Emergent Aspects of the Integration of Sensory and Motor Functions
Abstract
:1. Introduction
2. Sensory Integration
2.1. Superior Colliculus Functioning
2.2. Thalamus Functioning
2.3. Synchronization Drives Integration
3. Sensorimotor Integration
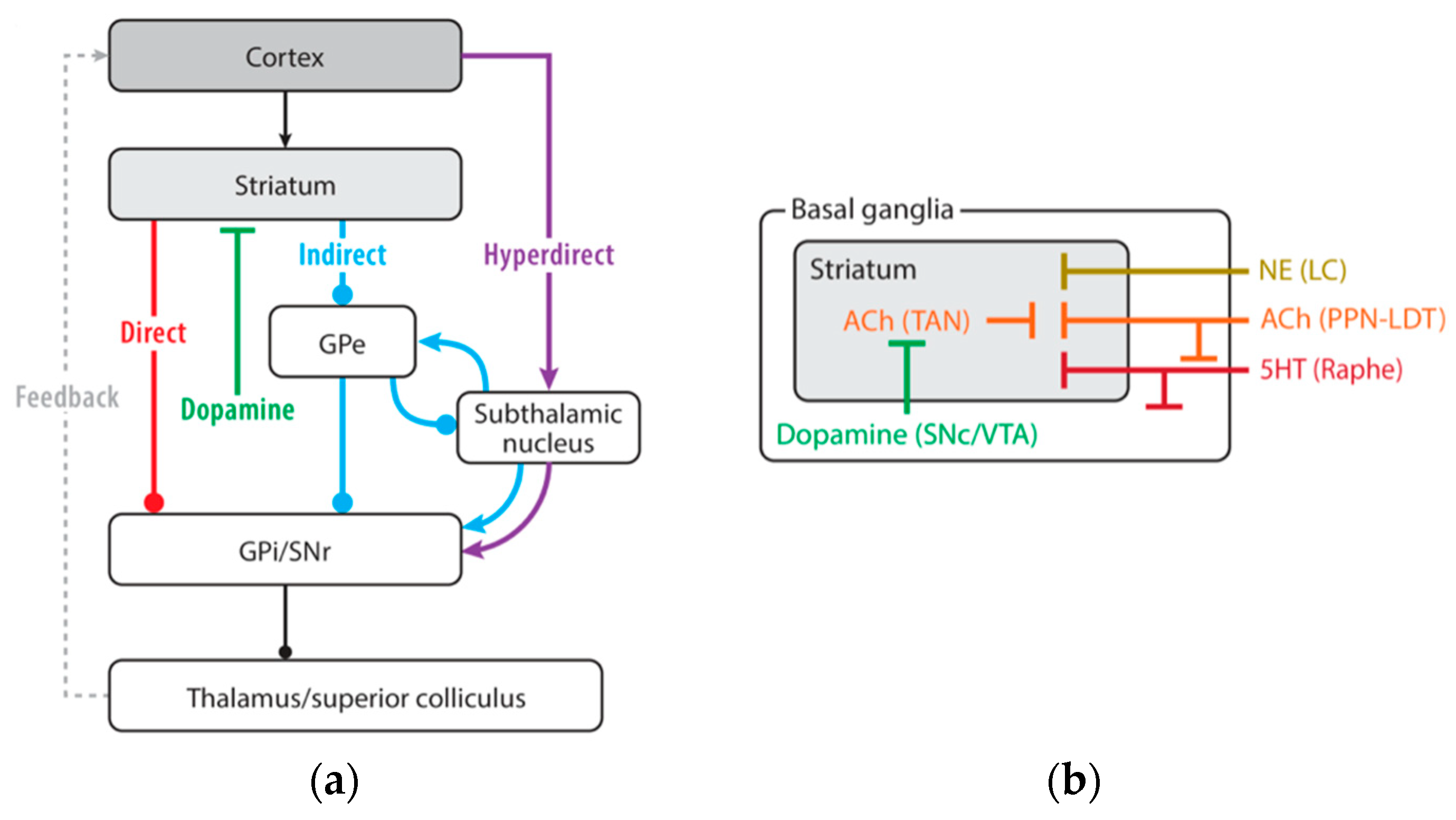
4. Basal Ganglia and Sensorimotor Integration
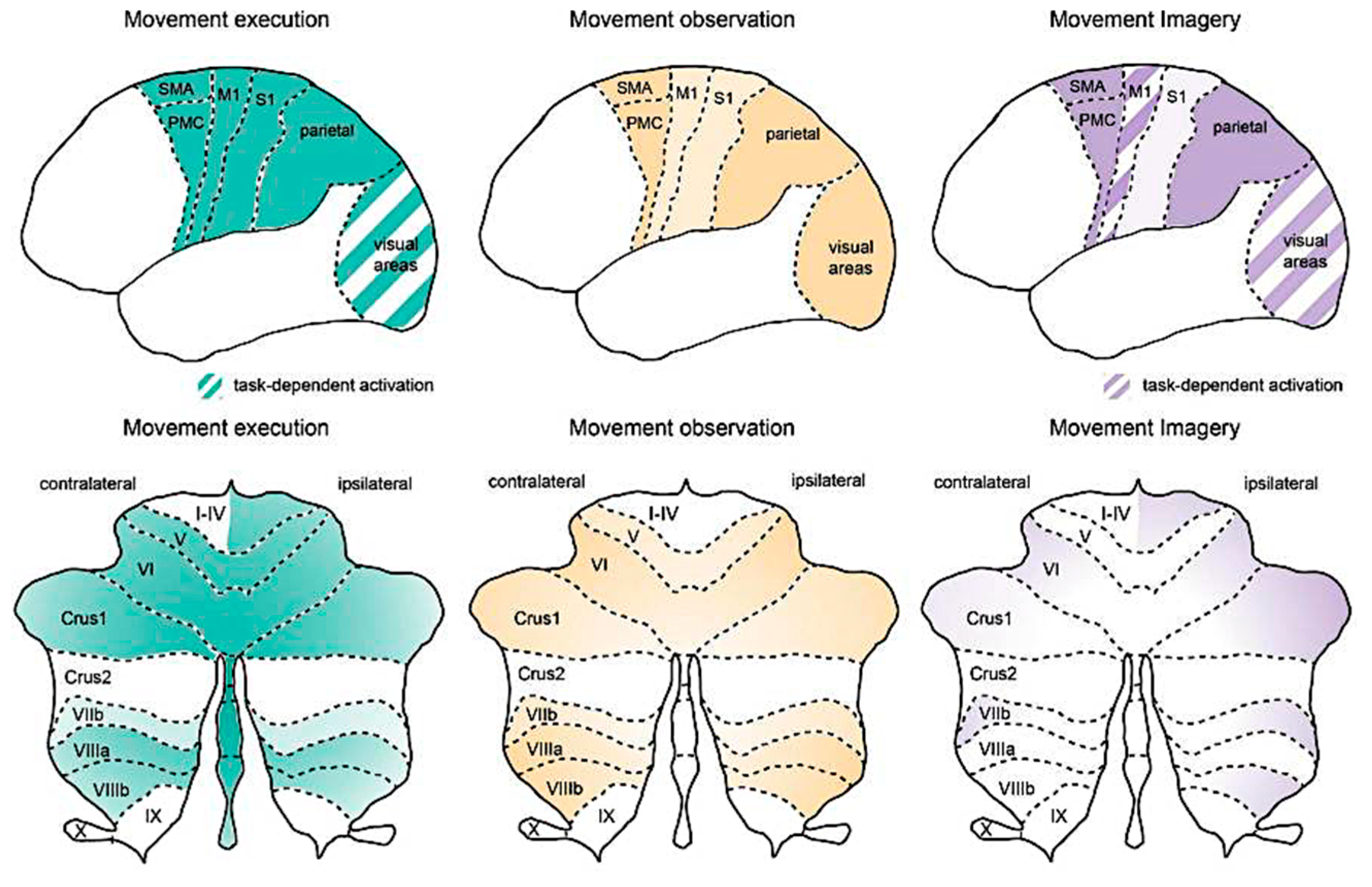
5. Cerebellum and Sensorimotor Integration
6. Cortical Areas and Sensorimotor Integration
7. Mirror System and Sensorimotor Integration
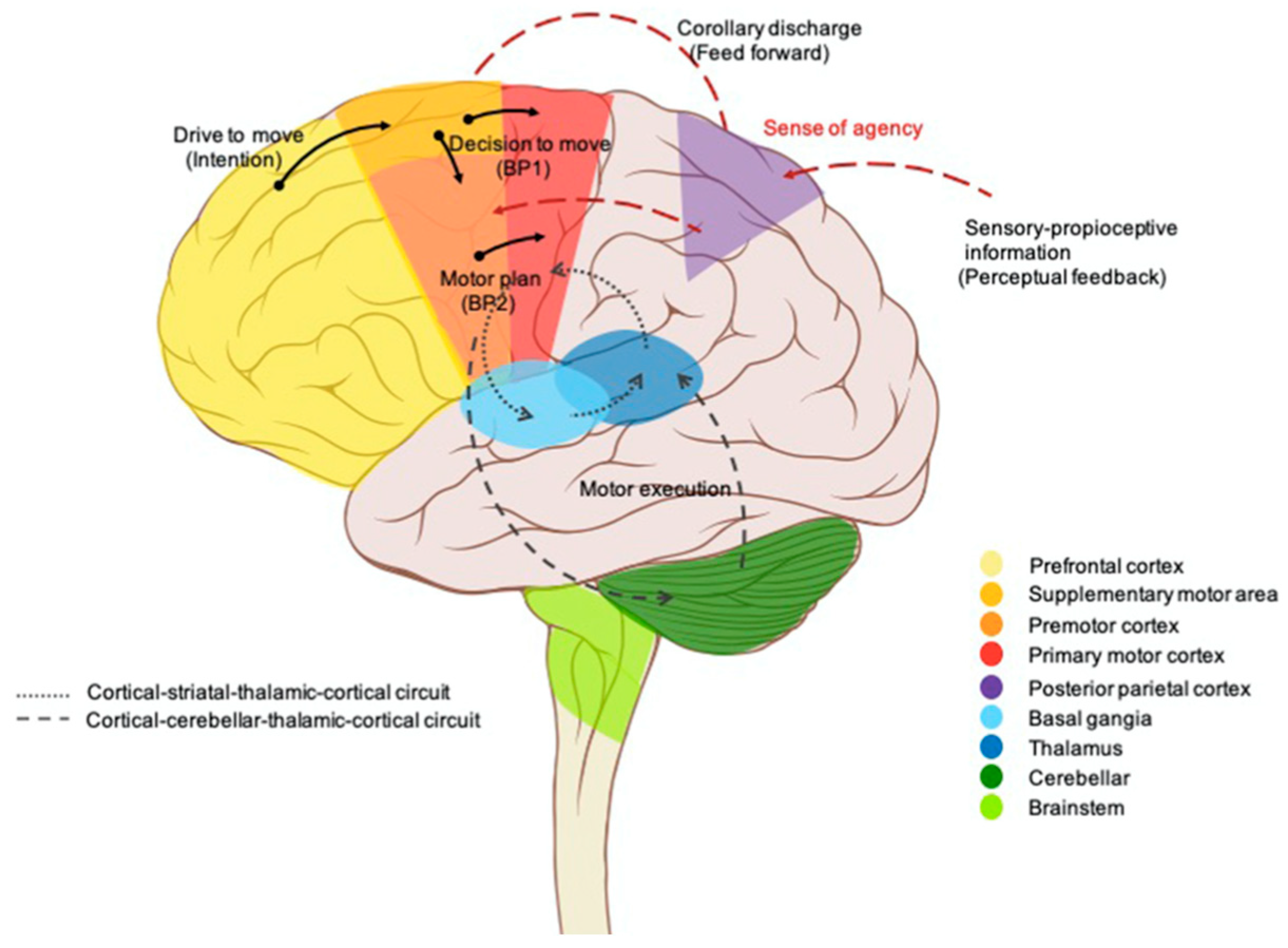
8. Inferences from Sensorimotor Integration
9. Mental Imagery and Sensorimotor Integration
10. Implications
11. Conclusions
Funding
Conflicts of Interest
References
- Stein, B.E.; Stanford, T.R.; Rowland, B.A. Multisensory integration and the society for neuroscience: Then and now. J. Neurosci. 2020, 40, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Angelaki, D.E.; Gu, Y.; DeAngelis, G.C. Multisensory integration: Psychophysics, neurophysiology, and computation. Curr. Opin. Neurobiol. 2009, 19, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Noel, J.P.; Blanke, O.; Serino, A. From multisensory integration in peripersonal space to bodily self-consciousness: From statistical regularities to statistical inference. Ann. N. Y. Acad. Sci. 2018, 1426, 146–165. [Google Scholar] [CrossRef]
- Stein, B.E.; Stanford, T.R. Multisensory integration: Current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 2008, 9, 255–266. [Google Scholar] [CrossRef]
- Turecek, J.; Ginty, D. How two intermingled sensory pathways combine to encode touch. Nature, 2022; online ahead of print. [Google Scholar]
- Kadunce, D.C.; Vaughan, W.J.; Wallace, M.T.; Stein, B.E. The influence of visual and auditory receptive field organization on multisensory integration in the superior colliculus. Exp. Brain Res. 2001, 139, 303–310. [Google Scholar] [CrossRef]
- Huey, E.L.; Turecek, J.; Delisle, M.M.; Mazor, O.; Romero, G.E.; Dua, M.; Sarafis, Z.K.; Hobble, A.; Booth, K.T.; Goodrich, L.V.; et al. The auditory midbrain mediates tactile vibration sensing. Cell 2025, 188, 104–120.e18. [Google Scholar] [CrossRef]
- Saal, H.P.; Bensmaia, S.J. Touch is a team effort: Interplay of submodalities in cutaneous sensibility. Trends Neurosci. 2014, 37, 689–697. [Google Scholar] [CrossRef]
- Isa, T.; Marquez-Legorreta, E.; Grillner, S.; Scott, E.K. The tectum/superior colliculus as the vertebrate solution for spatial sensory integration and action. Curr. Biol. 2021, 31, R741–R762. [Google Scholar] [CrossRef]
- Butler, B.E.; Chabot, N.; Lomber, S.G. A quantitative comparison of the hemispheric, areal, and laminar origins of sensory and motor cortical projections to the superior colliculus of the cat. J. Comp. Neurol. 2016, 524, 2623–2642. [Google Scholar] [CrossRef]
- Basso, M.A.; Bickford, M.E.; Cang, J. Unraveling circuits of visual perception and cognition through the superior colliculus. Neuron 2021, 109, 918. [Google Scholar] [CrossRef]
- May, P.J. The mammalian superior colliculus: Laminar structure and connections. Prog. Brain Res. 2006, 151, 321–378. [Google Scholar] [PubMed]
- Liu, X.; Huang, H.; Snutch, T.P.; Cao, P.; Wang, L.; Wang, F. The Superior Colliculus: Cell Types, Connectivity, and Behavior. Neurosci. Bull. 2022, 38, 1519–1540. [Google Scholar] [CrossRef]
