Prevalence and Clinical Correlates of Cerebrovascular Alterations in Fabry Disease: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Clinical Evaluation
2.2. CNS Involvement
- History of cerebral events (previous stroke or TIA).
- WMLs, quantified using the modified Fazekas score (mFS) [29] performed on the FLAIR axial sequences of conventional 1.5-tesla brain MRI in 32/40 patients suitable for the examination (not carriers of pacemaker (PMK) or implantable cardioverter device (ICD) as part of their routine follow-up assessment. WMLs were rated on axial FLAIR, ranging from 0 (no WMLs) to 3 (confluent WMLs).
- Cognitive assessment: 39/40 FD patients underwent neuropsychological assessment. The cognitive battery included the following tests with published standardized normative data from large control samples: the Mini-Mental State Examination (MMSE) [30], Rey’s auditory verbal learning task (RAVLT), including subtests of immediate and delayed recall and forced-choice recognition [31], the Rey–Osterrieth figure copy and delayed recall [32], an abstract reasoning test (Raven’s progressive matrices) [31], the Stroop test—short version [31], a demanding visual attention task (multiple features target cancellation) [33], phonological and semantic verbal fluency [31,34], and digit and spatial span forward and backward [35]. Individual test scores were considered either normal or altered based on the normative values. For statistical correlations, only values adjusted for age and education were used where applicable.
- Serum NfL levels were assessed in 22/40 FD patients and in a control group of 15 healthy age- and sex-matched subjects by single-molecule array (SiMoA, Quanterix, Billerica, MA, USA) as previously described [36]. Frozen serum samples were carried in dry ice to the Neuroimmunology Lab, Santa Lucia Foundation IRCCS, Rome (IT) for quantitative determination of NfL.
2.3. Other Systems Possibly Influencing CNS Impairment
- -
- Age at evaluation (AE), sex at birth, years of education.
- -
- Type of GLA variant.
- -
- Phenotype (classic or late onset).
- -
- Age at onset (AAO) of FD symptoms and years of disease duration (DD).
- -
- Type and years of treatment (enzymatic replacement therapy (ERT) or chaperone).
- -
- Plasma lyso-Gb3 levels 6 months before and 1 year after starting treatment. Plasma lyso-Gb3 levels were measured by tandem mass spectrometry [37] at an external laboratory (Centogene GmbH, Rostock, Germany).
- -
- Residual enzymatic alpha-Gal A activity (measured on leukocytes and expressed as percentage of the normal mean).
- -
- Renal function parameters (estimated glomerular filtration rate (eGFR, expressed in mL/min), creatinine (mg/dL), blood urea nitrogen (BUN, mg/dL), cystatin C (mg/dL), 24 h albuminuria (mg) and 24 h proteinuria (mg)), presence of renal dysfunction (eGFR < 90 mL/min or 24 h proteinuria > 300 mg/die or spot proteinuria >30 mg/dL), severe renal dysfunction (eGFR < 30 mL/min/1.73 m2, dialysis or renal transplantation). eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [38].
- -
- Common vascular risk factors and comorbidities (hypertension and use of antihypertensive medications, dyslipidemia and use of statins, type 2 diabetes mellitus, smoking).
- -
- Presence of cardiac hypertrophy and major cardiac events (atrial fibrillation or any major rhythm disturbance, congestive heart failure, implantation of an ICD or PMK, myocardial infarction, coronary artery bypass graft (CABG) surgery, or a percutaneous transluminal angioplasty). Echocardiographic parameters included: interventricular septum (IVS) and left ventricle (LV) posterior wall thickness, end-systolic and end-diastolic LV diameter, 2D guided B-mode calculated left ventricle mass index (LVMi), and relative wall thickness (RWT). Left ventricular hypertrophy was defined as a left ventricular mass index (LVMi) higher than the upper limit of normal (men ≥ 103 g/m2, women ≥ 89 g/m2) [39].
- -
- Presence of subjective depressive symptoms and use of antidepressants.
- -
- Diagnosis of migraine/tension headache.
- -
- Severity of pain assessed by VAS (visual analogue scale).
- -
- Use of medications for neuropathic pain, presence of acroparesthesias and/or dyshidrosis.
- -
- Presence of angiokeratomas and corneal abnormalities, tinnitus, and/or dizziness.
- -
- Presence of polyneuropathy detected by nerve conduction studies (NCSs) on lower limbs. NCSs were performed using Natus Keypoint EMG equipment (Middleton, WI, USA). Antidromic sural SNAP, tibial CMAP and F wave latency were measured and compared to our reference values [40].
