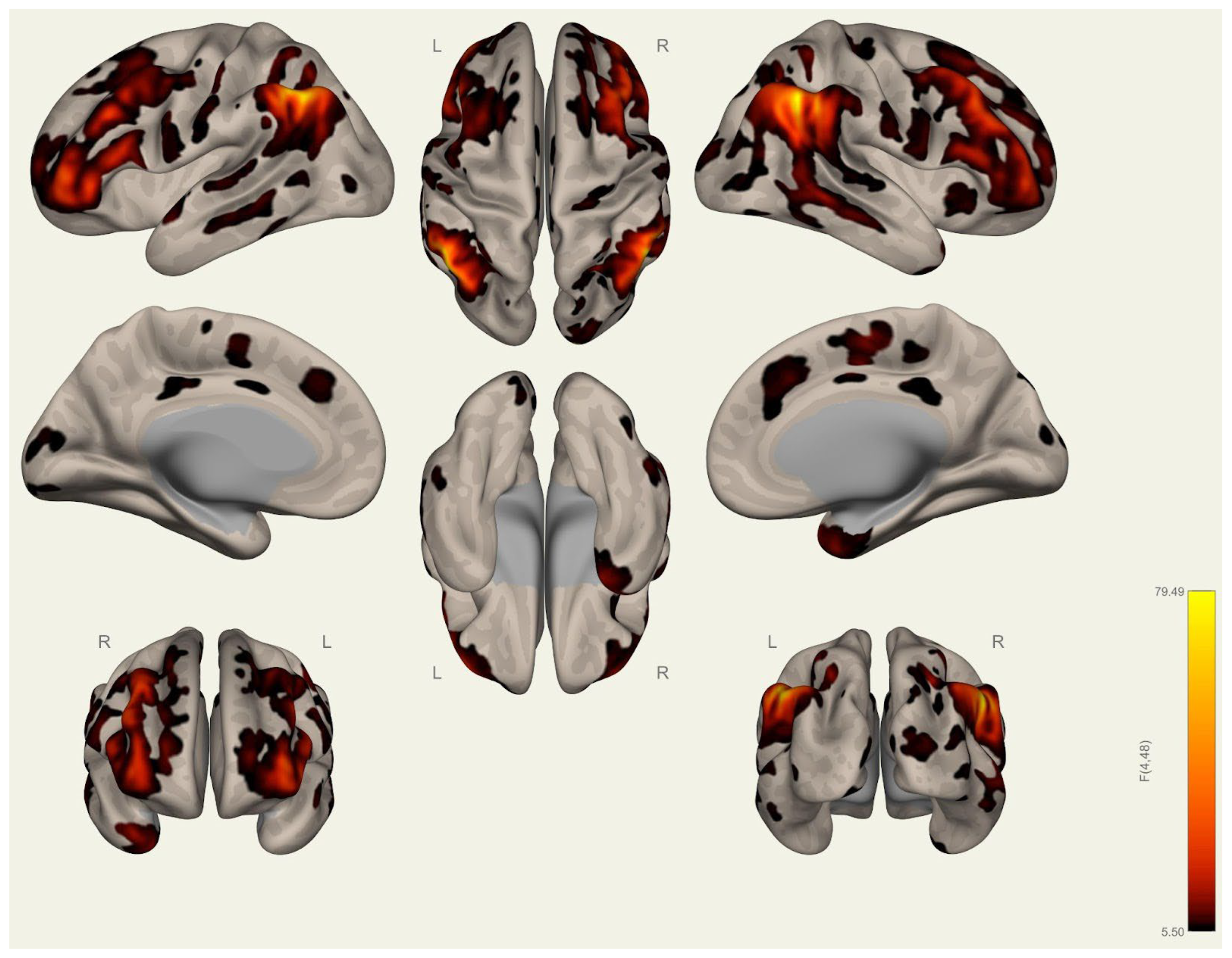
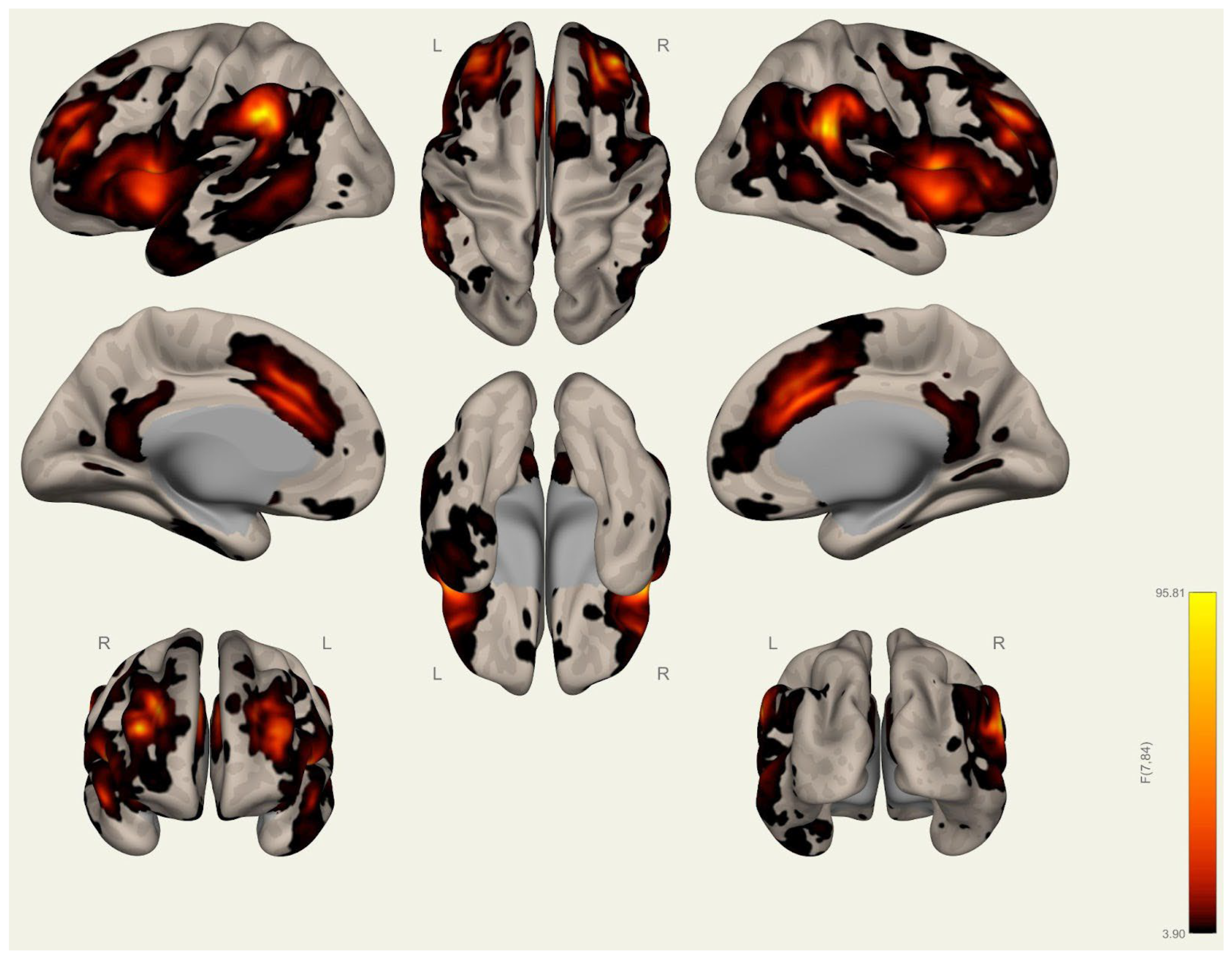
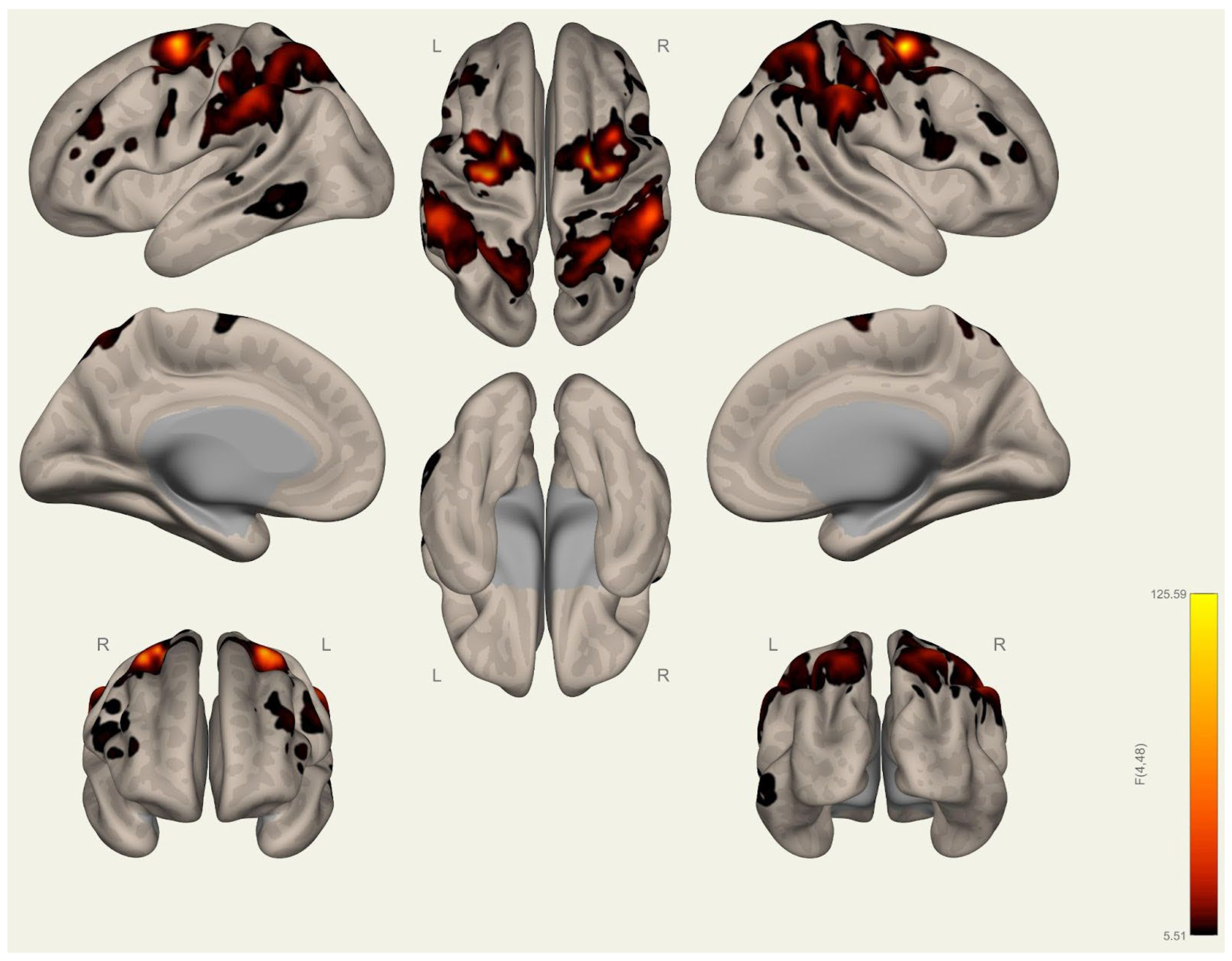
3.2. Resting-State Functional Connectivity Differences in Wrestlers and Handball Athletes
4. Discussion
4.1. Regional Grey Matter Differences
4.2. Cortical Thickness Differences
4.3. Functional Connectivity Differences
4.4. Integration of PPS Dynamics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BOLD | Blood Oxygenation Level-Dependent |
| CSF | cerebrospinal fluid |
| DARTEL | diffeomorphic anatomical registration through exponentiated lie algebra |
| EPI | echo-planar imaging |
| FOV | field of view |
| fMRI | functional magnetic resonance imaging |
| GM | gray matter |
| GMV | gray matter volume |
| MRI | magnetic resonance imaging |
| MFG | middle frontal gyrus |
| MTG | middle temporal gyrus |
| OFC | orbitofrontal cortex |
| PHG | parahippocampal gyrus |
| PPS | peripersonal space |
| PCC | posterior cingulate cortex |
| PCG | posterior cingulate gyrus |
| TE | echo time |
| TR | repetition time |
| ROI | region of interest |
| STG | superior temporal gyrus |
| STS | superior temporal sulcus |
| VBM | voxel-based morphometry |
| WM | white matter |
Appendix A


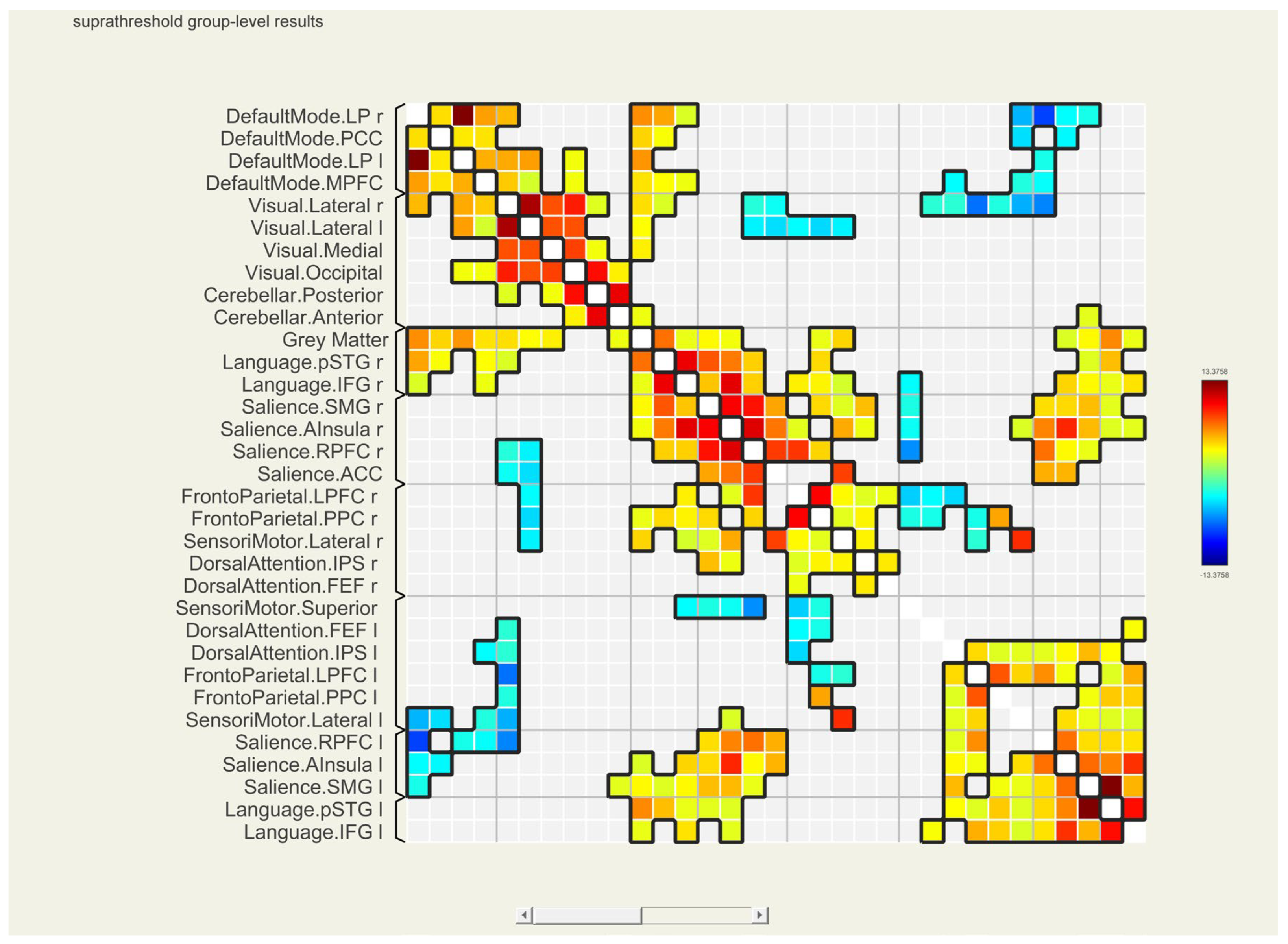







| Analysis Unit | T (25)-Value | p-unc | p-FDR | p-FWE | ||
|---|---|---|---|---|---|---|
| Cluster 1/34 | ||||||
| Score | 1378.23 | 0.000000 | 0.000000 | 0.000000 | ||
| Mass | 3402.43 | 0.000000 | 0.000000 | 0.000000 | ||
| Size | 48 | 0.000000 | 0.000000 | 0.000000 | ||
| DefaultMode LP (R) (47,−67,29)-DefaultMode LP (L) (−39,−77,33) | 16.11 | 0.000000 | 0.000000 | |||
| Cerebellar Posterior (0,−79,−32)-Cerebellar Anterior (0,−63,−30) | 14.69 | 0.000000 | 0.000000 | |||
| Visual Lateral (R) (38,−72,13)-Visual Medial (2,−79,12) | 12.61 | 0.000000 | 0.000000 | |||
| Visual Lateral (R) (38,−72,13)-Visual Occipital (0,−93,−4) | 11.85 | 0.000000 | 0.000000 | |||
| Visual Medial (2,−79,12)-Visual Occipital (0,−93,−4) | 11.78 | 0.000000 | 0.000000 | |||
| Visual Lateral (R) (38,−72,13)-Visual Lateral (L) (−37,−79,10) | 11.00 | 0.000000 | 0.000000 | |||
| Visual Lateral (L) (−37,−79,10)-Visual Occipital (0,−93,−4) | 9.21 | 0.000000 | 0.000000 | |||
| Visual Lateral (L) (−37,−79,10)-Visual Medial (2,−79,12) | 9.16 | 0.000000 | 0.000000 | |||
| Visual Occipital (0,−93,−4)-Cerebellar Posterior (0,−79,−32) | 8.74 | 0.000000 | 0.000000 | |||
| DefaultMode.MPFC (1,55,−3)-DefaultMode LP (L) (−39,−77,33) | 7.26 | 0.000000 | 0.000002 | |||
| DefaultMode.LP (R) (47,−67,29)-DefaultMode PCC (1,−61,38) | 6.94 | 0.000000 | 0.000003 | |||
| DefaultMode LP (L) (−39,−77,33)-Visual Lateral (L) (−37,−79,10) | 6.83 | 0.000000 | 0.000004 | |||
| DefaultMode LP (R) (47,−67,29)-DefaultMode MPFC (1,55,−3) | 6.51 | 0.000001 | 0.000007 | |||
| DefaultMode LP (R) (47,−67,29)-Visual Lateral (R) (38,−72,13) | 6.26 | 0.000002 | 0.000013 | |||
| DefaultMode MPFC (1,55,−3)-DefaultMode PCC (1,−61,38) | 5.62 | 0.000008 | 0.000055 | |||
| Visual Occipital (0,−93,−4)-Cerebellar Anterior (0,−63,−30) | 5.37 | 0.000015 | 0.000092 | |||
| DefaultMode PCC (1,−61,38)-DefaultMode LP (L) (−39,−77,33) | 5.36 | 0.000015 | 0.000092 | |||
| Visual Medial (2,−79,12)-Cerebellar Posterior (0,−79,−32) | 5.09 | 0.000030 | 0.000176 | |||
| Visual Medial (2,−79,12)-Cerebellar Anterior (0,−63,−30) | 4.51 | 0.000131 | 0.000619 | |||
| Visual Lateral (R) (38,−72,13)-Cerebellar Anterior (0,−63,−30) | 3.97 | 0.000537 | 0.002134 | |||
| DefaultMode LP (L) (−39,−77,33)-Visual Lateral (R) (38,−72,13) | 3.95 | 0.000569 | 0.002207 | |||
| Visual Lateral (L) (−37,−79,10)-Cerebellar Anterior (0,−63,−30) | 3.36 | 0.002532 | 0.007817 | |||
| Visual Lateral (R) (38,−72,13)-Cerebellar Posterior (0,−79,−32) | 3.19 | 0.003778 | 0.010782 | |||
| Visual Lateral (L) (−37,−79,10)-Cerebellar Posterior (0,−79,−32) | 2.17 | 0.039513 | 0.076421 | |||
| Cluster 2/34 | ||||||
| Score | 1146.55 | 0.000000 | 0.000000 | 0.000000 | ||
| Mass | 8454.38 | 0.000000 | 0.000000 | 0.000000 | ||
| Size | 226 | 0.000000 | 0.000000 | 0.000000 | ||
| Language pSTG (L) (−57,−47,15)-Salience SMG (L) (−60,−39,31) | 16.70 | 0.000000 | 0.000000 | |||
| Language IFG (L) (−51,26,2)-Salience AInsula (L) (−44,13,1) | 13.72 | 0.000000 | 0.000000 | |||
| Salience AInsula (R) (47,14,0)-Language IFG (R) (54,28,1) | 13.16 | 0.000000 | 0.000000 | |||
| FrontoParietal PPC (R) (52,−52,45)-FrontoParietal LPFC (R) (41,38,30) | 12.28 | 0.000000 | 0.000000 | |||
| Salience SMG (R) (62,−35,32)-Language pSTG (R) (59,−42,13) | 11.04 | 0.000000 | 0.000000 | |||
| Salience RPFC (R) (32,46,27)-FrontoParietal LPFC (R) (41,38,30) | 10.89 | 0.000000 | 0.000000 | |||
| Salience AInsula (L) (−44,13,1)-Salience AInsula (R) (47,14,0) | 10.19 | 0.000000 | 0.000000 | |||
| Grey Matter—Language pSTG (R) (59,−42,13) | 9.81 | 0.000000 | 0.000000 | |||
| Language pSTG (L) (−57,−47,15)-Language IFG (L) (−51,26,2) | 9.68 | 0.000000 | 0.000000 | |||
| FrontoParietal LPFC (L) (−43,33,28)-FrontoParietal PPC (L) (−46,58,49) | 9.56 | 0.000000 | 0.000000 | |||
| Salience AInsula (L) (−44,13,1)-Salience ACC (0,22,35) | 9.20 | 0.000000 | 0.000000 | |||
| Salience RPFC (R) (32,46,27)-Salience AInsula (R) (47,14,0) | 8.54 | 0.000000 | 0.000000 | |||
| Salience AInsula (R) (47,14,0)-Salience SMG (R) (62,−35,32) | 8.45 | 0.000000 | 0.000000 | |||
| Salience RPFC (L) (−32,45,27)-Salience RPFC (R) (32,46,27) | 8.44 | 0.000000 | 0.000000 | |||
| Salience ACC (0,22,35)—Salience RPFC (R) (32,46,27) | 8.40 | 0.000000 | 0.000000 | |||
| Language IFG (R) (54,28,1)-Language pSTG (R) (59,−42,13) | 8.32 | 0.000000 | 0.000000 | |||
| Salience ACC (0,22,35)—Salience AInsula (R) (47,14,0) | 8.18 | 0.000000 | 0.000000 | |||
| FrontoParietal PPC (L) (−46,−58,49)-FrontoParietal.PPC (R) (52,−52,45) | 7.92 | 0.000000 | 0.000000 | |||
| Salience.SMG (L) (−60,−39,31)-Salience.AInsula (L) (−44,13,1) | 7.86 | 0.000000 | 0.000001 | |||
| DorsalAttention.IPS (R) (39,−42,54)-Salience.SMG (R) (62,−35,32) | 7.85 | 0.000000 | 0.000001 | |||
| FrontoParietal.PPC (L) (−46,−58,49)-Language.pSTG (L) (−57,−47,15) | 7.82 | 0.000000 | 0.000001 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-Salience.AInsula (R) (47,14,0) | 7.77 | 0.000000 | 0.000001 | |||
| Salience.SMG (L) (−60,−39,31)-Salience.AInsula (R) (47,14,0) | 7.51 | 0.000000 | 0.000001 | |||
| Grey Matter—Language.pSTG (L) (−57,−47,15) | 7.47 | 0.000000 | 0.000001 | |||
| FrontoParietal.PPC (L) (−46,−58,49)-Grey Matter | 7.47 | 0.000000 | 0.000001 | |||
| Language.pSTG (L) (−57,−47,15)-Language.pSTG (R) (59,−42,13) | 7.42 | 0.000000 | 0.000001 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Language.IFG (L) (−51,26,2) | 7.37 | 0.000000 | 0.000001 | |||
| Salience.AInsula (L) (−44,13,1)-Salience.RPFC (L) (−32,45,27) | 7.33 | 0.000000 | 0.000001 | |||
| Language.pSTG (R) (59,−42,13)-FrontoParietal.PPC (R) (52,−52,45) | 7.26 | 0.000000 | 0.000002 | |||
| Salience.RPFC (L) (−32,45,27)-Salience.ACC (0,22,35) | 7.14 | 0.000000 | 0.000002 | |||
| Salience.SMG (R) (62,−35,32)-FrontoParietal.PPC (R) (52,−52,45) | 7.12 | 0.000000 | 0.000002 | |||
| Salience.RPFC (R) (32,46,27)-Salience.SMG (R) (62,−35,32) | 6.86 | 0.000000 | 0.000004 | |||
| Salience.SMG (R) (62,−35,32)-Language.IFG (R) (54,28,1) | 6.86 | 0.000000 | 0.000004 | |||
| Language.IFG (L) (−51,26,2)-Salience.SMG (L) (−60,−39,31) | 6.84 | 0.000000 | 0.000004 | |||
| DorsalAttentionIPS (R) (39,−42,54)-SensoriMotorLateral (R) (56,−10,29) | 6.70 | 0.000001 | 0.000005 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-Salience.SMG (L) (−60,−39,31) | 6.69 | 0.000001 | 0.000005 | |||
| Salience.AInsula (L) (−44,13,1)-Language.IFG (R) (54,28,1) | 6.52 | 0.000001 | 0.000007 | |||
| Salience.SMG (L) (−60,−39,31)-Salience.SMG (R) (62,−35,32) | 6.21 | 0.000002 | 0.000014 | |||
| Salience.AInsula (L) (−44,13,1)-Salience.SMG (R) (62,−35,32) | 6.10 | 0.000002 | 0.000018 | |||
| Salience.RPFC (L) (−32,45,27)-Salience.AInsula (R) (47,14,0) | 6.10 | 0.000002 | 0.000018 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Salience.RPFC (L) (−32,45,27) | 6.09 | 0.000002 | 0.000018 | |||
| Language.IFG (R) (54,28,1)-FrontoParietal.LPFC (R) (41,38,30) | 6.00 | 0.000003 | 0.000022 | |||
| Grey Matter—SensoriMotor.Lateral (R) (56,−10,29) | 5.98 | 0.000003 | 0.000023 | |||
| Language.pSTG (L) (−57,−47,15)-Salience.AInsula (L) (−44,13,1) | 5.66 | 0.000007 | 0.000051 | |||
| Salience.SMG (L) (−60,−39,31)-Language.IFG (R) (54,28,1) | 5.55 | 0.000009 | 0.000064 | |||
| Grey Matter—FrontoParietal.PPC (R) (52,−52,45) | 5.49 | 0.000011 | 0.000073 | |||
| Language.IFG (L) (−51,26,2)-Language.IFG (R) (54,28,1) | 5.40 | 0.000013 | 0.000087 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Salience.AInsula (L) (−44,13,1) | 5.39 | 0.000014 | 0.000089 | |||
| DorsalAttention.IPS (R) (39,−42,54)-FrontoParietal.PPC(R) (52,−52,45) | 5.25 | 0.000019 | 0.000121 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-SensoriMotor.Lateral (L) (−55,−12,29) | 5.25 | 0.000020 | 0.000121 | |||
| Salience.RPFC (R) (32,46,27)-Language.IFG (R) (54,28,1) | 5.08 | 0.000030 | 0.000177 | |||
| Language.IFG (R) (54,28,1)-FrontoParietal.PPC (R) (52,−52,45) | 4.96 | 0.000042 | 0.000235 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-Salience.SMG (R) (62,−35,32) | 4.92 | 0.000046 | 0.000257 | |||
| Salience.AInsula (L) (−44,13,1)-SensoriMotor.Lateral (R) (56,−10,29) | 4.90 | 0.000049 | 0.000267 | |||
| Grey Matter—Language.IFG (R) (54,28,1) | 4.87 | 0.000052 | 0.000282 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Grey Matter | 4.83 | 0.000058 | 0.000310 | |||
| Language.pSTG (L) (−57,−47,15)-Language.IFG (R) (54,28,1) | 4.77 | 0.000068 | 0.000360 | |||
| Salience.ACC (0,22,35)—Salience.SMG (R) (62,−35,32) | 4.74 | 0.000073 | 0.000377 | |||
| FrontoParietal.PPC (L) (−46,−58,49)-Salience.SMG (L) (−60,−39,31) | 4.73 | 0.000074 | 0.000377 | |||
| FrontoParietal.PPC (L) (−46,−58,49)-Language.IFG (L) (−51,26,2) | 4.72 | 0.000077 | 0.000388 | |||
| Language.IFG (L) (−51,26,2)-Salience.RPFC (L) (−32,45,27) | 4.55 | 0.000121 | 0.000576 | |||
| Salience.AInsula (R) (47,14,0)-Language.pSTG (R) (59,−42,13) | 4.41 | 0.000173 | 0.000785 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-FrontoParietal.PPC (L) (−46,−58,49) | 4.26 | 0.000256 | 0.001095 | |||
| Salience.RPFC (R) (32,46,27)-FrontoParietal.PPC (R) (52,−52,45) | 4.25 | 0.000257 | 0.001095 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Salience.SMG (L) (−60,−39,31) | 4.22 | 0.000281 | 0.001186 | |||
| Grey Matter—Salience.SMG (R) (62,−35,32) | 4.20 | 0.000293 | 0.001229 | |||
| Salience.SMG (R) (62,−35,32)-FrontoParietal.LPFC (R) (41,38,30) | 4.07 | 0.000419 | 0.001715 | |||
| DorsalAttention.IPS (R) (39,−42,54)-Salience.AInsula (R) (47,14,0) | 4.04 | 0.000449 | 0.001823 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-FrontoParietal.LPFC (L) (−43,33,28) | 3.97 | 0.000541 | 0.002134 | |||
| Salience.AInsula (L) (−44,13,1)-Salience.RPFC (R) (32,46,27) | 3.97 | 0.000542 | 0.002134 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-Language.IFG (R) (54,28,1) | 3.96 | 0.000543 | 0.002134 | |||
| DorsalAttention.IPS (R) (39,−42,54)-FrontoParietal.LPFC (R) (41,38,30) | 3.92 | 0.000613 | 0.002312 | |||
