Abstract
Amyotrophic lateral sclerosis (ALS) is a terminal complex neurodegenerative disease, with 10–15% of cases being familial and the majority being sporadic with no known cause. There are no animal models for the 85–90% of sporadic ALS cases. More creative, sophisticated models of ALS disease are required to unravel the mysteries of this complicated disease. While ALS patients urgently require new medications and treatments, suitable preclinical in vitro models for drug screening are lacking. Therefore, human-derived induced pluripotent stem cell (hiPSC) technology offers the opportunity to model diverse and unreachable cell types in a culture dish. In this review, we focus on recent hiPSC-derived ALS neuronal and non-neuronal models to examine the research progress of current ALS 2D monocultures, co-cultures, and more complex 3D-model organoids. Despite the challenges inherent to hiPSC-based models, their application to preclinical drug studies is enormous.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is characterized by the degeneration of upper and lower motor neurons (LMNs) in the motor cortex, brainstem, and spinal cord, resulting in the progressive weakness of voluntary and respiratory muscles. The disease’s rapid progression limits median survival to between 2 and 5 years, with no effective treatment available [1,2]. Between 85% and 90% of ALS cases are sporadic (sALS), with unknown causes that most likely combine environmental and polygenic factors [3]. Therefore, developing sALS disease models is very challenging. An estimated 10–15% of cases are familial ALS (fALS), which are linked to monogenic causes with primarily dominant inheritance [1,4]. ALS is now recognized as a multisystem neurodegenerative illness with heterogeneity in its clinical, genetic, and neuropathological aspects [1]. In fact, the recognized overlap with frontotemporal dementia (FTD) suggests ALS and FTD are now thought to be two ends of a spectrum due to the overlap in molecular mechanisms underlying both neurodegenerative disorders [5].
In 2007, Takahashi et al. demonstrated how to reprogram somatic cells into induced pluripotent stem cells (iPSCs), allowing researchers to differentiate these cells and model human disease processes [6]. Since then, iPSC biology has progressed rapidly, with the discovery of more advanced reprogramming techniques [7,8]. These developments have enabled in vitro investigations with human (h) iPSCs into the mechanisms of ALS, the most common adult motor neuron disease (MND) [9]. hiPSCs can differentiate into any somatic cell type found in the central and peripheral nervous systems (CNS and PNS), including neurons, astrocytes, oligodendrocytes, microglia, Schwann cells, and myocytes. The most notable feature of hiPSCs is the ability to be generated from somatic cells (usually skin fibroblasts or peripheral blood mononuclear cells) during adulthood (Figure 1). This enables the creation of iPSCs from ALS patients with varied phenotypes and genotypes, providing insights into the range of both familial and more common sporadic cases [10]. Recent whole-genome sequencing studies have shown that sporadic ALS cases are linked to several variants, suggesting that accumulating genetic mutations cause the disease to manifest [11,12]. The hiPSC containing all of the donor’s genetic material can be differentiated into a variety of cell types to be a powerful tool for simulating diverse sporadic disease pathogenic mechanisms in vitro. Importantly, hiPSC-based cellular models and their pathologies are driven by genetic information even in sporadic cases, because most epigenetic modifications in donor cells are reset to the embryonic state during the iPSC reprogramming process, with the exception of genetic imprinting and epigenetic memory [11].
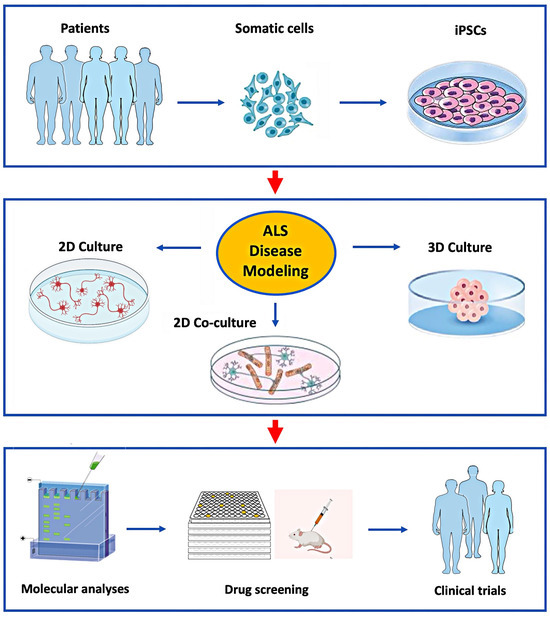
Figure 1.
Application of hiPSC’s to model ALS in vitro for studies of disease mechanisms and drug development.
Patient-derived somatic cells can be reprogrammed to iPSCs (top panel) and then differentiated into various cell types grown in 2D or 3D culture systems (middle panel). This allows for a detailed study of the molecular mechanisms causing ALS in specific patients for the development and preclinical testing of novel drugs selected for clinical trials (lower panel).
The more recent stem cell technologies, particularly those using hiPSCs, have enormous potential for modeling the distinct pathophysiology of an individual patient. Nevertheless, certain limitations exist, including phenotypic and genotypic variability and inadequate maturation, in comparison to in vivo models. In addition, the majority of preclinical ALS research to date has been conducted in vivo using mouse models [13,14]. Animal studies, however, are less than ideal for large-scale drug testing due to their high degree of variability and ethical issues [14], among other reasons. Although in vitro tissue culture models are popular methods for studying ALS pathogenic mechanisms and finding novel treatments because of their simplicity and lower cost, they cannot fully recapitulate human ALS. However, advancements with intricate in vitro cultures and co-cultures using organoids and microfluidic technology have made it possible to create large model systems for a more representative study of ALS.
No cure or effective treatment is currently available for ALS due to its great mechanistic complexity, genetic variability, and translational gaps between animal models and this human disease. The absence of proper preclinical in vitro models poses a hurdle to suitable clinical studies in ALS. Several recent reviews have covered different aspects of the hiPSC-derived in vitro modeling of ALS [10,15,16,17,18,19,20,21]. In this review, we discuss some of the most recent complex hiPSC-derived ALS models, such as monoculture differentiation, two-dimensional (2D) co-culture compositions, and three-dimensional (3D) organoids and their applications to understanding disease mechanisms and recent preclinical drug testing.
2. Human iPSC-Derived Motor Neuron Culturing
A variety of animal organisms, including yeast [22], worms [23], fruit flies [24], zebrafish [25], and rodents [26], have been applied to model ALS. hiPSCs have enormous potential in this effort, because they can be differentiated into multiple cell lineages, including motor neurons (MNs), while maintaining the patient’s genetic characteristics [11]. Such hiPSC technology can improve disease modeling, elucidate how drugs work, and possibly design new therapies. In vitro models are ideal for high-throughput screening and are advantageous in controlled, low-cost experimental settings. Even though there are many benefits to hiPSC disease modeling, several challenges exist, including obtaining disease-specific cell lines, preserving and differentiating cell cultures, and guaranteeing the models’ resilience and repeatability.
Since most ALS patients do not have a family history of the disease, modeling sALS is crucial to developing novel therapies. However, the absence of known monogenic mutations supports the notion of polygenic influences, along with environmental factors, in the causation of sALS. In a recent study, researchers at Keio University generated iPSCs from 32 sALS patients and six healthy individuals and established various in vitro cellular models as sALS resources [11]. They then differentiated these cells into MNs, which maintained the patient’s inherent genetic information and observed variations in the onset and development of various abnormalities, including the patterns of neuronal degeneration, aberrant protein aggregation, and mechanisms of cell death pathways. Finally, they created a case-clustering method to categorize these heterogeneous sALS models according to their in vitro characteristics. This study used multiple-patient sALS models to first categorize genetically and clinically heterogeneous sALS patients and then used a multiphenotypic analysis/screening system to identify potential drug candidates targeting pathogenic mechanisms in both sALS and fALS. As a result, ropinirole hydrochloride (ROPI), a dopamine D2 receptor (D2R) agonist, was identified as a promising drug candidate. Although ROPI showed protective effects in FUS- and TDP-43-mutated fALS models and the majority of sALS models, it did not suppress detected phenotypes in SOD1-mutant ALS models [11]. Whether the drug’s neuroprotective effect is mediated via a D2R-dependent or -independent mechanism, or both, is unclear, as this receptor is present on human spinal cord MNs and is expressed by ALS patient iPSC-derived spinal MNs. This finding raises the possibility that ROPI may protect cells from mitochondrial dysfunction occurring in ALS [27,28]. This study demonstrated ROPI’s neuroprotective effect on MNs derived from hiPSCs obtained from 9 out of 22 sALS patients as well as from patients carrying FUS (n = 9) and TDP-43 (n = 4) mutations compared to three healthy controls [11]. Lack of the drug’s benefit on cells from the six sALS patients supports the notion that pathogenic mechanisms, and therefore response to therapies, likely differ between individuals with sALS. Based on this hiPSC-related in vitro drug-discovery study, a phase 1/2a trial of ROPI was performed in patients with sALS [27]. Details of the clinical trial findings are beyond the scope of this hiPSC-related review, but several measures revealed a protective effect against ALS, including a slowed decline of ALSFRS-R scores and almost 28 weeks of disease-progression-free survival during the open-label extension period as well as a reduction in CSF neurofilament and lipid peroxide levels [27]. ROPI protected FUS-ALS, TDP-43 ALS, and sALS hiPSC-derived MNs by preventing reactive oxygen species formation, neurite degeneration (preserved length), neurotoxicity (reduced LDH leakage), apoptosis (decreased cleaved caspase-3), and TDP-43 aggregate formation (except in FUS-ALS MNs where FUS aggregates were prevented) [11]. Interestingly, ROPI showed no similar benefits on SOD1-positive iPSC-derived MNs except for improved mitochondrial dysfunction, suggesting the latter abnormality is common to both non-SOD1-positive and SOD1-positive forms of ALS. Consequently, these findings demonstrate the potential benefit of ROPI as an ALS treatment for a variety of clinical scenarios. The study also discovered that in most sALS models, lipid peroxidation and resultant ferroptosis are critical factors in the degeneration of MNs. Overall, the study highlights the utility of iPSC technology to provide new insights into pathogenic mechanisms underlying sALS and novel avenues for identifying effective treatments [11].