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of FD Patients
3.2. Prevalence and Correlates of Cerebrovascular Manifestation, WMLs, and Cognitive Impairment
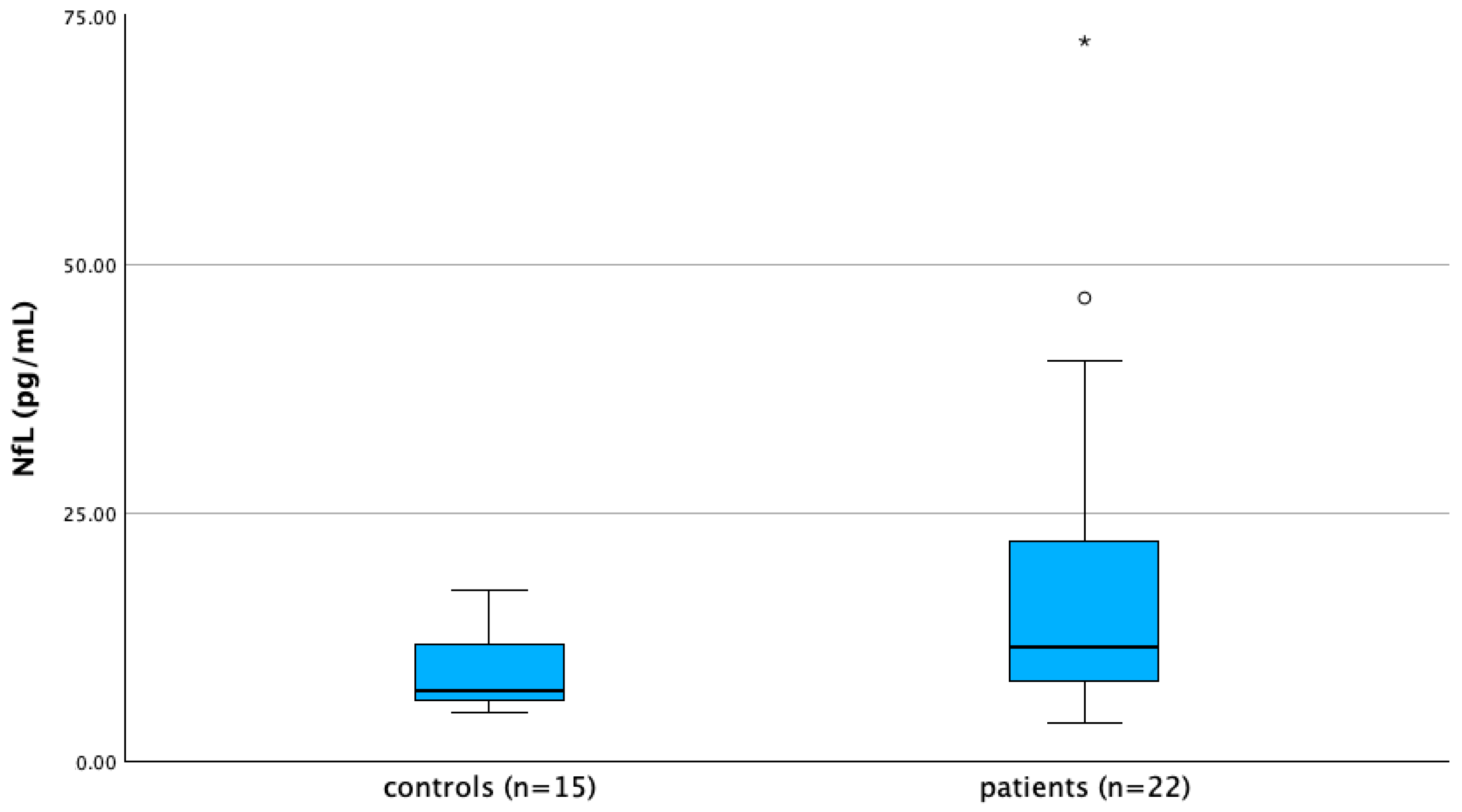
3.3. Serum NfL Levels
3.4. Correlation Analysis Regarding mFS and Serum NfL Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, A.; Hughes, D.A. Fabry Disease. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Simonetta, I.; Tuttolomondo, A.; Daidone, M.; Pinto, A. Biomarkers in Anderson-Fabry Disease. Int. J. Mol. Sci. 2020, 21, 8080. [Google Scholar] [CrossRef]
- Schaefer, E.; Mehta, A.; Gal, A. Genotype and Phenotype in Fabry Disease: Analysis of the Fabry Outcome Survey. Acta Paediatr. Suppl. 2005, 94, 87–92; discussion 79. [Google Scholar] [CrossRef]
- Waldek, S.; Patel, M.R.; Banikazemi, M.; Lemay, R.; Lee, P. Life Expectancy and Cause of Death in Males and Females with Fabry Disease: Findings from the Fabry Registry. Genet. Med. 2009, 11, 790–796. [Google Scholar] [CrossRef]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry Disease, an under-Recognized Multisystemic Disorder: Expert Recommendations for Diagnosis, Management, and Enzyme Replacement Therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef] [PubMed]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry Disease: Clinical Manifestations and Impact of Disease in a Cohort of 98 Hemizygous Males. J. Med. Genet. 2001, 38, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.J.; Marques, A.R.A.; Appelman, M.D.; Verhoek, M.; Strijland, A.; Mirzaian, M.; Scheij, S.; Ouairy, C.M.; Lahav, D.; Wisse, P.; et al. Lysosomal Glycosphingolipid Catabolism by Acid Ceramidase: Formation of Glycosphingoid Bases during Deficiency of Glycosidases. FEBS Lett. 2016, 590, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.M.; Groener, J.E.; Kuiper, S.; Donker-Koopman, W.E.; Strijland, A.; Ottenhoff, R.; van Roomen, C.; Mirzaian, M.; Wijburg, F.A.; Linthorst, G.E.; et al. Elevated Globotriaosylsphingosine Is a Hallmark of Fabry Disease. Proc. Natl. Acad. Sci. USA 2008, 105, 2812–2817. [Google Scholar] [CrossRef]
- Rombach, S.M.; Dekker, N.; Bouwman, M.G.; Linthorst, G.E.; Zwinderman, A.H.; Wijburg, F.A.; Kuiper, S.; Vd Bergh Weerman, M.A.; Groener, J.E.M.; Poorthuis, B.J.; et al. Plasma Globotriaosylsphingosine: Diagnostic Value and Relation to Clinical Manifestations of Fabry Disease. Biochim. Biophys. Acta 2010, 1802, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Togawa, T.; Kodama, T.; Suzuki, T.; Sugawara, K.; Tsukimura, T.; Ohashi, T.; Ishige, N.; Suzuki, K.; Kitagawa, T.; Sakuraba, H. Plasma Globotriaosylsphingosine as a Biomarker of Fabry Disease. Mol. Genet. Metab. 2010, 100, 257–261. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Li, F.; Sun, Y.; Ma, W.; Wu, Y.; Zhang, W.; Wang, Z.; Yuan, Y.; Huang, Y. Brain MRI Correlations with Disease Burden and Biomarkers in Fabry Disease. J. Neurol. 2023, 270, 4939–4948. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, L.; Kanber, B.; Prados, F.; Davagnanam, I.; Merwick, A.; Chan, E.; Williams, F.; Hughes, D.; Murphy, E.; Lachmann, R.H.; et al. White Matter Integrity Correlates with Cognition and Disease Severity in Fabry Disease. Brain 2020, 143, 3331–3342. [Google Scholar] [CrossRef] [PubMed]
