Abstract
Most brain development occurs in the “first 1000 days”, a critical period from conception to a child’s second birthday. Critical brain processes that occur during this time include synaptogenesis, myelination, neural pruning, and the formation of functioning neuronal circuits. Perturbations during the first 1000 days likely contribute to later-life neurodegenerative disease, including sporadic amyotrophic lateral sclerosis (ALS). Neurodevelopment is determined by many events, including the maturation and colonization of the infant microbiome and its metabolites, specifically neurotransmitters, immune modulators, vitamins, and short-chain fatty acids. Successful microbiome maturation and gut–brain axis function depend on maternal factors (stress and exposure to toxins during pregnancy), mode of delivery, quality of the postnatal environment, diet after weaning from breast milk, and nutritional deficiencies. While the neonatal microbiome is highly plastic, it remains prone to dysbiosis which, once established, may persist into adulthood, thereby inducing the development of chronic inflammation and abnormal excitatory/inhibitory balance, resulting in neural excitation. Both are recognized as key pathophysiological processes in the development of ALS.
1. Introduction
Amyotrophic lateral sclerosis (ALS/MND) is a complex, uniquely human neurodegenerative disease with a variety of phenotypes, including frontotemporal dementia [1,2,3]. ALS phenotypes (age of onset, site of initial clinical presentation, and disease duration, amongst others) are predicated by genetic, environmental, lifestyle, and epigenetic influences [4]. There are no known naturally occurring animal models, and induced animal models whilst usefully mimicking anterior horn cell death, and to a lesser extent loss of upper motor neurons [5], cannot truly recapitulate all aspects of the disorder as seen in humans [6,7]. Many consider ALS to be a primary brain disorder [8].
Neurodegenerations, including amyotrophic lateral sclerosis (ALS), have increased over the past two centuries [9,10]. This time period is short, encompassing a limited number of generations, making genetic factors alone an unlikely explanation [11]. Post-industrial revolution changes in lifestyle and environmental factors compared with conditions experienced during the preceding evolutionary period are relevant to the increasing incidence of ALS and other neurodegenerations [12,13]. A steady decline in dietary fruits, vegetables, and fibers and increased consumption of animal products, saturated fats, and refined sugars has exerted evolutionary pressure on the gut microbiota [14,15] and adversely influenced the metabolic and inflammatory profile of brain cells [16]. Dietary and broader lifestyle and environmental changes together with increased longevity have further impacted the human microbiome [15,17,18].
The microbiome consists of bacterial, archaeal, fungal, viral, and microscopic eukaryotes and protozoan communities that colonize multiple body sites. They form an interface between the host and the outside world through the gastrointestinal tract, skin, respiratory tract, and urogenital tract [19,20]. Most of the microbiome is contained within the gastrointestinal tract, and the total genome of the organisms colonizing it is many times greater than that of the human genome [21,22].
Imbalance of the gut microbiome and dysfunction of the gut–brain axis may develop because of diet, metabolism, altered immunity, age, stress, lifestyle, antibiotics, and other therapeutic agents [11,18]. The effect of dysbiosis appears greatest in early life, particularly during “the first 1000 days”, which spans conception to a child’s second birthday [23]. During this period of maturation, the gut and brain interact, with the gut microbiome playing a key role in shaping processes that support neural health [24,25,26].
Inappropriate signals within the gut–brain axis may induce low-grade inflammation, oxidative stress, disturbed energy metabolism, impairment of the blood–brain barrier, and increased cellular aging [11,16,27,28,29]. These are shared pathophysiological mechanisms involved in all neurodegenerations including ALS [30,31]. Neurodegenerations may exhibit manifestations long before classic features appear [32,33]; this too is true of sporadic ALS [34]. This includes gastrointestinal symptomatology related to dysbiosis occurring prior to the onset of typical Alzheimer’s disease, Parkinson’s disease, and ALS [35,36,37]. But it is possible that sporadic ALS has its origins in early life [38]. The “First 1000 Days of Life” are critical to brain development, encompassing synaptogenesis, myelination, neuroplasticity, and the formation of functional neuronal circuits. Successful completion of these fetal and neonatal brain development processes may be impacted by a variety of processes, including the initial colonization and establishment of the gut–brain axis [39]. As such, the microbiome represents an essential environment that links the processes of human physiology, metabolism, and the immune system [40] during this vital period of neuro-development [41,42,43,44,45], with alterations potentially promoting future neurodegenerations such as ALS [46,47,48,49,50,51]. In further support of such concepts, there is growing recognition that early-life dysbiosis may be causative of later-life neuro-psychiatric diseases [52,53]. With these background concepts, the present review aims to explore current evidence that may suggest a role for the microbiome as a potential contributor to the origin of ALS and other neurodegenerations, most particularly during the perinatal period [38]. ALS is viewed as a multistep process in which the first step includes the genome, in utero, and maternal influences [54,55,56,57]. The maturation of the microbiome might be considered an early life contributor to this multistep process.
2. Maturation of the Microbiome
While adults have relatively stable gut microbiomes, the developing neonatal microbiome is unstable, with greater adaptability. At birth and through the initial years of life, the microbiome composition changes and expands [58]. Its composition undergoes rapid evolution during the first 1000 days of life, [59] with changes related to altered modes of delivery [60,61], early life nutrition [62], and deficiencies in maternal diet such as folate, iron, or omega-3 fatty acids with a potentially negative impact on mitochondrial function [63] and antibiotic exposure [64] (Figure 1).
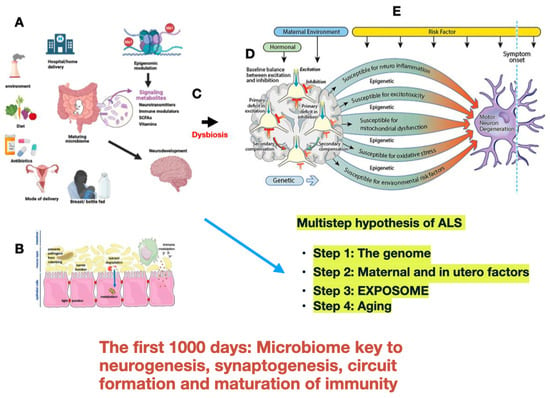
Figure 1.
Modified from Kiernan M.C., Ziemann U., and Eisen A. Amyotrophic lateral sclerosis: Origins traced to impaired balance between neural excitation and inhibition in the neonatal period [38]. Various factors influence the maturation of the fetal/neonatal microbiome (A). Establishment progresses rapidly during delivery, breastfeeding, and with the institution of a diet. Metabolites (neurotransmitters, vitamins, short-chain fatty acids, and amino acids) produced in the gut are key to neurodevelopment through a variety of neurobiological processes chiefly acting via the gut–brain axis and are modulated through epigenetic interaction which determines individual sensitivity and susceptibility. (B). Neonatal dysbiosis can set the stage for progressive neuroinflammation, excitotoxicity, mitochondrial dysfunction, and excessive oxidative stress to which motor neurons are susceptible (C). During neurodevelopment, neural network establishment and functioning are sensitive to excitatory (green) and inhibitory (red) balance (D). After decades and with aging, the motor system fails as further risk factors take effect in the multistep process hypothesized for ALS to become symptomatic and relentlessly progressive (E).
