The Inflammatory Mechanism of Parkinson’s Disease: Gut Microbiota Metabolites Affect the Development of the Disease Through the Gut–Brain Axis
Abstract
1. Introduction
2. Dysbiosis of Gut Microbiota and Parkinson’s Disease
2.1. The Symptoms of Parkinson’s Disease
2.2. Dysbiosis of Gut Microbiota in Parkinson’s Disease
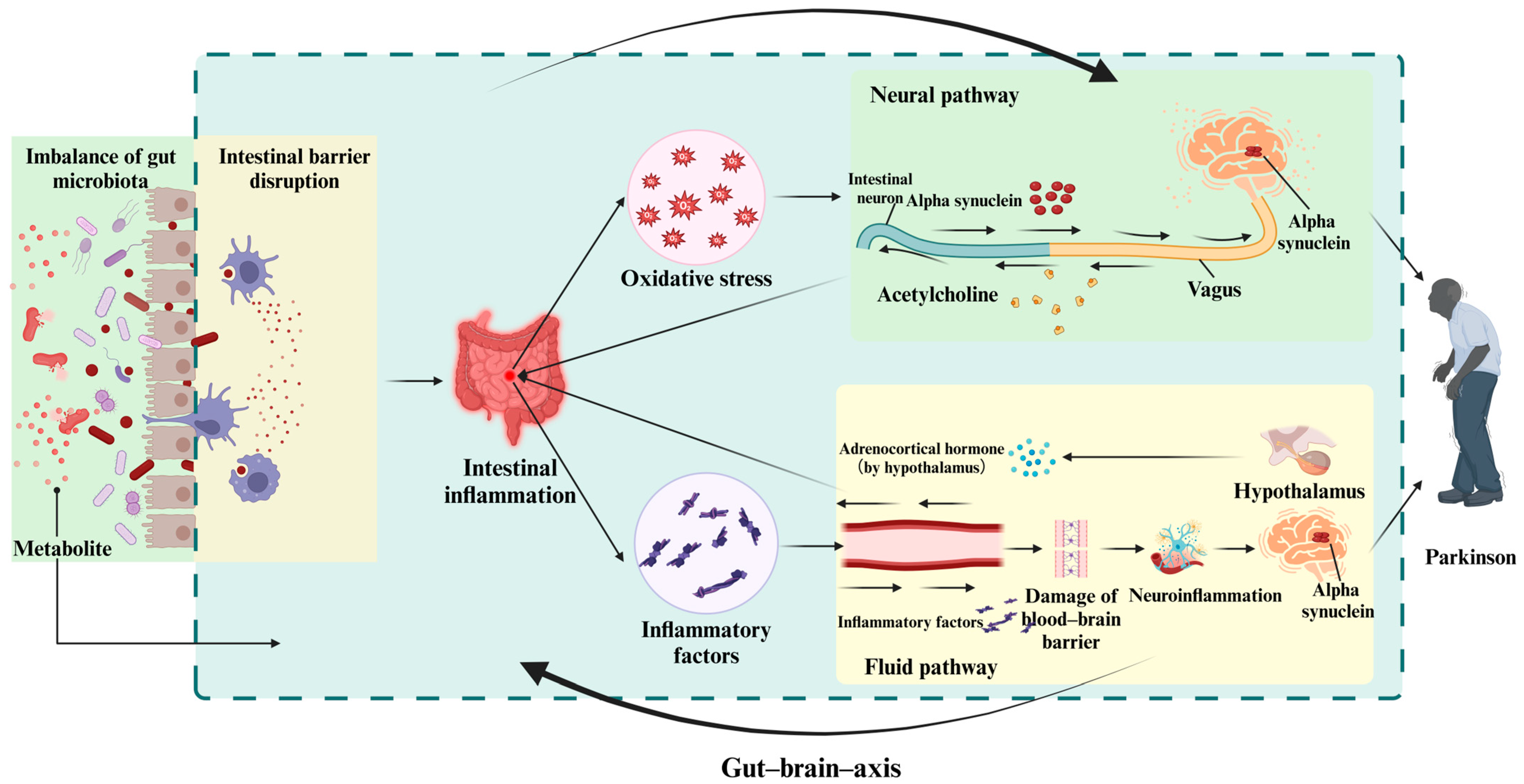
3. Gut–Brain Axis
3.1. Anatomical Pathway
3.1.1. The Neural Pathway
3.1.2. Systemic Circulation Pathway
3.2. The Bidirectional Transmission of Inflammatory Signals
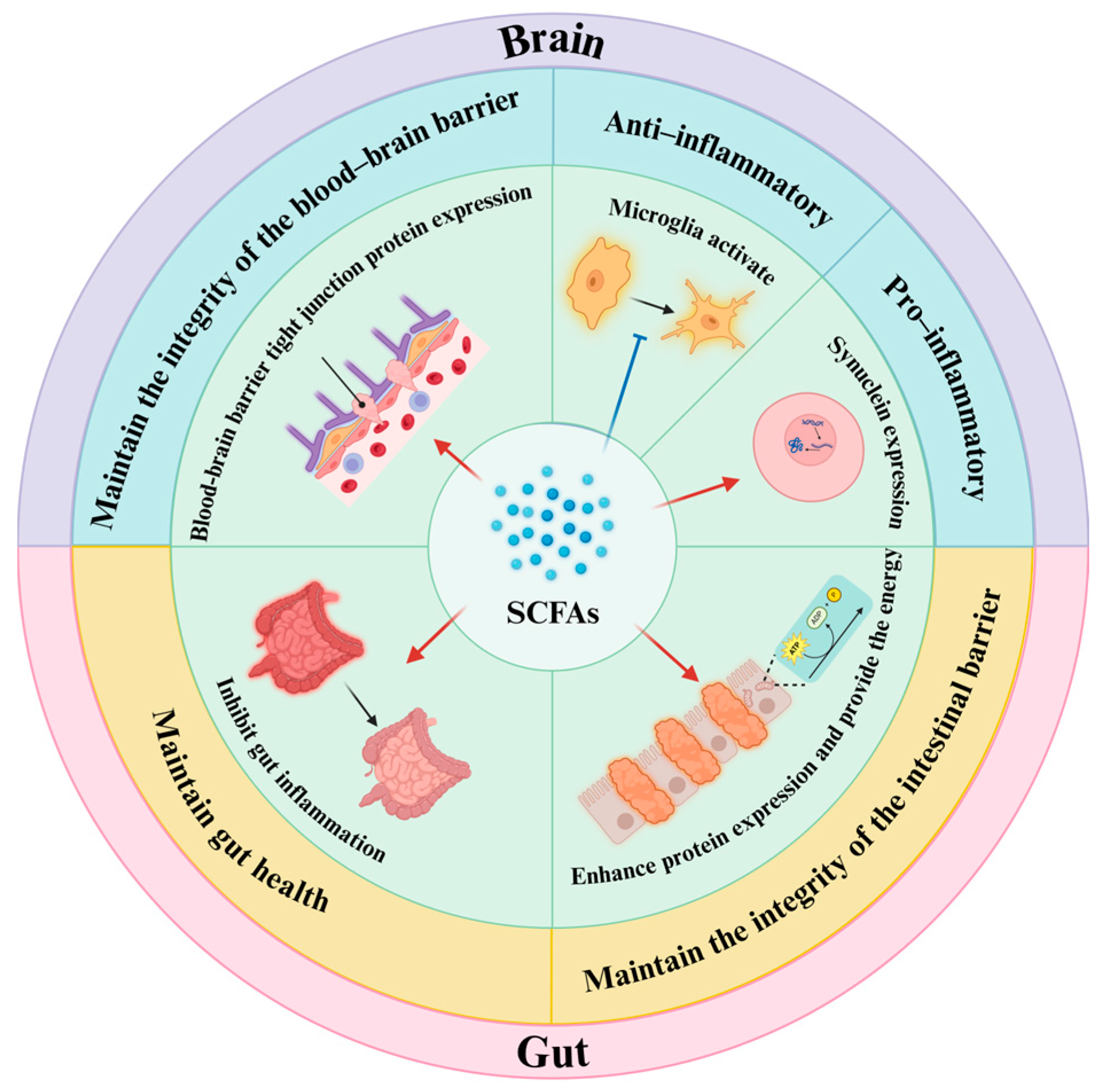
4. Gut Metabolites Affect Gut–Brain Axis
4.1. Key Links of the Bidirectional Inflammatory Signal Transmission
4.2. Metabolites Affect the Bidirectional Transmission of Inflammatory Signals
4.2.1. Metabolites Resulting from Dietary Elements
4.2.2. Metabolites Produced by the Host but Modified by Gut Microbiota
4.2.3. Metabolites Synthesized Autonomously by the Gut Microbiota
5. Research Methods and Techniques Based on the Metabolites of Gut Microbiota
5.1. Detection Method of Gut Microbiota Metabolites
5.2. Animal Models
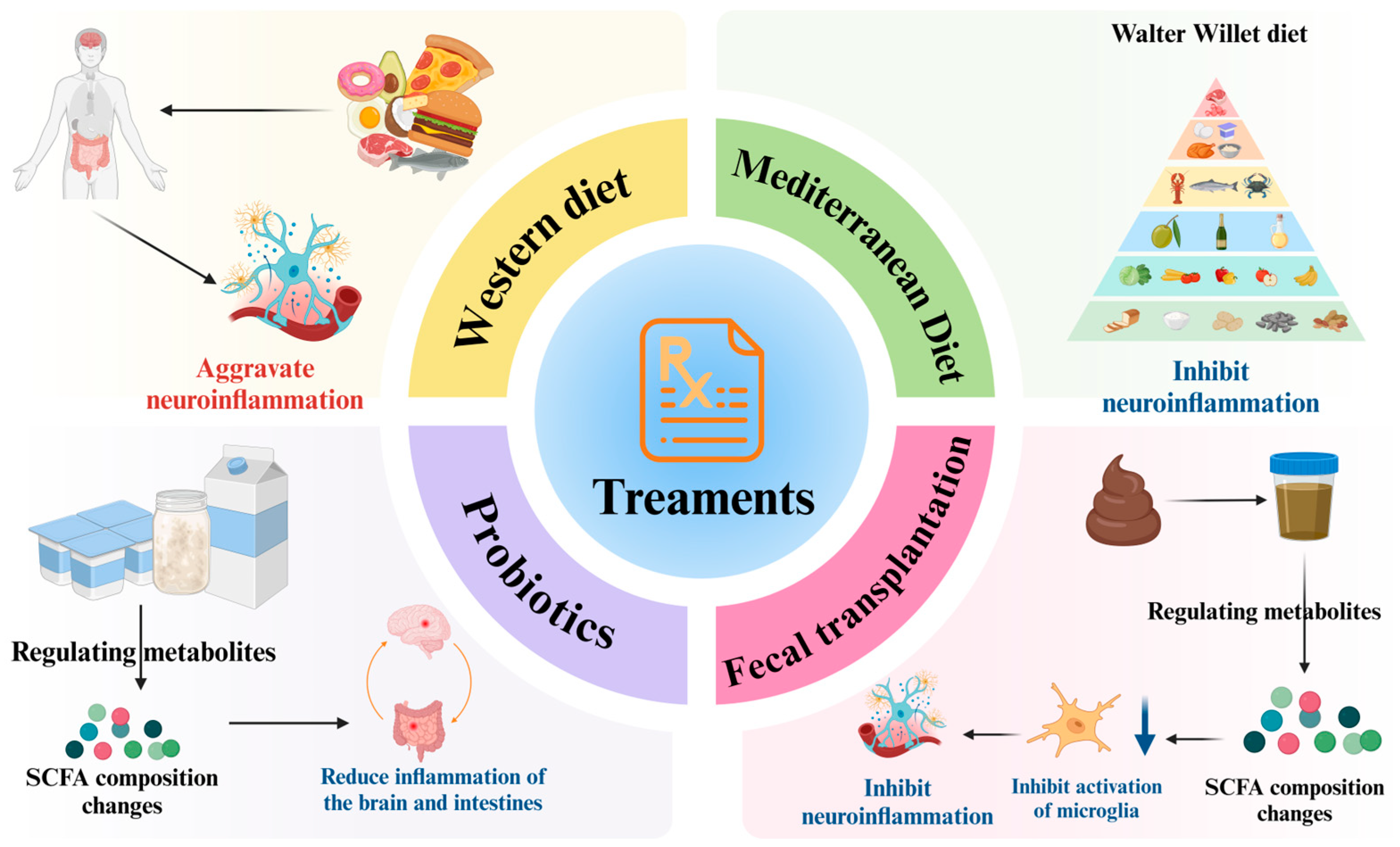
6. Treatment Methods for Parkinson’s Disease
6.1. Therapeutic Prospects Based on the Metabolites of Gut Microbiota
6.1.1. Dietary Intervention
6.1.2. Probiotic Intervention to Regulate Metabolites
6.1.3. Fecal Transplantation
6.2. Treatment Methods Based on Parkinson’s Comorbidity Patterns
6.2.1. Parkinson’s Disease and Cognitive Impairment of the Nervous System
6.2.2. Parkinson’s Disease and Gastrointestinal Disorders
7. Atypical Parkinson’s Syndrome
8. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| α-syn | α-synuclein |
| SCFAs | short-chain fatty acids |
| BBB | blood-brain barrier |
| CNS | central nervous system |
| HPA | hypothalamic–pituitary–adrenal axis |
| KYNA | Kynurenic acid |
| AA | anthranilic acid |
| QA | quinolinic acid |
| 5-HT | 5-hydroxytryptamine |
| NMDA | N-methyl-D-aspartate |
| HFD | high-fat diet |
| Bas | bile acids |
| TGR5 | Takeda G-protein-coupled receptor 5 |
| TUDCA | tauroursodeoxycholic acid |
| FXR | farnesoid X receptor |
| DCs | dendritic cells |
| SMVT | sodium-dependent multivitamin transporter |
| BCAAs | Branched-Chain Amino Acids |
| GABA | gamma-aminobutyric acid |
| LC | Liquid Chromatography |
| GC | Gas Chromatography |
| NMR | Nuclear Magnetic Resonance |
| MS | Mass Spectrometry |
| PGA | pyroglutamate |
| NMS | non-motor symptoms |
| PD-MCT | PD multimodal comprehensive therapy |
| SIBO | small intestinal bacterial overgrowth |
| ω-3 FAs | omega-3 fatty acids |
| CI | cognitive impairment |
| DBS | deep-brain stimulation |
| APS | atypical Parkinson’s syndrome |
| DLB | Lewy body dementia |
| PSP | progressive supranuclear palsy |
| MSA | multiple system atrophy |
| CBD | cortical basal ganglia degeneration |
References
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conte, C. Possible link between SARS-CoV-2 infection and Parkinson’s disease: The role of toll-like receptor 4. Int. J. Mol. Sci. 2021, 22, 7135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallen, Z.D.; Demirkan, A.; Twa, G.; Cohen, G.; Dean, M.N.; Standaert, D.G.; Sampson, T.R.; Payami, H. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat. Commun. 2022, 13, 6958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Zheng, J.; Pettersson, S.; Reynolds, R.; Tan, E.-K. The link between neuroinflammation and the neurovascular unit in synucleinopathies. Sci. Adv. 2023, 9, eabq1141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perez-Pardo, P.; Dodiya, H.B.; Broersen, L.M.; Douna, H.; van Wijk, N.; Lopes da Silva, S.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. Gut–brain and brain–gut axis in Parkinson’s disease models: Effects of a uridine and fish oil diet. Nutr. Neurosci. 