- Sims, K.; Politei, J.; Banikazemi, M.; Lee, P. Stroke in Fabry Disease Frequently Occurs before Diagnosis and in the Absence of Other Clinical Events: Natural History Data from the Fabry Registry. Stroke 2009, 40, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Banerjee, A.; Gandhi, A.B.; Kaleem, I.; Alexander, J.; Hisbulla, M.; Kannichamy, V.; Valaiyaduppu Subas, S.; Hamid, P. Stroke and Fabry Disease: A Review of Literature. Cureus 2020, 12, e12083. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Cloonan, L.; Kanakis, A.S.; Fitzpatrick, K.M.; Azzariti, D.R.; Clarke, V.; Lourenco, C.M.; Germain, D.P.; Politei, J.M.; Homola, G.A.; et al. Determinants of White Matter Hyperintensity Burden in Patients with Fabry Disease. Neurology 2016, 86, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Körver, S.; Vergouwe, M.; Hollak, C.E.M.; van Schaik, I.N.; Langeveld, M. Development and Clinical Consequences of White Matter Lesions in Fabry Disease: A Systematic Review. Mol. Genet. Metab. 2018, 125, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.F.; Kaneski, C.R.; Askari, H.; Schiffmann, R. The Cerebral Vasculopathy of Fabry Disease. J. Neurol. Sci. 2007, 257, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.F.; Parwani, J.; Millhouse, P.W.; Eissa-Garcés, A.; Hassen, G.; Cuenca, V.D.; Alzamora, I.M.; Khurana, M.; Herrera-Bucheli, D.; Altamimi, A.; et al. Prevalence of Fabry Disease in Patients With Cryptogenic Strokes: A Systematic Review. Cureus 2021, 13, e19358. [Google Scholar] [CrossRef]
- Bolsover, F.E.; Murphy, E.; Cipolotti, L.; Werring, D.J.; Lachmann, R.H. Cognitive Dysfunction and Depression in Fabry Disease: A Systematic Review. J. Inherit. Metab. Dis. 2014, 37, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Loeb, J.; Feldt-Rasmussen, U.; Madsen, C.V.; Vogel, A. Cognitive Impairments and Subjective Cognitive Complaints in Fabry Disease: A Nationwide Study and Review of the Literature. JIMD Rep. 2018, 41, 73–80. [Google Scholar] [CrossRef]
- Schermuly, I.; Müller, M.J.; Müller, K.-M.; Albrecht, J.; Keller, I.; Yakushev, I.; Beck, M.; Fellgiebel, A. Neuropsychiatric Symptoms and Brain Structural Alterations in Fabry Disease. Eur. J. Neurol. 2011, 18, 347–353. [Google Scholar] [CrossRef]
- Körver, S.; Geurtsen, G.J.; Hollak, C.E.M.; van Schaik, I.N.; Longo, M.G.F.; Lima, M.R.; Vedolin, L.; Dijkgraaf, M.G.W.; Langeveld, M. Predictors of Objective Cognitive Impairment and Subjective Cognitive Complaints in Patients with Fabry Disease. Sci. Rep. 2019, 9, 188. [Google Scholar] [CrossRef]
- Cole, A.L.; Lee, P.J.; Hughes, D.A.; Deegan, P.B.; Waldek, S.; Lachmann, R.H. Depression in Adults with Fabry Disease: A Common and under-Diagnosed Problem. J. Inherit. Metab. Dis. 2007, 30, 943–951. [Google Scholar] [CrossRef]
- Mroczek, M.; Maniscalco, I.; Sendel, M.; Baron, R.; Seifritz, E.; Nowak, A. Neuropsychiatric Symptoms and Their Association With Sex, Age, and Enzyme Replacement Therapy in Fabry Disease: A Systematic Review. Front. Psychiatry 2022, 13, 829128. [Google Scholar] [CrossRef]
- Okeda, R.; Nisihara, M. An Autopsy Case of Fabry Disease with Neuropathological Investigation of the Pathogenesis of Associated Dementia. Neuropathology 2008, 28, 532–540. [Google Scholar] [CrossRef]
- Lelieveld, I.M.; Böttcher, A.; Hennermann, J.B.; Beck, M.; Fellgiebel, A. Eight-Year Follow-Up of Neuropsychiatric Symptoms and Brain Structural Changes in Fabry Disease. PLoS ONE 2015, 10, e0137603. [Google Scholar] [CrossRef]