Similarly, microorganisms begin to colonize the skin [65] and mucosal cavities (oral [66], nasal [67], vaginal [68,69], and pulmonary [70,71]). However, the greatest colonization occurs within the gastrointestinal tract [72]. Microbial exposure precedes conception and gestation within the male and female reproductive tracts [73] and maturation in the fetal gut microbiome starts in utero [22,74].
At this stage, data from studies of fetal microbiome composition remain inconsistent [75], with controversy as to the existence of a placental microbiome [76]. Nevertheless, the maternal microbiome is implicated in placental structure and function [77,78]. The maternal microbiome drives the maturation of the offspring’s gut microbiome by means of local transmission during birth, with further exposure during maternal diet and breastfeeding [69,79]. It is accepted that breast milk contains important bioactive components that affect the establishment of the infant microbiome, including immunoglobulins, oligosaccharides, complement lactoferrin, lysozymes, hormones, and cytokines [80,81]. Human milk oligosaccharides are essential bioactive constituents and serve as prebiotics, mucosal signalling agents, and immunomodulators and significantly contribute to the enrichment of the gut microbiota, the enhancement of intestinal epithelial barrier integrity, and the support of immune function [81]. Brain development and the gut microbiota co-evolve, suggesting a bi-directional influence between the brain and commensal bacteria [82], while the developing gut–brain axis impacts the physiological and structural development of the central nervous system [83,84]. In terms of this bidirectional influence, the change from breast feeding to a solid diet is pivotal to the maturation of the gut flora [85,86], occurring at a time that coincides with intense synaptogenesis within the brain [79,87,88,89,90].
3. Brain Development and Gut Microbiome
The adult gut microbiome has diverse functions (see the review by Nandwana et al. [91]). During the “First 1000 days”, bacteria may exert an influence on the fine-tuning of synaptogenesis and the formation of neuronal circuits. In turn, neuromodulation is altered by chemical exchange through the gut–brain axis, manipulated via the vagal nerve, immune signalling, and bacterial production of metabolites [92]. Microbiota signalling also contributes to myelinization, especially in the prefrontal cortex [52,53,82,93].
Of further relevance, a large variety of metabolites are produced within the gut lumen, including neurotransmitters (gamma-aminobutyric acid, serotonin, dopamine, acetylcholine, and noradrenaline), several vitamins, short-chain fatty acids (propionate, acetate, and butyrate), and amino acids and their derivatives [94]. After absorption, some of these molecules cross the blood–brain barrier to reach and influence brain function [95]. But the mechanisms underlying the complex interactions of gut-produced metabolites remain to be determined [43,96]. Similarly, dysbiosis increases intestinal permeability with the translocation of harmful microbial products into the bloodstream that are able to promote an inflammatory response and damage the blood–brain barrier.
4. Neonatal Gut Microbiome and Immunity
The adult central nervous immune system is specialized and tightly regulated. Primary immune cells include microglia, astrocytes, T cells, and B cells, responding together to phagocytose debris, release cytokines, and recruit other immune cells. The immune responses are balanced to prevent excessive inflammation that induces neuronal damage and neurodegenerative diseases.
Maternal antibodies can be transferred placentally prior to birth. This passively derived immunity protects for the first few months of life through the transfer of IgG antibodies [97]. After birth, maternal milk provides the first source of antibody-mediated protection in the intestinal tract of infants against infection. In newborns, the immune system undergoes a rapid transition from dependency on maternal protection to becoming self-sufficient. Maternal microbes are transferred during delivery, then through maternal milk [98], and later when weaning to solid food occurs. Each of these steps advances colonization with microbe adherence to the intestinal epithelium [99] and maturation of the immune system [97]. The immune system becomes educated and expanded through infancy and early childhood through interactions with the gut microbiota [100], and if dysbiosis occurs during early development, it substantially impairs immune system elaboration [101].
Different signals and mechanisms derive from the developing microbiome, which controls immune activation [102]. This includes microbiome epigenetic remodelling and altered gene expression, the production of tissue-protective anti-inflammatory cytokines (e.g., IL-10), and curtailment of pro-inflammatory cytokines (e.g., IL-6 and TNF-α), which prevents excessive inflammation. Inflammatory control is mediated through microglia, the immune cells of the brain parenchyma [103]. But glias’ role extends much beyond immune functions [104]. They also sense neuronal activity, regulate neuronal synaptic pruning [105,106], affect developmental patterning and homeostatic functions in the central nervous system, including cell and/or debris clearance, synaptic maturation, neural circuit function, angio-/vasculogenesis, myelination, neurotransmission, and help maintain the integrity of the blood–brain barrier [103].
5. Neonatal Dysbiosis Induces Neurodegeneration
There are different but interacting mechanisms through which dysbiosis arising in the neonatal microbiome may affect neurodevelopment, with the subsequent potential for later-life neurodegenerative disease [31,107] (Table 1). Given that the neonatal gut microbiome is critical to training the immune system during early development [79,108,109], dysbiosis during this period can impair immune regulation, thereby causing overactivation of inflammatory pathways and resulting in low-grade chronic inflammation. Chronic inflammation activated through pro-inflammatory cytokines such as IL-6 and TNF-α is a major contributor to processes linked to ALS and related neurodegenerations [110,111,112,113,114], which may in turn suggest novel considerations for therapy [115].

Table 1.
Mechanisms by which neonatal dysbiosis could impact neurodegeneration.
At a pathological level, mislocalization and aggregation of the TAR DNA binding protein 43 (TDP-43) within the cytoplasm of neurons and glia is a pathological hallmark of ALS [116,117,118]. Growing evidence has associated neuroinflammation immune-mediated mechanisms with TDP-43 toxicity [119]. Cytoplasmic aggregates of TD for P-43 have also been implicated in neuronal excitotoxicity [120], considered a prime pathogenic mechanism in ALS [121,122,123]. Interleukin-1beta (IL-1beta) is a proinflammatory cytokine that contributes to the pathogenesis of both acute and chronic neurological disorders and mechanistically links the pro-inflammatory response to glutamate excitotoxicity [124]. TDP-43 increases blood barrier permeability and leukocyte recruitment, indicating complex intermolecular interactions between systemic inflammation and pathological TDP-43 protein, promoting disease progression [125].
There is scientific support for the role of the gut microbiome in preserving the integrity of the gut barrier [126,127]. Gastrointestinal barrier dysfunction (“leaky gut”) contributes to the development and progression of chronic low-grade systemic inflammation and age-related diseases such as ALS [128]. Gut bacteria may release metabolites into the blood that readily cross the blood–brain barrier [95]. Most gut microbes reside within the intestinal lumen lined by epithelial cells. Disruption of this gut epithelial barrier, as caused by pathogens, allows unregulated translocation of microbes into the lamina propria where gut immune cells reside [129].