| Grey Matter—Language.IFG (L) (−51,26,2) | 3.91 | 0.000618 | 0.002315 | |||
| Salience.RPFC (R) (32,46,27)-Language.pSTG (R) (59,−42,13) | 3.88 | 0.000677 | 0.002496 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-FrontoParietal.PPC (R) (52,−52,45) | 3.88 | 0.000681 | 0.002496 | |||
| Grey Matter—Salience.SMG (L) (−60,−39,31) | 3.83 | 0.000766 | 0.002769 | |||
| Salience.SMG (L) (−60,−39,31)-Salience.RPFC (L) (−32,45,27) | 3.78 | 0.000859 | 0.003066 | |||
| Grey Matter—FrontoParietal.LPFC (R) (41,38,30) | 3.75 | 0.000930 | 0.003296 | |||
| Language.IFG (L) (−51,26,2)-Language.pSTG (R) (59,−42,13) | 3.68 | 0.001112 | 0.003913 | |||
| Salience.ACC (0,22,35)—SensoriMotor.Lateral (R) (56,−10,29) | 3.65 | 0.001213 | 0.004227 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-Salience.AInsula (L) (−44,13,1) | 3.65 | 0.001217 | 0.004227 | |||
| Language.IFG (L) (−51,26,2)-Salience.AInsula (R) (47,14,0) | 3.59 | 0.001390 | 0.004736 | |||
| Salience.AInsula (R) (47,14,0)-FrontoParietal.LPFC (R) (41,38,30) | 3.59 | 0.001407 | 0.004763 | |||
| Grey Matter—Salience.AInsula (R) (47,14,0) | 3.38 | 0.002369 | 0.007357 | |||
| Grey Matter—DorsalAttention.IPS (R) (39,−42,54) | 3.22 | 0.003542 | 0.010276 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-FrontoParietal.LPFC (R) (41,38,30) | 3.21 | 0.003588 | 0.010353 | |||
| Salience.RPFC (L) (−32,45,27)-Salience.SMG (R) (62,−35,32) | 3.14 | 0.004253 | 0.011944 | |||
| Grey Matter—Salience.AInsula (L) (−44,13,1) | 3.11 | 0.004589 | 0.012667 | |||
| Salience.SMG (L) (−60,−39,31)-Language.pSTG (R) (59,−42,13) | 3.08 | 0.004972 | 0.013394 | |||
| Salience.AInsula (L) (−44,13,1)-Language.pSTG (R) (59,−42,13) | 3.07 | 0.005150 | 0.013733 | |||
| Language.pSTG (L) (−57,−47,15)-Salience.AInsula (R) (47,14,0) | 2.96 | 0.006682 | 0.017295 | |||
| Salience.AInsula (L) (−44,13,1)-DorsalAttention.IPS (R) (39,−42,54) | 2.90 | 0.007595 | 0.019373 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Language.IFG (L) (−51,26,2) | 2.84 | 0.008857 | 0.021956 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Salience.AInsula (L) (−44,13,1) | 2.81 | 0.009504 | 0.023125 | |||
| DorsalAttention.IPS(L) (−39,−43,52)-FrontoParietal.LPFC (L) (−43,33,28) | 2.79 | 0.009868 | 0.023901 | |||
| DorsalAttention.FEF (L) (−27,−9,64)-DorsalAttention.IPS (L) (−39,−43,52) | 2.67 | 0.013260 | 0.030708 | |||
| Salience.ACC (0,22,35)—Language.IFG (R) (54,28,1) | 2.65 | 0.013653 | 0.031479 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Language.pSTG (L) (−57,−47,15) | 2.61 | 0.015160 | 0.034627 | |||
| Salience.RPFC (R) (32,46,27)-DorsalAttention.IPS (R) (39,−42,54) | 2.59 | 0.015644 | 0.035299 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-Salience.RPFC (L) (−32,45,27) | 2.52 | 0.018576 | 0.041039 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Salience.RPFC (L) (−32,45,27) | 2.37 | 0.025557 | 0.054632 | |||
| Salience.SMG (L) (−60,−39,31)-Salience.RPFC (R) (32,46,27) | 2.37 | 0.025732 | 0.054785 | |||
| DorsalAttention.IPS (R) (39,−42,54)-Language.IFG (R) (54,28,1) | 2.37 | 0.026029 | 0.055122 | |||
| DorsalAttention.FEF (L) (−27,−9,64)-Salience.RPFC (L) (−32,45,27) | 2.36 | 0.026369 | 0.055249 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Language.pSTG (L) (−57,−47,15) | 2.32 | 0.028845 | 0.059495 | |||
| Salience.SMG (L) (−60,−39,31)-Salience.ACC (0,22,35) | 2.30 | 0.029968 | 0.061329 | |||
| Language.pSTG (L) (−57,−47,15)-Salience.SMG (R) (62,−35,32) | 2.27 | 0.032417 | 0.065081 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Grey Matter | 2.25 | 0.033526 | 0.066614 | |||
| Language.pSTG (R) (59,−42,13)-FrontoParietal.LPFC (R) (41,38,30) | 2.24 | 0.034150 | 0.067334 | |||
| DorsalAttention.FEF (L) (−27,−9,64)-Salience.ACC (0,22,35) | 2.24 | 0.034177 | 0.067334 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Salience.SMG (L) (−60,−39,31) | 2.16 | 0.040530 | 0.078101 | |||
| Salience.AInsula (R) (47,14,0)-FrontoParietal.PPC (R) (52,−52,45) | 2.10 | 0.045912 | 0.086268 | |||
| Salience.RPFC (R) (32,46,27)-SensoriMotor.Lateral (R) (56,−10,29) | 2.10 | 0.046229 | 0.086556 | |||
| Cluster 3/34 | ||||||
| Score | 355.71 | 0.000000 | 0.000000 | 0.000000 | ||
| Mass | 916.48 | 0.000000 | 0.000000 | 0.000000 | ||
| Size | 26 | 0.000000 | 0.000000 | 0.000000 | ||
| DefaultMode.LP (R) (47,−67,29)-Grey Matter | 8.77 | 0.000000 | 0.000000 | |||
| DefaultMode.LP (L) (−39,−77,33)-Grey Matter | 8.43 | 0.000000 | 0.000000 | |||
| DefaultMode.LP (L) (−39,−77,33)-FrontoParietal.PPC (L) (−46,−58,49) | 7.41 | 0.000000 | 0.000001 | |||
| DefaultMode.PCC (1,−61,38)-Grey Matter | 7.00 | 0.000000 | 0.000003 | |||
| Visual.Lateral (R) (38,−72,13)-Grey Matter | 6.59 | 0.000001 | 0.000006 | |||
| Visual.Medial (2,−79,12)-Grey Matter | 6.15 | 0.000002 | 0.000016 | |||
| DefaultMode.MPFC (1,55,−3)-Grey Matter | 5.69 | 0.000006 | 0.000047 | |||
| Visual.Lateral (L) (−37,−79,10)-Language.pSTG (L) (−57,−47,15) | 4.75 | 0.000072 | 0.000376 | |||
| Visual.Lateral (L) (−37,−79,10)-Grey Matter | 4.74 | 0.000074 | 0.000377 | |||
| DefaultMode.LP (L) (−39,−77,33)-Language.pSTG (L) (−57,−47,15) | 4.62 | 0.000101 | 0.000490 | |||
| DefaultMode.MPFC (1,55,−3)-Language.IFG (L) (−51,26,2) | 3.61 | 0.001322 | 0.004534 | |||
| DefaultMode.MPFC (1,55,−3)-Language.pSTG (L) (−57,−47,15) | 2.79 | 0.009933 | 0.023904 | |||
| Visual.Lateral (L) (−37,−79,10)-Language.IFG (L) (−51,26,2) | 2.36 | 0.026219 | 0.055155 | |||
| Cluster 4/34 | ||||||
| Score | 188.22 | 0.000000 | 0.000000 | 0.000000 | ||
| Mass | 396.83 | 0.000000 | 0.000000 | 0.000000 | ||
| Size | 14 | 0.002785 | 0.011835 | 0.055000 | ||
| DefaultMode.LP (R) (47,−67,29)-Language.pSTG (R) (59,−42,13) | 8.53 | 0.000000 | 0.000000 | |||
| DefaultMode.LP (R) (47,−67,29)-FrontoParietal.PPC (R) (52,−52,45) | 8.39 | 0.000000 | 0.000000 | |||
| DefaultMode.LP (R) (47,−67,29)-FrontoParietal.LPFC (R) (41,38,30) | 4.40 | 0.000175 | 0.000785 | |||
| DefaultMode.MPFC (1,55,−3)-Language.pSTG (R) (59,−42,13) | 3.91 | 0.000626 | 0.002329 | |||
| DefaultMode.PCC (1,−61,38)-Language.pSTG (R) (59,−42,13) | 3.15 | 0.004203 | 0.011867 | |||
| DefaultMode.LP (R) (47,−67,29)-Salience.SMG (R) (62,−35,32) | 2.32 | 0.028569 | 0.059387 | |||
| DefaultMode.MPFC (1,55,−3)-Language.IFG (R) (54,28,1) | 2.31 | 0.029582 | 0.060775 | |||
| Cluster 5/34 | ||||||
| Score | 125.54 | 0.000000 | 0.000000 | 0.000000 | ||
| Mass | 384.25 | 0.000000 | 0.000000 | 0.000000 | ||
| Size | 20 | 0.000349 | 0.001979 | 0.008000 | ||
| DefaultMode.PCC (1,−61,38)-SensoriMotor.Lateral (R) (56,−10,29) | −8.41 | 0.000000 | 0.000000 | |||
| DefaultMode.PCC (1,−61,38)-Salience.AInsula (R) (47,14,0) | −5.11 | 0.000028 | 0.000170 | |||
| DefaultMode.LP (L) (−39,−77,33)-Salience.AInsula (R) (47,14,0) | −4.97 | 0.000040 | 0.000229 | |||
| DefaultMode.PCC (1,−61,38)-Language.IFG (R) (54,28,1) | −4.46 | 0.000150 | 0.000694 | |||
| DefaultMode.LP (L) (−39,−77,33)-Salience.SMG (R) (62,−35,32) | −3.56 | 0.001514 | 0.004995 | |||
| DefaultMode.LP (L) (−39,−77,33)-Language.IFG (R) (54,28,1) | −3.24 | 0.003374 | 0.009898 | |||
| DefaultMode.LP (L) (−39,−77,33)-SensoriMotor.Lateral (R) (56,−10,29) | −3.08 | 0.004954 | 0.013394 | |||
| DefaultMode.LP (R) (47,−67,29)-Salience.AInsula (R) (47,14,0) | −2.74 | 0.011175 | 0.026340 | |||
| DefaultMode.MPFC (1,55,−3)-Salience.SMG (R) (62,−35,32) | −2.49 | 0.019981 | 0.043775 | |||
| DefaultMode.PCC (1,−61,38)-Salience.SMG (R) (62,−35,32) | −2.08 | 0.048206 | 0.089309 | |||
| Cluster 6/34 | ||||||
| Score | 118.81 | 0.000000 | 0.000000 | 0.000000 | ||
| Mass | 853.83 | 0.000000 | 0.000000 | 0.000000 | ||
| Size | 70 | 0.000000 | 0.000000 | 0.000000 | ||
| DefaultMode.LP (R) (47,−67,29)-Salience.RPFC (L) (−32,45,27) | −6.32 | 0.000001 | 0.000011 | |||
| Cerebellar.Anterior (0,−63,−30)-Salience.ACC (0,22,35) | −5.51 | 0.000010 | 0.000069 | |||
| DefaultMode.LP (R) (47,−67,29)-Salience.AInsula (L) (−44,13,1) | −4.66 | 0.000089 | 0.000445 | |||
| DefaultMode.PCC (1,−61,38)-Salience.AInsula (L) (−44,13,1) | −4.65 | 0.000092 | 0.000453 | |||
| Visual.Lateral (R) (38,−72,13)-Salience.RPFC (L) (−32,45,27) | −4.47 | 0.000148 | 0.000692 | |||
| DefaultMode.LP (R) (47,−67,29)-Salience.SMG (L) (−60,−39,31) | −4.38 | 0.000186 | 0.000820 | |||
| DefaultMode.PCC (1,−61,38)-Language.IFG (L) (−51,26,2) | −4.29 | 0.000235 | 0.001016 | |||
| Visual.Lateral (R) (38,−72,13)-Salience.ACC (0,22,35) | −4.14 | 0.000344 | 0.001430 | |||
| DefaultMode.LP (R) (47,−67,29)-Salience.ACC (0,22,35) | −3.96 | 0.000546 | 0.002134 | |||
| Visual.Lateral (L) (−37,−79,10)-Salience.ACC (0,22,35) | −3.87 | 0.000688 | 0.002507 | |||
| Visual.Lateral (L) (−37,−79,10)-Salience.RPFC (L) (−32,45,27) | −3.63 | 0.001272 | 0.004389 | |||
| Visual.Lateral (L) (−37,−79,10)-SensoriMotor.Lateral (R) (56,−10,29) | −3.56 | 0.001525 | 0.005000 | |||
| DefaultMode.LP (L) (−39,−77,33)-Salience.RPFC (R) (32,46,27) | −3.45 | 0.001983 | 0.006271 | |||
| DefaultMode.LP (L) (−39,−77,33)-Salience.AInsula (L) (−44,13,1) | −3.44 | 0.002057 | 0.006465 | |||
| Visual.Occipital (0,−93,−4)-SensoriMotor.Lateral (R) (56,−10,29) | −3.32 | 0.002745 | 0.008331 | |||
| Visual.Occipital (0,−93,−4)-Salience.ACC (0,22,35) | −3.29 | 0.002947 | 0.008790 | |||
| Visual.Occipital (0,−93,−4)-Salience.SMG (R) (62,−35,32) | −3.22 | 0.003525 | 0.010276 | |||
| Visual.Occipital (0,−93,−4)-Salience.RPFC (R) (32,46,27) | −3.12 | 0.004528 | 0.012611 | |||
| DefaultMode.MPFC (1,55,−3)-Salience.RPFC (L) (−32,45,27) | −3.11 | 0.004606 | 0.012667 | |||
| Visual.Lateral (L) (−37,−79,10)-Salience.SMG (R) (62,−35,32) | −2.94 | 0.006940 | 0.017788 | |||
| Cerebellar.Posterior (0,−79,−32)-Salience.ACC (0,22,35) | −2.82 | 0.009323 | 0.022941 | |||
| DefaultMode.LP (L) (−39,−77,33)-Salience.ACC (0,22,35) | −2.81 | 0.009385 | 0.022941 | |||
| DefaultMode.PCC (1,−61,38)-Salience.SMG (L) (−60,−39,31) | −2.79 | 0.009960 | 0.023904 | |||
| Visual.Lateral (R) (38,−72,13)-Salience.SMG (L) (−60,−39,31) | −2.79 | 0.010011 | 0.023919 | |||
| Visual.Medial (2,−79,12)-Salience.ACC (0,22,35) | −2.77 | 0.010344 | 0.024491 | |||
| Visual.Lateral (L) (−37,−79,10)-Salience.RPFC (R) (32,46,27) | −2.68 | 0.012817 | 0.029812 | |||
| Visual.Medial (2,−79,12)-Salience.SMG (R) (62,−35,32) | −2.63 | 0.014391 | 0.033036 | |||
| Cerebellar.Anterior (0,−63,−30)-DorsalAttention.IPS (R) (39,−42,54) | −2.57 | 0.016571 | 0.036992 | |||
| DefaultMode.LP (L) (−39,−77,33)-Salience.RPFC (L) (−32,45,27) | −2.57 | 0.016604 | 0.036992 | |||
| Visual.Lateral (R) (38,−72,13)-Salience.RPFC (R) (32,46,27) | −2.45 | 0.021611 | 0.046956 | |||
| DefaultMode.LP (R) (47,−67,29)-Language.IFG (L) (−51,26,2) | −2.38 | 0.025499 | 0.054632 | |||
| Cerebellar.Posterior (0,−79,−32)-SensoriMotor.Lateral (R) (56,−10,29) | −2.29 | 0.031057 | 0.063314 | |||
| Visual.Occipital (0,−93,−4)-Salience.AInsula (R) (47,14,0) | −2.27 | 0.031800 | 0.064567 | |||
| Visual.Medial (2,−79,12)-DorsalAttention.IPS (R) (39,−42,54) | −2.23 | 0.034611 | 0.067612 | |||
| Cerebellar.Posterior (0,−79,−32)-DorsalAttention.IPS (R) (39,−42,54) | −2.12 | 0.044535 | 0.084890 | |||
| Cluster 7/34 | ||||||
| Score | 90.26 | 0.000000 | 0.000000 | 0.000000 | ||
| Mass | 439.25 | 0.000000 | 0.000000 | 0.000000 | ||
| Size | 34 | 0.000000 | 0.000000 | 0.000000 | ||