- Germain, D.P.; Fouilhoux, A.; Decramer, S.; Tardieu, M.; Pillet, P.; Fila, M.; Rivera, S.; Deschênes, G.; Lacombe, D. Consensus Recommendations for Diagnosis, Management and Treatment of Fabry Disease in Paediatric Patients. Clin. Genet. 2019, 96, 107–117. [Google Scholar] [CrossRef]
- Whybra, C.; Kampmann, C.; Krummenauer, F.; Ries, M.; Mengel, E.; Miebach, E.; Baehner, F.; Kim, K.; Bajbouj, M.; Schwarting, A.; et al. The Mainz Severity Score Index: A New Instrument for Quantifying the Anderson-Fabry Disease Phenotype, and the Response of Patients to Enzyme Replacement Therapy. Clin. Genet. 2004, 65, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR Signal Abnormalities at 1.5 T in Alzheimer’s Dementia and Normal Aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; Caltagirone, C.; Gainotti, G. The Mental Deterioration Battery: Normative Data, Diagnostic Reliability and Qualitative Analyses of Cognitive Impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur. Neurol. 1996, 36, 378–384. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-Osterrieth Complex Figure: Normative Values in an Italian Population Sample. Neurol. Sci. 2002, 22, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.; Gainotti, G.; Scaricamazza, E.; Piccininni, C.; Ferraccioli, M.; Quaranta, D. The Multiple Features Target Cancellation (MFTC): An Attentional Visual Conjunction Search Test. Normative Values for the Italian Population. Neurol. Sci. 2013, 34, 173–180. [Google Scholar] [CrossRef]
- Quaranta, D.; Caprara, A.; Piccininni, C.; Vita, M.G.; Gainotti, G.; Marra, C. Standardization, Clinical Validation, and Typicality Norms of a New Test Assessing Semantic Verbal Fluency. Arch. Clin. Neuropsychol. 2016, 31, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and Backward Span for Verbal and Visuo-Spatial Data: Standardization and Normative Data from an Italian Adult Population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef]
- Nicoletti, T.F.; Rossi, S.; Vita, M.G.; Perna, A.; Guerrera, G.; Lino, F.; Iacovelli, C.; Di Natale, D.; Modoni, A.; Battistini, L.; et al. Elevated Serum Neurofilament Light Chain (NfL) as a Potential Biomarker of Neurological Involvement in Myotonic Dystrophy Type 1 (DM1). J. Neurol. 2022, 269, 5085–5092. [Google Scholar] [CrossRef] [PubMed]
- Boutin, M.; Lavoie, P.; Abaoui, M.; Auray-Blais, C. Tandem Mass Spectrometry Quantitation of Lyso-Gb3 and Six Related Analogs in Plasma for Fabry Disease Patients. Curr. Protoc. Hum. Genet. 2016, 90, 17.23.1–17.23.9. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Luigetti, M.; Quaranta, D.; Modoni, A.; Mereu, M.L.; Lo Monaco, M. Nerve Conduction Studies of the Sural Nerve: Normative Data from a Single-Center Experience. Clin. Neurophysiol. 2012, 123, 1891–1892. [Google Scholar] [CrossRef]
- Bornhorst, J.A.; Figdore, D.; Campbell, M.R.; Pazdernik, V.K.; Mielke, M.M.; Petersen, R.C.; Algeciras-Schimnich, A. Plasma Neurofilament Light Chain (NfL) Reference Interval Determination in an Age-Stratified Cognitively Unimpaired Cohort. Clin. Chim. Acta 2022, 535, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.M.; Fletcher, J.; Wilcox, W.R.; Waldek, S.; Scott, C.R.; Sillence, D.O.; Breunig, F.; Charrow, J.; Germain, D.P.; Nicholls, K.; et al. Fabry Disease: Baseline Medical Characteristics of a Cohort of 1765 Males and Females in the Fabry Registry. J. Inherit. Metab. Dis. 2007, 30, 184–192. [Google Scholar] [CrossRef]