6. Neurotransmitters and the Excitatory/Inhibitory Balance
Resident gut microbes, particularly bacteria, produce and utilize a variety of chemical messengers, including neurotransmitters critical for communication with the nervous system [130]. They include dopamine (metabolized through tyrosine), noradrenaline, serotonin (converted through tryptophan), GABA, and acetylcholine [131]. The pro-excitatory neurotransmitter glutamate and anti-excitatory neurotransmitter gamma-aminobutyric acid (GABA) plays a crucial role in regulating neuronal excitability [132]. Glutamate can be acquired from the diet and eukaryotic cells, synthesized by the microbiome, and can also be converted into GABA [132]. As demonstrated in animal studies and in humans, the manipulation of bacterial neurotransmitters impacts host physiology [94]. Dysbiosis may adversely alter or reduce neurotransmitter production, with a variety of deleterious secondary effects [133].
Of the variety of neurotransmitters produced by bacteria, GABA may be the most crucial because of the role of GABAergic inhibitory circuitry in excitatory/inhibitory (E/I) balance [134]. GABA is one of the earliest and most highly evolutionary conserved neurotransmitters [135,136]. During development, GABA is excitatory, while embryonic GABA signalling is the main excitatory drive for developing cortical networks [137,138,139]. The ability of embryonic GABA to depolarize primitive neurons arises because of a high intracellular chloride concentration. Excitatory GABAergic neurons migrate into the cerebral cortex via the white matter throughout the second half of gestation, and the switch to the typical adult inhibitory phenotype is greatest during the first postnatal year [140]. However, the change from excitatory (E) to inhibitory (I) continues to mature for several years before it is complete. Impaired maturation of E/I balance, particularly during embryogenesis, can exert lasting effects [134,141]. Maturation of E/I balance normally results in a decrease in overall excitatory tone in concert with sophisticated inhibitory control of neural activity. Failure of this normal maturation results in a net excitation recognized as a key pathophysiological factor driving the development of ALS [136].
The predominant excitatory neurotransmitter glutamate is essential for maintaining the metabolic performance of neurons and glia and the maintenance of proper E/I balance. After taking up glutamate via excitatory amino acid transporters (EAAT1 and EAAT2), astrocytic glutaminase hydrolyzes glutamate to glutamine, which is then transported to neurons [142]. Overall evidence points to loss of inhibition rather than excess glutamatergic excitation as the main driver behind ALS excitotoxicity [123,143,144].
7. Changes in Brain Permeability
Gut microbiome metabolites are important in the formation of the BBB during embryonic and neonatal life [145]. For example, the microbiome-derived fat-soluble vitamin K2 can diffuse freely across the BBB and regulate a wide spectrum of molecular mitochondrial functions [146]. During pregnancy, the maternal gut microbiota regulates the developing fetal BBB by upregulating the expression of proteins such as claudin-5 [29]. Claudin-5 (CLDN5) is a protein that helps form tight junctions, particularly in the blood–brain barrier (BBB) [147]. It is a key component of the BBB cell membrane and is involved in regulating the permeability of the barrier. Microbiome-induced inflammation can alter the permeability of the BBB, disrupting the tightly packed endothelial lining of the capillaries supplying the brain [148]. Proteins sealing the gaps between brain vascular endothelial cells are critical to its regulation [149,150], and these same molecules mediate intestinal permeability [151].
Several bacterial metabolites can cross the BBB through various transport mechanisms and accumulate in the brain, directly impacting its function. Detrimental gut–brain interactions may occur when intermediaries including bacteria, toxic digestive metabolites, bacterial toxins, and other virulent factors such as cytokines ‘leak’ into the bloodstream [152]. For this to happen, they must cross through both the intestinal epithelium barrier and the blood–brain barrier (BBB). Altered permeability of the intestinal epithelium results from a dysbiotic gut microbiome, which damages intestinal epithelial cells and facilitates the translocation of gut microbiota across the lumen to the mesenteric lymph and peripheral circulation. Bioactive metabolites that breach the BBB may impair the regulation of mitochondrial oxidation and microglia activation and induce pathogenic protein aggregation, important steps in the pathophysiology of neurodegeneration [132].
8. Mitochondrial Dysfunction and the Microbiome
Mitochondria are the metabolic hubs underlying a wide range of cellular processes, with mitochondrial dysfunction linked to the processes of neuronal death in ALS [153]. Mitochondria and the microbiome are closely related through their shared evolutionary background, maternal inheritance patterns, and overlapping roles in the maintenance of health and disease (“mitochondria-microbiome crosstalk”) [154]. Of relevance, increased levels of reactive oxygen species (ROS) may potentially damage DNA, lipids, and proteins, thereby contributing to age-related neurodegenerations, with the interplay between mitochondria and gut microbes through reactive oxygen species (ROS) [155]. Mitochondrial dysfunction caused by dysbiosis represents a major contributing factor to disruption of the gut epithelial barrier, with the potential for subsequent impairment of blood–brain barrier permeability, leading to neuroinflammation [156,157].
9. Future Considerations
Defective maturation of the neonatal gut microbiome and gut–brain axis may represent an initial brain insult, with only subtle deviations from a normal neonatal microbiome required to initiate a trajectory of chronic inflammation and excitotoxicity, both major contributors to the ultimate molecular cascade resulting in clinical ALS [123,158]. Both immunity and inflammation relate specifically to TDP-43, whose mislocalization and aggregation are hallmarks of ALS [119]. Over a lifetime, other interacting factors contribute to the effects of inflammation, and Betz cell neurons and the corticomotoneuronal system have been proposed as the nidus of origin of ALS and they are particularly vulnerable to inflammatory and excitotoxic insults and effects of aging [8]. The adult microbiome is readily manipulated through diets designed to introduce specific beneficial strains of bacteria or fecal microbiota transplants. However, the potential therapeutic benefits of these approaches in neurodegenerations, including ALS, have fallen short (see the review by Loh et al. [159]). Such procedures are not without risk, especially during early neurodevelopment [160,161]. Since most studies have been undertaken in rodent models, similar effectiveness does not necessarily translate to human disease [162].
There are further evolutionary considerations, given that the gut microbiota of hunter–gatherers and populations consuming a rural agrarian diet harbor greater diversity than the microbiota of the modern Western world [163,164]. Humans have experienced major dietary changes from gathered to farmed foods to the now mass consumption of processed meals. Each dietary shift has been accompanied by a concomitant adjustment in the microbiota and has induced a loss of microbiota diversity, postulated to be magnified over generations [163]. Much of the vast biochemical potential of the microbiome is distinct from that of the host, with the ability to modify phenotypes where host organisms gain new physiological abilities as a result of contributions from their microbial partners [165].
The idea that commensal microbiota can modulate the expression of the human genome is new, with information derived through the introduction of microbes into germ-free animals [166]. Intestinal gene expression undergoes dramatic reprogramming with microbial colonization after birth [167]. The microbiome encodes many more genes than the host genome, and interactions with it may alter the host genotype–phenotype [168]. Combining the microbiome’s consortium of genomes extends the genetic repertoire of the host, forming an “Extended Genotype” [168,169]. Wilde et al. [170] reviewed the host control of the microbiome in-depth, stressing that the generation time of symbionts is typically extremely short relative to their host’s, which enables rapid shifts in the species composition of microbiotas. Over evolutionary history, the incorporation of new species into the microbiota may have allowed a host species to take behavioral and social leaps, often ascribed to factors like altered brain morphology [165], and adaptations are most evident when microbes are first introduced in the neonatal period [171]. Technical improvements will allow for in-depth analysis of the microbiome and host genomes and how they influence each other [172].