2018, 21, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.R.; Chang, P.V. Deciphering the chemical lexicon of host–gut microbiota interactions. Trends Pharmacol. Sci. 2019, 40, 430–445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Tang, B.; Guo, J. Parkinson’s disease and gut microbiota: From clinical to mechanistic and therapeutic studies. Transl. Neurodegener. 2023, 12, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson‘s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corbillé, A.G.; Coron, E.; Neunlist, M.; Derkinderen, P.; Lebouvier, T. Appraisal of the dopaminergic and noradrenergic innervation of the submucosal plexus in PD. J. Parkinsons Dis. 2014, 4, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Wasner, G.; Deuschl, G. Pains in Parkinson disease—Many syndromes under one umbrella. Nat. Rev. Neurol. 2012, 8, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Dellapina, E.; Gerdelat-Mas, A.; Ory-Magne, F.; Pourcel, L.; Galitzky, M.; Calvas, F.; Simonetta-Moreau, M.; Thalamas, C.; Payoux, P.; Brefel-Courbon, C. Apomorphine effect on pain threshold in Parkinson’s disease: A clinical and positron emission tomography study. Mov. Disord. 2011, 26, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Snider, S.R.; Fahn, S.; Isgreen, W.P.; Cote, L.J. Primary sensory symptoms in parkinsonism. Neurology 1976, 26, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Lewis, D.R.; Wiegand, S.J.; Lindsay, R.M. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol. Biochem. Behav. 1997, 56, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ehrt, U.; Larsen, J.P.; Aarsland, D. Pain and its relationship to depression in Parkinson disease. Am. J. Geriatr. Psychiatry 2009, 17, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Q.; Doré, J.; Emmanuel, A.; Guarner, F.; Quigley, E.M. Gut microbiota and gastrointestinal health: Current concepts and future directions. Neurogastroenterol. Motil. 2013, 25, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Scherbaum, R.; Hartelt, E.; Kinkel, M.; Gold, R.; Muhlack, S.; Tönges, L. Parkinson’s Disease Multimodal Complex Treatment improves motor symptoms, depression and quality of life. J. Neurol. 2020, 267, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Heetun, Z.S.; Quigley, E.M. Gastroparesis and Parkinson‘s disease: A systematic review. Parkinsonism Relat. Disord. 2012, 18, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.T.; Munoz, D.G.; Schlossmacher, M.G.; Gray, D.A.; Woulfe, J.M. Protective effect of vagotomy suggests source organ for Parkinson disease. Ann. Neurol. 2015, 78, 834–835. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lucas, M.; Tobías, A.; Saz, P.; Sebastián, J.J. Effect of probiotic species on irritable bowel syndrome symptoms: A bring up to date meta-analysis. Rev. Esp. Enferm. Dig. 2013, 105, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.J.; O’Keeffe, G.W.; Sullivan, A.M. Viral vector delivery of neurotrophic factors for Parkinson’s disease therapy. Expert. Rev. Mol. Med. 2015, 17, e8. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Ohno, K. Parkinson’s disease and gut microbiota. Ann. Nutr. Metab. 2021, 77 (Suppl. 2), 28–35. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.-U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.-H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Grabrucker, S.; Marizzoni, M.; Silajdžić, E.; Lopizzo, N.; Mombelli, E.; Nicolas, S.; Dohm-Hansen, S.; Scassellati, C.; Moretti, D.V.; Rosa, M. Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain 2023, 146, 4916–4934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parodi, B.; Kerlero de Rosbo, N. The gut-brain axis in multiple sclerosis: Is. its dysfunction a pathological trigger or a consequence of the disease? Front. Immunol. 2021, 12, 718220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, A.H.; Lim, S.Y.; Lang, A.E. The microbiome–gut–brain axis in Parkinson disease—From basic research to the clinic. Nat. Rev. Neurol. 