- Wanner, C.; Ortiz, A.; Wilcox, W.R.; Hopkin, R.J.; Johnson, J.; Ponce, E.; Ebels, J.T.; Batista, J.L.; Maski, M.; Politei, J.M.; et al. Global Reach of over 20 Years of Experience in the Patient-Centered Fabry Registry: Advancement of Fabry Disease Expertise and Dissemination of Real-World Evidence to the Fabry Community. Mol. Genet. Metab. 2023, 139, 107603. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, F.E.; de Groot, J.C.; Achten, E.; Oudkerk, M.; Ramos, L.M.; Heijboer, R.; Hofman, A.; Jolles, J.; van Gijn, J.; Breteler, M.M. Prevalence of Cerebral White Matter Lesions in Elderly People: A Population Based Magnetic Resonance Imaging Study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 2001, 70, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.; Chen, X.; Wen, W. White Matter Hyperintensities in Mid-Adult Life. Curr. Opin. Psychiatry 2008, 21, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Uçeyler, N.; Homola, G.A.; Guerrero González, H.; Kramer, D.; Wanner, C.; Weidemann, F.; Solymosi, L.; Sommer, C. Increased Arterial Diameters in the Posterior Cerebral Circulation in Men with Fabry Disease. PLoS ONE 2014, 9, e87054. [Google Scholar] [CrossRef] [PubMed]
- Fellgiebel, A.; Müller, M.J.; Mazanek, M.; Baron, K.; Beck, M.; Stoeter, P. White Matter Lesion Severity in Male and Female Patients with Fabry Disease. Neurology 2005, 65, 600–602. [Google Scholar] [CrossRef]
- Korsholm, K.; Feldt-Rasmussen, U.; Granqvist, H.; Højgaard, L.; Bollinger, B.; Rasmussen, A.K.; Law, I. Positron Emission Tomography and Magnetic Resonance Imaging of the Brain in Fabry Disease: A Nationwide, Long-Time, Prospective Follow-Up. PLoS ONE 2015, 10, e0143940. [Google Scholar] [CrossRef]
- Lee, H.-J.; Hsu, T.-R.; Hung, S.-C.; Yu, W.-C.; Chu, T.-H.; Yang, C.-F.; Bizjajeva, S.; Tiu, C.-M.; Niu, D.-M. A Comparison of Central Nervous System Involvement in Patients with Classical Fabry Disease or the Later-Onset Subtype with the IVS4+919G>A Mutation. BMC Neurol. 2017, 17, 25. [Google Scholar] [CrossRef]
- Arends, M.; Wanner, C.; Hughes, D.; Mehta, A.; Oder, D.; Watkinson, O.T.; Elliott, P.M.; Linthorst, G.E.; Wijburg, F.A.; Biegstraaten, M.; et al. Characterization of Classical and Nonclassical Fabry Disease: A Multicenter Study. J. Am. Soc. Nephrol. 2017, 28, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Wu, L.; Nalleballe, K.; Sharma, R.; Brown, A.; Ranabothu, S.; Kapoor, N.; Onteddu, S. Fabry’s Disease and Stroke: Effectiveness of Enzyme Replacement Therapy (ERT) in Stroke Prevention, a Review with Meta-Analysis. J. Clin. Neurosci. 2019, 65, 83–86. [Google Scholar] [CrossRef]
- Ginsberg, L. Nervous System Manifestations of Fabry Disease: Data from FOS—The Fabry Outcome Survey. In Fabry Disease: Perspectives from 5 Years of FOS; Mehta, A., Beck, M., Sunder-Plassmann, G., Eds.; Oxford PharmaGenesis: Oxford, UK, 2006; ISBN 978-1-903539-03-3. [Google Scholar]
- Ginsberg, L.; Manara, R.; Valentine, A.R.; Kendall, B.; Burlina, A.P. Magnetic Resonance Imaging Changes in Fabry Disease. Acta Paediatr. Suppl. 2006, 95, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.; de la Sierra, A.; Paré, J.C.; Gómez-Angelats, E.; Coca, A. Correlation between Silent Cerebral White Matter Lesions and Left Ventricular Mass and Geometry in Essential Hypertension. Am. J. Hypertens. 2002, 15, 507–512. [Google Scholar] [CrossRef]
- Selvetella, G.; Notte, A.; Maffei, A.; Calistri, V.; Scamardella, V.; Frati, G.; Trimarco, B.; Colonnese, C.; Lembo, G. Left Ventricular Hypertrophy Is Associated with Asymptomatic Cerebral Damage in Hypertensive Patients. Stroke 2003, 34, 1766–1770. [Google Scholar] [CrossRef]