It has been proposed that ALS reflects a multistep process in which a series of biological processes result in a tipping point where the disease becomes manifest [54,55,56,57,173]. There may be six distinct processes, but the number is variable; for example, if there is a large effect mutation, there would be fewer steps [55]. The steps include not only genetic and epigenetic factors but also environmental exposures, as encompassed in the ALS exosome [12]. An unrecognized step of the multistep process may relate to the neonatal microbiome and its interactions with the host, considered another clue to the causation of neurodegenerations, and most specifically ALS [2]. Important to such a consideration, there remain potential therapeutic strategies to modulate the gut microbiota, including antibiotics and probiotics, in addition to nutritional interventions that may support the function of beneficial microbes, and microRNAs to enhance targeted microbial populations [174]. Clearly, such considerations should be formally evaluated through clinical trials, through a precision medicine approach, with biomarker evaluation [13].
10. Conclusions
Early life environmental influences may have a profound impact on neurodevelopment and subsequently be a factor in the development of neurodegeneration, including ALS [113,175]. One such environmental factor is the gut microbiota, which, immediately after birth, rapidly and densely populate the newborn with complex forms of microbes. As we have described above, the effects of the maturing microbiome are diverse and have an influence on many neurobiological processes that may be impaired by dysbiosis during its maturation. Modern lifestyles, including refined diets, antibiotic intake, exposure to air pollutants, microplastics, and stress all negatively affect the diversity and composition of the gut microbiota [15]. Any combination of these factors during the first 1000 days, including pregnancy, delivery, and neonatal life, may result in infant dysbiosis, which, if unattended, becomes a potential risk factor for ALS and other neurodegenerations. Further confirmation of this concept is important as implicating very early-life dysfunction as a clue to later-life neurodegenerations opens a large time scale of possible interventions.
Author Contributions
Conceptualization, A.E. and M.C.K.; original draft preparation, A.E.; writing—review and editing, A.E. and M.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hardiman, O.; van den Berg, L.H.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chio, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 465–479. [Google Scholar] [CrossRef]
- Grad, L.I.; Rouleau, G.A.; Ravits, J.; Cashman, N.R. Clinical Spectrum of Amyotrophic Lateral Sclerosis (ALS). Cold Spring Harb. Perspect. Med. 2017, 7, a024117. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Burg, T.; Scekic-Zahirovic, J.; Fischer, M.; Rouaux, C. Upper and Lower Motor Neuron Degenerations Are Somatotopically Related and Temporally Ordered in the Sod1 Mouse Model of Amyotrophic Lateral Sclerosis. Brain Sci. 2021, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.J.; Talbot, K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog. Neurobiol. 2008, 85, 94–134. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, T.; Zerbo, R.A.; Balbi, M.; Torazza, C.; Frumento, G.; Fedele, E.; Bonanno, G.; Milanese, M. Nearly 30 Years of Animal Models to Study Amyotrophic Lateral Sclerosis: A Historical Overview and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 12236. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Vucic, S.; Kiernan, M.C. Amyotrophic lateral sclerosis represents corticomotoneuronal system failure. Muscle Nerve 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Levison, L.S.; Blicher, J.U.; Andersen, H. Incidence and mortality of ALS: A 42-year population-based nationwide study. J. Neurol. 2024, 272, 44. [Google Scholar] [CrossRef]
- McFarlane, R.; Peelo, C.; Galvin, M.; Heverin, M.; Hardiman, O. Epidemiologic Trends of Amyotrophic Lateral Sclerosis in Ireland, 1996–2021. Neurology 2023, 101, e1905–e1912. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Goutman, S.A.; Savelieff, M.G.; Jang, D.G.; Hur, J.; Feldman, E.L. The amyotrophic lateral sclerosis exposome: Recent advances and future directions. Nat. Rev. Neurol. 2023, 19, 617–634. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Vucic, S.; Talbot, K.; McDermott, C.J.; Hardiman, O.; Shefner, J.M.; Al-Chalabi, A.; Huynh, W.; Cudkowicz, M.; Talman, P.; et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2021, 17, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Gonzalez, A.; Fullaondo, A.; Odriozola, A. Impact of evolution on lifestyle in microbiome. Adv. Genet. 2024, 111, 149–198. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Banik, A.; Saurabh, S.; Maulik, M.; Khatri, S.N. Neuroimmunometabolism: A New Pathological Nexus Underlying Neurodegenerative Disorders. J. Neurosci. 2022, 42, 1888–1907. [Google Scholar] [CrossRef]
- Manske, S. Lifestyle Medicine and the Microbiome: Holistic Prevention and Treatment. Integr. Med. 2024, 23, 10–14. [Google Scholar]
- Kwao-Zigah, G.; Bediako-Bowan, A.; Boateng, P.A.; Aryee, G.K.; Abbang, S.M.; Atampugbire, G.; Quaye, O.; Tagoe, E.A. Microbiome Dysbiosis, Dietary Intake and Lifestyle-Associated Factors Involve in Epigenetic Modulations in Colorectal Cancer: A Narrative Review. Cancer Control 2024, 31, 10732748241263650. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, J.R.; Wu, G.D. The gut microbiota, environment and diseases of modern society. Gut Microbes 2012, 3, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O. Non-bacteria microbiome (virus, fungi, and archaea) in gastrointestinal cancer. J. Gastroenterol. Hepatol. 2022, 37, 256–262. [Google Scholar] [CrossRef]
- Kawano-Sugaya, T.; Arikawa, K.; Saeki, T.; Endoh, T.; Kamata, K.; Matsuhashi, A.; Hosokawa, M. A single amplified genome catalog reveals the dynamics of mobilome and resistome in the human microbiome. Microbiome 2024, 12, 188. [Google Scholar] [CrossRef]
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: A brief review. Front. Pediatr. 2015, 3, 17. [Google Scholar] [CrossRef]
- Brines, J.; Rigourd, V.; Billeaud, C. The First 1000 Days of Infant. Healthcare 2022, 10, 106. [Google Scholar] [CrossRef]
- Wang, J.; Qie, J.; Zhu, D.; Zhang, X.; Zhang, Q.; Xu, Y.; Wang, Y.; Mi, K.; Pei, Y.; Liu, Y.; et al. The landscape in the gut microbiome of long-lived families reveals new insights on longevity and aging—Relevant neural and immune function. Gut Microbes 2022, 14, 2107288. [Google Scholar] [CrossRef]
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Baffy, G.; Portincasa, P. Unraveling the Role of the Human Gut Microbiome in Health and Diseases. Microorganisms 2024, 12, 2333. [Google Scholar] [CrossRef]