| SensoriMotor.Superior (0,−31,67)-FrontoParietal.LPFC (R) (41,38,30) | −5.42 | 0.000012 | 0.000085 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-FrontoParietal.LPFC (R) (41,38,30) | −5.42 | 0.000013 | 0.000085 | |||
| DorsalAttention.FEF (L) (−27,−9,64)-FrontoParietal.PPC (R) (52,−52,45) | −4.61 | 0.000101 | 0.000490 | |||
| DorsalAttention.FEF (L) (−27,−9,64)-FrontoParietal.LPFC (R) (41,38,30) | −4.45 | 0.000156 | 0.000718 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Language.pSTG (R) (59,−42,13) | −4.40 | 0.000174 | 0.000785 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Language.pSTG (R) (59,−42,13) | −3.46 | 0.001953 | 0.006212 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Salience.SMG (R) (62,−35,32) | −3.40 | 0.002248 | 0.007023 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-Language.pSTG (R) (59,−42,13) | −3.30 | 0.002898 | 0.008714 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-FrontoParietal.PPC (R) (52,−52,45) | −3.17 | 0.003953 | 0.011221 | |||
| SensoriMotor.Superior (0,−31,67)-Language.IFG (R) (54,28,1) | −3.07 | 0.005103 | 0.013676 | |||
| SensoriMotor.Superior (0,−31,67)-Salience.AInsula (R) (47,14,0) | −2.99 | 0.006113 | 0.015980 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Language.IFG (R) (54,28,1) | −2.78 | 0.010188 | 0.024231 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Language.IFG (R) (54,28,1) | −2.73 | 0.011524 | 0.027042 | |||
| DorsalAttention.FEF (L) (−27,−9,64)-Salience.SMG (R) (62,−35,32) | −2.71 | 0.011970 | 0.027965 | |||
| DorsalAttention.FEF (L) (−27,−9,64)-Language.pSTG (R) (59,−42,13) | −2.27 | 0.031917 | 0.064567 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-FrontoParietal.LPFC (R) (41,38,30) | −2.25 | 0.033559 | 0.066614 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-FrontoParietal.PPC (R) (52,−52,45) | −2.17 | 0.039383 | 0.076421 | |||
| Cluster 8/34 | ||||||
| Score | 78.54 | 0.000061 | 0.000208 | 0.001000 | ||
| Mass | 199.95 | 0.000055 | 0.000232 | 0.001000 | ||
| Size | 12 | 0.005748 | 0.019542 | 0.106000 | ||
| DefaultMode.LP (R) (47,−67,29)-SensoriMotor.Lateral (L) (−55,−12,29) | −6.24 | 0.000002 | 0.000014 | |||
| DefaultMode.PCC (1,−61,38)-SensoriMotor.Lateral (L) (−55,−12,29) | −5.04 | 0.000033 | 0.000193 | |||
| DefaultMode.LP (R) (47,−67,29)-DorsalAttention.IPS (L) (−39,−43,52) | −3.79 | 0.000851 | 0.003057 | |||
| DefaultMode.LP (R) (47,−67,29)-FrontoParietal.LPFC (L) (−43,33,28) | −3.25 | 0.003298 | 0.009729 | |||
| DefaultMode.MPFC (1,55,−3)-DorsalAttention.IPS (L) (−39,−43,52) | −2.40 | 0.024261 | 0.052285 | |||
| DefaultMode.LP (R) (47,−67,29)-DorsalAttention.FEF (L) (−27,−9,64) | −2.23 | 0.034702 | 0.067612 | |||
| DefaultMode.LP (R) (47,−67,29)-SensoriMotor.Lateral (L) (−55,−12,29) | −6.24 | 0.000002 | 0.000014 | |||
| DefaultMode.PCC (1,−61,38)-SensoriMotor.Lateral (L) (−55,−12,29) | −5.04 | 0.000033 | 0.000193 | |||
| DefaultMode.LP (R) (47,−67,29)-DorsalAttention.IPS (L) (−39,−43,52) | −3.79 | 0.000851 | 0.003057 | |||
| DefaultMode.LP (R) (47,−67,29)-FrontoParietal.LPFC (L) (−43,33,28) | −3.25 | 0.003298 | 0.009729 | |||
| DefaultMode.MPFC (1,55,−3)-DorsalAttention.IPS (L) (−39,−43,52) | −2.40 | 0.024261 | 0.052285 | |||
| DefaultMode.LP (R) (47,−67,29)-DorsalAttention.FEF (L) (−27,−9,64) | −2.23 | 0.034702 | 0.067612 | |||
| Cluster 9/34 | ||||||
| Score | 143.43 | 0.000000 | 0.000000 | 0.000000 | ||
| Mass | 188.19 | 0.000062 | 0.000232 | 0.001000 | ||
| Size | 6 | 0.063172 | 0.113045 | 0.675000 | ||
| SensoriMotor.Lateral (L) (−55,−12,29)-SensoriMotor.Lateral (R)(56,−10,29) | 7.58 | 0.000000 | 0.000001 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-DorsalAttention.IPS (R) (39,−42,54) | 4.61 | 0.000104 | 0.000498 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Salience.AInsula (R) (47,14,0) | 3.92 | 0.000605 | 0.002300 | |||
| Cluster 10/34 | ||||||
| Score | 55.03 | 0.000438 | 0.001241 | 0.009000 | ||
| Mass | 174.82 | 0.000069 | 0.000232 | 0.001000 | ||
| Size | 14 | 0.002785 | 0.011835 | 0.055000 | ||
| Visual.Medial (2,−79,12)-DorsalAttention.IPS (L) (−39,−43,52) | −5.70 | 0.000006 | 0.000046 | |||
| Visual.Lateral (L) (−37,−79,10)-SensoriMotor.Superior (0,−31,67) | −3.51 | 0.001718 | 0.005572 | |||
| Visual.Medial (2,−79,12)-SensoriMotor.Superior (0,−31,67) | −3.48 | 0.001877 | 0.006007 | |||
| Visual.Occipital (0,−93,−4)-DorsalAttention.IPS (L) (−39,−43,52) | −3.12 | 0.004538 | 0.012611 | |||
| Visual.Lateral (R) (38,−72,13)-DorsalAttention.FEF (L) (−27,−9,64) | −2.87 | 0.008253 | 0.020749 | |||
| Visual.Medial (2,−79,12)-DorsalAttention.FEF (L) (−27,−9,64) | −2.84 | 0.008755 | 0.021909 | |||
| Visual.Lateral (R) (38,−72,13)-SensoriMotor.Superior (0,−31,67) | −2.10 | 0.045692 | 0.086233 | |||
| Cluster 11/34 | ||||||
| Score | 117.49 | 0.000000 | 0.000000 | 0.000000 | ||
| Mass | 164.76 | 0.000075 | 0.000232 | 0.001000 | ||
| Size | 6 | 0.063172 | 0.113045 | 0.675000 | ||
| Visual.Lateral (R) (38,−72,13)-SensoriMotor.Lateral (L) (−55,−12,29) | −5.55 | 0.000009 | 0.000064 | |||
| Visual.Lateral (R) (38,−72,13)-FrontoParietal.LPFC (L) (−43,33,28) | −5.37 | 0.000014 | 0.000092 | |||
| Visual.Lateral (R) (38,−72,13)-FrontoParietal.PPC (L) (−46,−58,49) | −4.78 | 0.000067 | 0.000355 | |||
| Cluster 12/34 | ||||||
| Score | 37.05 | 0.003830 | 0.010018 | 0.079000 | ||
| Mass | 121.17 | 0.001439 | 0.004076 | 0.028000 | ||
| Size | 12 | 0.005748 | 0.019542 | 0.106000 | ||
| Cerebellar.Anterior (0,−63,−30)-Grey Matter | 4.39 | 0.000183 | 0.000814 | |||
| Cerebellar.Posterior (0,−79,−32)-Language.IFG (L) (−51,26,2) | 3.57 | 0.001489 | 0.004944 | |||
| Visual.Occipital (0,−93,−4)-Language.IFG (L) (−51,26,2) | 3.10 | 0.004738 | 0.012962 | |||
| Cerebellar.Anterior (0,−63,−30)-Salience.SMG (L) (−60,−39,31) | 2.82 | 0.009367 | 0.022941 | |||
| Cerebellar.Anterior (0,−63,−30)-Language.pSTG (L) (−57,−47,15) | 2.37 | 0.026100 | 0.055122 | |||
| Cerebellar.Posterior (0,−79,−32)-Language.pSTG (L) (−57,−47,15) | 2.34 | 0.027347 | 0.057073 | |||
| Analysis Unit | T (12)−Value | p-unc | p-FDR | p-FWE | ||
|---|---|---|---|---|---|---|
| Cluster 1/40 | ||||||
| Mass | 3177.80 | 0.000000 | 0.000000 | 0.000000 | ||
| DefaultMode.LP (R) (47,−67,29)-DefaultMode.LP (L) (−39,−77,33) | 9.88 | 0.000000 | 0.000047 | |||
| Salience.ACC (0,22,35)—Salience.AInsula (L) (−44,13,1) | 8.70 | 0.000002 | 0.000139 | |||
| Salience.AInsula (R) (47,14,0)-Language.IFG (R) (54,28,1) | 8.40 | 0.000002 | 0.000171 | |||
| Salience.SMG (R) (62,−35,32)-Language.pSTG (R) (59,−42,13) | 7.58 | 0.000007 | 0.000372 | |||
| FrontoParietal.LPFC (R) (41,38,30)-FrontoParietal.PPC (R) (52,−52,45) | 7.52 | 0.000007 | 0.000372 | |||
| FrontoParietal.PPC (R) (52,−52,45)-Language.pSTG (R) (59,−42,13) | 6.61 | 0.000025 | 0.000865 | |||
| DefaultMode.LP (R) (47,−67,29)-DefaultMode.PCC (1,−61,38) | 6.59 | 0.000026 | 0.000865 | |||
| Language.pSTG (R) (59,−42,13)-Grey Matter | 6.58 | 0.000026 | 0.000865 | |||
| Language.pSTG (R) (59,−42,13)-DefaultMode.LP (R) (47,−67,29) | 6.47 | 0.000031 | 0.000958 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-DorsalAttention.IPS (R) (39,−42,54) | 6.40 | 0.000034 | 0.000963 | |||
| Grey Matter—DefaultMode.LP (L) (−39,−77,33) | 6.36 | 0.000036 | 0.000963 | |||
| Salience.SMG (R) (62,−35,32)-DorsalAttention.IPS (R) (39,−42,54) | 6.35 | 0.000036 | 0.000963 | |||
| FrontoParietal.PPC (R) (52,−52,45)-DefaultMode.LP (R) (47,−67,29) | 6.28 | 0.000041 | 0.001025 | |||
| Grey Matter—DefaultMode.LP (R) (47,−67,29) | 6.19 | 0.000047 | 0.001117 | |||
| Salience.SMG (R) (62,−35,32)-FrontoParietal.PPC (R) (52,−52,45) | 6.03 | 0.000059 | 0.001356 | |||
| Salience.AInsula (L) (−44,13,1)-Salience.AInsula (R) (47,14,0) | 5.90 | 0.000072 | 0.001525 | |||
| FrontoParietal.PPC (R) (52,−52,45)-Grey Matter | 5.55 | 0.000126 | 0.002384 | |||
| Salience.AInsula (R) (47,14,0)-SensoriMotor.Lateral (R) (56,−10,29) | 5.36 | 0.000170 | 0.002684 | |||
| Salience.SMG (L) (−60,−39,31)-Salience.AInsula (R) (47,14,0) | 5.35 | 0.000174 | 0.002684 | |||
| Grey Matter—DefaultMode.PCC (1,−61,38) | 5.34 | 0.000177 | 0.002684 | |||
| FrontoParietal.LPFC (R) (41,38,30)-Grey Matter | 5.15 | 0.000243 | 0.002916 | |||
| Salience.RPFC (R) (32,46,27)-Salience.RPFC (L) (−32,45,27) | 4.98 | 0.000318 | 0.003576 | |||
| DefaultMode.LP (L) (−39,−77,33)-DefaultMode.MPFC (1,55,−3) | 4.84 | 0.000404 | 0.004311 | |||
| Salience.ACC (0,22,35)—Salience.AInsula (R) (47,14,0) | 4.84 | 0.000408 | 0.004311 | |||
| Language.IFG (R) (54,28,1)-Salience.SMG (R) (62,−35,32) | 4.72 | 0.000498 | 0.005156 | |||
| Salience.AInsula (L) (−44,13,1)-Language.IFG (R) (54,28,1) | 4.69 | 0.000521 | 0.005291 | |||
| Grey Matter—DefaultMode.MPFC (1,55,−3) | 4.68 | 0.000534 | 0.005321 | |||
| Language.IFG (R) (54,28,1)-FrontoParietal.LPFC (R) (41,38,30) | 4.60 | 0.000610 | 0.005901 | |||
| Salience.SMG (R) (62,−35,32)-SensoriMotor.Lateral (R) (56,−10,29) | 4.60 | 0.000615 | 0.005901 | |||
| Salience.ACC (0,22,35)—Salience.RPFC (L) (−32,45,27) | 4.56 | 0.000656 | 0.006183 | |||
| Salience.RPFC (R) (32,46,27)-Salience.ACC (0,22,35) | 4.43 | 0.000827 | 0.007526 | |||
| Salience.SMG (L) (−60,−39,31)-Salience.AInsula (L) (−44,13,1) | 4.39 | 0.000874 | 0.007693 | |||
| Salience.SMG (R) (62,−35,32)-FrontoParietal.LPFC (R) (41,38,30) | 4.33 | 0.000974 | 0.008159 | |||
| Language.IFG (R) (54,28,1)-Grey Matter | 4.30 | 0.001025 | 0.008378 | |||
| Salience.AInsula (L) (−44,13,1)-Salience.SMG (R) (62,−35,32) | 4.30 | 0.001031 | 0.008378 | |||
| Salience.SMG (L) (−60,−39,31)-Language.IFG (R) (54,28,1) | 4.27 | 0.001085 | 0.008684 | |||
| Salience.AInsula (R) (47,14,0)-Salience.SMG (R) (62,−35,32) | 4.26 | 0.001112 | 0.008766 | |||
| Salience.RPFC (R) (32,46,27)-Salience.AInsula (R) (47,14,0) | 4.19 | 0.001247 | 0.009541 | |||
| DefaultMode.PCC (1,−61,38)-DefaultMode.MPFC (1,55,−3) | 4.03 | 0.001675 | 0.011973 | |||
| Language.IFG (R) (54,28,1)-Language.pSTG (R) (59,−42,13) | 4.03 | 0.001678 | 0.011973 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-Grey Matter | 3.91 | 0.002058 | 0.013662 | |||
| Salience.RPFC (L) (−32,45,27)-Salience.AInsula (L) (−44,13,1) | 3.91 | 0.002070 | 0.013662 | |||
| Salience.SMG (L) (−60,−39,31)-Salience.SMG (R) (62,−35,32) | 3.81 | 0.002483 | 0.016161 | |||
| DorsalAttention.IPS (R) (39,−42,54)-FrontoParietal.PPC (R) (52,−52,45) | 3.80 | 0.002510 | 0.016161 | |||
| Salience.AInsula (L) (−44,13,1)-SensoriMotor.Lateral (R) (56,−10,29) | 3.76 | 0.002716 | 0.016950 | |||
| DefaultMode.LP (L) (−39,−77,33)-DefaultMode.PCC (1,−61,38) | 3.71 | 0.002955 | 0.017963 | |||
| Language.IFG (R) (54,28,1)-FrontoParietal.PPC (R) (52,−52,45) | 3.65 | 0.003329 | 0.019315 | |||
| DefaultMode.LP (R) (47,−67,29)-DefaultMode.MPFC (1,55,−3) | 3.64 | 0.003395 | 0.019482 | |||
| Salience.RPFC (R) (32,46,27)-Salience.SMG (R) (62,−35,32) | 3.48 | 0.004539 | 0.023609 | |||
| DorsalAttention.IPS (R) (39,−42,54)-Grey Matter | 3.45 | 0.004829 | 0.024755 | |||
| Language.IFG (R) (54,28,1)-SensoriMotor.Lateral (R) (56,−10,29) | 3.36 | 0.005678 | 0.028281 | |||
| DorsalAttention.IPS (R) (39,−42,54)-FrontoParietal.LPFC (R) (41,38,30) | 3.24 | 0.007055 | 0.032963 | |||
| FrontoParietal.LPFC (R) (41,38,30)-DefaultMode.LP (R) (47,−67,29) | 3.01 | 0.010799 | 0.045223 | |||
| Salience.ACC (0,22,35)—Language.IFG (R) (54,28,1) | 2.98 | 0.011432 | 0.046431 | |||