Studying iPSC-derived MNs and related cells from patients with genetic and sporadic forms of ALS will help clarify its causative molecular mechanisms and provide a model for drug discovery. It may be possible to model genetic variants of ALS with fewer patient lines if isogenic lines can be produced as controls. Nonetheless, modeling sALS with varied etiologies becomes more complicated [29]. Although in the previous Japanese study, only 32 ALS patients and six controls were adequate to identify distinct differences between groups of sALS patients [11], hiPSC biology is likely to vary among different ethnicities. Differences in the distributions of genes causing fALS in various countries support this notion. For example, the C9orf72 mutation is the most common genetic cause of fALS in Caucasians (~40%) but is much less frequent in Asian populations. In a study of 563 Japanese ALS patients, only 0.4% of sALS and no fALS cases were linked to C9orf72 [30]. Conversely, SOD1 mutations are more prevalent in Asian cohorts compared to Western ones, at 27.9% of 86 Chinese fALS cases [31]. These variations highlight the importance of considering genetic diversity in ALS research and treatment approaches across different ethnic groups. Therefore, studies on iPSCs from more patients may be required to identify additional and personalized disease-specific processes.
Answer ALS, a comprehensive research initiative aimed at understanding and finding a cure for this disease, is developing a large-scale hiPSC repository of at least 1000 iPSC lines generated with a standardized protocol from both ALS patients and healthy controls, along with clinical and whole-genome sequencing information [29]. Four hundred and thirty-three hiPSC lines were used in a study to differentiate into MNs via a specific 32-day protocol with a subsequent examination of gene expression patterns and neural cell markers. Patient covariates were considered to identify the confounding variables and challenges in these extensive patient-derived iPSC differentiation studies, providing valuable resources to the community [29]. Characterizing this largest batch of hiPSCs, which were differentiated into MNs, followed by bulk RNA-seq and cell marker expression profiling, revealed that sex and cell composition are significant sources of heterogeneity that must be carefully controlled in future research. Interestingly, Islet-1 (ISL1)-positive MN staining was more common in ALS cases than in controls. Additionally, the sex of the hiPSCs separated the data into two distinct subgroups independent of disease status. Compared to females, male cells produced more MNs with several dysregulated genes that were enriched in stress-related pathways [29].
hiPSCs are traditionally programmed into MN lineages by adding growth hormones, pluripotency inhibitors, and other neuron patterning factors to the media [32]. An alternative method for a quicker neuronal differentiation of hiPSCs involves genetic reprogramming to express master regulators of MN development by reducing or removing intermediate progenitor stages [33]. According to a 2017 study, the development of MN identity in mouse embryonic stem cells (mESCs) is dependent on the expression of only three transcription factors called NIL factors: neurogenin 2 (NGN2), ISL1, and LIM homeobox proteins (LHX3) [33]. In order to initiate neural differentiation and survival programs in the CNS and PNS, NGN2 is produced in neuronal progenitor cells (NPCs); LHX3 and ISL1 form a heterodimer transcription regulator complex to activate genes driving postmitotic neuron specification [34,35]. Standard methods used to develop hiPSCs into MNs are time-consuming and often result in heterogeneous populations of neuronal subtypes. Thus, to achieve the therapeutic potential of hiPSCs, additional refinement is required. Several research groups have proposed the direct reprogramming of stem cells into MNs by inserting recombinant genes that encode such transcription master regulators [33,34,36]. In these studies, transgene cassettes are introduced into ESCs or hiPSCs via retroviral vectors. However, because transgenic integration into the recipient genome occurs randomly, the potential for clinical applications has raised safety concerns.
According to a 2023 study [35], scientists used CRISPR/Cas9 (clustered regularly interspersed short palindromic repeats/CRISPR-associated protein 9) gene editing techniques with high-fidelity recombinant SpCas9 to unidirectionally transform iPSCs into MNs. ISL1 and LHX3 transgenes were delivered into well-defined safe harbor locations (H11, ROSA26, and AAVS1) in the human iPSC genome by electroporation. This method significantly reduced off-target binding of the Cas9/gRNA complex, because it is transient and resulted in much lower levels of ribonucleoprotein complexes compared to methods using plasmid DNA transfection to encode Cas9 and sgRNA. It was discovered that transgene transcription factors become activated in the modified iPSCs even prior to adding a TRE-inducing agent (doxycycline). This established a genetic program dedicated to MNs’ fate, although it was unable to carry out the unidirectional transition to MNs in the absence of extrinsic neuron patterning factors provided in the differentiation media. A comparative global transcriptome analysis of MN formation in native and LHX3/ISL1-modified iPSC cultures revealed that the neuronal patterning process was facilitated by genetic factors. However, premature activation of genetic pathways typical of mature MNs was caused by leaky gene expression of the exogenous MN master regulators in iPSCs. In addition, dysregulation of metabolic and regulatory pathways within the developmental process altered MN electrophysiological responses. Premature activation of ISL1 and LHX3 transgenes in iPSCs impacted neuronal development, cell proliferation, and Ca2+ signaling pathways in mature MNs; it also changed gene expression patterns during all phases of differentiation. This indicates that early activation of MN genes interrupts the correct differentiation process. Overexpressing important transcription factors in hiPSCs with CRISPR/Cas9 technology prevents the expected unidirectional transition to MNs [35].
The in vitro generation of functional MNs involves exposing them to intricate formulations that include the following neuron patterning molecules: retinoic acid (RA), WNT/sonic hedgehog (SHH) pathway activators, and notch pathway inhibitors. These formulations are supplied at precisely defined concentrations and within specific time windows for effectiveness [32]. Phrenic MNs (phMNs) innervating the diaphragm almost always degenerate in ALS, which results in respiratory failure and death [37]. Our understanding of the mechanisms underlying phMN degeneration in ALS has been poor primarily because of limited human experimental models to investigate such MNs. phMNs differ from other spinal MNs in several aspects, including their developmental origin, unique architecture, and electrical characteristics. The most well-established and widely utilized hiPSC-based MN derivation techniques produce heterogeneous cultures composed of MNs from the lateral motor column (LMC) and median motor column (MMC). Because the absence of phMNs from these cultures prevents the study of their (patho) physiology, a technique using hiPSCs to produce phMN-enriched cultures in as little as 30 days has been developed. This highly reproducible technique employs multiple hiPSC lines, combines optimal concentrations of RA and the SHH agonist purmorphamin, and uses fluorescence-activated cell sorting (FACS) based on a cell-surface protein immunoglobulin superfamily DCC subclass member 3 (IGDCC3) to enrich for phMNs. The calibrated activation of RA and SHH signaling in hiPSC-derived neural progenitor cells (NPCs) facilitates a cervical identity of dorsal NPCs to produce phMN-like neurons. This novel methodology now allows for the study of disease-relevant cells to evaluate mechanisms of respiratory MN dysfunction in ALS [37].
Neurons form highly specialized functional units with neuroglia (i.e., astrocytes, oligodendrocytes, and microglia) with close interactions that influence their health and survival. For this reason, we will discuss recent neuronal–neuroglial co-culturing studies that aim to more closely model the degeneration occurring in the CNS of patients with ALS.
3. Co-Culturing hiPSC-Derived Motor Neurons with Neuroglia
In addition to MNs, other neuronal subtypes and neuroglia, including astrocytes, are affected by ALS. Approximately 20–40% of neuroglia in the CNS are astrocytes, making them one of the most prevalent glial cell types [38]. Their functions are multiple, including maintaining brain and spinal cord structural integrity, providing neurotrophic support and neurotransmitter control and modulating neural activity. By controlling the blood–brain barrier, water flux, and ion and pH homeostasis and eliminating reactive oxygen species, astrocytes help to maintain the ideal CNS environment. Additionally, they contribute to inflammatory and immunologic responses by secreting cytokines and phagocytozing cellular debris and helping to create boundaries after injury. Through a variety of mechanisms, including creating perisynaptic processes (PAPs) by their endfeet and secreting specific molecules such as thrombospondins (THBS1/2), glypicans (GPC4/6), transforming growth factor β1 (TGF-β1), and brain-derived neurotrophic factor (BDNF), astrocytes modulate the plasticity and function of neuronal synapses [38]. In the CNS, these chemicals facilitate the development and maturation of inhibitory synapses, excitatory synapses, and tumor necrosis factor-α (TNF-α). In addition, astrocytes and microglia work together to dynamically modulate neuroplasticity, which is crucial for memory and learning [38]. Recent human embryonic stem cell experiments co-culturing MNs with astrocytes expressing the mutant SOD1 enzyme reduce MN survival [39,40,41,42].
Microglia, another type of neuroglia in the brain and spinal cord, receive signals from astrocytes and are required to properly assemble complex neural networks [43]. They account for about 10–15% of all cells in the brain [44]. Although derived from blood-borne macrophages, microglia differ from other tissue macrophages due to their specific homeostatic nature and multiple roles, including controlling brain growth, maintaining neural networks, and repairing injuries [45]. They remove bacteria, dead cells, unnecessary synapses, aggregated proteins, and other soluble and particulate antigens that could harm the CNS. Microglia are also important mediators of neuroinflammation and have the ability to initiate or influence a wide range of cellular responses, as they are the main source of proinflammatory cytokines. Brain aging and neurodegeneration are associated with changes in microglial functioning [45]. Microglia have also been found to express significant levels of SOD1 and C9orf72, two important ALS-associated genes [46]. There are several species-specific characteristics of microglia that are different between humans and rodents [47], with several genes linked to neurodegenerative diseases of microglia lacking orthologs in mice [48]. Furthermore, the precise function of microglia in the pathophysiology of ALS remains unclear, and, therefore, more realistic human disease models of microglial dysfunction are needed.
3.1. Astrocyte Co-Cultures
In the past, astroglia were thought of as simply passive satellite cells in the CNS, supporting neurons metabolically and controlling extracellular homeostasis. However, recent research has demonstrated their capacity to receive signals from neurons and release neuroactive chemicals as well as actively regulate neuronal functions, communication pathways, and plasticity [49].