- Stuphorn, V.; Bauswein, E.; Hoffmann, K.P. Neurons in the primate superior colliculus coding for arm movements in gaze-related coordinates. J. Neurophysiol. 2000, 83, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Klier, E.M.; Wang, H.; Crawford, J.D. The superior colliculus encodes gaze commands in retinal coordinates. Nat. Neurosci. 2001, 4, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Melleu, F.F.; Canteras, N.S. Neural Circuits of Fear and Anxiety: Insights from a Neuroethological Perspective. Physiology, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Benavidez, N.L.; Bienkowski, M.S.; Zhu, M.; Garcia, L.H.; Fayzullina, M.; Gao, L.; Bowman, I.; Gou, L.; Khanjani, N.; Cotter, K.R.; et al. Organization of the inputs and outputs of the mouse superior colliculus. Nat. Commun. 2021, 12, 4004. [Google Scholar] [CrossRef] [PubMed]
- Masullo, L.; Mariotti, L.; Alexandre, N.; Freire-Pritchett, P.; Boulanger, J.; Tripodi, M. Genetically Defined Functional Modules for Spatial Orienting in the Mouse Superior Colliculus. Curr. Biol. 2019, 29, 2892–2904.e8. [Google Scholar] [CrossRef]
- Peysakhovich, B.; Zhu, O.; Tetrick, S.M.; Shirhatti, V.; Silva, A.A.; Li, S.; Ibos, G.; Rosen, M.C.; Johnston, W.J.; Freedman, D.J. Primate superior colliculus is causally engaged in abstract higher-order cognition. Nat. Neurosci. 2024, 27, 1999–2008. [Google Scholar] [CrossRef]
- Alex Meredith, M.; Stein, B.E. Interactions Among Converging Sensory Inputs in the Superior Colliculus. Science 1983, 221, 389–391. [Google Scholar]
- Rowland, B.A.; Quessy, S.; Stanford, T.R.; Stein, B.E. Multisensory Integration Shortens Physiological Response Latencies. J. Neurosci. 2007, 27, 5879–5884. [Google Scholar] [CrossRef]
- Laurienti, P.J.; Perrault, T.J.; Stanford, T.R.; Wallace, M.T.; Stein, B.E. On the use of superadditivity as a metric for characterizing multisensory integration in functional neuroimaging studies. Exp. Brain Res. 2005, 166, 289–297. [Google Scholar]
- Werner, S.; Noppeney, U. Superadditive Responses in Superior Temporal Sulcus Predict Audiovisual Benefits in Object Categorization. Cereb. Cortex 2010, 20, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.M. Thalamic relays and cortical functioning. Prog. Brain Res. 2005, 149, 107–126. [Google Scholar] [PubMed]
- Hwang, K.; Bertolero, M.A.; Liu, W.B.; D’Esposito, M. The Human Thalamus Is an Integrative Hub for Functional Brain Networks. J. Neurosci. 2017, 37, 5594–5607. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Bagarinao, E.; Watanabe, H.; Naganawa, S.; Ozaki, N.; Sobue, G. Bridging large-scale cortical networks: Integrative and function-specific hubs in the thalamus. iScience 2021, 24, 103106. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Lewis, L.D.; Garrett, D.D.; Hwang, K. The impact of the human thalamus on brain-wide information processing. Nat. Rev. Neurosci. 2023, 24, 416. [Google Scholar] [CrossRef]
- Halassa, M.M.; Chen, Z.; Wimmer, R.D.; Brunetti, P.M.; Zhao, S.; Zikopoulos, B.; Wang, F.; Brown, E.N.; Wilson, M.A. State-dependent architecture of thalamic reticular subnetworks. Cell 2014, 158, 808–821. [Google Scholar] [CrossRef]
- Lam, Y.W.; Sherman, S.M. Behavioral/Systems/Cognitive Functional Organization of the Thalamic Input to the Thalamic Reticular Nucleus. J. Neurosci. 2011, 31, 6791–6799. [Google Scholar] [CrossRef]
- Min, B.K. A thalamic reticular networking model of consciousness. Theor. Biol. Med. Model. 2010, 7, 10. [Google Scholar] [CrossRef]
- Ueta, Y.; Miyata, M. Functional and structural synaptic remodeling mechanisms underlying somatotopic organization and reorganization in the thalamus. Neurosci. Biobehav. Rev. 2023, 152, 105332. [Google Scholar] [CrossRef]
- Wolff, M.; Morceau, S.; Folkard, R.; Martin-Cortecero, J.; Groh, A. A thalamic bridge from sensory perception to cognition. Neurosci. Biobehav. Rev. 2021, 120, 222–235. [Google Scholar] [CrossRef]