- Keshet, A.; Segal, E. Identification of gut microbiome features associated with host metabolic health in a large population-based cohort. Nat. Commun. 2024, 15, 9358. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, P.; Ding, N.; Jia, W.; Huang, C.; Tang, Y. Oxidative Stress, Inflammation, Gut Dysbiosis: What Can Polyphenols Do in Inflammatory Bowel Disease? Antioxidants 2023, 12, 967. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Toth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, M.; Varshney, N.; Rawal, K.S.; Jha, H.C. Gut dysbiosis and neurological modalities: An engineering approach via proteomic analysis of gut-brain axis. Adv. Protein Chem. Struct. Biol. 2024, 140, 199–248. [Google Scholar] [CrossRef]
- Pan, I.; Issac, P.K.; Rahman, M.M.; Guru, A.; Arockiaraj, J. Gut-Brain Axis a Key Player to Control Gut Dysbiosis in Neurological Diseases. Mol. Neurobiol. 2024, 61, 9873–9891. [Google Scholar] [CrossRef]
- Cavaleri, F. Paradigm shift redefining molecular, metabolic and structural events in Alzheimer’s disease involves a proposed contribution by transition metals. Defined lengthy preclinical stage provides new hope to circumvent advancement of disease- and age-related neurodegeneration. Med. Hypotheses 2015, 84, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W. Parkinson’s disease and the quest for preclinical diagnosis: An interview with Professor Werner Poewe. Neurodegener. Dis. Manag. 2017, 7, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Kiernan, M.; Mitsumoto, H.; Swash, M. Amyotrophic lateral sclerosis: A long preclinical period? J. Neurol. Neurosurg. Psychiatry 2014, 85, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, R.; Schmid, E.T.; Walker, D.W. Gut mitochondrial defects drive neurodegeneration. Nat. Aging 2022, 2, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Gao, Y. Gut-brain axis and neurodegeneration: Mechanisms and therapeutic potentials. Front. Neurosci. 2024, 18, 1481390. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, F.; Lamminpaa, I.; Niccolai, E.; Amedei, A. Nutritional and Microbiota-Based Approaches in Amyotrophic Lateral Sclerosis: From Prevention to Treatment. Nutrients 2024, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Ziemann, U.; Eisen, A. Amyotrophic lateral sclerosis: Origins traced to impaired balance between neural excitation and inhibition in the neonatal period. Muscle Nerve 2019, 60, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, M.; Li, S.; Lei, Y.; Xiang, X.; Guo, Z.; Wang, Q. Shaping oral and intestinal microbiota and the immune system during the first 1,000 days of life. Front. Pediatr. 2025, 13, 1471743. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Devkota, S.; Ghosh, T.S. Gut microbiome: A biomedical revolution. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 830–833. [Google Scholar] [CrossRef]
- Taddei, C.R.; Neu, J. Editorial: Microbiome in the first 1000 days: Multi-omic interactions, physiological effects, and clinical implications. Front. Cell. Infect. Microbiol. 2023, 13, 1242626. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. How gut microbes could drive brain disorders. Nature 2021, 590, 22–25. [Google Scholar] [CrossRef]
- Rykalo, N.; Riehl, L.; Kress, M. The gut microbiome and the brain. Curr. Opin. Support. Palliat. Care 2024, 17, 1261–1272. [Google Scholar] [CrossRef]
- Molinero, N.; Anton-Fernandez, A.; Hernandez, F.; Avila, J.; Bartolome, B.; Moreno-Arribas, M.V. Gut Microbiota, an Additional Hallmark of Human Aging and Neurodegeneration. Neuroscience 2023, 518, 141–161. [Google Scholar] [CrossRef]
- Kargbo, R.B. Microbiome-Gut-Brain Axis Modulation: New Approaches in Treatment of Parkinson’s Disease and Amyotrophic Lateral Sclerosis. ACS Med. Chem. Lett. 2023, 14, 886–888. [Google Scholar] [CrossRef]
- Boddy, S.L.; Giovannelli, I.; Sassani, M.; Cooper-Knock, J.; Snyder, M.P.; Segal, E.; Elinav, E.; Barker, L.A.; Shaw, P.J.; McDermott, C.J. The gut microbiome: A key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 2021, 19, 13. [Google Scholar] [CrossRef]
- Hertzberg, V.S.; Singh, H.; Fournier, C.N.; Moustafa, A.; Polak, M.; Kuelbs, C.A.; Torralba, M.G.; Tansey, M.G.; Nelson, K.E.; Glass, J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, T.; Debelius, J.W.; Fang, F. Gut microbiome and amyotrophic lateral sclerosis: A systematic review of current evidence. J. Intern. Med. 2021, 290, 758–788. [Google Scholar] [CrossRef]
- Seguella, L.; Sarnelli, G.; Esposito, G. Leaky gut, dysbiosis, and enteric glia activation: The trilogy behind the intestinal origin of Parkinson’s disease. Neural Regen. Res. 2020, 15, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the Role of the Gut Microbiome in Brain Development and Its Association With Neurodevelopmental Psychiatric Disorders. Front. Cell Dev. Biol. 2022, 10, 880544. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, A.; Calvo, A.; Chio, A.; Colville, S.; Ellis, C.M.; Hardiman, O.; Heverin, M.; Howard, R.S.; Huisman, M.H.B.; Keren, N.; et al. Analysis of amyotrophic lateral sclerosis as a multistep process: A population-based modelling study. Lancet Neurol. 2014, 13, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Chio, A.; Mazzini, L.; D’Alfonso, S.; Corrado, L.; Canosa, A.; Moglia, C.; Manera, U.; Bersano, E.; Brunetti, M.; Barberis, M.; et al. The multistep hypothesis of ALS revisited: The role of genetic mutations. Neurology 2018, 91, e635–e642. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Higashihara, M.; Sobue, G.; Atsuta, N.; Doi, Y.; Kuwabara, S.; Kim, S.H.; Kim, I.; Oh, K.W.; Park, J.; et al. ALS is a multistep process in South Korean, Japanese, and Australian patients. Neurology 2020, 94, e1657–e1663. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Westeneng, H.J.; Al-Chalabi, A.; Van Den Berg, L.H.; Talman, P.; Kiernan, M.C. Amyotrophic lateral sclerosis as a multi-step process: An Australia population study. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.G.; Wingert, R.A. Forever young by Alpha(diversity)ville: Restricting intestinal microbiome maturation stunts immune system development and increases susceptibility to infection. Tissue Barriers 2024, 12, 2281209. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.A.; Kellermayer, R. Disturbed Pediatric Gut Microbiome Maturation in the Developmental Origins of Subsequent Chronic Disease. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 123–127. [Google Scholar] [CrossRef]
- Leech, S.M.; Borg, D.J.; Rae, K.M.; Kumar, S.; Clifton, V.L.; Dekker Nitert, M. Delivery mode is a larger determinant of infant gut microbiome composition at 6 weeks than exposure to peripartum antibiotics. Microb. Genom. 2024, 10, 001269. [Google Scholar] [CrossRef]