Astrocytes have been implicated in contributing to MN toxicity and death in fALS by enhancing the release of toxic substances, decreasing lactate synthesis, and diminishing glutamate uptake [42]. FUS-ALS iPSC-derived astrocytes were studied in a monoculture and co-culture with FUS-ALS MNs in a microfluidic device with healthy skeletal myocytes to examine their interactions [50]. Compared to isogenic control astrocytes, FUS-ALS astrocytes were found by a variety of techniques, including immunocytochemistry, RNA sequencing, and calcium transient hyperactivity, to display elevated glial fibrillary acidic protein (GFAP) expression, cytoplasmic FUS mislocalization, the production of inflammatory cytokines, and enhanced spontaneous reactivity. Such co-cultures of hiPSC-derived cells mimicking in vivo multicellular networks revealed the deleterious effects of FUS astrocytes on MN neurite outgrowth and network integration, as well as on neuromuscular junction (NMJ) formation and functionality. Overall, FUS-ALS astrocytes, especially those expressing a mutation associated with an earlier onset and more aggressive clinical course, contributed to disease via multiple gain-of-toxicity and loss-of-support mechanisms. The increased secretion of proinflammatory cytokines by mutant-expressing astrocytes indicates that this is not dependent on stimulation by microglia [40], since the latter were missing from the cultures, and supports astrocytic contribution to neuroinflammation in ALS [51]. The study also found that activation of the WNT/β-catenin pathway plays a significant, although complex, role in FUS-ALS, since it may have some neuroprotective functions and is normally involved in the development of NMJs, axonal guidance, and cell survival [52]. The author’s multicellular microfluidics model provides a platform for more research with additional hiPSC lines to further explore the etiology of astrocyte cytotoxic characteristics and for novel drug development and testing.
ALS with frontotemporal dementia (ALS-FTD) is often caused by a polymorphic hexanucleotide repeat expansion (HRE) of GGGGCC (G4C2) in the first intron of the C9orf72 gene from normally less than 30 repeats into the hundreds or thousands, pathologically. The unusual repeat-associated non-AUG (RAN) translation of the HRE into five toxic dipeptide repeat (DPR) species—poly-PA, poly-GA, poly-PR, poly-GR, and poly-GP—appears to be a key factor driving the pathophysiology of C9orf72-related ALS-FTD. The most hazardous of the five DPRs are believed to be those containing arginine, specifically, poly-GR and poly-PR [53,54]. It has been shown that these DPRs affect the development of membrane-less organelles such as stress granules and can result in DNA damage and mitochondrial malfunction. Their expression is toxic to mouse and hiPSC-derived cortical and MNs [55,56].
Co-culturing iPSC-derived human astrocytes (iAstrocytes) and Hb9-GFP mouse MNs investigated differences in the cell-to-cell propagation of C9orf72-related poly-GA oligomers and fibrils [55]. In this system, DPR species are promptly internalized by astrocytes and spread to neuronal units with fibrils transmitting to MNs six times more efficiently than oligomers. Poly-GA DPRs are internalized through both dynamin-dependent and -independent endocytosis, ultimately localizing in lysosomes and causing axonal swelling. These findings point to a significant involvement of astrocytes in the transfer of DPRs produced from mutated C9orf72 to nearby MNs. It should be noted that results obtained in cell culture may not directly apply to humans or in vivo animal models. For example, the heterogeneous pattern of C9orf72 expression endogenously in vivo may result in significant regional variations in DPR concentrations in patients. In addition, the short-length DPR used in this study may affect cellular functions differently from the longer DPR repetitions found in patients with C9orf72 ALS. Nonetheless, this research sheds light on the basic principles of the biology behind poly-GA aggregation, binding, absorption, and cell-to-cell propagation between glia and neurons [55].
A number of investigations of the C9orf72 HRE mutation in humans or animals have shown an increased tendency to develop autoimmune diseases [57,58]. These studies collectively imply that C9orf72-induced ALS could arise from either the direct or indirect dysregulation of one or more inflammatory pathways. Previous research has revealed that a pathological feature of ALS is neuroinflammation, which is already present in the early stages of the disease. According to autopsy data, activated microglia and astrocytes are primarily responsible for inducing such inflammatory changes [59]. Although neurons may not have major roles in the inflammatory process of ALS, changes in their cellular homeostasis increase their susceptibility to inflammation and subsequent neurotoxicity [60].
3.2. Microglia Co-Cultures
According to recent research, in vitro pluripotent stem cell-derived microglia-like cells have been generated from multiple disease-specific cell lines and accurately replicate the expected ontogeny and characteristics of their in vivo counterparts in organotypic 3D cultures [61]. A cell model to explore human MN–microglia crosstalk has been recently reported by using a co-culture system of iPSC-derived microglia and spinal MNs [46,62,63]. In a co-culture, such MNs exhibit appropriate cellular markers, including neuronal electrophysiology by whole-cell patch-clamp and calcium imaging, both spontaneously and in response to potassium chloride stimulation. In addition to expressing important ALS-associated genes, co-cultured microglia display direct interactions with MNs and their neurites, exhibit ramifications with extremely dynamic remodeling, and have a gene profile similar to primary human microglia. This model can be used to investigate both cell-autonomous and non-cell-autonomous phenotypes, is relatively simple and highly efficient, and allows for easy scaling up for large-scale and drug-screening experiments [46].
Widespread microglial activation has been linked to the progression of ALS, especially in patients with C9orf72 HRE, the most prevalent genetic cause of the disease [64]. In neurons, the C9orf72 HRE causes haploinsufficiency, the formation of RNA foci and DPRs, and cytoplasmic mislocalization of TDP-43. In an RNA sequencing analysis of hiPSC-derived C9orf72 HRE mutant microglia, pathways associated with immune cell activation and the production of cytokine CXCL1 and CXCL6 activity were shown to be enriched, particularly after lipopolysaccharides (LPS) priming. LPS is one of several triggers that can activate microglia and cause a pro-inflammatory phenotype that increases neurotoxicity [64]. LPS-primed C9orf72 HRE mutant microglia consistently upregulate matrix metalloproteinase-9 (MMP9) production and release. MMP9 is an endopeptidase that cleaves cell-surface receptors as well as a number of extracellular matrix components. Multiple neurodegenerative diseases share MMP9 dysregulation, including ALS, where MMP9 has been shown to have a neurotoxic effect [65,66]. Unstimulated C9orf72 mutant microglia in an hiPSC-derived microglia–MN co-culture assay exhibit a dysregulated supernatant profile, although without impacting the survival or activity of normal/healthy spinal MNs. After LPS exposure and priming, however, C9orf72 mutant microglia co-cultures cause obvious neurodegeneration and trigger apoptosis in healthy MNs. The application of an MMP9 inhibitor concurrently with LPS-stimulated C9orf72 mutant microglia reduces their neurotoxic properties apparently mediated via the dipeptidyl peptidase-4 (DPP4) they release [64].
Although individual astrocyte or microglia cultures in vitro are effective strategies to explore molecular pathways involved in neuroinflammation, they cannot capture the in vivo interactions between neurons, astrocytes, and microglia. To better understand the effects of cellular crosstalk on neuroinflammation, new multicellular co-culture models are needed. Recently, more complex co-cultures have been developed using 2D triple co-culture (tri-culture) models of neurons, astrocytes, and microglia to study intercellular interactions and crosstalk. An example is a murine tri-culture system to study Alzheimer’s disease (AD) and recapitulate the pathophysiological characteristics lost in typical primary cultures [43]. In a tri-culture paradigm, these cells behave more physiologically than when cultivated in primary cultures: microglia exhibit less inflammation, astrocytes are less reactive, and neurons have a more mature morphology [43,67]. This 2D tri-culture model, which was found to closely mimic the in vivo CNS environment in a study of AD processes, should be considered for other neurological diseases such as ALS [43].
In comparison to hiPSC-derived 2D co-culture or tri-culture models, 3D organoids have enormous potential in studying disease mechanisms and neuronal development, although they require more resources than murine cultures and are methodologically more difficult. However, developments in culture technologies, biomaterials, and microfluidic devices have made it easier to move from 2D cultures to multicellular 3D model systems [21].
4. HiPSC-Derived Organoids
In contrast to previously discussed 2D models, iPSC-derived organoids present a prospective means of creating humanized 3D tissue with a variety of cell types and can provide a more accurate representation of the physiological environment [68]. Three-dimensional cell culture organoids, which can be generated from hiPSCs; multipotent adult stem cells (ASCs); or embryonic stem cells (ESCs) could create precise human disease models and provide patient-specific tissue sources for regenerative medicine. Typically, organoids are made up of several stem cells that have been cultivated with a variety of growth factors or media mixtures, causing the cells to take on specific fates that resemble the organ in question. Organoids offer a wide range of experimental applications to investigate human development and diseases, since they can be utilized to establish clinical models for tissue engineering and medication testing [69,70]. These in vitro culture systems can replicate certain activities of the represented organ and are distinguished by the self-organization of several organ-specific cell types into an in vivo-like spatial arrangement [71]. Organoids produced from patient iPSCs are more effective in expressing disease phenotypes, especially for complicated diseases in which there are no suitable disease models or when there is restricted access to presymptomatic patient samples (Figure 2).
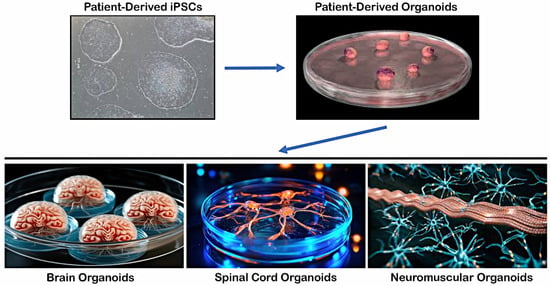
Figure 2.
Differentiation of hiPSCs into 3D organoids and tissue-specific structures.
Patient-derived iPSCs can be differentiated into multiple lineages upon exposure to specific signaling pathways and growth factors to form various tissue-specific structures, such as brain organoids resembling the cerebral cortex, hippocampus, or midbrain regions, spinal cord organoids containing anterior horn cells and neuroglia with central canal structures, and neuromuscular organoids in which anterior horn MNs with neuroglia form neuromuscular junctions on skeletal myocytes.
4.1. Brain Organoids
In 2013, Lancaster et al. applied a 3D culture to create the first human brain organoids that included cell lineages from the retina, choroid plexus, midbrain, and forebrain [72]. Brain organoids offer a useful model for researching neurodegenerative diseases. ALS patients’ brain organoids have been the subject of numerous studies, and the majority of these studies have focused on corticoid brain organoids [72,73,74,75].