- Smith, J.B.; Smith, Y.; Venance, L.; Watson, G.D.R. Editorial: Thalamic Interactions With the Basal Ganglia: Thalamostriatal System and Beyond. Front. Syst. Neurosci. 2022, 16, 883094. [Google Scholar] [CrossRef] [PubMed]
- Sillito, A.M.; Jones, H.E. Corticothalamic interactions in the transfer of visual information. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1739–1752. [Google Scholar] [CrossRef] [PubMed]
- Born, G.; Schneider-Soupiadis, F.A.; Erisken, S.; Vaiceliunaite, A.; Lao, C.L.; Mobarhan, M.H.; Spacek, M.A.; Einevoll, G.T.; Busse, L. Corticothalamic feedback sculpts visual spatial integration in mouse thalamus. Nat. Neurosci. 2021, 24, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.G.; Leow, Y.N.; Le, N.M.; Adam, E.; Huda, R.; Sur, M. Cortical-subcortical interactions in goal-directed behavior. Physiol. Rev. 2023, 103, 347–389. [Google Scholar] [CrossRef] [PubMed]
- Crandall, S.R.; Cruikshank, S.J.; Connors, B.W. A Corticothalamic Switch: Controlling the Thalamus with Dynamic Synapses. Neuron 2015, 86, 768–782. [Google Scholar] [CrossRef]
- Bauer, A.K.R.; Debener, S.; Nobre, A.C. Synchronisation of Neural Oscillations and Cross-modal Influences. Trends Cogn. Sci. 2020, 24, 481–495. [Google Scholar] [CrossRef]
- Singer, W. Neuronal oscillations: Unavoidable and useful? Eur. J. Neurosci. 2018, 48, 2389–2398. [Google Scholar] [CrossRef]
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Thut, G.; Miniussi, C.; Gross, J. The functional importance of rhythmic activity in the brain. Curr. Biol. 2012, 22, R658–R663. [Google Scholar] [CrossRef]
- Neske, G.T. The slow oscillation in cortical and thalamic networks: Mechanisms and functions. Front. Neural Circuits 2016, 9, 88. [Google Scholar] [CrossRef]
- Keil, J.; Senkowski, D. Neural Oscillations Orchestrate Multisensory Processing. Neuroscientist 2018, 24, 609–626. [Google Scholar] [CrossRef]
- Parker, M.; Spennemann, D.H.R.; Bond, J. Sensory and multisensory perception—Perspectives toward defining multisensory experience and heritage. J. Sens. Stud. 2024, 39, e12940. [Google Scholar] [CrossRef]
- Michail, G.; Senkowski, D.; Holtkamp, M.; Wächter, B.; Keil, J. Early beta oscillations in multisensory association areas underlie crossmodal performance enhancement. NeuroImage 2022, 257, 119307. [Google Scholar] [CrossRef] [PubMed]
- Newell, F.N.; McKenna, E.; Seveso, M.A.; Devine, I.; Alahmad, F.; Hirst, R.J.; O’Dowd, A. Multisensory perception constrains the formation of object categories: A review of evidence from sensory-driven and predictive processes on categorical decisions. Philos. Trans. R. Soc. B 2023, 378, 20220342. [Google Scholar] [CrossRef]
- González-Rueda, A.; Jensen, K.; Noormandipour, M.; de Malmazet, D.; Wilson, J.; Ciabatti, E.; Kim, J.; Williams, E.; Poort, J.; Hennequin, G.; et al. Kinetic features dictate sensorimotor alignment in the superior colliculus. Nature 2024, 631, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.G.E.; McDougle, S.D. Context is key for learning motor skills. Nature 2021, 600, 387–388. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Sinigaglia, C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 2010, 11, 264–274. [Google Scholar] [CrossRef]
- Luppino, G.; Murata, A.; Govoni, P.; Matelli, M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp. Brain Res. 1999, 128, 181–187. [Google Scholar] [CrossRef]