2022, 18, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.C.; Lima, M.P.P.; de Castro Brito, G.A.; de Barros Viana, G.S. Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res. Rev. 2023, 84, 101812. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aburto, M.R.; Cryan, J.F. Gastrointestinal and brain barriers: Unlocking gates of communication across the microbiota–gut–brain axis. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 222–247. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Kaddurah-Daouk, R.; Mazmanian, S.K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 2020, 21, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The microbiota–gut–brain Axis in psychiatric disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yarandi, S.S.; Kulkarni, S.; Saha, M.; Sylvia, K.E.; Sears, C.L.; Pasricha, P.J. Intestinal bacteria maintain adult enteric nervous system and nitrergic neurons via toll-like receptor 2-induced neurogenesis in mice. Gastroenterology 2020, 159, 200–213.e8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durgan, D.J.; Lee, J.; McCullough, L.D.; Bryan, R.M., Jr. Examining the role of the microbiota-gut-brain axis in stroke. Stroke 2019, 50, 2270–2277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin. Microbiol. Rev. 2022, 35, e00338-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarangi, A.N.; Goel, A.; Singh, A.; Sasi, A.; Aggarwal, R. Faecal bacterial microbiota in patients with cirrhosis and the effect of lactulose administration. BMC Gastroenterol. 2017, 17, 125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonaz, B. The gut-brain axis in Parkinson’s disease. Rev. Neurol. 2024, 180, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Lobbestael, E.; Vermeire, S.; Sabino, J.; Cleynen, I. Inflammatory bowel disease and Parkinson’s disease: Common pathophysiological links. Gut 2021, 70, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Fornai, M.; D’Antongiovanni, V.; Antonioli, L.; Bernardini, N.; Derkinderen, P. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol. Hepatol. 2023, 8, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The gut–brain axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Collins, S.; Verdu, E. Microbes and the gut-brain axis. Neurogastroenterol. Motil. 2012, 24, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonaz, B.; Bazin, T.; Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018, 12, 336468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campos-Acuña, J.; Elgueta, D.; Pacheco, R. T-cell-driven inflammation as a mediator of the gut-brain axis involved in Parkinson’s disease. Front. Immunol. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Xiao, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J. Adv. Res. 2022, 36, 27–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. npj Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, A.; Kumar, A.; Kulkarni, S. Licofelone attenuates MPTP-induced neuronal toxicity: Behavioral, biochemical and cellular evidence. Inflammopharmacology 2010, 18, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can. we shift the blame to gut microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef] [PubMed]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Yao, X.; Zhao, H.; Yang, W.; Zou, X.; Peng, F.; Li, B.; Cui, R. Gut microbiota and neuropsychiatric disorders: Implications for neuroendocrine-immune regulation. Pharmacol. Res. 2021, 173, 105909. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.; Houser, M.C.; Pereira, P.A.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iftikhar, P.M.; Anwar, A.; Saleem, S.; Nasir, S.; Inayat, A. Traumatic brain injury causing intestinal dysfunction: A review. J. Clin. Neurosci. 2020, 79, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2022, 231, 107988. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Alam, M.T.; Dey, J.; Sasidharan, B.C.P.; Ray, U.; Srivastava, A.K.; Gandhi, S.; Tripathi, P.P. Healthy gut, healthy brain: The gut microbiome in neurodegenerative disorders. Curr. Top. Med. Chem. 2020, 20, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, W.J.; Han, Y.Y.; Xu, X.; Yang, A.X.; Wei, J.; Hong, D.J.; Fang, X.; Chen, T.T. Neuroprotective effect of engineered Clostridium butyricum-pMTL007-GLP-1 on Parkinson‘s disease mice models via promoting mitophagy. Bioeng. Transl. Med. 2023, 8, e10505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Lv, X.; Ye, T.; Zhao, M.; Chen, Z.; Zhang, Y.; Yang, W.; Xie, H.; Zhan, L.; Chen, L. Microbiota-microglia crosstalk between Blautia producta and neuroinflammation of Parkinson’s disease: A bench-to-bedside translational approach. Brain Behav. Immun. 2024, 117, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.; Xia, H.; Wang, Z.; Li, B.; Xue, H.; Jin, S.; Xiao, L.; Wu, Y.; Guo, Q. Butyrate attenuates sympathetic activation in rats with chronic heart failure by inhibiting microglial inflammation in the paraventricular nucleus: Butyrate and sympathetic activation in chronic heart failure. Acta Biochim. Biophys. Sin. 2024, 56, 1823. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.-X.; Wang, F.; Liu, J.-Y.; Liu, C.-F. Relationship between short-chain fatty acids and Parkinson’s disease: A review from pathology to clinic. Neurosci. Bull. 2024, 40, 500–516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Pedicord, V.A.; Peng, T.; Hang, H.C. Site-specific acylation of a bacterial virulence regulator attenuates infection. Nat. Chem. Biol. 2020, 16, 95–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhai, L.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.; Wainwright, D.A. IDO1 in cancer: A Gemini of immune checkpoints. Cell. Mol. Immunol. 2018, 15, 447–457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, C.K.; Fernandez-Gomez, F.J.; Braidy, N.; Estrada, C.; Costa, C.; Costa, S.; Bessede, A.; Fernandez-Villalba, E.; Zinger, A.; Herrero, M.T. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Progress. Neurobiol. 2017, 155, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant. 2012, 5, 334–338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Cai, Y.; Guan, T.; Zhang, Y.; Huang, K.; Zhang, Z.; Cao, W.; Guan, X. Quinic acid alleviates high-fat diet-induced neuroinflammation by inhibiting DR3/IKK/NF-κB signaling via gut microbial tryptophan metabolites. Gut Microbes 2024, 16, 2374608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gershon, M. Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment. Pharmacol. Ther. 1999, 13, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jia, W.; Li, Y.; Cheung, K.C.; Zheng, X. Bile acid signaling in the regulation of whole body metabolic and immunological homeostasis. Sci. China Life Sci. 2024, 67, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-G.; Zheng, J.-X.; Xu, X.; Hu, Y.-M.; Ma, Y.-M. Hippocampal FXR plays a role in the pathogenesis of depression: A preliminary study based on lentiviral gene modulation. Psychiatry Res. 2018, 264, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. Neurosteroids in hepatic encephalopathy: Novel insights and new therapeutic opportunities. J. Steroid Biochem. Mol. Biol. 2016, 160, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lv, Y.-G.; Du, Y.-F.; Chen, F.; Reed, M.N.; Hu, M.; Suppiramaniam, V.; Tang, S.-S.; Hong, H. Neuroprotective effects of INT-777 against Aβ1–42-induced cognitive impairment, neuroinflammation, apoptosis, and synaptic dysfunction in mice. Brain Behav. Immun. 2018, 73, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiao, T.; Liu, W.; Luo, Y.; Wang, J.; Guo, X.; Tong, X.; Lin, Z.; Sun, C.; Wang, K. Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell 2022, 29, 1366–1381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, P.; Killinger, B.A.; Ensink, E.; Beddows, I.; Yilmaz, A.; Lubben, N.; Lamp, J.; Schilthuis, M.; Vega, I.E.; Woltjer, R. Gut microbiota dysbiosis is associated with elevated bile acids in Parkinson’s disease. Metabolites 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Pugin, B.; Barcik, W.; Westermann, P.; Heider, A.; Wawrzyniak, M.; Hellings, P.; Akdis, C.A.; O’Mahony, L. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb. Ecol. Health Dis. 2017, 28, 1353881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Pan, J.; Guo, M.; Duan, H.; Zhang, H.; Narbad, A.; Zhai, Q.; Tian, F.; Chen, W. Gut microbiota and anti-aging: Focusing on spermidine. Crit. Rev. Food Sci. Nutr. 2024, 64, 10419–10437. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishiwaki, H.; Ueyama, J.; Ito, M.; Hamaguchi, T.; Takimoto, K.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Mori, H.; Kurokawa, K. Meta-analysis of shotgun sequencing of gut microbiota in Parkinson’s disease. npj Park. Dis. 2024, 10, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fritsch, S.D.; Sukhbaatar, N.; Gonzales, K.; Sahu, A.; Tran, L.; Vogel, A.; Mazic, M.; Wilson, J.L.; Forisch, S.; Mayr, H. Metabolic support by macrophages sustains colonic epithelial homeostasis. Cell Metab. 2023, 35, 1931–1943.e8. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B vitamins and their role in immune regulation and cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agrawal, S.; Agrawal, A.; Said, H.M. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am. J. Physiol. Cell Physiol. 2016, 311, C386–C391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takechi, R.; Pallebage-Gamarallage, M.M.; Lam, V.; Giles, C.; Mamo, J.C. Nutraceutical agents with anti-inflammatory properties prevent dietary saturated-fat induced disturbances in blood–brain barrier function in wild-type mice. J. Neuroinflamm. 2013, 10, 842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabui, S.; Kapadia, R.; Ghosal, A.; Schneider, M.; Lambrecht, N.W.; Said, H.M. Biotin and pantothenic acid oversupplementation to conditional SLC5A6 KO mice prevents the development of intestinal mucosal abnormalities and growth defects. Am. J. Physiol. Cell Physiol. 2018, 315, C73–C79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, Y.; Ben, J.; Noto, A.; Kashiwaya, S.; Aoki, Y.; Watanabe, N.; Tsumoto, H.; Miura, Y.; Fukui, K. Tocotrienols Prevent the Decline of Learning Ability in High-Fat, High-Sucrose Diet-Fed C57BL/6 Mice. Int. J. Mol. Sci. 2024, 25, 3561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamontagne-Proulx, J.; Coulombe, K.; Dahhani, F.; Côté, M.; Guyaz, C.; Tremblay, C.; Di Marzo, V.; Flamand, N.; Calon, F.; Soulet, D. Effect of docosahexaenoic acid (DHA) at the enteric level in a synucleinopathy mouse model. Nutrients 2021, 13, 4218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mi, Y.; Qi, G.; Vitali, F.; Shang, Y.; Raikes, A.C.; Wang, T.; Jin, Y.; Brinton, R.D.; Gu, H.; Yin, F. Loss of fatty acid degradation by astrocytic mitochondria triggers neuroinflammation and neurodegeneration. Nat. Metab. 2023, 5, 445–465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, D.; Wang, X.; Zhang, L.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Chen, S.; Ying, J.; Hua, F. Lipid metabolism and storage in neuroglia: Role in brain development and neurodegenerative diseases. Cell Biosci. 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schönfeld, P.; Reiser, G. How the brain fights fatty acids’ toxicity. Neurochem. Int. 2021, 148, 105050. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Tippireddy, S.; Feriante, J.; Woltjer, R.L. Augmented frontal cortex diacylglycerol levels in Parkinson’s disease and Lewy Body Disease. PLoS ONE 2018, 13, e0191815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, B.; Ba, Y.; Li, Z.; Yang, C.; Su, K.; Qi, H.; Zhang, D.; Liu, X.; Wu, Y.; Chen, Y. Targeting dysregulated lipid metabolism for the treatment of Alzheimer’s disease and Parkinson‘s disease: Current advancements and future prospects. Neurobiol. Dis. 2024, 196, 106505. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hao, Y.; Pan, X.; You, J.; Li, G.; Xu, M.; Rao, Z. Microbial production of branched chain amino acids: Advances and perspectives. Bioresour. Technol. 2024, 397, 130502. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhao, G. The Associations Among Gut Microbiota, Branched Chain Amino Acids, and Parkinson’s Disease: Mendelian Randomization Study. J. Park. Dis. 2024, 14, 1129–1138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, Z.; Yang, F.; Sun, L.; Yu, J.; Sun, L.; Si, Y.; Yao, L. Role of gut microbiota-derived branched-chain amino acids in the pathogenesis of Parkinson’s disease: An animal study. Brain Behav. Immun. 2022, 106, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, L.-X.; Li, Y.-L.; Wu, J.-T.; Song, J.-Z.; Li, X.-M. Glutamatergic neurons in the caudal zona incerta regulate parkinsonian motor symptoms in mice. Neurosci. Bull. 2022, 38, 1–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correale, J.; Hohlfeld, R.; Baranzini, S.E. The role of the gut microbiota in multiple sclerosis. Nat. Rev. Neurol. 2022, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Heilman, P.L.; Wang, E.W.; Lewis, M.M.; Krzyzanowski, S.; Capan, C.D.; Burmeister, A.R.; Du, G.; Escobar Galvis, M.L.; Brundin, P.; Huang, X. Tryptophan metabolites are associated with symptoms and nigral pathology in Parkinson’s disease. Mov. Disord. 2020, 35, 2028–2037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted metabolomics strategies—Challenges and emerging directions. J. Am. Soc. Mass. Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keshavan, M.S. Characterizing transdiagnostic premorbid biotypes can help progress in selective prevention in psychiatry. World Psychiatry 2021, 20, 231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alarcon-Barrera, J.C.; Kostidis, S.; Ondo-Mendez, A.; Giera, M. Recent advances in metabolomics analysis for early drug development. Drug Discov. Today 2022, 27, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.C.; Zhang, K.; Walker, D.I.; Sinsheimer, J.; Yu, Y.; Kusters, C.; Del Rosario, I.; Folle, A.D.; Keener, A.M.; Bronstein, J. Untargeted serum metabolomics reveals novel metabolite associations and disruptions in amino acid and lipid metabolism in Parkinson’s disease. Mol. Neurodegener. 2023, 18, 100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.; Meng, X.-H.; Qiu, C.; Shen, H.; Zhao, Q.; Zhao, L.-J.; Tian, Q.; Sun, C.-Q.; Deng, H.-W. Integrative analysis of multi-omics data to detect the underlying molecular mechanisms for obesity in vivo in humans. Hum. Genom. 2022, 16, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, C.T.; Chao, B.N.; Barajas, R.; Haznadar, M.; Maruvada, P.; Nicastro, H.L.; Ross, S.A.; Verma, M.; Rogers, S.; Zanetti, K.A. An evaluation of the National Institutes of Health grants portfolio: Identifying opportunities and challenges for multi-omics research that leverage metabolomics data. Metabolomics 2022, 18, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Filippo, M.; Pescini, D.; Galuzzi, B.G.; Bonanomi, M.; Gaglio, D.; Mangano, E.; Consolandi, C.; Alberghina, L.; Vanoni, M.; Damiani, C. INTEGRATE: Model-based multi-omics data integration to characterize multi-level metabolic regulation. PLoS Comput. Biol. 2022, 18, e1009337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopes, F.M.; Bristot, I.J.; Da Motta, L.L.; Parsons, R.B.; Klamt, F. Mimicking Parkinson’s disease in a dish: Merits and pitfalls of the most commonly used dopaminergic in vitro models. NeuroMolecular Med. 2017, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, J.M.; Robertson, H.A. The BSSG rat model of Parkinson’s disease: Progressing towards a valid, predictive model of disease. Epma J. 2017, 8, 261–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verstraeten, A.; Theuns, J.; Van Broeckhoven, C. Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet. 2015, 31, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.; Chopra, K. Experimental models of Parkinson’s disease: Challenges and opportunities. Eur. J. Pharmacol. 2024, 980, 176819. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Q.; Yu, L.-l.; Chen, W.; Tian, F.-W.; Zhai, Q.-X. Dietary patterns affect Parkinson’s disease via the microbiota-gut-brain axis. Trends Food Sci. Technol. 2021, 116, 90–101. [Google Scholar] [CrossRef]
- Ross, F.C.; Mayer, D.E.; Gupta, A.; Gill, C.I.; Del Rio, D.; Cryan, J.F.; Lavelle, A.; Ross, R.P.; Stanton, C.; Mayer, E.A. Existing and future strategies to manipulate the gut microbiota with diet as a potential adjuvant treatment for psychiatric disorders. Biol. Psychiatry 2024, 95, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Schulfer, A.; Santiago-Rodriguez, T.M.; Ly, M.; Borin, J.M.; Chopyk, J.; Blaser, M.J.; Pride, D.T. Fecal viral community responses to high-fat diet in mice. MSphere 2020, 5, e00833-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliver, A.; Chase, A.B.; Weihe, C.; Orchanian, S.B.; Riedel, S.F.; Hendrickson, C.L.; Lay, M.; Sewall, J.M.; Martiny, J.B.H.; Whiteson, K. High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids. mSystems 2021, 6, e00115–e00121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.L.; Jia, X.B.; Sun, M.F.; Zhu, Y.L.; Qiao, C.M.; Zhang, B.P.; Zhao, L.P.; Yang, Q.; Cui, C.; Chen, X.; et al. Neuroprotection of Fasting Mimicking Diet on MPTP-Induced Parkinson’s Disease Mice via Gut Microbiota and Metabolites. Neurotherapeutics 2019, 16, 741–760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pietrzak, D.; Kasperek, K.; Rękawek, P.; Piątkowska-Chmiel, I. The Therapeutic Role of Ketogenic Diet in Neurological Disorders. Nutrients 2022, 14, 1952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, X.; Cheng, B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J. Mol. Neurosci. 2010, 42, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, M.; Liu, X.; Ye, Y.; Yan, X.; Cheng, Y.; Zhao, L.; Chen, F.; Ling, Z. Gut microbiota: A novel therapeutic target for Parkinson’s disease. Front. Immunol. 2022, 13, 937555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balmer, M.L.; Ma, E.H.; Thompson, A.J.; Epple, R.; Unterstab, G.; Lötscher, J.; Dehio, P.; Schürch, C.M.; Warncke, J.D.; Perrin, G. Memory CD8+ T cells balance pro-and anti-inflammatory activity by reprogramming cellular acetate handling at sites of infection. Cell Metab. 2020, 32, 457–467.e5. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yip, W.; Hughes, M.R.; Li, Y.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate shapes immune cell fate and function in allergic asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Powell, D.N.; Swimm, A.; Sonowal, R.; Bretin, A.; Gewirtz, A.T.; Jones, R.M.; Kalman, D. Indoles from the commensal microbiota act via the AHR and IL-10 to tune the cellular composition of the colonic epithelium during aging. Proc. Natl. Acad. Sci. USA 2020, 117, 21519–21526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, T.; Chu, C.; Yu, L.; Zhai, Q.; Wang, S.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Neuroprotective effects of Bifidobacterium breve CCFM1067 in MPTP-induced mouse models of Parkinson’s disease. Nutrients 2022, 14, 4678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanssen, N.M.; de Vos, W.M.; Nieuwdorp, M. Fecal microbiota transplantation in human metabolic diseases: From a murky past to a bright future? Cell Metab. 2021, 33, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-F.; Zhu, Y.-L.; Zhou, Z.-L.; Jia, X.-B.; Xu, Y.-D.; Yang, Q.; Cui, C.; Shen, Y.-Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Shen, X.; Leng, B.; Zhang, S.; Kwok, L.Y.; Zhao, F.; Zhao, J.; Sun, Z.; Zhang, J. Secondary analysis reveals gut microbiota differences in patients with Parkinson’s disease and/or cognitive impairment. Microbiome Res. Rep. 2024, 3, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Novikov, N.I.; Brazhnik, E.S.; Kitchigina, V.F. Pathological Correlates of Cognitive Decline in Parkinson’s Disease: From Molecules to Neural Networks. Biochemistry 2023, 88, 1890–1904. [Google Scholar] [CrossRef] [PubMed]