- Esposito, R.; Russo, C.; Santoro, C.; Cocozza, S.; Riccio, E.; Sorrentino, R.; Pontillo, G.; Luciano, F.; Imbriaco, M.; Brunetti, A.; et al. Association between Left Atrial Deformation and Brain Involvement in Patients with Anderson-Fabry Disease at Diagnosis. J. Clin. Med. 2020, 9, 2741. [Google Scholar] [CrossRef]
- Steinicke, R.; Gaertner, B.; Grittner, U.; Schmidt, W.; Dichgans, M.; Heuschmann, P.U.; Tanislav, C.; Putaala, J.; Kaps, M.; Endres, M.; et al. Kidney Function and White Matter Disease in Young Stroke Patients: Analysis of the Stroke in Young Fabry Patients Study Population. Stroke 2012, 43, 2382–2388. [Google Scholar] [CrossRef]
- Wang, N.; Liang, C.; Zhang, X.; Sui, C.; Gao, Y.; Guo, L.; Wen, H. Brain Structure-Function Coupling Associated with Cognitive Impairment in Cerebral Small Vessel Disease. Front. Neurosci. 2023, 17, 1163274. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J. Neuropsychiatric and Psychosocial Aspects of Fabry Disease. In Fabry Disease: Perspectives from 5 Years of FOS; Mehta, A., Beck, M., Sunder-Plassmann, G., Eds.; Oxford PharmaGenesis: Oxford, UK, 2006; ISBN 978-1-903539-03-3. [Google Scholar]
- Chertcoff, A.; Cejas, L.L.; Marchesoni, C.; Reisin, R. Depression: The Hidden Problem in Fabry Disease. A Review. J. Inborn Errors Metab. Screen. 2021, 9, e20210015. [Google Scholar] [CrossRef]
- Pinto, L.L.C.; Vieira, T.A.; Giugliani, R.; Schwartz, I.V.D. Expression of the Disease on Female Carriers of X-Linked Lysosomal Disorders: A Brief Review. Orphanet J. Rare Dis. 2010, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as Biomarkers in Neurological Disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Baka, P.; Steenken, L.; Escolano-Lozano, F.; Steffen, F.; Papagianni, A.; Sommer, C.; Pogatzki-Zahn, E.; Hirsch, S.; Protopapa, M.; Bittner, S.; et al. Studying Serum Neurofilament Light Chain Levels as a Potential New Biomarker for Small Fiber Neuropathy. Eur. J. Neurol. 2024, 31, e16192. [Google Scholar] [CrossRef]
- Gaetani, L.; Salvadori, N.; Lisetti, V.; Eusebi, P.; Mancini, A.; Gentili, L.; Borrelli, A.; Portaccio, E.; Sarchielli, P.; Blennow, K.; et al. Cerebrospinal Fluid Neurofilament Light Chain Tracks Cognitive Impairment in Multiple Sclerosis. J. Neurol. 2019, 266, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Kalatha, T.; Arnaoutoglou, M.; Koukoulidis, T.; Hatzifilippou, E.; Bouras, E.; Baloyannis, S.; Koutsouraki, E. Does Cognitive Dysfunction Correlate with Neurofilament Light Polypeptide Levels in the CSF of Patients with Multiple Sclerosis? J. Int. Med. Res. 2019, 47, 2187–2198. [Google Scholar] [CrossRef]
- Kuhle, J.; Gaiottino, J.; Leppert, D.; Petzold, A.; Bestwick, J.P.; Malaspina, A.; Lu, C.-H.; Dobson, R.; Disanto, G.; Norgren, N.; et al. Serum Neurofilament Light Chain Is a Biomarker of Human Spinal Cord Injury Severity and Outcome. J. Neurol. Neurosurg. Psychiatry 2015, 86, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hołub, T.; Kędzierska, K.; Muras-Szwedziak, K.; Nowicki, M. Serum Neurofilament Light Chain Is Not a Useful Biomarker of Central Nervous System Involvement in Women with Fabry Disease. Intractable Rare Dis. Res. 2021, 10, 276–282. [Google Scholar] [CrossRef] [PubMed]

| n (%) | Mean | SD | Median | IQR | |
|---|---|---|---|---|---|
| Males | 17 (42.5%) | ||||
| AE, y | 45.38 | 16.93 | 44.17 | 25.00 | |