- Iacoboni, M.; Dapretto, M. The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci. 2006, 7, 942–951. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Luppino, G. The cortical motor system. Neuron 2001, 31, 889–901. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Cattaneo, L.; Fabbri-Destro, M.; Rozzi, S. Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol. Rev. 2014, 94, 655–706. [Google Scholar] [CrossRef]
- Steriade, M. Grouping of brain rhythms in corticothalamic systems. Neuroscience 2006, 137, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.R. When brain rhythms aren’t ‘rhythmic’: Implication for their mechanisms and meaning. Curr. Opin. Neurobiol. 2016, 40, 72–80. [Google Scholar] [CrossRef]
- Foxe, J.J.; Snyder, A.C. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2011, 2, 10747. [Google Scholar] [CrossRef]
- Romei, V.; Driver, J.; Schyns, P.G.; Thut, G. Report Rhythmic TMS over Parietal Cortex Links Distinct Brain Frequencies to Global versus Local Visual Processing. Curr. Biol. 2011, 21, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Ruiz, M.H.; Kilavik, B.E.; Lundqvist, M.; Starr, P.A.; Aron, A.R. Beta oscillations in working memory, executive control of movement and thought, and sensorimotor function. J. Neurosci. 2019, 39, 8231–8238. [Google Scholar] [CrossRef] [PubMed]
- Blenkinsop, A.; Anderson, S.; Gurney, K. Frequency and function in the basal ganglia: The origins of beta and gamma band activity. J. Physiol. 2017, 595, 4525–4548. [Google Scholar] [CrossRef]
- Barone, J.; Rossiter, H.E. Understanding the Role of Sensorimotor Beta Oscillations. Front. Syst. Neurosci. 2021, 15, 655886. [Google Scholar] [CrossRef]
- Tatti, E.; Cacciola, A.; Carrara, F.; Luciani, A.; Quartarone, A.; Ghilardi, M.F. Movement-related ERS and connectivity in the gamma frequency decrease with practice. NeuroImage 2023, 284, 120444. [Google Scholar] [CrossRef]
- Villalobos, N.; Almazán-Alvarado, S.; Magdaleno-Madrigal, V.M. Elevation of GABA levels in the globus pallidus disinhibits the thalamic reticular nucleus and desynchronized cortical beta oscillations. J. Physiol. Sci. 2022, 72, 17. [Google Scholar] [CrossRef]
- Jenkinson, N.; Brown, P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011, 34, 611–618. [Google Scholar] [CrossRef]
- Brittain, J.S.; Brown, P. Oscillations and the basal ganglia: Motor control and beyond. NeuroImage 2014, 85, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Dostrovsky, J.O.; Hodaie, M.; Lozano, A.M.; Hutchison, W.D. Spatial extent of beta oscillatory activity in and between the subthalamic nucleus and substantia nigra pars reticulata of Parkinson’s disease patients. Exp. Neurol. 2013, 245, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.H.; Haumesser, J.K.; Kühn, J.; Altschüler, J.; Kühn, A.A.; van Riesen, C. Short- and long-term dopamine depletion causes enhanced beta oscillations in the cortico-basal ganglia loop of parkinsonian rats. Exp. Neurol. 2016, 286, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Iskhakova, L.; Rappel, P.; Deffains, M.; Fonar, G.; Marmor, O.; Paz, R.; Israel, Z.; Eitan, R.; Bergman, H. Modulation of dopamine tone induces frequency shifts in cortico-basal ganglia beta oscillations. Nat. Commun. 2021, 12, 7026. [Google Scholar] [CrossRef]
- Merel, J.; Botvinick, M.; Wayne, G. Hierarchical motor control in mammals and machines. Nat. Commun. 2019, 10, 5489. [Google Scholar] [CrossRef]
- Da Cunha, C.; Gomez-A, A.; Blaha, C.D. The role of the basal ganglia in motivated Behaviour. Rev. Neurosci. 2012, 23, 747–767. [Google Scholar] [CrossRef]
- Gigi, I.; Senatore, R.; Marcelli, A. Neurocomputational Modeling of the Basal Ganglia in Motor Learning at Mesoscopic Scale: An Overview. 2021. Available online: https://engrxiv.org/preprint/view/1902 (accessed on 3 February 2025).