Using the air–liquid interface (ALI) method to culture brain organoids has resulted in extended neuronal survival (e.g., >12 months), axon outgrowth with a variety of morphologies, and active neural networks, presumably because of an enhanced supply of oxygen [70]. ALI-brain organoids enable the investigation of neurodevelopmental disorders of the corpus callosum, imbalances in neural circuits, and other defects where connectivity is believed to be involved. Szebényi et al. created a human cortical organoid (CO) model that can simulate the early molecular pathology of ALS/FTD over an extended period of time [75]. COs with the C9orf72 HRE mutation (C9 ALI-COs) were developed from iPSCs generated from individuals with ALS/FTD up to 240 days in vitro at the ALI. ALI-COs develop a consistent microarchitecture and mature cortical circuit-forming disease-relevant phenotypes. Although lacking microglia and vasculatures, C9 ALI-COs show abnormalities specific to neurons and astrocytes. By using a combination of single-cell RNA sequencing and biological assays, disturbances were noted in distinct transcriptional, proteostasis, and DNA repair processes in neurons and astroglia. Deep-layer neurons showed the toxic DPR species poly-GA, DNA damage, and cell death that was pharmacologically induced by enhancing proteostasis and that was inhibited by GSK2606414. GSK260414 is a selective inhibitor of the enzyme protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), which mediates the unfolded protein response (UPR) pathway that is implicated in the pathogenesis of various neurodegenerative diseases [76]. The autophagy signaling protein p62 was also elevated in astrocytes. In this model, long-term ALI-CO cultures offer an opportunity to investigate the timing and causative relationships between synapse and neuronal loss, which previously could not be examined mechanistically in the setting of human diseases [75].
4.2. Spinal Cord Organoids
Spinal cord organoids (SCOs) have been created from hiPSCs to study the LMN components of ALS [77]. The cellular and molecular abnormalities in ALS spinal cord can be modeled over time in vitro and studied by RNA-sequencing (RNA-seq), single-cell (sc) RNA-seq, and other high-throughput sequencing approaches. Creating ALS SCOs offers a unique opportunity to study LMN disease mechanisms involving both neuronal and non-neuronal cell types. In a study generating wild-type (WT) iPSC lines from healthy donor fibroblasts, Guo et al. [77] suppressed C9orf72 expression by lentivirus transfection of silencing short hairpin RNA (shRNA). Stable C9-knockdown hiPSC colonies (C9-hiPSCs) were selected and differentiated into MNs, astrocytes, and SCOs to examine gene expression of inflammation using RNA-seq (with data analysis using NCBI Gene Expression Omnibus) and qRT-PCR. Grown in a 3D culture environment, WT-iPSCs and C9-hiPSCs both retained a comparable capacity to differentiate into astrocytes, MNs, and the three germ layers to generate SCOs. In order to optimally construct mature and stable C9-hiPSC-derived SCOs, the bone morphogenetic protein pathway was blocked, and the Wnt pathway was activated; cultures were caudalized using retinoic acid and ventralized using the sonic hedgehog pathway agonist, purmorphamine. The SCOs created with lower levels of the C9orf72 protein showed characteristic cellular compositions that were similar to those in the spinal cord, including motor, sensory, and other neurons. However, the expression of inflammatory factors (e.g., IL-6 and TNF-a) in C9-SCOs was significantly higher than in astrocyte or MN 2D monolayers, indicating the involvement of numerous other nerve cell types in C9-induced neuroinflammation. Bioinformatics analyses confirmed elevated proinflammatory factors in C9-hiPSC-derived 2D cells and 3D-cultured SCOs. By using these methods, spinal neurogenesis was effectively duplicated, including the formation of a central canal, and a useful in vitro neural model for examining the LMN pathophysiology of C9-ALS was produced [77].
4.3. Neuromuscular Organoids
In the early stages of ALS, skeletal muscle and neuromuscular junction (NMJ) changes have been documented, which may be responsible for metabolic dysfunctions and in the “dying back” phenomenon of MNs [78]. The so-called “dying back” hypothesis suggests that retrograde signals contribute to the centripetal MN degeneration in ALS [78]. Skeletal muscle has gained importance in the past few years, both in the etiology and therapy of ALS [79]. Gaining insight into the molecular processes behind skeletal muscle degeneration could help in the creation of treatment strategies that maintain muscle function, halt disease progression, and enhance the quality of life for ALS patients [79]. In a recent study, hiPSC-derived neuromuscular organoids (NMOs) simulated the spinal neuromuscular characteristics of ALS patients with the C9orf72 HRE mutation [80]. ALS NMOs replicated peripheral abnormalities in ALS, such as muscle contraction weakness, neural denervation, and the loss of Schwann cells. DPRs and RNA foci were seen in neurons and astrocytes of ALS NMOs with more prominent DPR aggregation in astrocytes. Interestingly, brain organoids derived from C9orf72-hiPSCs developed poly-GA aggregates in only cortical neurons; future research should explore whether this is so. The potential use of NMOs for preclinical drug testing was demonstrated after acute administration of the UPR inhibitor GSK2606414 resulted in a two-fold increase in muscle contraction and decreased autophagy and DPR aggregation [80]. Further examination of ALS NMOs generated with iPSCs from individuals with sALS or fALS due to C9orf72-HRE and other mutations is necessary to ensure their broad applicability to understanding pathogenic mechanisms of the LMN component of ALS.
The distinct anatomical and physiological characteristics of the human NMJ make it susceptible to pathogenic processes [72,73]. There is evidence that the pathophysiological cascade of ALS begins at the NMJ because of synaptic dysfunction and elimination occurring prior to MN loss [81]. NMJs offer the most significant pathological and functional disease readouts, and they appear to be an early and vulnerable target for ALS and spinal muscular atrophy, a genetic LMN-predominant MND [82]. Consequently, cell culture techniques facilitating NMJ development and connection to their target muscle cells will enhance the study of human MNs in health and disease [81]. Although several studies have demonstrated significant advances in the creation of skeletal muscle–spinal MN 2D and 3D co-culture procedures, modeling NMJs using hiPSCs has proven to be challenging [83,84]. Several studies demonstrated a great deal of advancement in the creation of 2D and 3D co-culture procedures. Bakooshli et al. [85] developed a 3D co-culture of healthy human muscle progenitors from a muscle biopsy mixed with human pluripotent stem cell-derived MNs that self-organized and formed healthy functional NMJs in vitro. However, NMJs derived from iPSCs of ALS patients have not yet been successfully produced [86,87,88,89,90]. To create and have a better understanding of NMJs’ mechanism, MNs produced from hiPSCs were co-cultured with myoblasts grown from a single control cell line in a new microfluidic technique previously [91]. The success of using in vitro neural networks derived from hiPSC-derived neuronal cultures and organoids to understand pathogenic mechanisms in ALS and other MNDs, as well as for preclinical drug screening, will likely depend on the ability to reproduce physiologic synapses.
In an interesting study, sensorimotor organoids containing physiologically functional NMJs were generated from five iPSC lines obtained from two healthy controls and three individuals with ALS [92]. After growing in suspension to form spheres, free-floating sphere cultures were fused, plated at a density of 46 spheres/cm2, and grown under adherent conditions for up to 15 weeks. Organoids containing NMJs along with skeletal muscle, MNs, astrocytes, microglia, and vasculatures, were derived and characterized using a variety of molecular, genomic, and physiological approaches [92]. Demonstrating abnormal NMJs in organoid cultures made from hiPSC lines carrying various gene mutations by physiological (contraction) and immunocytochemical approaches is significant, since NMJ loss is an early and crucial component of ALS models. Creating isogenic control pairings of such iPSC lines carrying TARDBP, SOD1, and PFN1 mutations in this study allowed for more detailed investigations and further verified the robustness of these sensorimotor organoids. Compared to either the single parental or other lines, for example, the variation in both sphere makeup and derived cell types was dramatically reduced in the isogenic lines [92]. Human in vitro disease modeling of the NMJ may be useful in testing mechanistic theories of early pathogenesis in ALS, facilitating the identification and validation of targets and developing personalized preclinical therapeutic candidates. In this regard, the NMJ may already offer the first and most significant pathological and functional readouts of ALS [92,93].
In a comparable study, a 3D hydrogel-based in vitro ALS model was created with hiPSC-derived MNs and myoblast-derived human skeletal muscle tissue, which consistently produced axons and NMJs connecting with myotubes [81]. Human skeletal myoblasts differentiated into fully developed muscle tissue, with calcium imaging and acetylcholine-induced contraction used to verify muscle function. Two iPSC lines generated from ALS patients with SOD1 mutations (heterozygous R115G mutation and homozygous D90A mutation) were differentiated into MNs and then co-cultured with muscle tissue. Interestingly, the 3D co-cultures containing MNs from patients or healthy controls were identical in appearance with comparable glutamate-induced muscular contraction after 14 days in a culture. This suggests functional integration of the ALS MNs and NMJs with typical morphological characteristics such as postsynaptic folding. After 21 days, however, reduced muscle contraction was observed in co-cultures made from both SOD1 mutations; nonetheless, co-cultures were viable and functional for at least six weeks. These findings prove that MNs with SOD1 mutations successfully adapt into the system initially and only later exhibit a pathogenic phenotype [81]. This MN–muscle co-culture model appears suitable for researching both the development and disorders of the human NMJ. However, there is still a need for a more functional, generic NMJ model applicable to a variety of conditions. Key findings in select iPSC-derived ALS model studies are shown in the Table 1.

Table 1.
Summary of findings in recent iPSC-derived ALS model studies.
Organoid culture techniques have advanced recently, although important challenges remain when using this multicellular approach to model neurodegenerative disorders like ALS. In addition to variability in cell composition, morphology, and maturity across preparations, the lack of the vascularization restricting provision of nutrients and oxygen, limits growth, survival, and possibly the final stages of cell maturation and differentiation. Other approaches, such as microfluidic chip (“organ-on-a-chip”) methodologies, can circumvent some of the limitations of organoids, although they are more complicated to prepare and may support less 3D analysis [94].