| AAO, y | 26.90 | 17.53 | 24.00 | 25.00 | |
| AAD, y | 35.35 | 17.83 | 35.50 | 27.00 | |
| DD, y | 19.41 | 17.30 | 12.50 | 25.80 | |
| Diagnostic delay, y | 10.53 | 12.91 | 5.00 | 18.00 | |
| Phenotype | classic 24 (60.00%) | ||||
| late-onset 16 (40%) | |||||
| GLA variant | missense 31 (77.50%) | ||||
| truncating 9 (22.50%) | |||||
| Treatment (n = 32) | ERT 25 (78.10%) | ||||
| chaperone 6 (18.80%) | |||||
| Treatment duration, y (n = 32) | 8.50 | 5.53 | 8.00 | 5.00 | |
| MSSI | 17.80 | 14.30 | 14.50 | 14.00 | |
| general | 4.08 | 3.17 | 3.00 | 4.00 | |
| renal | 3.55 | 5.44 | 1.00 | 4.00 | |
| cardiological | 5.48 | 5.79 | 3.00 | 9.00 | |
| neurological | 4.38 | 3.49 | 3.50 | 6.00 | |
| Lyso-Gb3 (before treatment, ng/mL (n = 34) | 11.27 | 14.58 | 5.35 | 8.38 | |
| Lyso-Gb3 over the upper limit (before treatment) n = 34 | 31 (91.20%) | ||||
| Lyso-Gb3 (after treatment, ng/mL (n = 34) | 8.90 | 12.08 | 4.10 | 6.90 | |
| Lyso-Gb3 over the upper Limit (after treatment) n = 34 | 27 (79.40%) | ||||
| Alpha-Gal A activity, % (n = 39) | 28.86 | 21.68 | 20.00 | 41.56 | |
| Renal features | |||||
| Renal dysfunction | 20 (50.00%) | ||||
| Creatinine, mg/dL | 1.01 | 0.47 | 0.83 | 0.53 | |
| Cystatin C, mg/dL | 0.99 | 0.52 | 0.86 | 0.37 | |
| BUN, mg/dL | 19.45 | 10.91 | 15.50 | 12.00 | |
| eGFR, ml/min | 89.12 | 33.83 | 90.40 | 54.60 | |
| 24 h proteinuria, mg (n = 26) | 63.48 | 126.33 | 0.12 | 74.05 | |
| 24 h albuminuria, mg (n = 37) | 130.64 | 290.63 | 27.00 | 83.50 | |
| Microalbuminuria (n = 36) | 17 (47.20%) | ||||
| Major renal events | 4 (10.00%) | ||||
| Cardiovascular features | |||||
| Loop-recorder implantation | 3 (7.50%) | ||||
| PMK/ICD | 8 (20.00%) | ||||
| Major cardiovascular events | 9 (22.50%) | ||||
| sAH | 22 (55.00%) | ||||
| Dyslipidemia | 12 (30.00%) | ||||
| Statin treatment | 11 (27.50%) | ||||
| Antithrombotic drug treatment | 9 (22.50%) | ||||
| Smoking habit | 16 (40.00%) | ||||
| Diabetes mellitus | 2 (5.00%) | ||||
| Lower limb edema | 4 (10.00%) | ||||
| IVS thickness, mm | 13.29 | 5.57 | 12.00 | 6.00 | |
| LV end-diastolic diameter, mm | 45.50 | 5.20 | 46.00 | 7.90 | |
| LV end-systolic diameter, mm | 27.06 | 4.60 | 26.70 | 5.90 | |
| LV posterior wall thickness, mm | 11.52 | 3.55 | 10.50 | 5.00 | |
| LVMi g/m2 | 123.50 | 60.86 | 102.50 | 62.00 | |
| LV hypertrophy | 18 (45.00%) | ||||
| RWT (n = 35) | 0.45 | 0.16 | 0.41 | 0.31 | |
| Neurological features | |||||
| Previous stroke | 4 (10%) | ||||
| 2 (50.00%) lacunar stroke | |||||
| 2 (50.00%) large-vessel stroke | |||||
| Brain MRI pulvinar hyperintensity (n = 32) | 1 (3.13%) | ||||
| Brain MRI basilar dolichoectasia (n = 32) | 3 (2.88%) | ||||
| Fazekas score (n = 32) | |||||
| 0 | 19 (59.38%) | ||||
| 1 | 7 (21.88%) | ||||
| 2 | 3 (9.38%) | ||||
| 3 | 3 (9.38%) | ||||
| Serum NfL (pg/mL) (n = 22) | 45.59 | 129.36 | 11.50 | 17.65 | |
| PNP | 1 (2.5%) | ||||
| Depression | 12 (30.00%) | ||||
| Antidepressive treatment | 3 (7.50%) | ||||
| Headache | 15 (37.50%) | ||||
| VAS score | 1.68 | 1.70 | 1.00 | 3.00 | |
| Tinnitus | 11 (27.50%) | ||||
| Vertigo | 9 (22.50%) | ||||
| Hearing loss | 13 (32.50%) | ||||
| Acroparesthesias | 29 (72.50%) | ||||
| Dyshidrosis | 23 (57.50%) | ||||
| Other | |||||
| Recurrent fever | 13 (32.50%) | ||||
| Angiokeratomas | 13 (32.50%) | ||||
| Corneal abnormalities | 12 (30.00%) | ||||
| Gastrointestinal manifestations | 21 (52.50%) | ||||