| Salience.RPFC (L) (−32,45,27)-Salience.AInsula (R) (47,14,0) | 2.96 | 0.011839 | 0.047357 | |||
| Salience.RPFC (R) (32,46,27)-Language.IFG (R) (54,28,1) | 2.93 | 0.012604 | 0.050035 | |||
| Salience.AInsula (R) (47,14,0)-DorsalAttention.IPS (R) (39,−42,54) | 2.84 | 0.014821 | 0.056705 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-FrontoParietal.PPC (R) (52,−52,45) | 2.73 | 0.018135 | 0.065140 | |||
| Salience.SMG (R) (62,−35,32)-Grey Matter | 2.57 | 0.024741 | 0.083871 | |||
| Salience.AInsula (L) (−44,13,1)-DorsalAttention.IPS (R) (39,−42,54) | 2.50 | 0.027935 | 0.090488 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-Language.pSTG (R) (59,−42,13) | 2.48 | 0.028934 | 0.092032 | |||
| Salience.AInsula (R) (47,14,0)-FrontoParietal.LPFC (R) (41,38,30) | 2.45 | 0.030374 | 0.095067 | |||
| Language.IFG (R) (54,28,1)-DorsalAttention.IPS (R) (39,−42,54) | 2.40 | 0.033823 | 0.103229 | |||
| Salience.ACC (0,22,35)—Salience.SMG (L) (−60,−39,31) | 2.38 | 0.034910 | 0.105329 | |||
| Salience.RPFC (R) (32,46,27)-Salience.AInsula (L) (−44,13,1) | 2.34 | 0.037116 | 0.110718 | |||
| FrontoParietal.LPFC (R) (41,38,30)-Language.pSTG (R) (59,−42,13) | 2.28 | 0.041394 | 0.121422 | |||
| Language.pSTG (R) (59,−42,13)-DefaultMode.MPFC (1,55,−3) | 2.25 | 0.043840 | 0.127887 | |||
| Cluster 2/40 | ||||||
| Mass | 1038.87 | 0.000000 | 0.000000 | 0.000000 | ||
| Visual.Medial (2,−79,12)- Visual.Lateral (R) (38,−72,13) | 9.89 | 0.000000 | 0.000047 | |||
| Cerebellar.Posterior (0,−79,−32)-Cerebellar.Anterior (0,−63,−30) | 9.81 | 0.000000 | 0.000047 | |||
| Visual.Occipital (0,−93,−4)-Visual.Medial (2,−79,12) | 8.18 | 0.000003 | 0.000198 | |||
| Visual.Occipital (0,−93,−4)-Visual.Lateral (R) (38,−72,13) | 7.42 | 0.000008 | 0.000387 | |||
| Cerebellar.Anterior (0,−63,−30)-Visual.Medial (2,−79,12) | 5.99 | 0.000063 | 0.001387 | |||
| Visual.Lateral (L) (−37,−79,10)-Visual.Lateral (R) (38,−72,13) | 5.72 | 0.000095 | 0.001865 | |||
| Visual.Occipital (0,−93,−4)-Visual.Lateral (L) (−37,−79,10) | 5.34 | 0.000177 | 0.002684 | |||
| Visual.Medial (2,−79,12)-Visual.Lateral (L) (−37,−79,10) | 5.33 | 0.000178 | 0.002684 | |||
| Cerebellar.Posterior (0,−79,−32)-Visual.Occipital (0,−93,−4) | 4.48 | 0.000755 | 0.006990 | |||
| Cerebellar.Posterior (0,−79,−32)-Visual.Medial (2,−79,12) | 4.23 | 0.001174 | 0.009117 | |||
| Cerebellar.Anterior (0,−63,−30)-Visual.Lateral (R) (38,−72,13) | 3.93 | 0.002011 | 0.013662 | |||
| Cerebellar.Anterior (0,−63,−30)-Visual.Occipital (0,−93,−4) | 3.66 | 0.003265 | 0.019315 | |||
| Cerebellar.Anterior (0,−63,−30)-Visual.Lateral (L) (−37,−79,10) | 3.34 | 0.005868 | 0.028956 | |||
| Cluster 3/40 | ||||||
| Mass | 910.51 | 0.000000 | 0.000000 | 0.000000 | ||
| Salience.SMG (L) (−60,−39,31)-Language.pSTG (L) (−57,−47,15) | 13.91 | 0.000000 | 0.000005 | |||
| Salience.AInsula (L) (−44,13,1)-Language.IFG (L) (−51,26,2) | 10.51 | 0.000000 | 0.000047 | |||
| Language.IFG (R) (54,28,1)-Language.pSTG (L) (−57,−47,15) | 4.92 | 0.000353 | 0.003883 | |||
| Salience.SMG (L) (−60,−39,31)-Language.IFG (L) (−51,26,2) | 4.37 | 0.000917 | 0.007940 | |||
| Salience.SMG (L) (−60,−39,31)-DorsalAttention.IPS (L) (−39,−43,52) | 4.35 | 0.000944 | 0.008035 | |||
| Salience.SMG (L) (−60,−39,31)-FrontoParietal.PPC (L) (−46,−58,49) | 3.76 | 0.002729 | 0.016950 | |||
| Language.IFG (R) (54,28,1)-Language.IFG (L) (−51,26,2) | 3.61 | 0.003608 | 0.020051 | |||
| Salience.AInsula (L) (−44,13,1)-SensoriMotor.Lateral (L) (−55,−12,29) | 3.52 | 0.004243 | 0.022862 | |||
| Salience.AInsula (R) (47,14,0)-SensoriMotor.Lateral (L) (−55,−12,29) | 3.31 | 0.006229 | 0.030264 | |||
| Salience.SMG (L) (−60,−39,31)-SensoriMotor.Lateral (L) (−55,−12,29) | 3.20 | 0.007671 | 0.035146 | |||
| Salience.RPFC (L) (−32,45,27)-FrontoParietal.LPFC (L) (−43,33,28) | 3.05 | 0.010060 | 0.042494 | |||
| Salience.AInsula (L) (−44,13,1)-Language.pSTG (L) (−57,−47,15) | 2.68 | 0.020155 | 0.070014 | |||
| Salience.RPFC (L) (−32,45,27)-Language.IFG (L) (−51,26,2) | 2.48 | 0.028738 | 0.091961 | |||
| Salience.AInsula (R) (47,14,0)-Language.IFG (L) (−51,26,2) | 2.43 | 0.031639 | 0.098253 | |||
| Cluster 4/40 | ||||||
| Mass | 371.40 | 0.000126 | 0.001262 | 0.002000 | ||
| Salience.SMG (R) (62,−35,32)-DefaultMode.LP (L) (−39,−77,33) | −5.25 | 0.000204 | 0.002730 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-DefaultMode.PCC (1,−61,38) | −5.24 | 0.000207 | 0.002730 | |||
| Salience.AInsula (R) (47,14,0)-DefaultMode.LP (L) (−39,−77,33) | −4.01 | 0.001723 | 0.012133 | |||
| Salience.ACC (0,22,35)—DefaultMode.LP (R) (47,−67,29) | −3.50 | 0.004397 | 0.023218 | |||
| Language.IFG (R) (54,28,1)-DefaultMode.LP (L) (−39,−77,33) | −3.48 | 0.004561 | 0.023609 | |||
| Salience.SMG (L) (−60,−39,31)-DefaultMode.PCC (1,−61,38) | −3.28 | 0.006602 | 0.031547 | |||
| Salience.SMG (L) (−60,−39,31)-DefaultMode.LP (R) (47,−67,29) | −3.25 | 0.006973 | 0.032874 | |||
| Language.IFG (R) (54,28,1)-DefaultMode.PCC (1,−61,38) | −3.15 | 0.008328 | 0.037200 | |||
| Salience.AInsula (L) (−44,13,1)-DefaultMode.LP (R) (47,−67,29) | −3.15 | 0.008384 | 0.037200 | |||
| Salience.AInsula (R) (47,14,0)-DefaultMode.PCC (1,−61,38) | −3.11 | 0.008997 | 0.039586 | |||
| Salience.AInsula (R) (47,14,0)-DefaultMode.LP (R) (47,−67,29) | −3.10 | 0.009192 | 0.040112 | |||
| Salience.AInsula (L) (−44,13,1)-DefaultMode.PCC (1,−61,38) | −2.89 | 0.013555 | 0.053015 | |||
| Salience.AInsula (L) (−44,13,1)-DefaultMode.LP (L) (−39,−77,33) | −2.82 | 0.015480 | 0.057826 | |||
| Salience.RPFC (L) (−32,45,27)-DefaultMode.LP (R) (47,−67,29) | −2.68 | 0.020053 | 0.070014 | |||
| DorsalAttention.IPS (R) (39,−42,54)-DefaultMode.PCC (1,−61,38) | −2.50 | 0.027781 | 0.090488 | |||
| Cluster 5/40 | ||||||
| Mass | 299.41 | 0.000352 | 0.002818 | 0.008000 | ||
| Language.pSTG (L) (−57,−47,15)-FrontoParietal.PPC (L) (−46,−58,49) | 7.03 | 0.000014 | 0.000605 | |||
| FrontoParietal.PPC (L) (−46,−58,49)-FrontoParietal.LPFC (L) (−43,33,28) | 5.82 | 0.000082 | 0.001674 | |||
| Language.IFG (L) (−51,26,2)-FrontoParietal.LPFC (L) (−43,33,28) | 5.32 | 0.000182 | 0.002684 | |||
| Language.IFG (L) (−51,26,2)-Language.pSTG (L) (−57,−47,15) | 5.19 | 0.000227 | 0.002910 | |||
| Language.pSTG (L) (−57,−47,15)-Cerebellar.Posterior (0,−79,−32) | 2.40 | 0.033319 | 0.102283 | |||
| Language.IFG (L) (−51,26,2)-FrontoParietal.PPC (L) (−46,−58,49) | 2.34 | 0.037644 | 0.111663 | |||
| Cluster 6/40 | ||||||
| Mass | 254.00 | 0.000515 | 0.003435 | 0.011000 | ||
| Language.pSTG (R) (59,−42,13)-Language.pSTG (L) (−57,−47,15) | 5.32 | 0.000183 | 0.002684 | |||
| FrontoParietal.PPC (R) (52,−52,45)-FrontoParietal.PPC (L) (−46,−58,49) | 5.30 | 0.000188 | 0.002684 | |||
| Grey Matter—FrontoParietal.PPC (L) (−46,−58,49) | 5.25 | 0.000204 | 0.002730 | |||
| Grey Matter—Language.pSTG (L) (−57,−47,15) | 4.42 | 0.000841 | 0.007526 | |||
| Language.pSTG (R) (59,−42,13)-Language.IFG (L) (−51,26,2) | 3.09 | 0.009410 | 0.040394 | |||
| Grey Matter—Language.IFG (L) (−51,26,2) | 2.76 | 0.017323 | 0.063518 | |||
| Grey Matter—SensoriMotor.Lateral (L) (−55,−12,29) | 2.53 | 0.026353 | 0.086966 | |||
| Cluster 7/40 | ||||||
| Mass | 205.24 | 0.000956 | 0.005135 | 0.019000 | ||
| FrontoParietal.LPFC (R) (41,38,30)-SensoriMotor.Lateral (L) (−55,−12,29) | −4.08 | 0.001520 | 0.011147 | |||
| FrontoParietal.PPC (R) (52,−52,45)-DorsalAttention.FEF (L) (−27,−9,64) | −3.61 | 0.003558 | 0.019984 | |||
| Grey Matter—SensoriMotor.Superior (0,−31,67) | −3.58 | 0.003766 | 0.020715 | |||
| FrontoParietal.PPC (R) (52,−52,45)-SensoriMotor.Lateral (L) (−55,−12,29) | −3.28 | 0.006632 | 0.031547 | |||
| FrontoParietal.LPFC (R) (41,38,30)-SensoriMotor.Superior (0,−31,67) | −3.19 | 0.007721 | 0.035146 | |||
| Grey Matter—DorsalAttention.FEF (L) (−27,−9,64) | −3.17 | 0.008047 | 0.036313 | |||
| Language.pSTG (R) (59,−42,13)-DorsalAttention.IPS (L) (−39,−43,52) | −2.88 | 0.013836 | 0.053718 | |||
| FrontoParietal.LPFC (R) (41,38,30)-DorsalAttention.FEF (L) (−27,−9,64) | −2.82 | 0.015552 | 0.057826 | |||
| Language.pSTG (R) (59,−42,13)-DorsalAttention.FEF (L) (−27,−9,64) | −2.60 | 0.023087 | 0.079157 | |||
| DefaultMode.LP (R) (47,−67,29)-DorsalAttention.FEF (L) (−27,−9,64) | −2.46 | 0.029823 | 0.094292 | |||
| Cluster 8/40 | ||||||
| Mass | 198.47 | 0.001027 | 0.005135 | 0.020000 | ||
| Grey Matter—Visual.Lateral (R) (38,−72,13) | 5.17 | 0.000231 | 0.002910 | |||
| Grey Matter—Visual.Medial (2,−79,12) | 5.15 | 0.000241 | 0.002916 | |||
| DefaultMode.LP (L) (−39,−77,33)-Visual.Lateral (L) (−37,−79,10) | 3.91 | 0.002070 | 0.013662 | |||
| DefaultMode.LP (R) (47,−67,29)-Visual.Lateral (R) (38,−72,13) | 3.76 | 0.002729 | 0.016950 | |||
| Grey Matter—Visual.Lateral (L) (−37,−79,10) | 3.41 | 0.005207 | 0.026435 | |||
| Language.pSTG (R) (59,−42,13)-Visual.Lateral (R) (38,−72,13) | 2.22 | 0.046641 | 0.133840 | |||
| Analysis Unit | T (12)-Value | p-unc | p-FDR | p-FWE | ||
|---|---|---|---|---|---|---|
| Cluster 1/36 | ||||||
| Mass | 5664.04 | 0.000000 | 0.000000 | 0.000000 | ||
| DefaultMode.LP (R) (47,−67,29)-DefaultMode.LP (L) (−39,−77,33) | 13.38 | 0.000000 | 0.000004 | |||
| Visual.Lateral (R) (38,−72,13)-Visual.Lateral (L) (−37,−79,10) | 12.27 | 0.000000 | 0.000007 | |||
| Salience.AInsula (R) (47,14,0)-Salience.RPFC (R) (32,46,27) | 10.86 | 0.000000 | 0.000017 | |||
| Language.IFG (R) (54,28,1)-Salience.AInsula (R) (47,14,0) | 10.75 | 0.000000 | 0.000017 | |||
| Cerebellar.Posterior (0,−79,−32)-Cerebellar.Anterior (0,−63,−30) | 10.61 | 0.000000 | 0.000017 | |||
| Language.pSTG (R) (59,−42,13)-Language.IFG (R) (54,28,1) | 10.45 | 0.000000 | 0.000017 | |||
| Salience.SMG (R) (62,−35,32)-Salience.AInsula (R) (47,14,0) | 10.10 | 0.000000 | 0.000021 | |||
| FrontoParietal.LPFC (R) (41,38,30)-FrontoParietal.PPC (R) (52,−52,45) | 10.00 | 0.000000 | 0.000021 | |||
| Visual.Occipital (0,−93,−4)-Cerebellar.Posterior (0,−79,−32) | 9.69 | 0.000001 | 0.000024 | |||
| Salience.SMG (R) (62,−35,32)-Salience.RPFC (R) (32,46,27) | 9.59 | 0.000001 | 0.000025 | |||
| Visual.Lateral (R) (38,−72,13)-Visual.Occipital (0,−93,−4) | 9.31 | 0.000001 | 0.000031 | |||
| Salience.RPFC (R) (32,46,27)-FrontoParietal.LPFC (R) (41,38,30) | 8.63 | 0.000002 | 0.000053 | |||
| Salience.RPFC (R) (32,46,27)-Salience.ACC (0,22,35) | 8.51 | 0.000002 | 0.000055 | |||
| Visual.Medial (2,−79,12)-Visual.Occipital (0,−93,−4) | 8.35 | 0.000002 | 0.000063 | |||
| Salience.ACC (0,22,35)—SensoriMotor.Lateral (R) (56,−10,29) | 8.33 | 0.000002 | 0.000063 | |||
| Visual.Lateral (L) (−37,−79,10)-Visual.Occipital (0,−93,−4) | 7.97 | 0.000004 | 0.000093 | |||
| Visual.Lateral (R) (38,−72,13)-Visual.Medial (2,−79,12) | 7.91 | 0.000004 | 0.000097 | |||
| Visual.Lateral (L) (−37,−79,10)-Visual.Medial (2,−79,12) | 7.88 | 0.000004 | 0.000097 | |||
| Language.pSTG (R) (59,−42,13)-Salience.SMG (R) (62,−35,32) | 7.72 | 0.000005 | 0.000110 | |||
| Grey Matter—Language.pSTG (R) (59,−42,13) | 7.04 | 0.000014 | 0.000232 | |||
| Salience.AInsula (R) (47,14,0)-Salience.ACC (0,22,35) | 6.88 | 0.000017 | 0.000281 | |||
| Language.pSTG (R) (59,−42,13)-Salience.AInsula (R) (47,14,0) | 6.85 | 0.000018 | 0.000282 | |||
| DefaultMode.LP (R) (47,−67,29)-Grey Matter | 6.25 | 0.000043 | 0.000556 | |||
| DefaultMode.LP (L) (−39,−77,33)-Grey Matter | 6.02 | 0.000060 | 0.000755 | |||