5. Discussion
Advances in hiPSC technology have unlocked new opportunities in ALS research, allowing patient-specific cells to be used for disease modeling and drug discovery. For over a century, conventional 2D cell culture techniques have contributed significantly to the in vitro research of various human diseases. Because such models allow for only side-by-side (single plane) contact between cells and lack 3D tissue complexity, however, they cannot accurately represent the complex nature of human cells and their interactions in vivo. As a result, such restrictions in the modeling process affect cell morphology, survival, proliferation, and differentiation, and consequently the mechanisms underlying human disease, including ALS [21].
Cell co-culture models allow for the study of cellular interactions with one another and the surrounding microenvironment. Besides direct contact, chemical signals within the microenvironment also play a significant role in cell–cell interactions [21]. The advent of 2D co-culture models in ALS has replicated some of the intricate connections between neuronal and neuroglial cells in vitro. Such studies provide proof-of-concept methods to investigate astrocyte and microglial effects on neuronal biology. They also pave the way for investigations into differing aspects of neuro-immune interactions within the framework of disease biology, using astrocytes, microglia, and neurons derived from patients suffering from various neurodevelopmental, neuropsychiatric, and neurodegenerative disorders. To fully understand the development of human MN degeneration in ALS, the roles of microglia and astrocytes must be considered. This is especially important given the non-cell-autonomous nature of ALS [95]. For example, in addition to being resident immune cells of the CNS, microglia play roles in synapse formation, the regulation of synaptic plasticity, and neural circuit maturation. Co-culturing hiPSC-derived microglia with neurons has demonstrated that microglia are phagocytically competent, express markers specific to human microglia and genes linked to neurodegenerative diseases, downregulate pathogen-response pathways, upregulate homeostatic pathways, and stimulate anti-inflammatory responses [15,45,56].
Multi-dimensional triple co-cultures of neurons with astrocytes and microglia more accurately mimic in vivo neuroinflammatory reactions than do conventional monocultures [43]. In 2D triple co-cultures, neurons become more complex in morphology and express higher levels of postsynaptic markers. Microglia express more anti-inflammatory markers and less pro-inflammatory markers with astrocytes, reducing the expression of pro-inflammatory markers [43]. Beyond 2D cell culture models, 3D organoid models may be the bridge to animal models by more precisely simulating in vivo neuronal architecture. It is likely that 3D neurodegenerative disease models are more realistic than triple co-culture 2D models, in part because of their organ-like intercellular relationships. Although techniques to reproducibly create and evaluate 3D cultures still need to be refined, they have the potential to model diseases with a higher degree of complexity and permit more intricate and in vivo-like communication between cells and the microenvironment. Lack of vascularization and restricted circulation to the organoid core for the delivery of nutrients and the elimination of waste products is a limitation for 3D culture models, often resulting in cell death at the center. Mixing cells of interest with mesenchymal and endothelial cells to encourage vascularization is one potential tactic to overcome this limitation [15]. Other challenges of creating such heterogeneous 3D culture models are their cost, the need for specialized equipment and optimization and consistent reproducibility from batch to batch. Nonetheless, producing more complex phenotypes is essential to recreating the most in vivo-like ALS disease cell culture models [15].
Our general understanding of the fundamental disease pathways of ALS is derived from years of research using both in vivo and in vitro models as well as post-mortem tissue. Important insights have been gained using these approaches, albeit with limited effect on therapeutic drug development. Because of the disease’s high mechanistic complexity, genetic heterogeneity, and translational gaps between model and patient, there is still no known cure or clinically effective treatment for ALS. Although the Food and Drug Administration (FDA) previously approved riluzole and edaravone for all forms of ALS and tofersen for SOD1-positive ALS, additional pharmacotherapies are urgently needed [96]. Drug development in neurological diseases and nervous system disorders has an estimated success rate of 8% from Phase I to FDA approval, whereas for ALS drug development, the failure rate is close to 100%. In addition, the translational potential of preclinical animal models to human clinical trial outcomes has been very poor in ALS. The hope is that many of the limitations of preclinical drug testing in animal models and the challenges of differential disease mechanisms in ALS can be overcome by using ALS patient-derived iPSCs to generate MNs and other relevant cell types to model diseases in vitro (Figure 3). As explained in different parts of this and other reviews [10,21,97,98,99], the development of hiPSC technology has made it possible to replicate many 2D and 3D ALS model diseases for drug development and also by using cell co-culture models to observe how cells interact with one another or with their surrounding microenvironment.
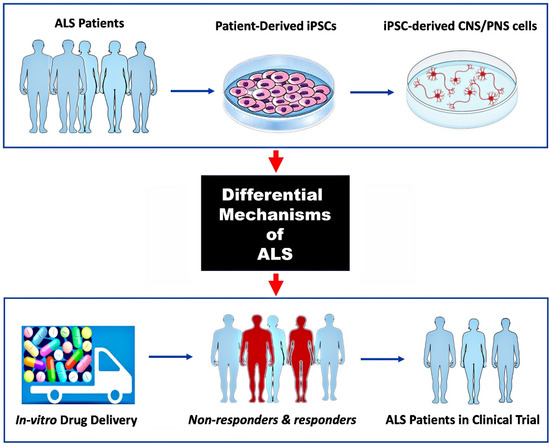
Figure 3.
Use of hiPSC-derived ALS models for preclinical drug screening.
Previous availability of only animal models and the molecular heterogeneity of ALS disease pathogenesis are two of the greatest obstacles to clinical trial success. Generating MNs and other relevant CNS and/or PNS cells from ALS patient-derived iPSCs can reveal specific molecular mechanisms of disease to be targeted (top and middle panels). Screening multiple drug therapies on such 2D or 3D tissue culture models can identify patient subgroups that are more likely to respond to a particular therapy (bottom panel; non-responders in red).
This relatively recent utilization of patient-derived iPSCs to develop in vitro stem cell models has already advanced our molecular understanding of ALS. Because of their increasing complexity and great potential, hiPSC-derived CNS and PNS cell models represent invaluable resources to understand the basic science of and to develop novel therapies for ALS and related disorders [38]. However, several limitations in hiPSC-derived models persist, including cellular variability, lack of complexity in 2D models, and challenges in mimicking ALS-specific phenotypes [100]. One of the key challenges with hiPSC-derived ALS models is the inherent variability between different patient iPSC lines, primarily when derived from individuals with genetic diversity. Genome editing tools, such as CRISPR-Cas9 and newer base-editing technologies, offer the potential to standardize ALS models and reduce variability by precisely manipulating genetic mutations associated with ALS, such as C9orf72 expansions, SOD1 mutations, or TDP-43 mislocalization. Researchers can create more homogeneous disease models by introducing specific ALS-related mutations into isogenic iPSC lines, which reduces gene-related variability across individual cell lines and promotes reproducible phenotypic analysis [100].
Furthermore, traditional 2D cultures of iPSCs often fail to fully recapitulate the complexity of tissue architecture and cellular interactions seen in vivo. Three-dimensional culture systems, including organoids, spheroids, and microfluidic devices, offer more advanced models for ALS that can mimic the in vivo environment more closely. So, generating 3D MN organoids from iPSCs can create more representative disease models. These organoids can incorporate glial cells like astrocytes and microglia, which are involved in ALS pathogenesis, providing a more complete model of disease pathology. More recently, devices such as lab-on-a-chip platforms allow for the integration of multiple cell types (MNs, astrocytes, and microglia) in a dynamic 3D environment, enabling the modeling of disease progression, cellular interactions, and drug responses in real time [101,102]. Utilizing 3D bioprinting technology to create customized tissue models can improve the spatial organization of ALS disease models, enabling more precise studies of neuronal networks, axonal degeneration, and glial involvement. Additionally, Omics approaches, including genomics, transcriptomics, proteomics, and metabolomics, play a crucial role in providing a systems-level view of ALS biology and disease progression. Integrating these technologies with iPSC-derived ALS models offers several advantages for drug discovery and mechanistic insights [103].
6. Conclusions and Future Direction
The advent of hiPSCs to model human neurodegenerative diseases in vitro, including ALS, is accelerating our understanding of the molecular mechanisms underlying neurodegeneration in genetic and sporadic forms of a disease, where animal models are unavailable. HiPSC-derived single-cell-type and 2D co-culture systems have already revealed essential insights into ALS-specific cellular and molecular pathways. They are transforming the future of ALS research by personalizing disease modeling, uncovering pathogenic mechanisms, personalizing approaches to therapy, accelerating drug discovery, and opening doors to regenerative medicine. Recent developments of 3D organoid systems recapitulate a more in vivo-like environment of cell–cell interactions where the complexity of multiple cell types contributing to ALS causation can be closely analyzed. We are only beginning to see the potential of such in vitro disease models to unravel the complexities of ALS and to develop targeted therapies. As hiPSC technology advances to address the current limitations of 2D and, especially, 3D co-culture systems, it will enable more accurate modeling of human diseases, paving the way for a precision medicine approach to understanding and solving the puzzle of ALS.
Author Contributions
All authors contributed equally to the conceptualization, preparation, and writing of this review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by funding from PROJECT HOPE and the ALS Society of BC.
Data Availability Statement
No new data were created or analyzed in the preparation of this review. Data sharing is not applicable to this article.