| Mean | SD | Median | Min. | Max. | |
|---|---|---|---|---|---|
| MMSE | 28.79 | 1.64 | 29.00 | 22.00 | 30.00 |
| RAVLT immediate recall (adj) | 40.67 (36.77) | 12.75 (8.90) | 42.00 (35.02) | 10.00 (19.14) | 65.00 (57.80) |
| RAVLT delayed recall (adj) | 8.87 (7.74) | 3.17 (2.17) | 10.00 (7.81) | 0 (0) | 14.00 (11.80) |
| RAVLT forced-choice recognition (adj) | 0.91 | 0.11 | 0.95 | 0.53 | 1.00 |
| Rey’s complex figure recall (adj) | 17.53 (13.95) | 7.03 (6.07) | 17.50 (15.71) | 2.50 (0.34) | 29.00 (25.40) |
| Digit span forward (adj) | 5.97 (5.74) | 1.20 (1.11) | 6.00 (5.63) | 4 (3.66) | 8 (7.73) |
| Digit span backward (adj) | 4.08 (3.77) | 1.04 (0.96) | 4.00 (3.62) | 2 (1.53) | 6 (5.81) |
| Spatial span forward (adj) | 5.00 (4.74) | 0.89 (0.82) | 5.00 (4.68) | 4 (3.35) | 7 (6.46) |
| Spatial span backward (adj) | 4.46 (4.21) | 0.79 (0.73) | 4.00 (3.94) | 3 (3.26) | 7 (6.29) |
| Raven’s colored progressive matrices (adj) | 29.69 (27.35) | 30.00 (27.31) | 4.92 (3.89) | 16 (18.37) | 35 (34.35) |
| Rey’s complex figure copy (adj) | 31.33 (30.43) | 4.67 (3.73) | 33.00 (31.40) | 18.00 (19.22) | 36.00 (37.24) |
| MFTC accuracy adj. | 0.91 | 0.15 | 0.96 | 0.09 | 1.00 |
| MFTC false (adj) | 0.10 (0.53) | 0.31 (0.48) | 0.00 (0.42) | 0 (0) | 1 (2.85) |
| MFTC time (adj) | 40.45 (40.43) | 12.27 (18.20) | 37.00 (40.78) | 24.00 (0.82) | 72.00 (77.34) |
| Phonological verbal fluency (adj) | 36.36 (32.39) | 14.32 (13.17) | 38.00 (31.38) | 11.00 (3.70) | 71.00 (71.07) |
| Categorial verbal fluency—total (adj) | 18.72 (14.58) | 5.82 (5.12) | 20.00 (14.13) | 7.00 (4.77) | 28.00 (23.59) |
| Stroop test time (adj) | 19.96 (23.55) | 11.50 (10.32) | 18.00 (21.74) | 6.50 (3.29) | 58.00 (60.57) |
| Stroop test errors (adj) | 0.15 (0.94) | 0.49 (0.93) | 0 (0.81) | 0 (0) | 2 (3.25) |
| MCST—category | 5.47 | 1.18 | 6.00 | 1 | 6 |
| MCST—perseverative errors (adj) | 2.29 (2.56) | 3.72 (3.43) | 1.00 (1.29) | 0 (0) | 16.00 (15.19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Natale, D.; Rossi, S.; Dalla Zanna, G.; Funcis, A.; Nicoletti, T.F.; Sicignano, L.L.; Verrecchia, E.; Romano, A.; Vita, M.G.; Caraglia, N.; et al. Prevalence and Clinical Correlates of Cerebrovascular Alterations in Fabry Disease: A Cross-Sectional Study. Brain Sci. 2025, 15, 166. https://doi.org/10.3390/brainsci15020166
Di Natale D, Rossi S, Dalla Zanna G, Funcis A, Nicoletti TF, Sicignano LL, Verrecchia E, Romano A, Vita MG, Caraglia N, et al. Prevalence and Clinical Correlates of Cerebrovascular Alterations in Fabry Disease: A Cross-Sectional Study. Brain Sciences. 2025; 15(2):166. https://doi.org/10.3390/brainsci15020166
Chicago/Turabian StyleDi Natale, Daniele, Salvatore Rossi, Gianmarco Dalla Zanna, Antonio Funcis, Tommaso Filippo Nicoletti, Ludovico Luca Sicignano, Elena Verrecchia, Angela Romano, Maria Gabriella Vita, Naike Caraglia, and et al. 2025. "Prevalence and Clinical Correlates of Cerebrovascular Alterations in Fabry Disease: A Cross-Sectional Study" Brain Sciences 15, no. 2: 166. https://doi.org/10.3390/brainsci15020166
APA StyleDi Natale, D., Rossi, S., Dalla Zanna, G., Funcis, A., Nicoletti, T. F., Sicignano, L. L., Verrecchia, E., Romano, A., Vita, M. G., Caraglia, N., Graziani, F., Re, F., Guerrera, G., Battistini, L., & Silvestri, G. (2025). Prevalence and Clinical Correlates of Cerebrovascular Alterations in Fabry Disease: A Cross-Sectional Study. Brain Sciences, 15(2), 166. https://doi.org/10.3390/brainsci15020166