| DefaultMode.LP (L) (−39,−77,33)-Visual.Lateral (L) (−37,−79,10) | 5.87 | 0.000076 | 0.000928 | |||
| DefaultMode.LP (R) (47,−67,29)-DefaultMode.MPFC (1,55,−3) | 5.86 | 0.000078 | 0.000932 | |||
| Salience.SMG (R) (62,−35,32)-Salience.ACC (0,22,35) | 5.69 | 0.000101 | 0.001158 | |||
| DefaultMode.LP (R) (47,−67,29)-Language.pSTG (R) (59,−42,13) | 5.61 | 0.000114 | 0.001282 | |||
| DefaultMode.LP (L) (−39,−77,33)-Visual.Lateral (R) (38,−72,13) | 5.50 | 0.000137 | 0.001473 | |||
| Salience.AInsula (R) (47,14,0)-SensoriMotor.Lateral (R) (56,−10,29) | 5.47 | 0.000144 | 0.001522 | |||
| DefaultMode.LP (L) (−39,−77,33)-DefaultMode.MPFC (1,55,−3) | 5.40 | 0.000161 | 0.001571 | |||
| DefaultMode.LP (R) (47,−67,29)-Visual.Lateral (R) (38,−72,13) | 5.05 | 0.000284 | 0.002481 | |||
| Salience.SMG (R) (62,−35,32)-DorsalAttention.IPS (R) (39,−42,54) | 4.91 | 0.000357 | 0.002899 | |||
| Language.IFG (R) (54,28,1)-Salience.SMG (R) (62,−35,32) | 4.80 | 0.000435 | 0.003427 | |||
| DefaultMode.MPFC (1,55,−3)-Visual.Lateral (R) (38,−72,13) | 4.70 | 0.000518 | 0.003908 | |||
| Language.IFG (R) (54,28,1)-Salience.RPFC (R) (32,46,27) | 4.60 | 0.000607 | 0.004512 | |||
| Language.pSTG (R) (59,−42,13)-Salience.RPFC (R) (32,46,27) | 4.50 | 0.000730 | 0.005075 | |||
| DefaultMode.PCC (1,−61,38)-Grey Matter | 4.46 | 0.000785 | 0.005320 | |||
| Salience.RPFC (R) (32,46,27)-FrontoParietal.PPC (R) (52,−52,45) | 4.46 | 0.000786 | 0.005320 | |||
| Grey Matter—SensoriMotor.Lateral (R) (56,−10,29) | 4.43 | 0.000825 | 0.005340 | |||
| Salience.SMG (R) (62,−35,32)-FrontoParietal.PPC (R) (52,−52,45) | 4.28 | 0.001077 | 0.006614 | |||
| Language.pSTG (R) (59,−42,13)-FrontoParietal.PPC (R) (52,−52,45) | 4.22 | 0.001180 | 0.007000 | |||
| DefaultMode.LP (R) (47,−67,29)-DefaultMode.PCC (1,−61,38) | 4.15 | 0.001343 | 0.007462 | |||
| Visual.Lateral (R) (38,−72,13)-Grey Matter | 4.07 | 0.001543 | 0.008228 | |||
| DefaultMode.MPFC (1,55,−3)-Grey Matter | 4.06 | 0.001586 | 0.008373 | |||
| DefaultMode.PCC (1,−61,38)-DefaultMode.MPFC (1,55,−3) | 3.99 | 0.001791 | 0.009271 | |||
| DorsalAttention.IPS (R) (39,−42,54)-DorsalAttention.FEF (R) (30,−6,64) | 3.83 | 0.002401 | 0.012075 | |||
| DefaultMode.PCC (1,−61,38)-DefaultMode.LP (L) (−39,−77,33) | 3.78 | 0.002614 | 0.012857 | |||
| Visual.Occipital (0,−93,−4)-Cerebellar.Anterior (0,−63,−30) | 3.78 | 0.002642 | 0.012857 | |||
| Language.IFG (R) (54,28,1)-FrontoParietal.LPFC (R) (41,38,30) | 3.77 | 0.002678 | 0.012857 | |||
| Visual.Medial (2,−79,12)-Grey Matter | 3.73 | 0.002885 | 0.013723 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-DorsalAttention.IPS (R) (39,−42,54) | 3.54 | 0.004059 | 0.018120 | |||
| FrontoParietal.PPC (R) (52,−52,45)-DorsalAttention.IPS (R) (39,−42,54) | 3.54 | 0.004084 | 0.018120 | |||
| Language.IFG (R) (54,28,1)-FrontoParietal.PPC (R) (52,−52,45) | 3.52 | 0.004190 | 0.018382 | |||
| Grey Matter—Salience.SMG (R) (62,−35,32) | 3.41 | 0.005163 | 0.020639 | |||
| FrontoParietal.LPFC (R) (41,38,30)-SensoriMotor.Lateral (R) (56,−10,29) | 3.40 | 0.005235 | 0.020639 | |||
| DefaultMode.MPFC (1,55,−3)-Language.pSTG (R) (59,−42,13) | 3.26 | 0.006853 | 0.025131 | |||
| Visual.Lateral (L) (−37,−79,10)-Grey Matter | 3.25 | 0.006913 | 0.025174 | |||
| DefaultMode.PCC (1,−61,38)-Language.pSTG (R) (59,−42,13) | 3.14 | 0.008497 | 0.029711 | |||
| DefaultMode.MPFC (1,55,−3)-Visual.Occipital (0,−93,−4) | 3.07 | 0.009674 | 0.032915 | |||
| DefaultMode.LP (L) (−39,−77,33)-Visual.Occipital (0,−93,−4) | 3.07 | 0.009757 | 0.032915 | |||
| Grey Matter—Salience.AInsula (R) (47,14,0) | 3.03 | 0.010401 | 0.034540 | |||
| Visual.Medial (2,−79,12)-Cerebellar.Posterior (0,−79,−32) | 2.96 | 0.011894 | 0.038766 | |||
| Cerebellar.Anterior (0,−63,−30)-Grey Matter | 2.94 | 0.012383 | 0.039626 | |||
| DefaultMode.MPFC (1,55,−3)-Language.IFG (R) (54,28,1) | 2.93 | 0.012498 | 0.039752 | |||
| Salience.AInsula (R) (47,14,0)-DorsalAttention.IPS (R) (39,−42,54) | 2.84 | 0.014905 | 0.046022 | |||
| FrontoParietal.LPFC (R) (41,38,30)-DorsalAttention.FEF (R) (30,−6,64) | 2.84 | 0.015005 | 0.046062 | |||
| Grey Matter—Language.IFG (R) (54,28,1) | 2.71 | 0.019005 | 0.054834 | |||
| Grey Matter—FrontoParietal.PPC (R) (52,−52,45) | 2.69 | 0.019668 | 0.056440 | |||
| FrontoParietal.PPC (R) (52,−52,45)-SensoriMotor.Lateral (R) (56,−10,29) | 2.66 | 0.020889 | 0.058668 | |||
| Salience.AInsula (R) (47,14,0)-FrontoParietal.LPFC (R) (41,38,30) | 2.58 | 0.023945 | 0.064505 | |||
| Salience.SMG (R) (62,−35,32)-SensoriMotor.Lateral (R) (56,−10,29) | 2.52 | 0.027042 | 0.071751 | |||
| Visual.Lateral (R) (38,−72,13)-Cerebellar.Posterior (0,−79,−32) | 2.47 | 0.029499 | 0.076351 | |||
| FrontoParietal.LPFC (R) (41,38,30)-DorsalAttention.IPS (R) (39,−42,54) | 2.39 | 0.034250 | 0.085706 | |||
| Visual.Lateral (R) (38,−72,13)-Language.pSTG (R) (59,−42,13) | 2.32 | 0.038735 | 0.094909 | |||
| DefaultMode.LP (R) (47,−67,29)-Language.IFG (R) (54,28,1) | 2.27 | 0.042268 | 0.100984 | |||
| DefaultMode.MPFC (1,55,−3)-Visual.Lateral (L) (−37,−79,10) | 2.24 | 0.044879 | 0.106261 | |||
| Language.IFG (R) (54,28,1)-SensoriMotor.Lateral (R) (56,−10,29) | 2.22 | 0.046358 | 0.109272 | |||
| Cluster 2/36 | ||||||
| Mass | 1837.04 | 0.000000 | 0.000000 | 0.000000 | ||
| Salience.SMG (L) (−60,−39,31)-Language.pSTG (L) (−57,−47,15) | 13.37 | 0.000000 | 0.000004 | |||
| Language.pSTG (L) (−57,−47,15)-Language.IFG (L) (−51,26,2) | 9.77 | 0.000000 | 0.000024 | |||
| Salience.AInsula (L) (−44,13,1)-Language.IFG (L) (−51,26,2) | 8.80 | 0.000001 | 0.000046 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-FrontoParietal.PPC (L) (−46,−58,49) | 7.72 | 0.000005 | 0.000110 | |||
| Salience.AInsula (L) (−44,13,1)-Salience.SMG (L) (−60,−39,31) | 7.18 | 0.000011 | 0.000211 | |||
| Salience.RPFC (L) (−32,45,27)-Salience.AInsula (L) (−44,13,1) | 7.04 | 0.000014 | 0.000232 | |||
| Salience.AInsula (L) (−44,13,1)-Language.pSTG (L) (−57,−47,15) | 6.48 | 0.000030 | 0.000430 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Salience.RPFC (L) (−32,45,27) | 6.24 | 0.000043 | 0.000556 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Language.IFG (L) (−51,26,2) | 5.42 | 0.000155 | 0.001564 | |||
| Salience.SMG (L) (−60,−39,31)-Language.IFG (L) (−51,26,2) | 5.24 | 0.000207 | 0.001914 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-Salience.SMG (L) (−60,−39,31) | 5.05 | 0.000287 | 0.002481 | |||
| FrontoParietal.PPC (L) (−46,−58,49)-Language.pSTG (L) (−57,−47,15) | 4.73 | 0.000488 | 0.003791 | |||
| FrontoParietal.PPC (L) (−46,−58,49)-Language.IFG (L) (−51,26,2) | 4.55 | 0.000670 | 0.004783 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-SensoriMotor.Lateral (L) (−55,−12,29) | 4.42 | 0.000829 | 0.005340 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-FrontoParietal.LPFC (L) (−43,33,28) | 4.28 | 0.001077 | 0.006614 | |||
| Salience.RPFC (L) (−32,45,27)-Language.pSTG (L) (−57,−47,15) | 4.22 | 0.001187 | 0.007000 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Salience.AInsula (L) (−44,13,1) | 4.14 | 0.001378 | 0.007578 | |||
| Salience.RPFC (L) (−32,45,27)-Salience.SMG (L) (−60,−39,31) | 4.10 | 0.001460 | 0.007887 | |||
| Salience.RPFC (L) (−32,45,27)-Language.IFG (L) (−51,26,2) | 3.95 | 0.001926 | 0.009779 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-Salience.AInsula (L) (−44,13,1) | 3.41 | 0.005168 | 0.020639 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-Language.pSTG (L) (−57,−47,15) | 3.34 | 0.005848 | 0.022056 | |||
| DorsalAttention.FEF (L) (−27,−9,64)-Language.IFG (L) (−51,26,2) | 3.09 | 0.009312 | 0.031926 | |||
| FrontoParietal.PPC (L) (−46,−58,49)-Salience.SMG (L) (−60,−39,31) | 3.07 | 0.009787 | 0.032915 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Salience.AInsula (L) (−44,13,1) | 2.95 | 0.012192 | 0.039253 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Salience.SMG (L) (−60,−39,31) | 2.74 | 0.018032 | 0.053390 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-Salience.RPFC (L) (−32,45,27) | 2.66 | 0.020841 | 0.058668 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-SensoriMotor.Lateral (L) (−55,−12,29) | 2.63 | 0.022131 | 0.061247 | |||
| DorsalAttention.IPS (L) (−39,−43,52)-FrontoParietal.PPC (L) (−46,−58,49) | 2.44 | 0.031052 | 0.079589 | |||
| FrontoParietal.LPFC (L) (−43,33,28)-Language.pSTG (L) (−57,−47,15) | 2.39 | 0.034182 | 0.085706 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Language.pSTG (L) (−57,−47,15) | 2.38 | 0.034450 | 0.085801 | |||
| SensoriMotor.Lateral (L) (−55,−12,29)-Language.IFG (L) (−51,26,2) | 2.31 | 0.039670 | 0.096080 | |||
| Cluster 3/36 | ||||||
| Mass | 1040.56 | 0.000000 | 0.000000 | 0.000000 | ||
| Salience.AInsula (R) (47,14,0)-Salience.AInsula (L) (−44,13,1) | 9.06 | 0.000001 | 0.000039 | |||
| Salience.RPFC (R) (32,46,27)- Salience.RPFC (L) (−32,45,27) | 7.10 | 0.000012 | 0.000227 | |||
| Salience.AInsula (R) (47,14,0)-Salience.RPFC (L) (−32,45,27) | 6.51 | 0.000029 | 0.000424 | |||
| Grey Matter –Language.pSTG (L) (−57,−47,15) | 6.38 | 0.000035 | 0.000485 | |||
| Salience.ACC (0,22,35)—Salience.RPFC (L) (−32,45,27) | 5.41 | 0.000157 | 0.001564 | |||
| Salience.ACC (0,22,35)—Salience.AInsula (L) (−44,13,1) | 5.22 | 0.000216 | 0.001964 | |||
| Salience.AInsula (R) (47,14,0)-Salience.SMG (L) (−60,−39,31) | 5.12 | 0.000255 | 0.002282 | |||
| Language.pSTG (R) (59,−42,13)-Language.pSTG (L) (−57,−47,15) | 5.01 | 0.000302 | 0.002569 | |||
| Salience.SMG (R) (62,−35,32)-Salience.SMG (L) (−60,−39,31) | 5.00 | 0.000311 | 0.002569 | |||
| Language.IFG (R) (54,28,1)-Salience.AInsula (L) (−44,13,1) | 4.36 | 0.000931 | 0.005850 | |||
| Salience.SMG (R) (62,−35,32)-Salience.AInsula (L) (−44,13,1) | 4.22 | 0.001199 | 0.007000 | |||
| Salience.SMG (R) (62,−35,32)-Salience.RPFC (L) (−32,45,27) | 4.20 | 0.001239 | 0.007110 | |||
| Language.IFG (R) (54,28,1)-Language.IFG (L) (−51,26,2) | 3.98 | 0.001811 | 0.009282 | |||
| Language.IFG (R) (54,28,1)-Salience.SMG (L) (−60,−39,31) | 3.48 | 0.004551 | 0.019695 | |||
| Salience.RPFC (R) (32,46,27)-Salience.AInsula (L) (−44,13,1) | 3.46 | 0.004703 | 0.020067 | |||
| Grey Matter—Salience.SMG (L) (−60,−39,31) | 3.40 | 0.005228 | 0.020639 | |||
| Language.IFG (R) (54,28,1)-Language.pSTG (L) (−57,−47,15) | 2.77 | 0.016927 | 0.051073 | |||
| Salience.RPFC (R) (32,46,27)-Salience.SMG (L) (−60,−39,31) | 2.77 | 0.017074 | 0.051223 | |||
| Grey Matter—Language.IFG (L) (−51,26,2) | 2.73 | 0.018100 | 0.053390 | |||
| Salience.SMG (R) (62,−35,32)-Language.pSTG (L) (−57,−47,15) | 2.66 | 0.020775 | 0.058668 | |||
| Cerebellar.Anterior (0,−63,−30)-Salience.SMG (L) (−60,−39,31) | 2.58 | 0.023878 | 0.064505 | |||
| Salience.AInsula (R) (47,14,0)-Language.IFG (L) (−51,26,2) | 2.57 | 0.024527 | 0.065404 | |||
| Salience.AInsula (R) (47,14,0)-Language.pSTG (L) (−57,−47,15) | 2.50 | 0.027914 | 0.073327 | |||
| Language.pSTG (R) (59,−42,13)-Salience.SMG (L) (−60,−39,31) | 2.41 | 0.032858 | 0.083408 | |||
| Grey Matter—Salience.AInsula (L) (−44,13,1) | 2.33 | 0.038176 | 0.094192 | |||
| Salience.AInsula (R) (47,14,0)-SensoriMotor.Lateral (L) (−55,−12,29) | 2.27 | 0.042137 | 0.100984 | |||
| Cluster 4/36 | ||||||
| Mass | 668.06 | 0.000000 | 0.000000 | 0.000000 | ||
| DefaultMode.LP (R) (47,−67,29)-Salience.RPFC (L) (−32,45,27) | −8.54 | 0.000002 | 0.000055 | |||