- Aagaard, K.M. Mode of delivery and pondering potential sources of the neonatal microbiome. eBioMedicine 2020, 51, 102554. [Google Scholar] [CrossRef] [PubMed]
- Ames, S.R.; Lotoski, L.C.; Azad, M.B. Comparing early life nutritional sources and human milk feeding practices: Personalized and dynamic nutrition supports infant gut microbiome development and immune system maturation. Gut Microbes 2023, 15, 2190305. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.N.; Madan, J.C.; Emond, J.A.; Morrison, H.G.; Christensen, B.C.; Karagas, M.R.; Hoen, A.G. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.D.; Lieber, A.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra382. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Roux, P.F.; Boireau-Adamezyk, E.; Lboukili, I.; Oddos, T. Skin maturation from birth to 10 years of age: Structure, function, composition and microbiome. Exp. Dermatol. 2023, 32, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Monteiro-Silva, F. Acquisition and maturation of oral microbiome throughout childhood: An update. Dent. Res. J. 2014, 11, 291–301. [Google Scholar]
- Xiao, S.; Zhou, W.; Caldwell, R.; Decker, S.; Oh, J.; Milstone, A.M. Association of Neonatal and Maternal Nasal Microbiome Among Neonates in the Intensive Care Unit. Open Forum Infect. Dis. 2024, 11, ofae644. [Google Scholar] [CrossRef] [PubMed]
- Jaspan, H.B.; Mitchell, C.M.; Happel, A.U. The vagina question: Can maternal vaginal fluid impact the infant gut microbiome and neurodevelopment? Cell Host Microbe 2023, 31, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, C.; Barman, D.; Tripathi, D.; Dutta, S.; Bhattacharya, C.; Alam, M.; Choudhury, P.; Devi, U.; Mahanta, J.; Rasaily, R.; et al. Influence of Maternal Breast Milk and Vaginal Microbiome on Neonatal Gut Microbiome: A Longitudinal Study during the First Year. Microbiol. Spectr. 2023, 11, e0496722. [Google Scholar] [CrossRef] [PubMed]
- Zemanick, E.T.; Rosas-Salazar, C. The Role of the Microbiome in Pediatric Respiratory Diseases. Clin. Chest Med. 2024, 45, 587–597. [Google Scholar] [CrossRef]
- Kloepfer, K.M.; Kennedy, J.L. Childhood respiratory viral infections and the microbiome. J. Allergy Clin. Immunol. 2023, 152, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Ratsika, A.; Codagnone, M.C.; O’Mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients 2021, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Selma-Royo, M.; Tarrazo, M.; Garcia-Mantrana, I.; Gomez-Gallego, C.; Salminen, S.; Collado, M.C. Shaping Microbiota During the First 1000 Days of Life. Adv. Exp. Med. Biol. 2019, 1125, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Beharry, K.D.; Latkowska, M.; Valencia, A.M.; Allana, A.; Soto, J.; Cai, C.L.; Golombek, S.; Hand, I.; Aranda, J.V. Factors Influencing Neonatal Gut Microbiome and Health with a Focus on Necrotizing Enterocolitis. Microorganisms 2023, 11, 2528. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; de Goffau, M.C.; Perez-Munoz, M.E.; Arrieta, M.C.; Backhed, F.; Bork, P.; Braun, T.; Bushman, F.D.; Dore, J.; de Vos, W.M.; et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature 2023, 613, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Papaevangelou, V.; Malamitsi-Puchner, A. The jury is still out on the existence of a placental microbiome. Acta Paediatr. 2021, 110, 2958–2963. [Google Scholar] [CrossRef]
- Stupak, A.; Geca, T.; Kwasniewska, A.; Mlak, R.; Piwowarczyk, P.; Nawrot, R.; Gozdzicka-Jozefiak, A.; Kwasniewski, W. Comparative Analysis of the Placental Microbiome in Pregnancies with Late Fetal Growth Restriction versus Physiological Pregnancies. Int. J. Mol. Sci. 2023, 24, 6922. [Google Scholar] [CrossRef] [PubMed]
- Zakis, D.R.; Paulissen, E.; Kornete, L.; Kaan, A.M.M.; Nicu, E.A.; Zaura, E. The evidence for placental microbiome and its composition in healthy pregnancies: A systematic review. J. Reprod. Immunol. 2022, 149, 103455. [Google Scholar] [CrossRef]
- Davis, E.C.; Castagna, V.P.; Sela, D.A.; Hillard, M.A.; Lindberg, S.; Mantis, N.J.; Seppo, A.E.; Jarvinen, K.M. Gut microbiome and breast-feeding: Implications for early immune development. J. Allergy Clin. Immunol. 2022, 150, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wan, F. Breastfeeding and infant gut microbiota: Influence of bioactive components. Gut Microbes 2025, 17, 2446403. [Google Scholar] [CrossRef]
- Duman, H.; Bechelany, M.; Karav, S. Human Milk Oligosaccharides: Decoding Their Structural Variability, Health Benefits, and the Evolution of Infant Nutrition. Nutrients 2024, 17, 118. [Google Scholar] [CrossRef]
- Tognini, P. Gut Microbiota: A Potential Regulator of Neurodevelopment. Front. Cell. Neurosci. 2017, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Tapiainen, T.; Paalanne, N.; Tejesvi, M.V.; Koivusaari, P.; Korpela, K.; Pokka, T.; Salo, J.; Kaukola, T.; Pirttila, A.M.; Uhari, M.; et al. Maternal influence on the fetal microbiome in a population-based study of the first-pass meconium. Pediatr. Res. 2018, 84, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef]
- Samarra, A.; Flores, E.; Bernabeu, M.; Cabrera-Rubio, R.; Bauerl, C.; Selma-Royo, M.; Collado, M.C. Shaping Microbiota During the First 1000 Days of Life. Adv. Exp. Med. Biol. 2024, 1449, 1–28. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Dejardin, F.; Sparwasser, T.; Berard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288.e1275. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, V.; Carta, M.; Accomando, S.; Giuffre, M. The First 1000 Days of Life: How Changes in the Microbiota Can Influence Food Allergy Onset in Children. Nutrients 2023, 15, 4014. [Google Scholar] [CrossRef]
- Romano-Keeler, J.; Sun, J. The First 1000 Days: Assembly of the Neonatal Microbiome and Its Impact on Health Outcomes. Newborn 2022, 1, 219–226. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal development of brain circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The Role of Microbiome in Brain Development and Neurodegenerative Diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef]