Acknowledgments
L.D.N is grateful to P.E. Aryan for her encouragement.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 2D | Two dimensional |
| 3D | Three dimensional |
| ALI-COs | Air–liquid interface–cerebral organoids |
| ALS | Amyotrophic lateral sclerosis |
| ALS-FTD | Amyotrophic lateral sclerosis with frontotemporal dementia |
| CNS | Central nervous system |
| C RISPR-Cas9 | Clustered regularly interspersed short palindromic repeats |
| DPR | Dipeptide protein repeat |
| ESCs | Embryonic stem cells |
| fALS | Familial amyotrophic lateral sclerosis |
| FUS | Fused in sarcoma |
| FTD | Frontotemporal dementia |
| h iPSCs | Human induced pluripotent stem cells |
| HRE | Hexanucleotide repeat expansion |
| iPSCs | Induced pluripotent stem cells |
| LMC | Lateral motor columns |
| MMC | Median motor columns |
| MNs | Motor neurons |
| NMOs | Neuromuscular organoids |
| NPCs | Neuronal progenitor cells |
| PNS | Peripheral nervous system |
| phMNs | Phrenic motor neurons |
| RAN | Repeat-associated non-AUG |
| ROPI | Ropinirole hydrochloride |
| sALS | Sporadic amyotrophic lateral sclerosis |
References
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Lomen-Hoerth, C.; Anderson, T.; Miller, B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 2002, 59, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Vasta, R.; Chia, R.; Traynor, B.J.; Chiò, A. Unraveling the complex interplay between genes, environment, and climate in ALS. EBioMedicine 2022, 75, 103795. [Google Scholar] [CrossRef] [PubMed]
- Pham, J.; Keon, M.; Brennan, S.; Saksena, N. Connecting RNA-Modifying Similarities of TDP-43, FUS, and SOD1 with MicroRNA Dysregulation Amidst A Renewed Network Perspective of Amyotrophic Lateral Sclerosis Proteinopathy. Int. J. Mol. Sci. 2020, 21, 3464. [Google Scholar] [CrossRef]
- Burrell, J.R.; Kiernan, M.C.; Vucic, S.; Hodges, J.R. Motor Neuron dysfunction in frontotemporal dementia. Brain 2011, 134, 2582–2594. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- González, F.; Boué, S.; Belmonte, J.C.I. Methods for making induced pluripotent stem cells: Reprogramming à la carte. Nat. Rev. Genet. 2011, 12, 231–242. [Google Scholar] [CrossRef]
- Ragagnin, A.M.G.; Shadfar, S.; Vidal, M.; Jamali, S.; Atkin, J.D. Motor Neuron Susceptibility in ALS/FTD. Front. Neurosci. 2019, 13, 532. [Google Scholar] [CrossRef]
- Ferraiuolo, L.; Maragakis, N.J. Mini-Review: Induced Pluripotent Stem Cells and the Search for New Cell-Specific ALS Thera-peutic Targets. Neurosci. Lett. 2021. [CrossRef]
- Fujimori, K.; Ishikawa, M.; Otomo, A.; Atsuta, N.; Nakamura, R.; Akiyama, T.; Hadano, S.; Aoki, M.; Saya, H.; Sobue, G.; et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat. Med. 2018, 24, 1579–1589. [Google Scholar] [CrossRef]
- Manolio, T.A.; Collins, F.S.; Cox, N.J.; Goldstein, D.B.; Hindorff, L.A.; Hunter, D.J.; McCarthy, M.I.; Ramos, E.M.; Cardon, L.R.; Chakravarti, A.; et al. Finding the missing heritability of complex diseases. Nature 2009, 461, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, T.; Zerbo, R.A.; Balbi, M.; Torazza, C.; Frumento, G.; Fedele, E.; Bonanno, G.; Milanese, M. Nearly 30 Years of Animal Models to Study Amyotrophic Lateral Sclerosis: A Historical Overview and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 12236. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.M.C.; Greensmith, L.; Malaspina, A.; Fratta, P.; Hanna, M.G.; Schiavo, G.; Isaacs, A.M.; Orrell, R.W.; Cunningham, T.J.; Arozena, A.A. Opinion: More Mouse Models and More Translation Needed for ALS. Mol. Neurodegener. 2023, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Centeno, E.G.Z.; Cimarosti, H.; Bithell, A. 2D versus 3D Human Induced Pluripotent Stem Cell-Derived Cultures for Neurodegenerative Disease Modelling. Mol. Neurodegener. 2018, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huo, Z.; Chen, Y.; Zhao, Z.; Meng, F.; Wang, X.; Liu, S.; Zhang, H.; Zhou, F.; Liu, J.; et al. Induced Pluripotent Stem Cells and Their Applications in Amyotrophic Lateral Sclerosis. Cells 2023, 12, 971. [Google Scholar] [CrossRef]
- Giacomelli, E.; Vahsen, B.F.; Calder, E.L.; Xu, Y.; Scaber, J.; Gray, E.; Dafinca, R.; Talbot, K.; Studer, L. Human stem cell models of neurodegeneration: From basic science of amyotrophic lateral sclerosis to clinical translation. Cell Stem Cell 2022, 29, 11–35. [Google Scholar] [CrossRef]
- Guo, W.; Fumagalli, L.; Prior, R.; Van Den Bosch, L. Current Advances and Limitations in Modeling ALS/FTD in a Dish Using Induced Pluripotent Stem Cells. Front. Neurosci. 2017, 11, 671. [Google Scholar] [CrossRef]
- Van Damme, P.; Robberecht, W.; Van Den Bosch, L. Modelling amyotrophic lateral sclerosis: Progress and possibilities. Dis. Model. Mech. 2017, 10, 537–549. [Google Scholar] [CrossRef]
- Okano, H.; Morimoto, S.; Kato, C.; Nakahara, J.; Takahashi, S. Induced Pluripotent Stem Cells-Based Disease Modeling, Drug Screening, Clinical Trials, and Reverse Translational Research for Amyotrophic Lateral Sclerosis. J. Neurochem. 2023, 167, 603–614. [Google Scholar] [CrossRef]
- Liu, R.; Meng, X.; Yu, X.; Wang, G.; Dong, Z.; Zhou, Z.; Qi, M.; Yu, X.; Ji, T.; Wang, F. From 2D to 3D Co-Culture Systems: A Review of Co-Culture Models to Study the Neural Cells Interaction. Int. J. Mol. Sci. 2022, 23, 13116. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R. A Yeast Model for Understanding ALS: Fast, Cheap, and Easy to Control. PLoS Biol. 2011, 9, e1001053. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Farr, G.W.; Hall, D.H.; Li, F.; Furtak, K.; Dreier, L.; Horwich, A.L. An ALS-Linked Mutant SOD1 Produces a Locomotor Defect Associated with Aggregation and Synaptic Dysfunction When Expressed in Neurons of Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000350. [Google Scholar] [CrossRef] [PubMed]
- Casci, I.; Pandey, U.B. A fruitful endeavor: Modeling ALS in the fruit fly. Brain Res. 2015, 1607, 47–74. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Lyon, A.N.; Pineda, R.H.; Wang, C.; Janssen, P.M.L.; Canan, B.D.; Burghes, A.H.M.; Beattie, C.E. A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease. Dis. Model. Mech. 2010, 3, 652–662. [Google Scholar] [CrossRef]
- Stephenson, J.; Amor, S. Modelling amyotrophic lateral sclerosis in mice. Drug Discov. Today Dis. Model. 2017, 25-26, 35–44. [Google Scholar] [CrossRef]
- Morimoto, S.; Takahashi, S.; Ito, D.; Daté, Y.; Okada, K.; Kato, C.; Nakamura, S.; Ozawa, F.; Chyi, C.M.; Nishiyama, A.; et al. Phase 1/2a clinical trial in ALS with ropinirole, a drug candidate identified by iPSC drug discovery. Cell Stem Cell 2023, 30, 766–780.e9. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. The Link between Oxidative Stress, Redox Status, Bioenergetics and Mitochondria in the Pathophysiology of ALS. Int. J. Mol. Sci. 2021, 22, 6352. [Google Scholar] [CrossRef]
- Workman, M.J.; Lim, R.G.; Wu, J.; Frank, A.; Ornelas, L.; Panther, L.; Galvez, E.; Perez, D.; Meepe, I.; Lei, S.; et al. Large-scale differentiation of iPSC-derived motor neurons from ALS and control subjects. Neuron 2023, 111, 1191–1204.e5. [Google Scholar] [CrossRef]
- Ogaki, K.; Li, Y.; Atsuta, N.; Tomiyama, H.; Funayama, M.; Watanabe, H.; Nakamura, R.; Yoshino, H.; Yato, S.; Tamura, A.; et al. Analysis of C9orf72 repeat expansion in 563 Japanese patients with amyotrophic lateral sclerosis. Neurobiol. Aging 2012, 33, 2527.e11–2527.e16. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, Q.; Chen, Y.; Ou, R.; Cao, B.; Xu, Y.; Yang, J.; Shang, H.-F. Analysis of SOD1 mutations in a Chinese population with amyotrophic lateral sclerosis: A case-control study and literature review. Sci. Rep. 2017, 7, srep44606. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-W.; Chen, H.; Liu, H.; Lu, J.; Qian, K.; Huang, C.-L.; Zhong, X.; Fan, F.; Zhang, S.-C. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun. 2015, 6, 6626. [Google Scholar] [CrossRef] [PubMed]
- Velasco, S.; Ibrahim, M.M.; Kakumanu, A.; Garipler, G.; Aydin, B.; Al-Sayegh, M.A.; Hirsekorn, A.; Abdul-Rahman, F.; Satija, R.; Ohler, U.; et al. A Multi-Step Transcriptional and Chromatin State Cascade Underlies Motor Neuron Programming from Embryonic Stem Cells. Cell Stem Cell 2017, 20, 205–217.e8. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Pfaff, S.L. Synchronization of Neurogenesis and Motor Neuron Specification by Direct Coupling of bHLH and Homeodomain Transcription Factors. Neuron 2003, 38, 731–745. [Google Scholar] [CrossRef]