| Visual.Lateral (R) (38,−72,13)-FrontoParietal.LPFC (L) (−43,33,28) | −7.18 | 0.000011 | 0.000211 | |||
| Visual.Lateral (R) (38,−72,13)-Salience.RPFC (L) (−32,45,27) | −6.70 | 0.000022 | 0.000339 | |||
| Visual.Lateral (R) (38,−72,13)-SensoriMotor.Lateral (L) (−55,−12,29) | −5.56 | 0.000123 | 0.001356 | |||
| DefaultMode.LP (R) (47,−67,29)-SensoriMotor.Lateral (L) (−55,−12,29) | −5.36 | 0.000171 | 0.001639 | |||
| DefaultMode.PCC (1,−61,38)-SensoriMotor.Lateral (L) (−55,−12,29) | −4.21 | 0.001206 | 0.007000 | |||
| DefaultMode.PCC (1,−61,38)-Salience.AInsula (L) (−44,13,1) | −3.61 | 0.003594 | 0.016358 | |||
| DefaultMode.LP (R) (47,−67,29)-Salience.AInsula (L) (−44,13,1) | −3.40 | 0.005230 | 0.020639 | |||
| DefaultMode.MPFC (1,55,−3)-DorsalAttention.IPS (L) (−39,−43,52) | −3.37 | 0.005605 | 0.021292 | |||
| DefaultMode.MPFC (1,55,−3)-Salience.RPFC (L) (−32,45,27) | −3.26 | 0.006854 | 0.025131 | |||
| DefaultMode.LP (L) (−39,−77,33)-Salience.RPFC (L) (−32,45,27) | −2.89 | 0.013679 | 0.042991 | |||
| DefaultMode.LP (R) (47,−67,29)-Salience.SMG (L) (−60,−39,31) | −2.84 | 0.014817 | 0.046020 | |||
| Visual.Lateral (R) (38,−72,13)-FrontoParietal.PPC (L) (−46,−58,49) | −2.64 | 0.021398 | 0.059779 | |||
| DefaultMode.MPFC (1,55,−3)-SensoriMotor.Lateral (L) (−55,−12,29) | −2.63 | 0.022156 | 0.061247 | |||
| Visual.Lateral (R) (38,−72,13)-DorsalAttention.IPS (L) (−39,−43,52) | −2.32 | 0.039048 | 0.095011 | |||
| Visual.Lateral (R) (38,−72,13)-DorsalAttention.FEF (L) (−27,−9,64) | −2.25 | 0.044029 | 0.104718 | |||
| Cluster 5/36 | ||||||
| Mass | 223.06 | 0.000255 | 0.001838 | 0.007000 | ||
| SensoriMotor.Lateral (R) (56,−10,29)-SensoriMotor.Lateral (L) (−55,−12,29) | 8.88 | 0.000001 | 0.000045 | |||
| FrontoParietal.PPC (R) (52,−52,45)-FrontoParietal.PPC (L) (−46,−58,49) | 5.72 | 0.000096 | 0.001127 | |||
| Cluster 6/36 | ||||||
| Mass | 184.98 | 0.000662 | 0.003971 | 0.017000 | ||
| Visual.Lateral (L) (−37,−79,10)-FrontoParietal.PPC (R) (52,−52,45) | −4.38 | 0.000898 | 0.005711 | |||
| Visual.Lateral (L) (−37,−79,10)-Salience.ACC (0,22,35) | −4.23 | 0.001173 | 0.007000 | |||
| Visual.Lateral (L) (−37,−79,10)-SensoriMotor.Lateral (R) (56,−10,29) | −3.78 | 0.002625 | 0.012857 | |||
| Visual.Lateral (L) (−37,−79,10)-Salience.RPFC (R) (32,46,27) | −3.69 | 0.003084 | 0.014411 | |||
| Visual.Lateral (L) (−37,−79,10)-FrontoParietal.LPFC (R) (41,38,30) | −3.44 | 0.004880 | 0.020450 | |||
| Visual.Lateral (R) (38,−72,13)-Salience.ACC (0,22,35) | −3.15 | 0.008334 | 0.029486 | |||
| Visual.Lateral (R) (38,−72,13)-Salience.RPFC (R) (32,46,27) | −2.40 | 0.033697 | 0.085130 | |||
| Cluster 7/36 | ||||||
| Mass | 163.64 | 0.001003 | 0.005158 | 0.027000 | ||
| FrontoParietal.LPFC (R) (41,38,30)-SensoriMotor.Superior (0,−31,67) | −4.72 | 0.000497 | 0.003800 | |||
| FrontoParietal.LPFC (R) (41,38,30)-DorsalAttention.IPS (L) (−39,−43,52) | −4.43 | 0.000817 | 0.005340 | |||
| FrontoParietal.LPFC (R) (41,38,30)-DorsalAttention.FEF (L) (−27,−9,64) | −3.40 | 0.005316 | 0.020639 | |||
| FrontoParietal.PPC (R) (52,−52,45)-DorsalAttention.FEF (L) (−27,−9,64) | −2.83 | 0.015138 | 0.046203 | |||
| FrontoParietal.PPC (R) (52,−52,45)-FrontoParietal.LPFC (L) (−43,33,28) | −2.81 | 0.015800 | 0.047946 | |||
| FrontoParietal.PPC (R) (52,−52,45)-SensoriMotor.Superior (0,−31,67) | −2.66 | 0.020742 | 0.058668 | |||
| SensoriMotor.Lateral (R) (56,−10,29)-FrontoParietal.LPFC (L) (−43,33,28) | −2.32 | 0.038826 | 0.094909 | |||
| Cluster 8/36 | ||||||
| Mass | 139.18 | 0.001634 | 0.007355 | 0.039000 | ||
| Salience.RPFC (R) (32,46,27)-SensoriMotor.Superior (0,−31,67) | −6.34 | 0.000037 | 0.000504 | |||
| Language.IFG (R) (54,28,1)-SensoriMotor.Superior (0,−31,67) | −3.43 | 0.004953 | 0.020593 | |||
| Salience.AInsula (R) (47,14,0)-SensoriMotor.Superior (0,−31,67) | −3.20 | 0.007703 | 0.027858 | |||
| Salience.SMG (R) (62,−35,32)-SensoriMotor.Superior (0,−31,67) | −2.72 | 0.018543 | 0.053796 | |||
References
- Festa, F.; Medori, S.; Macrì, M. Move your body, boost your brain: The positive impact of physical activity on cognition across all age groups. Biomedicines 2023, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, S.; Qi, Y.; Zhou, Q.; Wang, J.; Wang, Y.; Zhou, C. Differential resting-state brain characteristics of skeleton athletes and non-athletes: A preliminary resting-state fMRI study. Brain Sci. 2024, 14, 1016. [Google Scholar] [CrossRef] [PubMed]
- Hofman, A.; Rodriguez-Ayllon, M.; Vernooij, M.W.; Croll, P.H.; Luik, A.I.; Neumann, A.; Niessen, W.J.; Ikram, M.A.; Voortman, T.; Muetzel, R.L. Physical activity levels and brain structure in middle-aged and older adults: A bidirectional longitudinal population-based study. Neurobiol. Aging 2023, 121, 28–37. [Google Scholar] [CrossRef]
- Duru, A.D.; Balcioglu, T.H. Functional and structural plasticity of brain in elite karate athletes. J. Healthc. Eng. 2018, 2018, 8310975. [Google Scholar] [CrossRef] [PubMed]
- Augusto-Oliveira, M.; Arrifano, G.P.; Leal-Nazaré, C.G.; Santos-Sacramento, L.; Lopes-Araújo, A.; Royes, L.F.F.; Crespo-Lopez, M.E. Exercise reshapes the brain: Molecular, cellular, and structural changes associated with cognitive improvements. Mol. Neurobiol. 2023, 60, 6950–6974. [Google Scholar] [CrossRef]
- Saviola, F.; Deste, G.; Barlati, S.; Vita, A.; Gasparotti, R.; Corbo, D. The effect of physical exercise on people with psychosis: A qualitative critical review of neuroimaging findings. Brain Sci. 2023, 13, 923. [Google Scholar] [CrossRef]
- De Bosscher, V.; Descheemaeker, K.; Shibli, S. Starting and specialisation ages of elite athletes across olympic sports: An international cross-sectional study. Eur. J. Sport Sci. 2023, 3, 9–19. [Google Scholar]
- Wu, H.; Yan, H.; Yang, Y.; Xu, M.; Shi, Y.; Zeng, W.; Li, J.; Zhang, J.; Chang, C.; Wang, N. Occupational neuroplasticity in the human brain: A critical review and meta-analysis of neuroimaging studies. Front. Hum. Neurosci. 2020, 14, 215. [Google Scholar] [CrossRef]
- Araújo, D.; Davids, K. Team synergies in sport: Theory and measures. Front. Psychol. 2016, 7, 1449. [Google Scholar] [CrossRef]
- Badau, D.; Badau, A.; Joksimović, M.; Manescu, C.O.; Manescu, D.C.; Dinciu, C.C.; Margarit, I.R.; Tudor, V.; Mujea, A.M.; Neofit, A. Identifying the Level of Symmetrization of Reaction Time According to Manual Lateralization between Team Sports Athletes, Individual Sports Athletes, and Non-Athletes. Symmetry 2023, 16, 28. [Google Scholar] [CrossRef]
- Tabben, M.; Chaouachi, A.; Mahfoudhi, M.; Aloui, A.; Habacha, H.; Tourny, C.; Franchini, E. Physical and physiological characteristics of high-level combat sport athletes. J. Combat Sports Martial Arts 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Krabben, K.; Orth, D.; van der Kamp, J. Combat as an interpersonal synergy: An ecological dynamics approach to combat sports. Sports Med. 2019, 49, 1825–1836. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Liu, J.; Liu, R.; Cao, C. Detecting structural and functional neuroplasticity in elite ice-skating athletes. Hum. Mov. Sci. 2021, 78, 102795. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Y.; Huang, R.; Li, L.; Xia, F.; Zou, L.; Yu, Q.; Lin, J.; Herold, F.; Perrey, S. Structural and functional brain signatures of endurance runners. Brain Struct. Funct. 2021, 226, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Xu, L.; Wang, H.; Song, T.; Shao, Y.; Liu, Q.; Weng, X. The lateralization of spatial cognition in table tennis players: Neuroplasticity in the dominant hemisphere. Brain Sci. 2022, 12, 1607. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Huang, M.; Qin, Z.; Lang, J. Progressive increase of brain gray matter volume in individuals with regular soccer training. Sci. Rep. 2024, 14, 7023. [Google Scholar] [CrossRef] [PubMed]
- Kurtoğlu, E.; Payas, A.; Düz, S.; Arık, M.; Uçar, I.; Tokmak, T.T.; Erbay, M.F.; Acer, N.; Unur, E. Analysis of changes in brain morphological structure of taekwondo athletes by diffusion tensor imaging. J. Chem. Neuroanat. 2023, 129, 102250. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.W.; Tae, W.S. Cerebral cortex changes in basketball players. J. Korean Med. Sci. 2022, 37, e86. [Google Scholar] [CrossRef]
- Fukuo, M.; Kamagata, K.; Kuramochi, M.; Andica, C.; Tomita, H.; Waki, H.; Sugano, H.; Tange, Y.; Mitsuhashi, T.; Uchida, W. Regional brain gray matter volume in world-class artistic gymnasts. J. Physiol. Sci. 2020, 70, 43. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Yao, Y.; Lin, J.; Mo, L. Neural correlates of basketball proficiency: An MRI study across skill levels. J. Exerc. Sci. Fit. 2025, 23, 14–20. [Google Scholar] [CrossRef]
- Yao, Z.-F.; Sligte, I.G.; Moreau, D.; Hsieh, S.; Yang, C.-T.; Ridderinkhof, K.R.; Muggleton, N.G.; Wang, C.-H. The brains of elite soccer players are subject to experience-dependent alterations in white matter connectivity. Cortex 2020, 132, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Topka, M.S.; Hänggi, J. Differences in cortical representation and structural connectivity of hands and feet between professional handball players and ballet dancers. Neural Plast. 2016, 2016, 6817397. [Google Scholar] [CrossRef] [PubMed]
- Schlaffke, L.; Lissek, S.; Lenz, M.; Brüne, M.; Juckel, G.; Hinrichs, T.; Platen, P.; Tegenthoff, M.; Schmidt-Wilcke, T. Sports and brain morphology—A voxel-based morphometry study with endurance athletes and martial artists. Neuroscience 2014, 259, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jan, Y.-K.; Liu, Y.; Zhao, T.; Zhang, L.; Liu, R.; Liu, J.; Cao, C. Exercise intensity and brain plasticity: What’s the difference of brain structural and functional plasticity characteristics between elite aerobic and anaerobic athletes? Front. Hum. Neurosci. 2022, 16, 757522. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhu, P.; Cai, Z.-L.; Xing, X.-X.; Wu, J.-J.; Zheng, M.-X.; Hua, X.-Y.; Gong, B.-M.; Xu, J.-G. Sports promote brain evolution: A resting-state fMRI study of volleyball athlete. Front. Sports Act. Living 2024, 6, 1393988. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vidaurre, D.; Beckmann, C.F.; Glasser, M.F.; Jenkinson, M.; Miller, K.L.; Nichols, T.E.; Robinson, E.C.; Salimi-Khorshidi, G.; Woolrich, M.W. Functional connectomics from resting-state fMRI. Trends Cogn. Sci. 2013, 17, 666–682. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Chicago, IL, USA, 2020. [Google Scholar]
- Hallquist, M.N.; Hwang, K.; Luna, B. The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 2013, 82, 208–225. [Google Scholar] [CrossRef]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef]
- Di, X.; Biswal, B.B. A functional MRI pre-processing and quality control protocol based on statistical parametric mapping (SPM) and MATLAB. Front. Neuroimaging 2023, 1, 1070151. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Castanon, A.; Whitfield-Gabrieli, S. CONN Functional Connectivity Toolbox: RRID SCR_009550; Version 22. Available online: https://www.researchgate.net/profile/Alfonso-Nieto-Castanon/publication/369070545_CONN_functional_connectivity_toolbox_RRID_SCR_009550_release_22/links/6413bb5a66f8522c38ae9623/CONN-functional-connectivity-toolbox-RRID-SCR-009550-release-22.pdf (accessed on 6 February 2025).