- Frerichs, N.M.; de Meij, T.G.J.; Niemarkt, H.J. Microbiome and its impact on fetal and neonatal brain development: Current opinion in pediatrics. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Dubey, H.; Roychoudhury, R.; Alex, A.; Best, C.; Liu, S.; White, A.; Carlson, A.; Azcarate-Peril, M.A.; Mansfield, L.S.; Knickmeyer, R. Effect of Human Infant Gut Microbiota on Mouse Behavior, Dendritic Complexity, and Myelination. bioRxiv 2023. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Banks, W.A. Gut reactions: How the blood-brain barrier connects the microbiome and the brain. Exp. Biol. Med. 2018, 243, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Sanidad, K.Z.; Amir, M.; Ananthanarayanan, A.; Singaraju, A.; Shiland, N.B.; Hong, H.S.; Kamada, N.; Inohara, N.; Nunez, G.; Zeng, M.Y. Maternal gut microbiome-induced IgG regulates neonatal gut microbiome and immunity. Sci. Immunol. 2022, 7, eabh3816. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.B.; Chou, W.C.; Conner, M.E.; Earl, A.M.; Knight, R.; Bjorkman, P.J.; et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Peng, W.; Mao, X. Glial polarization in neurological diseases: Molecular mechanisms and therapeutic opportunities. Ageing Res. Rev. 2024, 104, 102638. [Google Scholar] [CrossRef]
- Lopez-Ortiz, A.O.; Eyo, U.B. Astrocytes and microglia in the coordination of CNS development and homeostasis. J. Neurochem. 2024, 168, 3599–3614. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Iglesias, M.; Maldonado-Teixido, J.; Melero, A.; Piriz, J.; Galea, E.; Ransohoff, R.M.; Sierra, A. Microglia as hunters or gatherers of brain synapses. Nat. Neurosci. 2024, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Eyo, U.; Molofsky, A.V. Defining microglial-synapse interactions. Science 2023, 381, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Mukhopadhyay, S.; Lakshminrusimha, S.; Bevins, C.L. Neonatal intestinal dysbiosis. J. Perinatol. 2020, 40, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Apostol, A.C.; Jensen, K.D.C.; Beaudin, A.E. Training the Fetal Immune System Through Maternal Inflammation-A Layered Hygiene Hypothesis. Front. Immunol. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Jeurink, P.V.; Knipping, K.; Wiens, F.; Baranska, K.; Stahl, B.; Garssen, J.; Krolak-Olejnik, B. Importance of maternal diet in the training of the infant’s immune system during gestation and lactation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1311–1319. [Google Scholar] [CrossRef]
- Batty, G.D.; Kivimaki, M.; Frank, P.; Gale, C.R.; Wright, L. Systemic inflammation and subsequent risk of amyotrophic lateral sclerosis: Prospective cohort study. Brain Behav. Immun. 2023, 114, 46–51. [Google Scholar] [CrossRef]
- Appel, S.H.; Beers, D.R.; Zhao, W. Amyotrophic lateral sclerosis is a systemic disease: Peripheral contributions to inflammation-mediated neurodegeneration. Curr. Opin. Neurol. 2021, 34, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Beland, L.C.; Markovinovic, A.; Jakovac, H.; De Marchi, F.; Bilic, E.; Mazzini, L.; Kriz, J.; Munitic, I. Immunity in amyotrophic lateral sclerosis: Blurred lines between excessive inflammation and inefficient immune responses. Brain Commun. 2020, 2, fcaa124. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Pioro, E.P.; Goutman, S.A.; Kiernan, M.C. Nanoplastics and Neurodegeneration in ALS. Brain Sci. 2024, 14, 471. [Google Scholar] [CrossRef]
- Bright, F.; Werry, E.L.; Dobson-Stone, C.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kassiou, M.; et al. Neuroinflammation in frontotemporal dementia. Nat. Rev. Neurol. 2019, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Henderson, R.D.; Mathers, S.; Needham, M.; Schultz, D.; Kiernan, M.C.; The TEALS Study Group. Safety and efficacy of dimethyl fumarate in ALS: Randomised controlled study. Ann. Clin. Transl. Neurol. 2021, 8, 1991–1999. [Google Scholar] [CrossRef]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Del Tredici, K.; Braak, H. Neuropathology and neuroanatomy of TDP-43 amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2022, 35, 660–671. [Google Scholar] [CrossRef]
- Pongracova, E.; Buratti, E.; Romano, M. Prion-like Spreading of Disease in TDP-43 Proteinopathies. Brain Sci. 2024, 14, 1132. [Google Scholar] [CrossRef] [PubMed]
- Bright, F.; Chan, G.; van Hummel, A.; Ittner, L.M.; Ke, Y.D. TDP-43 and Inflammation: Implications for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Int. J. Mol. Sci. 2021, 22, 7781. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Urushitani, M. Molecular Dissection of TDP-43 as a Leading Cause of ALS/FTLD. Int. J. Mol. Sci. 2022, 23, 12508. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Park, S.B. Hyperexcitability, neurodegeneration, and disease progression in amyotrophic lateral sclerosis. Muscle Nerve 2023, 68, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Cheah, B.C.; Kiernan, M.C. Defining the mechanisms that underlie cortical hyperexcitability in amyotrophic lateral sclerosis. Exp. Neurol. 2009, 220, 177–182. [Google Scholar] [CrossRef]
- Odierna, G.L.; Vucic, S.; Dyer, M.; Dickson, T.; Woodhouse, A.; Blizzard, C. How do we get from hyperexcitability to excitotoxicity in amyotrophic lateral sclerosis? Brain 2024, 147, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Fogal, B.; Hewett, S.J. Interleukin-1beta: A bridge between inflammation and excitotoxicity? J. Neurochem. 2008, 106, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, F.; Loon, A.R.; Smeltzer, S.; Benyamine, K.; Navalpur Shanmugam, N.K.; Stewart, N.J.F.; Lee, D.C.; Nash, K.; Selenica, M.B. TDP-43 mediated blood-brain barrier permeability and leukocyte infiltration promote neurodegeneration in a low-grade systemic inflammation mouse model. J. Neuroinflamm. 2020, 17, 283. [Google Scholar] [CrossRef]
- Compare, D.; Sgamato, C.; Rocco, A.; Coccoli, P.; Ambrosio, C.; Nardone, G. The Leaky Gut and Human Diseases: “Can’t Fill the Cup if You Don’t Plug the Holes First”. Dig. Dis. 2024, 42, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. What is the leaky gut? Clinical considerations in humans. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Escalante, J.; Artaiz, O.; Diwakarla, S.; McQuade, R.M. Leaky gut in systemic inflammation: Exploring the link between gastrointestinal disorders and age-related diseases. Geroscience 2024. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Peng, M.; Liang, J.; Sun, H. The Role of Gut Microbiota in Blood-Brain Barrier Disruption after Stroke. Mol. Neurobiol. 2024, 61, 9735–9755. [Google Scholar] [CrossRef] [PubMed]
- Belelli, D.; Lambert, J.J.; Wan, M.L.Y.; Monteiro, A.R.; Nutt, D.J.; Swinny, J.D. From bugs to brain: Unravelling the GABA signalling networks in the brain-gut-microbiome axis. Brain 2024. [Google Scholar] [CrossRef]
- Zhong, J.G.; Lan, W.T.; Feng, Y.Q.; Li, Y.H.; Shen, Y.Y.; Gong, J.H.; Zou, Z.; Hou, X. Associations between dysbiosis gut microbiota and changes of neurotransmitters and short-chain fatty acids in valproic acid model rats. Front. Physiol. 2023, 14, 1077821. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Mastandrea, I.; Melekhova, A.; Elinav, E. Mechanisms by which microbiome-derived metabolites exert their impacts on neurodegeneration. Cell Chem. Biol. 2024, 32, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.; Jellali, A.; Crenner, F.; Aunis, D.; Angel, F. Functional evidence for a role of GABA receptors in modulating nerve activities of circular smooth muscle from rat colon in vitro. Life Sci. 2003, 72, 1481–1493. [Google Scholar] [CrossRef]