- Davis-Anderson, K.; Micheva-Viteva, S.; Solomon, E.; Hovde, B.; Cirigliano, E.; Harris, J.; Twary, S.; Iyer, R. CRISPR/Cas9 Directed Reprogramming of iPSC for Accelerated Motor Neuron Differentiation Leads to Dysregulation of Neuronal Fate Patterning and Function. Int. J. Mol. Sci. 2023, 24, 16161. [Google Scholar] [CrossRef]
- Liu, M.L.; Zang, T.; Zhang, C.L. Direct Lineage Reprogramming Reveals Disease-Specific Phenotypes of Motor Neurons from Human ALS Patients. Cell Rep. 2016, 14, 115–128. [Google Scholar] [CrossRef]
- Thiry, L.; Sirois, J.; Durcan, T.M.; Stifani, S. Generation of Human IPSC-Derived Phrenic-like Motor Neurons to Model Respiratory Motor Neuron Degeneration in ALS. Commun. Biol. 2024. [Google Scholar] [CrossRef]
- Stoklund Dittlau, K.; Van Den Bosch, L. Why Should We Care about Astrocytes in a Motor Neuron Disease? Front. Mol. Med. 2023, 3, 1047540. [Google Scholar] [CrossRef]
- Kunze, A.; Lengacher, S.; Dirren, E.; Aebischer, P.; Magistretti, P.J.; Renaud, P. Astrocyte-Neuron Co-Culture on Microchips Based on the Model of SOD Mutation to Mimic ALS. Integr. Biol. 2013, 5, 964–975. [Google Scholar] [CrossRef]
- Di Giorgio, F.P.; Carrasco, M.A.; Siao, M.C.; Maniatis, T.; Eggan, K. Non-Cell Autonomous Effect of Glia on Motor Neurons in an Embryonic Stem Cell-Based ALS Model. Nat. Neurosci. 2007, 10, 608–614. [Google Scholar] [CrossRef]
- Marchetto, M.C.N.; Muotri, A.R.; Mu, Y.; Smith, A.M.; Cezar, G.G.; Gage, F.H. Non-Cell-Autonomous Effect of Human SOD1G37R Astrocytes on Motor Neurons Derived from Human Embryonic Stem Cells. Cell Stem Cell 2008, 3, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Haidet-Phillips, A.M.; Hester, M.E.; Miranda, C.J.; Meyer, K.; Braun, L.; Frakes, A.; Song, S.; Likhite, S.; Murtha, M.J.; Foust, K.D.; et al. Astrocytes from Familial and Sporadic ALS Patients Are Toxic to Motor Neurons. Nat. Biotechnol. 2011, 29, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Luchena, C.; Zuazo-Ibarra, J.; Valero, J.; Matute, C.; Alberdi, E.; Capetillo-Zarate, E. A Neuron, Microglia, and Astrocyte Triple Co-culture Model to Study Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 844534. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.E.; Medeiros, M.; Porfirio, J.; Tavares, W.; Pessôa, L.; Grinberg, L.; Leite, R.E.; Ferretti-Rebustini, R.E.L.; Suemoto, C.K.; Filho, W.J.; et al. Similar Microglial Cell Densities across Brain Structures and Mammalian Species: Implications for Brain Tissue Function. J. Neurosci. 2020, 40, 4622–4643. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Vahsen, B.F.; Gray, E.; Candalija, A.; Cramb, K.M.L.; Scaber, J.; Dafinca, R.; Katsikoudi, A.; Xu, Y.; Farrimond, L.; Wade-Martins, R.; et al. Human iPSC co-culture model to investigate the interaction between microglia and motor neurons. Sci. Rep. 2022, 12, 12606. [Google Scholar] [CrossRef]
- Geirsdottir, L.; David, E.; Keren-Shaul, H.; Weiner, A.; Bohlen, S.C.; Neuber, J.; Balic, A.; Giladi, A.; Sheban, F.; Dutertre, C.-A.; et al. Cross-Species Single-Cell Analysis Reveals Divergence of the Primate Microglia Program. Cell 2019, 179, 1609–1622.e16. [Google Scholar] [CrossRef]
- Fattorelli, N.; Martinez-Muriana, A.; Wolfs, L.; Geric, I.; De Strooper, B.; Mancuso, R. Stem-cell-derived human microglia transplanted into mouse brain to study human disease. Nat. Protoc. 2021, 16, 1013–1033. [Google Scholar] [CrossRef]
- Kirchhoff, F.; Dringen, R.; Giaume, C. Pathways of neuron-astrocyte interactions and their possible role in neuroprotection. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 159–169. [Google Scholar] [CrossRef]
- Stoklund Dittlau, K.; Terrie, L.; Baatsen, P.; Kerstens, A.; De Swert, L.; Janky, R.; Corthout, N.; Masrori, P.; Van Damme, P.; Hyttel, P.; et al. FUS-ALS HiPSC-Derived Astrocytes Impair Human Motor Units through Both Gain-of-Toxicity and Loss-of-Support Mechanisms. Mol. Neurodegener. 2023, 18, 5. [Google Scholar] [CrossRef]
- McCauley, M.E.; Baloh, R.H. Inflammation in ALS/FTD Pathogenesis. Acta Neuropathol. 2019, 137, 715–730. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; González, P.; Rodríguez, F. New Insights into Wnt Signaling Alterations in Amyotrophic Lateral Sclerosis: A Potential Therapeutic Target? Neural Regen. Res. 2020, 15, 1580–1589. [Google Scholar] [CrossRef]
- Gagliardi, D.; Costamagna, G.; Taiana, M.; Andreoli, L.; Biella, F.; Bersani, M.; Bresolin, N.; Comi, G.P.; Corti, S. Insights into Disease Mechanisms and Potential Therapeutics for C9orf72-Related Amyotrophic Lateral Sclerosis/Frontotemporal Dementia. Ageing Res. Rev. 2020, 64, 101172. [Google Scholar] [CrossRef]
- Schmitz, A.; Pinheiro Marques, J.; Oertig, I.; Maharjan, N.; Saxena, S. Emerging Perspectives on Dipeptide Repeat Proteins in C9ORF72 ALS/FTD. Front. Cell. Neurosci. 2021, 15, 637548. [Google Scholar] [CrossRef]
- Marchi, P.M.; Marrone, L.; Brasseur, L.; Coens, A.; Webster, C.P.; Bousset, L.; Destro, M.; Smith, E.F.; Walther, C.G.; Alfred, V.; et al. C9ORF72-Derived Poly-GA DPRs Undergo Endocytic Uptake in IAstrocytes and Spread to Motor Neurons. Life Sci. Alliance 2022, 5, e202101276. [Google Scholar] [CrossRef]
- Nihei, Y.; Mori, K.; Werner, G.; Arzberger, T.; Zhou, Q.; Khosravi, B.; Japtok, J.; Hermann, A.; Sommacal, A.; Weber, M.; et al. Poly-Glycine–Alanine Exacerbates C9orf72 Repeat Expansion-Mediated DNA Damage via Sequestration of Phosphorylated ATM and Loss of Nuclear HnRNPA. Acta Neuropathol. 2020, 139, 99–118. [Google Scholar] [CrossRef]
- Li, C.Y.; Yang, T.M.; Ou, R.W.; Wei, Q.Q.; Shang, H.F. Genome-Wide Genetic Links between Amyotrophic Lateral Sclerosis and Autoimmune Diseases. BMC Med. 2021, 19, 27. [Google Scholar] [CrossRef]
- Burberry, A.; Suzuki, N.; Wang, J.Y.; Moccia, R.; Mordes, D.A.; Stewart, M.H.; Suzuki-Uematsu, S.; Ghosh, S.; Singh, A.; Merkle, F.T.; et al. Loss-of-Function Mutations in the C9ORF72 Mouse Ortholog Cause Fatal Autoimmune Disease. Sci. Transl. Med. 2016, 8, 347ra93. [Google Scholar] [CrossRef]
- Joshi, A.U.; Minhas, P.S.; Liddelow, S.A.; Haileselassie, B.; Andreasson, K.I.; Dorn, G.W.; Mochly-Rosen, D. Fragmented Mitochondria Released from Microglia Trigger A1 Astrocytic Response and Propagate Inflammatory Neurodegeneration. Nat. Neurosci. 2019, 22, 1635–1648. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, S.; Staats, K.A.; Li, Y.; Chang, W.H.; Hung, S.T.; Hendricks, E.; Linares, G.R.; Wang, Y.; Son, E.Y.; et al. Haploinsufficiency Leads to Neurodegeneration in C9ORF72 ALS/FTD Human Induced Motor Neurons. Nat. Med. 2018, 24, 313–325. [Google Scholar] [CrossRef]
- Muffat, J.; Li, Y.; Yuan, B.; Mitalipova, M.; Omer, A.; Corcoran, S.; Bakiasi, G.; Tsai, L.-H.; Aubourg, P.; Ransohoff, R.M.; et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 2016, 22, 1358–1367. [Google Scholar] [CrossRef]
- Lopez-Lengowski, K.; Kathuria, A.; Gerlovin, K.; Karmacharya, R. Co-Culturing Microglia and Cortical Neurons Differentiated from Human Induced Pluripotent Stem Cells. J. Vis. Exp. 2021, e62480. [Google Scholar] [CrossRef]
- Haenseler, W.; Sansom, S.N.; Buchrieser, J.; Newey, S.E.; Moore, C.S.; Nicholls, F.J.; Chintawar, S.; Schnell, C.; Antel, J.P.; Allen, N.D.; et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response. Stem Cell Rep. 2017, 8, 1727–1742. [Google Scholar] [CrossRef]
- Vahsen, B.F.; Nalluru, S.; Morgan, G.R.; Farrimond, L.; Carroll, E.; Xu, Y.; Cramb, K.M.L.; Amein, B.; Scaber, J.; Katsikoudi, A.; et al. C9orf72-ALS Human IPSC Microglia Are pro-Inflammatory and Toxic to Co-Cultured Motor Neurons via MMP. Nat. Commun. 2023, 14, 5898. [Google Scholar] [CrossRef]
- Spiller, K.J.; Khan, T.; Dominique, M.A.; Restrepo, C.R.; Cotton-Samuel, D.; Levitan, M.; Jafar-Nejad, P.; Zhang, B.; Soriano, A.; Rigo, F.; et al. Reduction of matrix metalloproteinase 9 (MMP-9) protects motor neurons from TDP-43-triggered death in rNLS8 mice. Neurobiol. Dis. 2019, 124, 133–140. [Google Scholar] [CrossRef]
- Kaplan, A.; Spiller, K.J.; Towne, C.; Kanning, K.C.; Choe, G.T.; Geber, A.; Akay, T.; Aebischer, P.; Henderson, C.E. Neuronal Matrix Metalloproteinase-9 Is a Determinant of Selective Neurodegeneration. Neuron 2014, 81, 333–348. [Google Scholar] [CrossRef]