- Worsley, K.J.; Marrett, S.; Neelin, P.; Vandal, A.C.; Friston, K.J.; Evans, A.C. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 1996, 4, 58–73. [Google Scholar] [CrossRef]
- Peelen, M.V.; Downing, P.E. Selectivity for the human body in the fusiform gyrus. J. Neurophysiol. 2005, 93, 603–608. [Google Scholar] [CrossRef]
- Herlin, B.; Navarro, V.; Dupont, S. The temporal pole: From anatomy to function—A literature appraisal. J. Chem. Neuroanat. 2021, 113, 101925. [Google Scholar] [CrossRef]
- Hocking, J.; Price, C.J. The role of the posterior superior temporal sulcus in audiovisual processing. Cereb. Cortex 2008, 18, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Rabellino, D.; Frewen, P.A.; McKinnon, M.C.; Lanius, R.A. Peripersonal space and bodily self-consciousness: Implications for psychological trauma-related disorders. Front. Neurosci. 2020, 14, 586605. [Google Scholar] [CrossRef]
- Grivaz, P.; Blanke, O.; Serino, A. Common and distinct brain regions processing multisensory bodily signals for peripersonal space and body ownership. Neuroimage 2017, 147, 602–618. [Google Scholar] [CrossRef]
- Roberts, S.D.; Wilson, A.; Rahimi, A.; Gorbet, D.; Sergio, L.; Stevens, W.D.; Wojtowicz, M. Investigation of baseline attention, executive control, and performance variability in female varsity athletes. Brain Imaging Behav. 2022, 16, 1636–1645. [Google Scholar] [CrossRef]
- Menon, V.; D’Esposito, M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 90–103. [Google Scholar] [CrossRef]
- Di Pellegrino, G.; Làdavas, E. Peripersonal space in the brain. Neuropsychologia 2015, 66, 126–133. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Tsai, J.H.-C.; Wang, C.-C.; Chang, E.C. Structural differences in basal ganglia of elite running versus martial arts athletes: A diffusion tensor imaging study. Exp. Brain Res. 2015, 233, 2239–2248. [Google Scholar] [CrossRef]
- Burles, F.; Umiltá, A.; McFarlane, L.H.; Potocki, K.; Iaria, G. Ventral—Dorsal functional contribution of the posterior cingulate cortex in human spatial orientation: A meta-analysis. Front. Hum. Neurosci. 2018, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Takahashi, N.; Ogawara, K.; Hattori, T. Pure topographical disorientation due to right posterior cingulate lesion. Cortex 1999, 35, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A.; Finch, D.M.; Olson, C.R. Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cereb. Cortex 1992, 2, 435–443. [Google Scholar] [CrossRef]
- Shima, K.; Aya, K.; Mushiake, H.; Inase, M.; Aizawa, H.; Tanji, J. Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J. Neurophysiol. 1991, 65, 188–202. [Google Scholar] [CrossRef]
- Xu, H.; Wang, P.; Ye, Z.E.; Di, X.; Xu, G.; Mo, L.; Lin, H.; Rao, H.; Jin, H. The role of medial frontal cortex in action anticipation in professional badminton players. Front. Psychol. 2016, 7, 1817. [Google Scholar] [CrossRef]
- He, M.; Qi, C.; Lu, Y.; Song, A.; Hayat, S.Z.; Xu, X. The sport expert’s attention superiority on skill-related scene dynamic by the activation of left medial frontal Gyrus: An ERP and LORETA study. Neuroscience 2018, 379, 93–102. [Google Scholar] [CrossRef]
- Martín-Luengo, B.; Vorobiova, A.N.; Feurra, M.; Myachykov, A.; Shtyrov, Y. Transcranial magnetic stimulation of the left middle frontal gyrus modulates the information people communicate in different social contexts. Sci. Rep. 2023, 13, 9995. [Google Scholar] [CrossRef]
- Risius, U.-M.; Staniloiu, A.; Piefke, M.; Maderwald, S.; Schulte, F.P.; Brand, M.; Markowitsch, H.J. Retrieval, monitoring, and control processes: A 7 tesla FMRI approach to memory accuracy. Front. Behav. Neurosci. 2013, 7, 24. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, B.; Luo, Q.; Qiu, L.; Wang, S. Gray matter structural alterations in social anxiety disorder: A voxel-based meta-analysis. Front. Psychiatry 2018, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Y.; Wang, X.; Yang, X.; Cheng, B.; Pan, N.; Suo, X.; Gong, Q. Emotional intelligence mediates the association between middle temporal gyrus gray matter volume and social anxiety in late adolescence. Eur. Child Adolesc. Psychiatry 2021, 30, 1857–1869. [Google Scholar] [CrossRef]
- Pluhar, E.; McCracken, C.; Griffith, K.L.; Christino, M.A.; Sugimoto, D.; Meehan, W.P., III. Team sport athletes may be less likely to suffer anxiety or depression than individual sport athletes. J. Sports Sci. Med. 2019, 18, 490. [Google Scholar]
- Silva, J.M., III; Shultz, B.B.; Haslam, R.W.; Murray, D. A psychophysiological assessment of elite wrestlers. Res. Q. Exerc. Sport 1981, 52, 348–358. [Google Scholar] [CrossRef]
- Ren, J.; Huang, F.; Zhou, Y.; Zhuang, L.; Xu, J.; Gao, C.; Qin, S.; Luo, J. The function of the hippocampus and middle temporal gyrus in forming new associations and concepts during the processing of novelty and usefulness features in creative designs. Neuroimage 2020, 214, 116751. [Google Scholar] [CrossRef] [PubMed]
- Taubert, M.; Wenzel, U.; Draganski, B.; Kiebel, S.J.; Ragert, P.; Krug, J.; Villringer, A. Investigating neuroanatomical features in top athletes at the single subject level. PLoS ONE 2015, 10, e0129508. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Haier, R.J. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behav. Brain Sci. 2007, 30, 135–154. [Google Scholar] [CrossRef]
- Bogousslavsky, J.; Miklossy, J.; Deruaz, J.-P.; Assal, G.; Regli, F. Lingual and fusiform gyri in visual processing: A clinico-pathologic study of superior altitudinal hemianopia. J. Neurol. Neurosurg. Psychiatry 1987, 50, 607–614. [Google Scholar] [CrossRef]
- Yau, Y.; Dadar, M.; Taylor, M.; Zeighami, Y.; Fellows, L.; Cisek, P.; Dagher, A. Neural correlates of evidence and urgency during human perceptual decision-making in dynamically changing conditions. Cereb. Cortex 2020, 30, 5471–5483. [Google Scholar] [CrossRef]
- Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005, 6, 533–544. [Google Scholar] [CrossRef]
- Taylor, K.S.; Seminowicz, D.A.; Davis, K.D. Two systems of resting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. 2009, 30, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Khairuddin, S.; Ngo, F.Y.; Lim, W.L.; Aquili, L.; Khan, N.A.; Fung, M.-L.; Chan, Y.-S.; Temel, Y.; Lim, L.W. A decade of progress in deep brain stimulation of the subcallosal cingulate for the treatment of depression. J. Clin. Med. 2020, 9, 3260. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.; Clarke, H.F.; Roberts, A.C. A focus on the functions of area 25. Brain Sci. 2019, 9, 129. [Google Scholar] [CrossRef]
- Casey, B.J.; Trainor, R.J.; Orendi, J.L.; Schubert, A.B.; Nystrom, L.E.; Giedd, J.N.; Castellanos, F.X.; Haxby, J.V.; Noll, D.C.; Cohen, J.D. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J. Cogn. Neurosci. 1997, 9, 835–847. [Google Scholar] [CrossRef]
- Kowalska, D.M.; Bachevalier, J.; Mishkin, M. The role of the inferior prefrontal convexity in performance of delayed nonmatching-to-sample. Neuropsychologia 1991, 29, 583–600. [Google Scholar] [CrossRef]
- Elliott, R.; Dolan, R.J.; Frith, C.D. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cereb. Cortex 2000, 10, 308–317. [Google Scholar] [CrossRef]
- Fink, G.R.; Markowitsch, H.J.; Reinkemeier, M.; Bruckbauer, T.; Kessler, J.; Heiss, W.-D. Cerebral representation of one’s own past: Neural networks involved in autobiographical memory. J. Neurosci. 1996, 16, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Frith, C.; Dolan, R.J. Differential neural response to positive and negative feedback in planning and guessing tasks. Neuropsychologia 1997, 35, 1395–1404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pu, Y.; Cornwell, B.R.; Cheyne, D.; Johnson, B.W. The functional role of human right hippocampal/parahippocampal theta rhythm in environmental encoding during virtual spatial navigation. Hum. Brain Mapp. 2017, 38, 1347–1361. [Google Scholar] [CrossRef]
- Baumann, O.; Chan, E.; Mattingley, J.B. Dissociable neural circuits for encoding and retrieval of object locations during active navigation in humans. Neuroimage 2010, 49, 2816–2825. [Google Scholar] [CrossRef]
- Baumann, O.; Mattingley, J.B. Extrahippocampal contributions to spatial navigation in humans: A review of the neuroimaging evidence. Hippocampus 2021, 31, 640–657. [Google Scholar] [CrossRef]
- Owen, A.M.; Milner, B.; Petrides, M.; Evans, A.C. A specific role for the right parahippocampal gyrus in the retrieval of object-location: A positron emission tomography study. J. Cogn. Neurosci. 1996, 8, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Aminoff, E.M.; Kveraga, K.; Bar, M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013, 17, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Hein, G.; Knight, R.T. Superior temporal sulcus—It’s my area: Or is it? J. Cogn. Neurosci. 2008, 20, 2125–2136. [Google Scholar] [CrossRef]
- Vander Wyk, B.C.; Hudac, C.M.; Carter, E.J.; Sobel, D.M.; Pelphrey, K.A. Action understanding in the superior temporal sulcus region. Psychol. Sci. 2009, 20, 771–777. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, P.; Tang, S.; Lu, L.; Bu, X.; Zhang, L.; Gao, Y.; Li, H.; Hu, X.; Wang, S. Left superior temporal sulcus morphometry mediates the impact of anxiety and depressive symptoms on sleep quality in healthy adults. Soc. Cogn. Affect. Neurosci. 2021, 16, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Molenberghs, P.; Brander, C.; Mattingley, J.B.; Cunnington, R. The role of the superior temporal sulcus and the mirror neuron system in imitation. Hum. Brain Mapp. 2010, 31, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Palacios, A. Training Proprioception and Touch for Ball Handling in Sports. 2016. Available online: https://osf.io/preprints/osf/tjefx (accessed on 27 January 2025).