- Blandini, F.; Nappi, G.; Tassorelli, C.; Martignoni, E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog. Neurobiol. 2000, 62, 63–88. [Google Scholar] [CrossRef]
- Grahn, J.A.; Parkinson, J.A.; Owen, A.M. The role of the basal ganglia in learning and memory: Neuropsychological studies. Behav. Brain Res. 2009, 199, 53–60. [Google Scholar] [CrossRef]
- Leisman, G.; Braun-Benjamin, O.; Melillo, R. Cognitive-motor interactions of the basal ganglia in development. Front. Syst. Neurosci. 2014, 8, 72254. [Google Scholar] [CrossRef]
- Hikosaka, O.; Kim, H.F.; Yasuda, M.; Yamamoto, S. Basal ganglia circuits for reward value-guided behavior. Annu. Rev. Neurosci. 2014, 37, 289–306. [Google Scholar] [CrossRef]
- Jin, X.; Costa, R.M. Shaping action sequences in basal ganglia circuits. Curr. Opin. Neurobiol. 2015, 33, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Coddington, L.T.; Dudman, J.T. Basal Ganglia Circuits for Action Specification. Annu. Rev. Neurosci. 2020, 43, 485–507. [Google Scholar] [CrossRef] [PubMed]
- Dhawale, A.K.; Wolff, S.B.E.; Ko, R.; Ölveczky, B.P. The basal ganglia control the detailed kinematics of learned motor skills. Nat. Neurosci. 2021, 24, 1256–1269. [Google Scholar] [CrossRef]
- Alexander, G.E.; Crutcher, M.D. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 1990, 13, 266–271. [Google Scholar] [CrossRef]
- Graybiel, A.M. A stereometric pattern of distribution of acetylthiocholinesterase in the deep layers of the superior colliculus. Nature 1978, 272, 539–541. [Google Scholar] [CrossRef]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef]
- de la Torre-Martinez, R.; Ketzef, M.; Silberberg, G. Ongoing movement controls sensory integration in the dorsolateral striatum. Nat. Commun. 2023, 14, 1004. [Google Scholar] [CrossRef]
- Wall, N.R.; DeLaParra, M.; Callaway, E.M.; Kreitzer, A.C. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 2013, 79, 347–360. [Google Scholar] [CrossRef]
- Nambu, A.; Tokuno, H.; Takada, M. Functional significance of the cortico–subthalamo–pallidal ‘hyperdirect’ pathway. Neurosci. Res. 2002, 43, 111–117. [Google Scholar] [CrossRef]
- Chen, W.; de Hemptinne, C.; Miller, A.M.; Leibbrand, M.; Little, S.J.; Lim, D.A.; Larson, P.S.; Starr, P.A. Prefrontal-Subthalamic Hyperdirect Pathway Modulates Movement Inhibition in Humans. Neuron 2020, 106, 579–588.e3. [Google Scholar] [CrossRef]
- Ding, L. Contributions of the Basal Ganglia to Visual Perceptual Decisions. Annu. Rev. Vis. Sci. 2023, 9, 385–407. [Google Scholar] [CrossRef] [PubMed]
- Condé, H. Organization and physiology of the substantia nigra. Exp. Brain Res. 1992, 88, 233–248. [Google Scholar] [CrossRef]
- Schultz, W. Dopamine reward prediction-error signalling: A two-component response. Nat. Rev. Neurosci. 2016, 17, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Matityahu, L.; Gilin, N.; Sarpong, G.A.; Atamna, Y.; Tiroshi, L.; Tritsch, N.X.; Wickens, J.R.; Goldberg, J.A. Acetylcholine waves and dopamine release in the striatum. Nat. Commun. 2023, 14, 6852. [Google Scholar] [CrossRef] [PubMed]
- Threlfell, S.; Lalic, T.; Platt, N.J.; Jennings, K.A.; Deisseroth, K.; Cragg, S.J. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 2012, 75, 58–64. [Google Scholar] [CrossRef]
- Apicella, P. The role of the intrinsic cholinergic system of the striatum: What have we learned from TAN recordings in behaving animals? Neuroscience 2017, 360, 81–94. [Google Scholar] [CrossRef]
- Moretti, R.; Caruso, P.; Crisman, E.; Gazzin, S. Basal ganglia: Their role in complex cognitive procedures in experimental models and in clinical practice. Neurol. India 2017, 65, 814–825. [Google Scholar]
- O’Rawe, J.F.; Leung, H.C. Topographic organization of the human caudate functional connectivity and age-related changes with resting-state fMRI. Front. Syst. Neurosci. 2022, 16, 966433. [Google Scholar] [CrossRef]
- Menon, V.; D’Esposito, M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 90–103. [Google Scholar] [CrossRef]
- Kosakowski, H.L.; Saadon-Grosman, N.; Du, J.; Eldaief, M.C.; Buckner, R.L. Human striatal association megaclusters. J. Neurophysiol. 2024, 131, 1083–1100. [Google Scholar] [CrossRef]
- Manzoni, D. The cerebellum and sensorimotor coupling: Looking at the problem from the perspective of vestibular reflexes. Cerebellum 2007, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Kazantsev, V.B.; Nekorkin, V.I.; Makarenko, V.I.; Llinás, R. Olivo-cerebellar cluster-based universal control system. Proc. Natl. Acad. Sci. USA 2003, 100, 13064–13068. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.H. Cerebellar learning mechanisms. Brain Res. 2015, 1621, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Cheron, G.; Márquez-Ruiz, J.; Dan, B. Oscillations, Timing, Plasticity, and Learning in the Cerebellum. Cerebellum 2016, 15, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Bastian, A.J. Cerebellar control of balance and locomotion. Neuroscientist 2004, 10, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.R. The olivo-cerebellar system: A key to understanding the functional significance of intrinsic oscillatory brain properties. Front. Neural Circuits 2014, 7, 96. [Google Scholar] [CrossRef]
- Pisotta, I.; Molinari, M. Cerebellar contribution to feedforward control of locomotion. Front. Hum. Neurosci. 2014, 8, 475. [Google Scholar] [CrossRef]
- Leech, K.A.; Roemmich, R.T.; Gordon, J.; Reisman, D.S.; Cherry-Allen, K.M. Updates in Motor Learning: Implications for Physical Therapist Practice and Education. Phys. Ther. 2022, 102, pzab250. [Google Scholar] [CrossRef]