- Goshi, N.; Morgan, R.K.; Lein, P.J.; Seker, E. A Primary Neural Cell Culture Model to Study Neuron, Astrocyte, and Microglia Interactions in Neuroinflammation. J. Neuroinflamm. 2020, 17, 155. [Google Scholar] [CrossRef]
- Suarez-Martinez, E.; Suazo-Sanchez, I.; Celis-Romero, M.; Carnero, A. 3D and Organoid Culture in Research: Physiology, Hereditary Genetic Diseases and Cancer. Cell Biosci. 2022, 12, 39. [Google Scholar] [CrossRef]
- Dawoody Nejad, L.; Julian, L.M. Stem Cell-Derived Organoid Models for SARS-CoV-2 and Its Molecular Interaction with Host Cells. Mol. Biol. Rep. 2023, 12, 10627–10635. [Google Scholar] [CrossRef]
- Agboola, O.S.; Hu, X.; Shan, Z.; Wu, Y.; Lei, L. Brain Organoid: A 3D Technology for Investigating Cellular Composition and Interactions in Human Neurological Development and Disease Models in Vitro. Stem Cell Res. Ther. 2021, 12, 430. [Google Scholar] [CrossRef]
- Huch, M.; Koo, B.K. Modeling Mouse and Human Development Using Organoid Cultures. Development 2015, 142, 3113–3125. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Mierau, S.B.; Gibbons, G.M.; Wenger, L.M.D.; Masullo, L.; Sit, T.; Sutcliffe, M.; Boulanger, J.; Tripodi, M.; Derivery, E.; et al. Cerebral Organoids at the Air–Liquid Interface Generate Diverse Nerve Tracts with Functional Output. Nat. Neurosci. 2019, 22, 669–679. [Google Scholar] [CrossRef]
- Eichmüller, O.L.; Knoblich, J.A. Human Cerebral Organoids - a New Tool for Clinical Neurology Research. Nat. Rev. Neurol. 2022, 18, 661–680. [Google Scholar] [CrossRef]
- Szebényi, K.; Wenger, L.M.D.; Sun, Y.; Dunn, A.W.E.; Limegrover, C.A.; Gibbons, G.M.; Conci, E.; Paulsen, O.; Mierau, S.B.; Balmus, G.; et al. Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nat. Neurosci. 2021, 24, 1542–1554. [Google Scholar] [CrossRef]
- Smedley, G.D.; Walker, K.E.; Yuan, S.H. The Role of PERK in Understanding Development of Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 8146. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Zhang, J.; Zhou, Z.; Feng, B.; Du, X.; Liu, X.; Ma, J.; Cui, H. Neural Differentiation and Spinal Cord Organoid Generation from Induced Pluripotent Stem Cells (IPSCs) for ALS Modelling and Inflammatory Screening. Mol. Neurobiol. 2023, 61, 4732–4749. [Google Scholar] [CrossRef]
- Dadon-Nachum, M.; Melamed, E.; Offen, D. The “Dying-Back” Phenomenon of Motor Neurons in ALS. J. Mol. Neurosci. 2011, 43, 470–477. [Google Scholar] [CrossRef]
- Scaricamazza, S.; Salvatori, I.; Ferri, A.; Valle, C. Skeletal Muscle in Als: An Unappreciated Therapeutic Opportunity? Cells 2021, 10, 525. [Google Scholar] [CrossRef]
- Gao, C.; Shi, Q.; Pan, X.; Chen, J.; Zhang, Y.; Lang, J.; Wen, S.; Liu, X.; Cheng, T.L.; Lei, K. Neuromuscular Organoids Model Spinal Neuromuscular Pathologies in C9orf72 Amyotrophic Lateral Sclerosis. Cell Rep. 2024, 43, 113892. [Google Scholar] [CrossRef]
- Massih, B.; Veh, A.; Schenke, M.; Mungwa, S.; Seeger, B.; Selvaraj, B.T.; Chandran, S.; Reinhardt, P.; Sterneckert, J.; Hermann, A.; et al. A 3D Cell Culture System for Bioengineering Human Neuromuscular Junctions to Model ALS. Front. Cell Dev. Biol. 2023, 11, 996952. [Google Scholar] [CrossRef]
- Murray, L.M.; Talbot, K.; Gillingwater, T.H. Review: Neuromuscular Synaptic Vulnerability in Motor Neurone Disease: Amyotrophic Lateral Sclerosis and Spinal Muscular Atrophy. Neuropathol. Appl. Neurobiol. 2010, 36, 133–156. [Google Scholar] [CrossRef]
- Xu, C.; Tabebordbar, M.; Iovino, S.; Ciarlo, C.; Liu, J.; Castiglioni, A.; Price, E.; Liu, M.; Barton, E.R.; Kahn, C.R.; et al. XA Zebrafish Embryo Culture System Defines Factors That Promote Vertebrate Myogenesis across Species. Cell 2013, 155, 909–921. [Google Scholar] [CrossRef]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced Pluripotent Stem Cells Generated from Patients with ALS Can Be Differentiated into Motor Neurons. Science 2008, 321, 1218–1221. [Google Scholar] [CrossRef]
- Bakooshli, M.A.; Lippmann, E.S.; Mulcahy, B.; Iyer, N.; Nguyen, C.T.; Tung, K.; Stewart, B.A.; Van Den Dorpel, H.; Fuehrmann, T.; Shoichet, M.; et al. A 3d Culture Model of Innervated Human Skeletal Muscle Enables Studies of the Adult Neuromuscular Junction. elife 2019, 8, e44530. [Google Scholar] [CrossRef]
- Lin, C.Y.; Yoshida, M.; Li, L.T.; Ikenaka, A.; Oshima, S.; Nakagawa, K.; Sakurai, H.; Matsui, E.; Nakahata, T.; Saito, M.K. IPSC-Derived Functional Human Neuromuscular Junctions Model the Pathophysiology of Neuromuscular Diseases. J. Clin. Investig. Insight 2019, 4, e124299. [Google Scholar] [CrossRef]
- Dittlau, K.S.; Krasnow, E.N.; Fumagalli, L.; Vandoorne, T.; Terrie, L.; Baatsen, P.; Giacomazzi, G.; Sampaolesi, M.; Thorrez, L.; Van Damme, P.; et al. Modeling ALS—Human Neuromuscular Junctions in a Dish. Amyotroph. Lateral Scler. Front. Degener. 2019, 11, 2304989. [Google Scholar]
- Faustino Martins, J.M.; Fischer, C.; Urzi, A.; Vidal, R.; Kunz, S.; Ruffault, P.L.; Kabuss, L.; Hube, I.; Gazzerro, E.; Birchmeier, C.; et al. Self-Organizing 3D Human Trunk Neuromuscular Organoids. Cell Stem Cell 2020, 26, 172–186.e6. [Google Scholar] [CrossRef]
- Andersen, J.; Revah, O.; Miura, Y.; Thom, N.; Amin, N.D.; Kelley, K.W.; Singh, M.; Chen, X.; Thete, M.V.; Walczak, E.M.; et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell 2020, 183, 1913–1929.e26. [Google Scholar] [CrossRef]
- Santhanam, N.; Kumanchik, L.; Guo, X.; Sommerhage, F.; Cai, Y.; Jackson, M.; Martin, C.; Saad, G.; McAleer, C.W.; Wang, Y.; et al. Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials 2018, 166, 64–78. [Google Scholar] [CrossRef]
- Osaki, T.; Uzel, S.G.M.; Kamm, R.D. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv. 2018, 4, eaat5847. [Google Scholar] [CrossRef]
- Pereira, J.D.; DuBreuil, D.M.; Devlin, A.C.; Held, A.; Sapir, Y.; Berezovski, E.; Hawrot, J.; Dorfman, K.; Chander, V.; Wainger, B.J. Human Sensorimotor Organoids Derived from Healthy and Amyotrophic Lateral Sclerosis Stem Cells Form Neuromuscular Junctions. Nat. Commun. 2021, 12, 4744. [Google Scholar] [CrossRef]
- McCampbell, A.; Cole, T.; Wegener, A.J.; Tomassy, G.S.; Setnicka, A.; Farley, B.J.; Schoch, K.M.; Hoye, M.L.; Shabsovich, M.; Sun, L.; et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J. Clin. Investig. 2018, 128, 3558–3567. [Google Scholar] [CrossRef]
- Pașca, A.M.; Park, J.-Y.; Shin, H.-W.; Qi, Q.; Revah, O.; Krasnoff, R.; O’hara, R.; Willsey, A.J.; Palmer, T.D.; Pașca, S.P. Human 3D cellular model of hypoxic brain injury of prematurity. Nat. Med. 2019, 25, 784–791. [Google Scholar] [CrossRef]
- Lobsiger, C.S.; Cleveland, D.W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat. Neurosci. 2007, 10, 1355–1360. [Google Scholar] [CrossRef]
- Jaiswal, M.K. Riluzole and Edaravone: A Tale of Two Amyotrophic Lateral Sclerosis Drugs. Med. Res. Rev. 2019, 39, 733–748. [Google Scholar] [CrossRef]
- Bonaventura, G.; Iemmolo, R.; Attaguile, G.A.; La Cognata, V.; Pistone, B.S.; Raudino, G.; D’agata, V.; Cantarella, G.; Barcellona, M.L.; Cavallaro, S. iPSCs: A Preclinical Drug Research Tool for Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 4596. [Google Scholar] [CrossRef]
- Beghini, D.G.; Kasai-Brunswick, T.H.; Henriques-Pons, A. Induced Pluripotent Stem Cells in Drug Discovery and Neurodegenerative Disease Modelling. Int. J. Mol. Sci. 2024, 25, 2392. [Google Scholar] [CrossRef]
- Pasteuning-Vuhman, S.; de Jongh, R.; Timmers, A.; Pasterkamp, R.J. Towards Advanced IPSC-Based Drug Development for Neurodegenerative Disease. Trends Mol. Med. 2021, 27, 263–279. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current Challenges of IPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- Monteduro, A.G.; Rizzato, S.; Caragnano, G.; Trapani, A.; Giannelli, G.; Maruccio, G. Organs-on-Chips Technologies—A Guide from Disease Models to Opportunities for Drug Development. Biosens. Bioelectron. 2023, 231, 115271. [Google Scholar] [CrossRef] [PubMed]
- Busek, M.; Aizenshtadt, A.; Amirola-Martinez, M.; Delon, L.; Krauss, S. Academic User View: Organ-on-a-Chip Technology. Biosensors 2022, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Morello, G.; Salomone, S.; D’agata, V.; Conforti, F.L.; Cavallaro, S. From Multi-Omics Approaches to Precision Medicine in Amyotrophic Lateral Sclerosis. Front. Neurosci. 2020, 14, 577755. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).