- Uliana, D.L.; Lisboa, J.R.F.; Gomes, F.V.; Grace, A.A. The excitatory-inhibitory balance as a target for the development of novel drugs to treat schizophrenia. Biochem. Pharmacol. 2024, 228, 116298. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, K.; Lin, Y. The contribution of GABAergic dysfunction to neurodevelopmental disorders. Trends Mol. Med. 2011, 17, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Taube, W.; Lauber, B. Changes in the cortical GABAergic inhibitory system with ageing and ageing-related neurodegenerative diseases. J. Physiol. 2024. early view. [Google Scholar] [CrossRef]
- Wang, D.D.; Kriegstein, A.R. Defining the role of GABA in cortical development. J. Physiol. 2009, 587, 1873–1879. [Google Scholar] [CrossRef]
- Kilb, W. Development of the GABAergic system from birth to adolescence. Neuroscientist 2012, 18, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Gaiarsa, J.L.; Tyzio, R.; Khazipov, R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef]
- McKeon, S.D.; Perica, M.I.; Parr, A.C.; Calabro, F.J.; Foran, W.; Hetherington, H.; Moon, C.H.; Luna, B. Aperiodic EEG and 7T MRSI evidence for maturation of E/I balance supporting the development of working memory through adolescence. Dev. Cogn. Neurosci. 2024, 66, 101373. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Geevasinga, N.; Menon, P.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Diagnostic utility of cortical excitability studies in amyotrophic lateral sclerosis. Eur. J. Neurol. 2014, 21, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Menon, P.; Geevasinga, N.; van den Bos, M.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Cortical hyperexcitability and disease spread in amyotrophic lateral sclerosis. Eur. J. Neurol. 2017, 24, 816–824. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Zhang, Y.; Xia, C.; Lai, Q.; Dong, Z.; Kuang, W.; Yang, C.; Su, D.; Li, H.; et al. Potential effects of antibiotic-induced gut microbiome alteration on blood-brain barrier permeability compromise in rhesus monkeys. Ann. N. Y. Acad. Sci. 2020, 1470, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef]
- Fisher, D.; Mentor, S. Are claudin-5 tight-junction proteins in the blood-brain barrier porous? Neural Regen. Res. 2020, 15, 1838–1839. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood-brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef] [PubMed]
- Zachos, K.A.; Gamboa, J.A.; Dewji, A.S.; Lee, J.; Brijbassi, S.; Andreazza, A.C. The interplay between mitochondria, the gut microbiome and metabolites and their therapeutic potential in primary mitochondrial disease. Front. Pharmacol. 2024, 15, 1428242. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.W.O.; Towarnicki, S.G. Mitochondria, the gut microbiome and ROS. Cell Signal. 2020, 75, 109737. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Shah, R.; Alford, N.; Mishra, S.P.; Jain, S.; Hansen, B.; Sanberg, P.; Molina, A.J.A.; Yadav, H. The Triple Alliance: Microbiome, Mitochondria, and Metabolites in the Context of Age-Related Cognitive Decline and Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2187–2202. [Google Scholar] [CrossRef]
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host mitochondria influence gut microbiome diversity: A role for ROS. Sci. Signal. 2019, 12, eaaw3159. [Google Scholar] [CrossRef] [PubMed]
- Wosiski-Kuhn, M.; Lyon, M.S.; Caress, J.; Milligan, C. Inflammation, immunity, and amyotrophic lateral sclerosis: II. immune-modulating therapies. Muscle Nerve 2019, 59, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, D.; Ahmed, A.; Shafiq, A.; McVeigh, C.; Chaari, A.; Zakaria, D.; Bendriss, G. Fecal microbiota transplants: A review of emerging clinical data on applications, efficacy, and risks (2015–2020). Qatar Med. J. 2021, 2021, 5. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T. We need more evidence about the risks and benefits of giving children faecal microbiota transplants. Acta Paediatr. 2024, 113, 1987–1988. [Google Scholar] [CrossRef]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or Exaggerating Causality for the Gut Microbiome: Lessons from Human Microbiota-Associated Rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- Lynch, J.B.; Hsiao, E.Y. Microbiomes as sources of emergent host phenotypes. Science 2019, 365, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Boccuto, L.; Tack, J.; Ianiro, G.; Abenavoli, L.; Scarpellini, E. Human Genes Involved in the Interaction between Host and Gut Microbiome: Regulation and Pathogenic Mechanisms. Genes 2023, 14, 857. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Dai, C.L.; Wilmanski, T.; Baloni, P.; Smith, B.; Rappaport, N.; Hood, L.; Magis, A.T.; Gibbons, S.M. Genome-microbiome interplay provides insight into the determinants of the human blood metabolome. Nat. Metab. 2022, 4, 1560–1572. [Google Scholar] [CrossRef]
- Henry, L.P.; Bruijning, M.; Forsberg, S.K.G.; Ayroles, J.F. The microbiome extends host evolutionary potential. Nat. Commun. 2021, 12, 5141. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.D.D. Extended genomes: Symbiosis and evolution. Interface Focus 2017, 7, 20170001. [Google Scholar] [CrossRef]
- Wilde, J.; Slack, E.; Foster, K.R. Host control of the microbiome: Mechanisms, evolution, and disease. Science 2024, 385, eadi3338. [Google Scholar] [CrossRef]
- Obeng, N.; Czerwinski, A.; Schutz, D.; Michels, J.; Leipert, J.; Bansept, F.; Garcia Garcia, M.J.; Schultheiss, T.; Kemlein, M.; Fuss, J.; et al. Bacterial c-di-GMP has a key role in establishing host-microbe symbiosis. Nat. Microbiol. 2023, 8, 1809–1819. [Google Scholar] [CrossRef]
- Tegegne, H.A.; Savidge, T.C. Leveraging human microbiomes for disease prediction and treatment. Trends Pharmacol. Sci. 2024, 46, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Ziser, L.; van Eijk, R.P.A.; Kiernan, M.C.; McRae, A.; Henderson, R.D.; Schultz, D.; Needham, M.; Mathers, S.; McCombe, P.; Talman, P.; et al. Amyotrophic lateral sclerosis established as a multistep process across phenotypes. Eur. J. Neurol. 2025, 32, e16532. [Google Scholar] [CrossRef]
- Kaul, M.; Mukherjee, D.; Weiner, H.L.; Cox, L.M. Gut microbiota immune cross-talk in amyotrophic lateral sclerosis. Neurotherapeutics 2024, 21, e00469. [Google Scholar] [CrossRef] [PubMed]
- Shefner, J.M.; Al-Chalabi, A.; Baker, M.R.; Cui, L.Y.; de Carvalho, M.; Eisen, A.; Grosskreutz, J.; Hardiman, O.; Henderson, R.; Matamala, J.M.; et al. A proposal for new diagnostic criteria for ALS. Clin. Neurophysiol. 2020, 131, 1975–1978. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).