- Streng, M.L.; Popa, L.S.; Ebner, T.J. Cerebellar Representations of Errors and Internal Models. Cerebellum 2022, 21, 814–820. [Google Scholar] [CrossRef]
- Gao, Z.; Davis, C.; Thomas, A.M.; Economo, M.N.; Abrego, A.M.; Svoboda, K.; De Zeeuw, C.I.; Li, N. A cortico-cerebellar loop for motor planning. Nature 2018, 563, 113–116. [Google Scholar] [CrossRef]
- Boven, E.; Cerminara, N.L. Cerebellar contributions across behavioural timescales: A review from the perspective of cerebro-cerebellar interactions. Front. Syst. Neurosci. 2023, 17, 1211530. [Google Scholar] [CrossRef] [PubMed]
- Boven, E.; Pemberton, J.; Chadderton, P.; Apps, R.; Costa, R.P. Cerebro-cerebellar networks facilitate learning through feedback decoupling. Nat. Commun. 2023, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Henschke, J.U.; Pakan, J.M.P. Engaging distributed cortical and cerebellar networks through motor execution, observation, and imagery. Front. Syst. Neurosci. 2023, 17, 1165307. [Google Scholar] [CrossRef] [PubMed]
- Debaere, F.; Wenderoth, N.; Sunaert, S.; Van Hecke, P.; Swinnen, S.P. Internal vs external generation of movements: Differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. NeuroImage 2003, 19, 764–776. [Google Scholar] [CrossRef]
- Virameteekul, S.; Bhidayasiri, R. We Move or Are We Moved? Unpicking the Origins of Voluntary Movements to Better Understand Semivoluntary Movements. Front. Neurol. 2022, 13, 834217. [Google Scholar] [CrossRef]
- Deecke, L. Planning, preparation, execution, and imagery of volitional action. Cogn. Brain Res. 1996, 3, 59–64. [Google Scholar] [CrossRef]
- Halsband, U.; Lange, R.K. Motor learning in man: A review of functional and clinical studies. J. Physiol.-Paris 2006, 99, 414–424. [Google Scholar] [CrossRef]
- Cona, G.; Semenza, C. Supplementary motor area as key structure for domain-general sequence processing: A unified account. Neurosci. Biobehav. Rev. 2017, 72, 28–42. [Google Scholar] [CrossRef]
- Thut, G.; Hauert, C.A.; Viviani, P.; Morand, S.; Spinelli, L.; Blanke, O.; Landis, T.; Michel, C. Internally driven vs. externally cued movement selection: A study on the timing of brain activity. Brain Res. Cogn. Brain Res. 2000, 9, 261–269. [Google Scholar] [CrossRef]
- Doyon, J.; Penhune, V.; Ungerleider, L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 2003, 41, 252–262. [Google Scholar] [CrossRef]
- Doyon, J.; Bellec, P.; Amsel, R.; Penhune, V.; Monchi, O.; Carrier, J.; Lehéricy, S.; Benali, H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009, 199, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Penhune, V.B.; Steele, C.J. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav. Brain Res. 2012, 226, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Klein-Flügge, M.C.; Bongioanni, A.; Rushworth, M.F.S. Medial and orbital frontal cortex in decision-making and flexible behavior. Neuron 2022, 110, 2743–2770. [Google Scholar] [CrossRef] [PubMed]
- Nick, Q.; Gale, D.J.; Areshenkoff, C.; De Brouwer, A.; Nashed, J.; Wammes, J.; Zhu, T.; Flanagan, R.; Smallwood, J.; Gallivan, J. Reconfigurations of cortical manifold structure during reward-based motor learning. eLife 2023, 12, RP91928. [Google Scholar] [CrossRef]
- Raymond, J.L. Research on the cerebellum yields rewards. Nature 2020, 579, 202–203. [Google Scholar] [CrossRef]
- Sendhilnathan, N.; Semework, M.; Goldberg, M.E.; Ipata, A.E. Neural Correlates of Reinforcement Learning in Mid-lateral Cerebellum. Neuron 2020, 106, 188–198. [Google Scholar] [CrossRef]
- Simonsmeier, B.A.; Andronie, M.; Buecker, S.; Frank, C. The effects of imagery interventions in sports: A meta-analysis. Int. Rev. Sport. Exerc. Psychol. 2021, 14, 186–207. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wang, T.; Cui, H. Neural Geometry from Mixed Sensorimotor Selectivity for Predictive Sensorimotor Control. 2024. Available online: https://elifesciences.org/reviewed-preprints/100064v1 (accessed on 17 August 2024).
- Rizzolatti, G.; Fogassi, L.; Gallese, V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001, 2, 661–670. [Google Scholar] [CrossRef]
- Fadiga, L.; Fogassi, L.; Pavesi, G.; Rizzolatti, G. Motor facilitation during action observation: A magnetic stimulation study. J. Neurophysiol. 1995, 73, 2608–2611. [Google Scholar] [CrossRef]
- Mukamel, R.; Ekstrom, A.D.; Kaplan, J.; Iacoboni, M.; Fried, I. Single-Neuron Responses in Humans during Execution and Observation of Actions. Curr. Biol. 2010, 20, 750–756. [Google Scholar] [CrossRef]
- Fabbri-Destro, M.; Rizzolatti, G. Mirror neurons and mirror systems in monkeys and humans. Physiology 2008, 23, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M. Neurobiology of imitation. Curr. Opin. Neurobiol. 2009, 19, 661–665. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Fabbri-Destro, M.; Cattaneo, L. Mirror neurons and their clinical relevance. Nat. Clin. Pract. Neurol. 2009, 5, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Fogassi, L. The mirror neuron system: How cognitive functions emerge from motor organization. J. Econ. Behav. Organ. 2011, 77, 66–75. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Ferrari, P.F.; Rozzi, S.; Fogassi, L. The inferior parietal lobule: Where action becomes perception. In Percept, Decision, Action: Bridging the Gaps; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 129–145. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470034989.ch11 (accessed on 28 December 2024).