- Bernasconi, F.; Noel, J.-P.; Park, H.D.; Faivre, N.; Seeck, M.; Spinelli, L.; Schaller, K.; Blanke, O.; Serino, A. Audio-tactile and peripersonal space processing around the trunk in human parietal and temporal cortex: An intracranial EEG study. Cereb. Cortex 2018, 28, 3385–3397. [Google Scholar] [CrossRef]
- Graziano, M.S.; Cooke, D.F. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia 2006, 44, 845–859. [Google Scholar] [CrossRef]
- Brozzoli, C.; Gentile, G.; Ehrsson, H.H. That’s near my hand! Parietal and premotor coding of hand-centered space contributes to localization and self-attribution of the hand. J. Neurosci. 2012, 32, 14573–14582. [Google Scholar] [CrossRef]
- Teraoka, R.; Kuroda, N.; Kojima, R.; Teramoto, W. Comparison of peripersonal space in front and rear spaces. Exp. Brain Res. 2024, 242, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, Z.; Lin, Z.; Nie, C.; Yang, T. A neural network model for the orbitofrontal cortex and task space acquisition during reinforcement learning. PLoS Comput. Biol. 2018, 14, e1005925. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Jefferies, E.; Embleton, K.V.; Lambon Ralph, M.A. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: Distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. J. Cogn. Neurosci. 2012, 24, 1766–1778. [Google Scholar] [CrossRef]
- Brett, B.L.; Walton, S.R.; Meier, T.B.; Nencka, A.S.; Powell, J.R.; Giovanello, K.S.; Guskiewicz, K.M.; McCrea, M.A. Head impact exposure, gray matter volume, and moderating effects of estimated intelligence quotient and educational attainment in former athletes at midlife. J. Neurotrauma 2022, 39, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.B.; Espana, L.Y.; Kirk, A.J.; Nader, A.M.; Powell, J.E.; Nelson, L.D.; Mayer, A.R.; Brett, B.L. Association of previous concussion with hippocampal volume and symptoms in collegiate-aged athletes. J. Neurotrauma 2021, 38, 1358–1367. [Google Scholar] [CrossRef]
- Garcia-Cordero, I.; Vasilevskaya, A.; Taghdiri, F.; Khodadadi, M.; Mikulis, D.; Tarazi, A.; Mushtaque, A.; Anssari, N.; Colella, B.; Green, R. Functional connectivity changes in neurodegenerative biomarker-positive athletes with repeated concussions. J. Neurol. 2024, 271, 4180–4190. [Google Scholar] [CrossRef]
- Major, B.; Symons, G.F.; Sinclair, B.; O’Brien, W.T.; Costello, D.; Wright, D.K.; Clough, M.; Mutimer, S.; Sun, M.; Yamakawa, G.R. White and gray matter abnormalities in Australian footballers with a history of sports-related concussion: An MRI study. Cereb. Cortex 2021, 31, 5331–5338. [Google Scholar] [CrossRef]
- Oliveira, T.G.; Ifrah, C.; Fleysher, R.; Stockman, M.; Lipton, M.L. Soccer heading and concussion are not associated with reduced brain volume or cortical thickness. PLoS ONE 2020, 15, e0235609. [Google Scholar] [CrossRef]
- Karimpoor, M.; Georgiadis, M.; Zhao, M.Y.; Goubran, M.; Moein Taghavi, H.; Mills, B.D.; Tran, D.; Mouchawar, N.; Sami, S.; Wintermark, M. Longitudinal Alterations of Cerebral Blood Flow in High-Contact Sports. Ann. Neurol. 2023, 94, 457–469. [Google Scholar] [CrossRef]
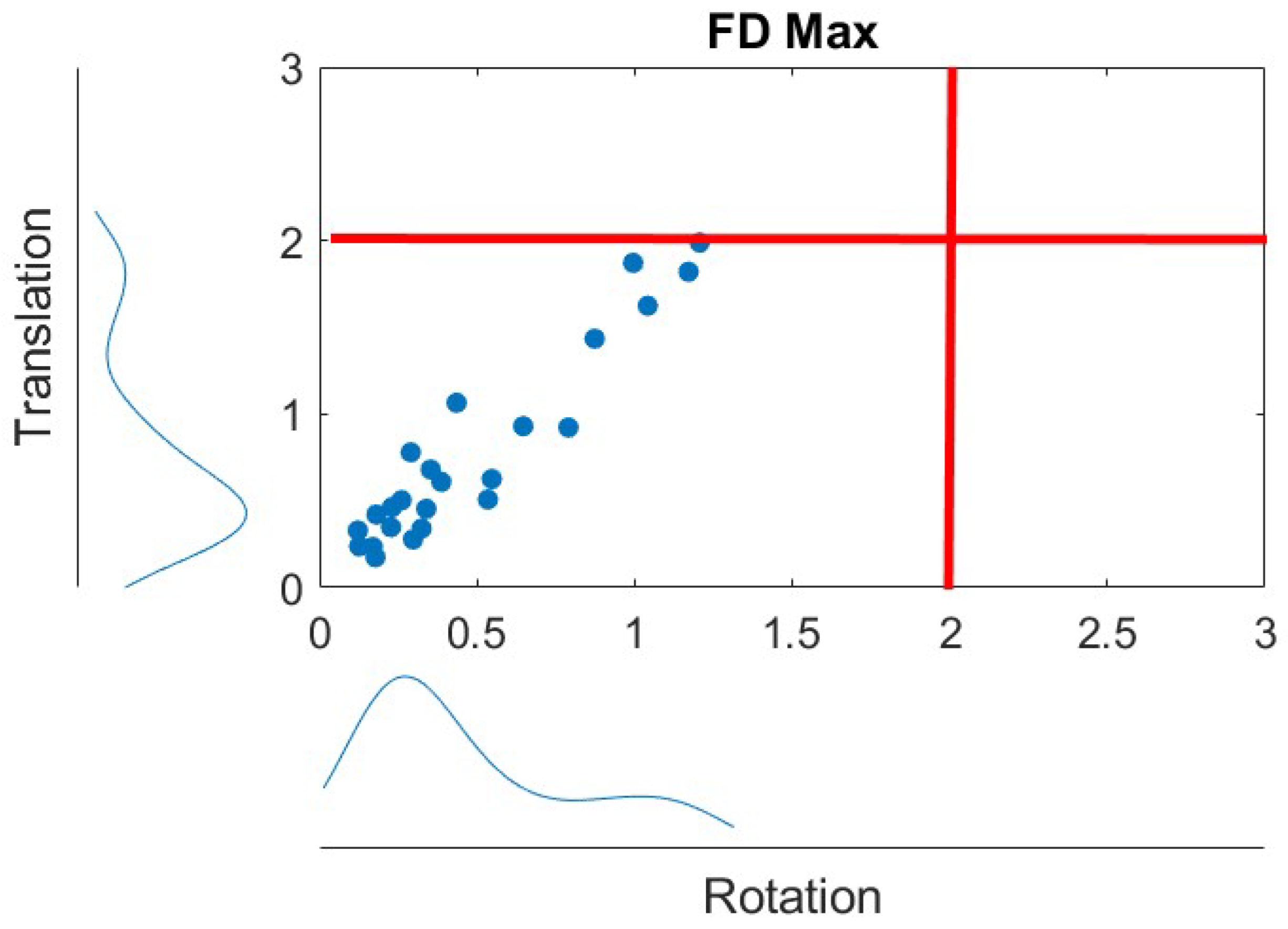
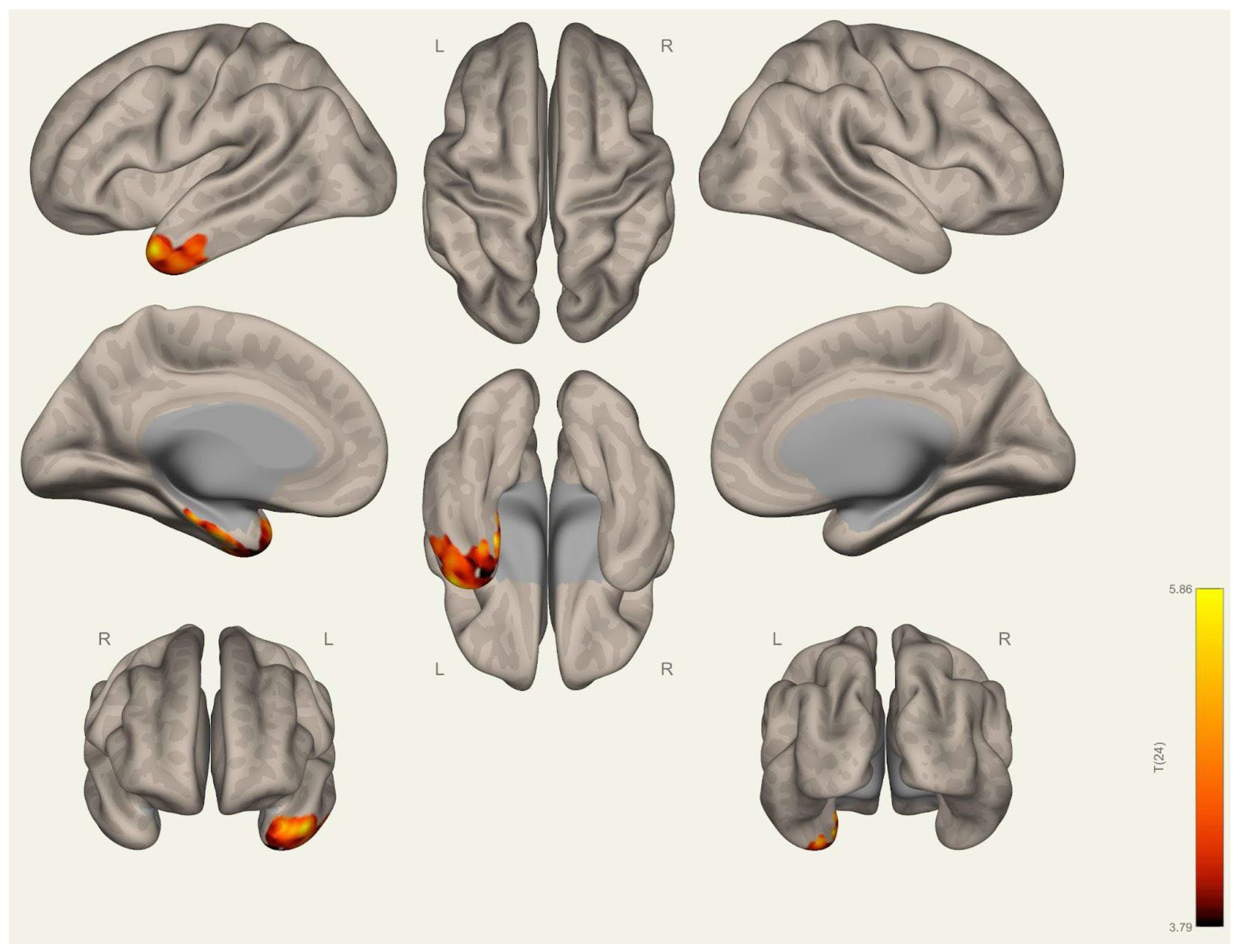
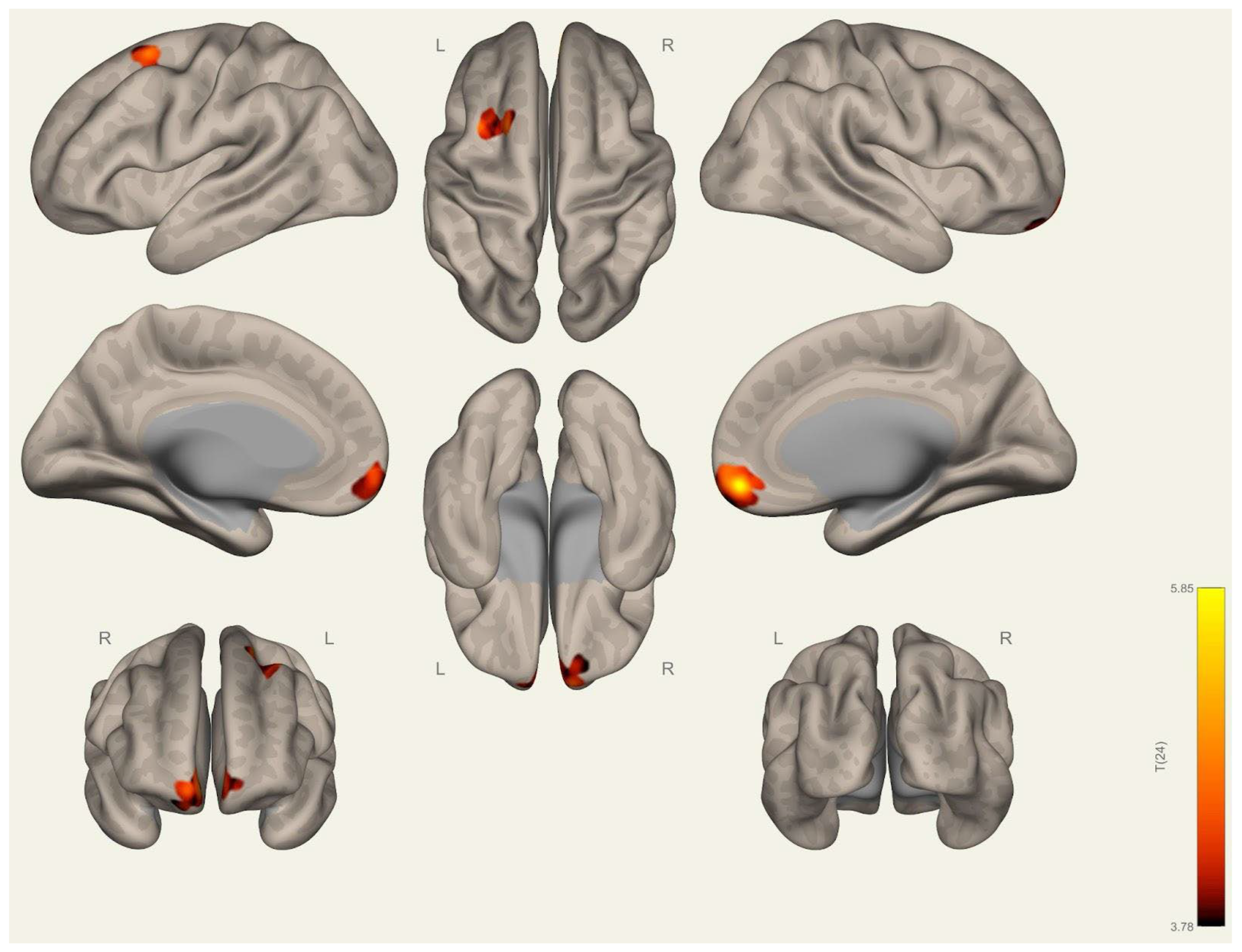
| Network | ROI | Coordinate |
|---|---|---|
| Cerebellar | Anterior | (0,−63,−30) |
| Cerebellar | Posterior | (0,−79,−32) |
| Frontoparietal | LPFC (L) | (−43,33,28) |
| Frontoparietal | PPC (L) | (−46,−58,49) |
| Frontoparietal | LPFC (R) | (41,38,30) |
| Frontoparietal | PPC (R) | (52,−52,45) |
| Default mode | MPFC | (1,55,−3) |
| Default mode | LP (L) | (−39,−77,33) |
| Default mode | LP (R) | (47,−67,29) |
| Default mode | PCC | (1,−61,38) |
| Sensorimotor | Lateral (L) | (−55,−12,29) |
| Sensorimotor | Lateral (R) | (56,−10,29) |
| Sensorimotor | Superior | (0,−31,67) |
| Dorsal attention | FEF (L) | (−27,−9,64) |
| Dorsal attention | FEF (R) | (30,−6,64) |
| Dorsal attention | IPS (L) | (−39,−43,52) |
| Dorsal attention | IPS (R) | (39,−42,54) |
| Language | IFG (L) | (−51,26,2) |
| Language | IFG (R) | (54,28,1) |
| Language | pSTG (L) | (−57,−47,15) |
| Language | pSTG (R) | (59,−42,13) |
| Salience | ACC | (0,22,35) |
| Salience | AInsula (L) | (−44,13,1) |
| Salience | AInsula (R) | (47,14,0) |
| Salience | RPFC (L) | (−32,45,27) |
| Salience | RPFC (R) | (32,46,27) |
| Salience | SMG (L) | (−60,−39,31) |
| Salience | SMG (R) | (62,−35,32) |
| Visual | Medial | (2,−79,12) |
| Visual | Occipital | (0,−93,−4) |
| Visual | Lateral (L) | (−37,−79,10) |
| Visual | Lateral (R) | (38,−72,13) |
| Parameter | Group | Mean | Median | SD | U | p | SE |
|---|---|---|---|---|---|---|---|
| CSF absolute volume cm3 | Wrestling | 237.77 | 259.00 | 76.91 | 69 | 0.442 | 19.5 |
| Handball | 263.62 | 262.00 | 48.68 | ||||
| CSF relative volume (%) | Wrestling | 15.98 | 16.20 | 1.99 | 69 | 0.442 | 19.5 |
| Handball | 16.77 | 16.90 | 2.71 | ||||
| GM absolute volume | Wrestling | 761.85 | 751.00 | 63.97 | 62 | 0.259 | 19.5 |
| Handball | 729.23 | 722.00 | 52.64 | ||||
| GM relative volume (%) | Wrestling | 48.28 | 47.40 | 1.75 | 37 | 0.016 | 19.5 |
| Handball | 46.55 | 46.30 | 1.73 | ||||
| WM absolute volume | Wrestling | 564.85 | 559.00 | 56.98 | 67 | 0.383 | 19.5 |
| Handball | 573.92 | 570.00 | 45.48 | ||||
| WM relative volume (%) | Wrestling | 35.74 | 35.50 | 1.19 | 67.5 | 0.397 | 19.5 |
| Handball | 36.67 | 35.90 | 2.41 | ||||
| TIV | Wrestling | 1580.08 | 1541.00 | 148.10 | 82 | 0.918 | 19.5 |
| Handball | 1566.77 | 1564.00 | 100.67 | ||||
| Thickness | Wrestling | 2.45 | 2.47 | 0.09 | 64 | 0.304 | 19.5 |
| Handball | 2.42 | 2.44 | 0.07 |
| Brain Region | (x, y, z) | Peak T | Peak p | Peak p-FWE |
|---|---|---|---|---|
| Wrestling > handball | ||||
| Middle temporal gyrus (R) | 60 −6 −27 | 4.41 | 0.0001 | 0.22 |
| 61.5 −15 −21 | 3.68 | 0.0006 | 0.67 | |
| Middle frontal gyrus (L) | −27 0 57 | 4.20 | 0.0002 | 0.32 |
| Posterior cingulate gyrus (R) | 10.5 −30 43.5 | 3.60 | 0.0007 | 0.72 |
| Brain Region | Group | Mean | Median | SD | U | p | SE |
|---|---|---|---|---|---|---|---|
| Left superior occipital sulcus and transverse occipital sulcus | Wrestling | 2.07 | 2.06 | 0.10 | 43.0 | 0.034 | 19.5 |
| Handball | 1.98 | 1.94 | 0.10 | ||||
| Left superior temporal sulcus | Wrestling | 2.41 | 2.41 | 0.09 | 30.5 | 0.006 | 19.5 |
| Handball | 2.33 | 2.32 | 0.01 | ||||
| Left fusiform | Wrestling | 2.69 | 2.68 | 0.07 | 41.0 | 0.027 | 19.5 |
| Handball | 2.61 | 2.61 | 0.08 | ||||
| Right fusiform | Wrestling | 2.74 | 2.73 | 0.10 | 37.0 | 0.014 | 19.5 |
| Handball | 2.62 | 2.63 | 0.10 | ||||
| Left temporal pole | Wrestling | 3.63 | 3.61 | 0.15 | 38.0 | 0.016 | 19.5 |
| Handball | 3.33 | 3.36 | 0.36 | ||||
| Right middle–posterior part of the cingulate gyrus and sulcus | Wrestling | 2.61 | 2.62 | 0.11 | 35.0 | 0.012 | 19.5 |
| Handball | 2.50 | 2.47 | 0.08 | ||||
| Right subcallosal area, subcallosal gyrus | Wrestling | 2.77 | 2.84 | 0.20 | 34.0 | 0.010 | 19.5 |
| Handball | 2.56 | 2.56 | 0.28 | ||||
| Right medial orbital sulcus (olfactory sulcus) | Wrestling | 2.33 | 2.32 | 0.12 | 43.0 | 0.035 | 19.5 |
| Handball | 2.20 | 2.14 | 0.19 | ||||
| Right lat. orbitofrontal | Wrestling | 2.73 | 2.73 | 0.07 | 45.5 | 0.048 | 19.5 |
| Handball | 2.64 | 2.68 | 0.11 | ||||
| Right posterior cingulate | Wrestling | 2.55 | 2.54 | 0.10 | 35.0 | 0.012 | 19.5 |
| Handball | 2.41 | 2.36 | 0.12 |
| Brain Region | Cluster (x, y, z) | Cluster p-FWE | Peak p-FWE | Peak T |
|---|---|---|---|---|
| Wrestling > handball | ||||
| Seed DMN MPFC | 1 55 −3 | |||
| Left superior temporal gyrus | −38 10 −32 | 0.001 | 0.022 | 6.82 |
| Left parahippocampal gyrus | −24 −14 −34 | 0.020 | 0.049 | 6.40 |
| Seed Visual Occipital | 0 −93 −4 | |||
| Left anterior orbital gyrus | −26 40 −14 | 0.005 | 0.029 | 6.71 |
| Right superior frontal gyrus–medial orbital | 10 52 −14 | 0.002 | 0.035 | 6.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahin Ozarslan, F.; Duru, A.D. Differences in Anatomical Structures and Resting-State Brain Networks Between Elite Wrestlers and Handball Athletes. Brain Sci. 2025, 15, 285. https://doi.org/10.3390/brainsci15030285
Sahin Ozarslan F, Duru AD. Differences in Anatomical Structures and Resting-State Brain Networks Between Elite Wrestlers and Handball Athletes. Brain Sciences. 2025; 15(3):285. https://doi.org/10.3390/brainsci15030285
Chicago/Turabian StyleSahin Ozarslan, Fatma, and Adil Deniz Duru. 2025. "Differences in Anatomical Structures and Resting-State Brain Networks Between Elite Wrestlers and Handball Athletes" Brain Sciences 15, no. 3: 285. https://doi.org/10.3390/brainsci15030285
APA StyleSahin Ozarslan, F., & Duru, A. D. (2025). Differences in Anatomical Structures and Resting-State Brain Networks Between Elite Wrestlers and Handball Athletes. Brain Sciences, 15(3), 285. https://doi.org/10.3390/brainsci15030285