- Rao, R.P.N. A sensory–motor theory of the neocortex. Nat. Neurosci. 2024, 27, 1221–1235. [Google Scholar] [CrossRef]
- Morris, R.W.; Dezfouli, A.; Griffiths, K.R.; Le Pelley, M.E.; Balleine, B.W. The Neural Bases of Action-Outcome Learning in Humans. J. Neurosci. 2022, 42, 3636–3647. [Google Scholar] [CrossRef]
- Hernández, L.F.; Redgrave, P.; Obeso, J.A. Habitual behavior and dopamine cell vulnerability in Parkinson disease. Front. Neuroanat. 2015, 9, 99. [Google Scholar] [CrossRef]
- Lohse, K.R.; Wadden, K.; Boyd, L.A.; Hodges, N.J. Motor skill acquisition across short and long time scales: A meta-analysis of neuroimaging data. Neuropsychologia 2014, 59, 130–141. [Google Scholar]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. The basal ganglia communicate with the cerebellum. Proc. Natl. Acad. Sci. USA 2010, 107, 8452–8456. [Google Scholar] [CrossRef]
- Antonioni, A.; Raho, E.M.; Straudi, S.; Granieri, E.; Koch, G.; Fadiga, L. The cerebellum and the Mirror Neuron System: A matter of inhibition? From neurophysiological evidence to neuromodulatory implications. A narrative review. Neurosci. Biobehav. Rev. 2024, 164, 105830. [Google Scholar] [CrossRef]
- Lee, B.C.; Choi, J.; Martin, B.J. Roles of the prefrontal cortex in learning to time the onset of pre-existing motor programs. PLoS ONE 2020, 15, e0241562. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, M. The Roles of the Cortical Motor Areas in Sequential Movements. Front. Behav. Neurosci. 2021, 15, 640659. [Google Scholar] [CrossRef] [PubMed]
- Land, W.M.; Volchenkov, D.; Bläsing, B.E.; Schack, T. From action representation to action execution: Exploring the links between cognitive and biomechanical levels of motor control. Front. Comput. Neurosci. 2013, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Spapé, M.M.; Serrien, D.J.; Ravaja, N. 3-2-1, action! A combined motor control-temporal reproduction task shows intentions, motions, and consequences alter time perception. Heliyon 2023, 9, e19728. [Google Scholar] [CrossRef]
- Keller, G.B.; Sterzer, P. Predictive Processing: A Circuit Approach to Psychosis. Annu. Rev. Neurosci. 2024, 47, 85–101. [Google Scholar] [CrossRef]
- Schmerwitz, C.; Kopp, B. The future of neuropsychology is digital, theory-driven, and Bayesian: A paradigmatic study of cognitive flexibility. Front. Psychol. 2024, 15, 1437192. [Google Scholar] [CrossRef]
- Köster, M.; Gruber, T. Rhythms of human attention and memory: An embedded process perspective. Front. Hum. Neurosci. 2022, 16, 905837. [Google Scholar] [CrossRef]
- Cowan, N. Attention and Memory: An Integrated Framework; Oxford University Press: Oxford, UK, 1998; pp. 1–336. Available online: https://academic.oup.com/book/32746 (accessed on 20 January 2025).
- Cowan, N.; Bao, C.; Bishop-Chrzanowski, B.M.; Costa, A.N.; Greene, N.R.; Guitard, D.; Li, C.; Musich, M.L.; Ünal, Z.E. The Relation Between Attention and Memory. Annu. Rev. Psychol. 2024, 75, 183–214. [Google Scholar] [CrossRef]
- Hobson, H.M.; Bishop, D.V.M. The interpretation of mu suppression as an index of mirror neuron activity: Past, present and future. R. Soc. Open Sci. 2017, 4, 160662. [Google Scholar] [CrossRef]
- Yin, S.; Liu, Y.; Ding, M. Amplitude of Sensorimotor Mu Rhythm Is Correlated with BOLD from Multiple Brain Regions: A Simultaneous EEG-fMRI Study. Front. Hum. Neurosci. 2016, 10, 189865. [Google Scholar] [CrossRef]
- Kemmerer, D. What modulates the Mirror Neuron System during action observation?: Multiple factors involving the action, the actor, the observer, the relationship between actor and observer, and the context. Prog. Neurobiol. 2021, 205, 102128. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.A. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Res. Rev. 2005, 50, 57–68. [Google Scholar] [CrossRef]
- Coll, M.P.; Press, C.; Hobson, H.; Catmur, C.; Bird, G. Crossmodal Classification of Mu Rhythm Activity during Action Observation and Execution Suggests Specificity to Somatosensory Features of Actions. J. Neurosci. 2017, 37, 5936–5947. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Brunner, C.; Schlögl, A.; Lopes da Silva, F.H. Mu rhythm [de]synchronization and EEG single-trial classification of different motor imagery tasks. NeuroImage 2006, 31, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.A.; Bakermans-Kranenburg, M.J.; Yoo, K.H.; Bowman, L.C.; Cannon, E.N.; Vanderwert, R.E.; Ferrari, P.F.; Van IJzendoorn, M.H. Assessing human mirror activity with EEG mu rhythm: A meta-analysis. Psychol. Bull. 2016, 142, 291–313. [Google Scholar] [CrossRef]
- Hobson, H.M.; Bishop, D.V.M. Mu suppression—A good measure of the human mirror neuron system? Cortex 2016, 82, 290–310. [Google Scholar] [CrossRef]
- Angelini, M.; Fabbri-Destro, M.; Lopomo, N.F.; Gobbo, M.; Rizzolatti, G.; Avanzini, P. Perspective-dependent reactivity of sensorimotor mu rhythm in alpha and beta ranges during action observation: An EEG study. Sci. Rep. 2018, 8, 12429. [Google Scholar] [CrossRef]
- Bolt, N.K.; Loehr, J.D. Motor-related cortical oscillations distinguish one’s own from a partner’s contributions to a joint action. Biol. Psychol. 2024, 190, 108804. [Google Scholar] [CrossRef]
- Lockhart, A.K.; Sharpley, C.F.; Bitsika, V. Mu Desynchronisation in Autistic Individuals: What We Know and What We Need to Know. Rev. J. Autism Dev. Disord. 2024, 11, 595–606. [Google Scholar] [CrossRef]
- Shams, L.; Beierholm, U. Bayesian causal inference: A unifying neuroscience theory. Neurosci. Biobehav. Rev. 2022, 137, 104619. [Google Scholar] [CrossRef]
- Friston, K. Functional integration and inference in the brain. Prog. Neurobiol. 2002, 68, 113–143. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. Learning and inference in the brain. Neural Netw. 2003, 16, 1325–1352. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. A theory of cortical responses. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 815–836. [Google Scholar] [CrossRef]
- Friston, K.J.; Daunizeau, J.; Kilner, J.; Kiebel, S.J. Action and behavior: A free-energy formulation. Biol. Cybern. 2010, 102, 227–260. [Google Scholar] [CrossRef]
- Parr, T.; Rees, G.; Friston, K.J. Computational neuropsychology and bayesian inference. Front. Hum. Neurosci. 2018, 12, 332197. [Google Scholar] [CrossRef]
- Kilner, J.M.; Friston, K.J.; Frith, C.D. Predictive coding: An account of the mirror neuron system. Cogn. Process 2007, 8, 159. [Google Scholar] [CrossRef]
- Kosslyn, S.M.; Ganis, G.; Thompson, W.L. Neural foundations of imagery. Nat. Rev. Neurosci. 2001, 2, 635–642. [Google Scholar] [CrossRef]
- Boccaccio, F.M.; Pennisi, A.; Guerrera, C.S.; Platania, G.A.; Torre, V.; Varrasi, S.; Vezzosi, V.F.; Coco, F.; Castellano, S.; Pirrone, C. Mental Imagery between Cognition and Emotion: A Narrative Review. Psychiatry Int. 2024, 5, 697–717. [Google Scholar] [CrossRef]
- Kreiman, G.; Koch, C.; Fried, I. Imagery neurons in the human brain. Nature 2000, 408, 357–361. [Google Scholar] [CrossRef]
- Muraki, E.J.; Speed, L.J.; Pexman, P.M. Insights into embodied cognition and mental imagery from aphantasia. Nat. Rev. Psychol. 2023, 2, 591–605. [Google Scholar] [CrossRef]
- Keller, P.E. Mental imagery in music performance: Underlying mechanisms and potential benefits. Ann. N. Y Acad. Sci. 2012, 1252, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.A.; Tamir, D.I. Neural representations of situations and mental states are composed of sums of representations of the actions they afford. Nat. Commun. 2024, 15, 620. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M. Mental imagery in the motor context. Neuropsychologia 1995, 33, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Hanakawa, T. Organizing motor imageries. Neurosci. Res. 2016, 104, 56–63. [Google Scholar] [CrossRef]
- Mulder, T. Motor imagery and action observation: Cognitive tools for rehabilitation. J. Neural Transm. 2007, 114, 1265–1278. [Google Scholar] [CrossRef]
- Raffin, E.; Mattout, J.; Reilly, K.T.; Giraux, P. Disentangling motor execution from motor imagery with the phantom limb. Brain 2012, 135, 582–595. [Google Scholar] [CrossRef]
- Kitamura, M.; Kamibayashi, K. Changes in corticospinal excitability during motor imagery by physical practice of a force production task: Effect of the rate of force development during practice. Neuropsychologia 2024, 201, 108937. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Fabbri-Destro, M.; Nuara, A.; Gatti, R.; Avanzini, P. The role of mirror mechanism in the recovery, maintenance, and acquisition of motor abilities. Neurosci. Biobehav. Rev. 2021, 127, 404–423. [Google Scholar] [CrossRef]
- Bassolino, M.; Campanella, M.; Bove, M.; Pozzo, T.; Fadiga, L. Training the motor cortex by observing the actions of others during immobilization. Cereb. Cortex 2014, 24, 3268–3276. [Google Scholar] [CrossRef]
- Driskell, J.E.; Copper, C.; Moran, A. Does Mental Practice Enhance Performance? J. Appl. Psychol. 1994, 79, 481–492. [Google Scholar] [CrossRef]
- Jeannerod, M. Neural Simulation of Action: A Unifying Mechanism for Motor Cognition. NeuroImage 2001, 14, S103–S109. [Google Scholar] [CrossRef] [PubMed]
- Herbort, O.; Butz, M.V. Too good to be true? Ideomotor theory from a computational perspective. Front. Psychol. 2012, 3, 33680. [Google Scholar] [CrossRef] [PubMed]
- Melcher, T.; Winter, D.; Hommel, B.; Pfister, R.; Dechent, P.; Gruber, O. The neural substrate of the ideomotor principle revisited: Evidence for asymmetries in action-effect learning. Neuroscience 2013, 231, 13–27. [Google Scholar] [CrossRef]
- Koch, I.; Keller, P.; Prinz, W. The Ideomotor approach to action control: Implications for skilled performance. Int. J. Sport Exerc. Psychol. 2004, 2, 362–375. [Google Scholar] [CrossRef]
- Moeller, B.; Pfister, R. Ideomotor learning: Time to generalize a longstanding principle. Neurosci. Biobehav. Rev. 2022, 140, 104782. [Google Scholar] [CrossRef]
- Frank, C.; Kraeutner, S.N.; Rieger, M.; Boe, S.G. Learning motor actions via imagery—Perceptual or motor learning? Psychol. Res. 2024, 88, 1820–1832. [Google Scholar] [CrossRef]
- Frank, C.; Guillot, A.; Vogt, S. Imagery and motor learning: A special issue on the neurocognitive mechanisms of imagery and imagery practice of motor actions. Psychol. Res. 2024, 88, 1785–1789. [Google Scholar] [CrossRef]
- Ida, H.; Fukuhara, K.; Ogata, T. Virtual reality modulates the control of upper limb motion in one-handed ball catching. Front. Sports Act. Living. 2022, 4, 926542. [Google Scholar] [CrossRef]
- Seitz, S.; Schuster-Amft, C.; Wandel, J.; Bonati, L.H.; Parmar, K.; Gerth, H.U.; Behrendt, F. Effect of concurrent action observation, peripheral nerve stimulation and motor imagery on dexterity in patients after stroke: A pilot study. Sci. Rep. 2024, 14, 14858. [Google Scholar] [CrossRef]
- Lambert, K.J.M.; Singhal, A.; Leung, A.W.S. The lateralized effects of Parkinson’s Disease on motor imagery: Evidence from mental chronometry. Brain Cogn. 2024, 178, 106181. [Google Scholar] [CrossRef]
- Li, S.; Kulvicius, T.; Tamosiunaite, M.; Wörgötter, F. Simulated mental imagery for robotic task planning. Front. Neurorobot. 2023, 17, 1218977. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Y.; Rajabi, N.; Taleb, F.; Yang, Q.; Kragic, D.; Li, Z. Shaping high-performance wearable robots for human motor and sensory reconstruction and enhancement. Nat. Commun. 2024, 15, 1760. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; You, Y.; He, Z.; Chen, J. An Avatar-Based Intervention System for Children with Autism Spectrum Disorder. In Pattern Recognition and Computer Vision—PRCV 2024; Lecture Notes in Computer Science; Springer: Singapore, 2025; Volume 15039, pp. 220–231. Available online: https://link.springer.com/chapter/10.1007/978-981-97-8692-3_16 (accessed on 3 February 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florio, T.M. Emergent Aspects of the Integration of Sensory and Motor Functions. Brain Sci. 2025, 15, 162. https://doi.org/10.3390/brainsci15020162
Florio TM. Emergent Aspects of the Integration of Sensory and Motor Functions. Brain Sciences. 2025; 15(2):162. https://doi.org/10.3390/brainsci15020162
Chicago/Turabian StyleFlorio, Tiziana M. 2025. "Emergent Aspects of the Integration of Sensory and Motor Functions" Brain Sciences 15, no. 2: 162. https://doi.org/10.3390/brainsci15020162
APA StyleFlorio, T. M. (2025). Emergent Aspects of the Integration of Sensory and Motor Functions. Brain Sciences, 15(2), 162. https://doi.org/10.3390/brainsci15020162