- Liepelt-Scarfone, I.; Ophey, A.; Kalbe, E. Cognition in prodromal Parkinson‘s disease. Prog. Brain Res. 2022, 269, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Sieg, E. Cognitive Impairment and Dementia in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Brønnick, K.; Fladby, T. Mild cognitive impairment in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2011, 11, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Foltynie, T.; Lewis, S.J.; Barker, R.A. Cognitive deficits and psychosis in Parkinson’s disease: A review of pathophysiology and therapeutic options. CNS Drugs 2006, 20, 477–505. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.A.; Doiron, M.; Talon-Croteau, J.; Dupré, N.; Simard, M. Effects of Antiparkinson Medication on Cognition in Parkinson’s Disease: A Systematic Review. Can. J. Neurol. Sci. 2018, 45, 375–404. [Google Scholar] [CrossRef] [PubMed]
- Frouni, I.; Kwan, C.; Belliveau, S.; Huot, P. Cognition and serotonin in Parkinson’s disease. Prog. Brain Res. 2022, 269, 373–403. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Cui, Y.; Li, S.; Le, W. Current Pharmaceutical Treatments and Alternative Therapies of Parkinson’s Disease. Curr. Neuropharmacol. 2016, 14, 339–355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Vedders, L.; Lue, L.; White, C.L., III; Akiyama, H.; Caviness, J.N.; Shill, H.A.; Sabbagh, M.N.; et al. Arizona Parkinson’s Disease Consortium. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010, 119, 689–702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Böttner, M.; Zorenkov, D.; Hellwig, I.; Barrenschee, M.; Harde, J.; Fricke, T.; Deuschl, G.; Egberts, J.H.; Becker, T.; Fritscher-Ravens, A.; et al. Expression pattern and localization of alpha-synuclein in the human enteric nervous system. Neurobiol. Dis. 2012, 48, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural. Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Parkinson’s disease and the gut: ‘the wheel is come full circle’. J. Parkinsons Dis. 2014, 4, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, A.; Kashihara, K. Increased frequencies of caries, periodontal disease and tooth loss in patients with Parkinson’s disease. J. Clin. Neurosci. 2009, 16, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Srivanitchapoom, P.; Pandey, S.; Hallett, M. Drooling in Parkinson’s disease: A review. Park. Relat. Disord. 2014, 20, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cecchini, M.P.; Osculati, F.; Ottaviani, S.; Boschi, F.; Fasano, A.; Tinazzi, M. Taste performance in Parkinson’s disease. J. Neural Transm. 2014, 121, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Noyce, A.J.; Silveira-Moriyama, L.; Gilpin, P.; Ling, H.; Howard, R.; Lees, A.J. Severe dysphagia as a presentation of Parkinson’s disease. Mov. Disord. 2012, 27, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Sheard, J.M.; Ash, S.; Silburn, P.A.; Kerr, G.K. Prevalence of malnutrition in Parkinson’s disease: A systematic review. Nutr. Rev. 2011, 69, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.H.; Qiu, J.; Friis, S.; Wermuth, L.; Ritz, B. Treatment for Helicobacter pylori infection and risk of Parkinson’s disease in Denmark. Eur. J. Neurol. 2012, 19, 864–869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gabrielli, M.; Bonazzi, P.; Scarpellini, E.; Bendia, E.; Lauritano, E.C.; Fasano, A.; Ceravolo, M.G.; Capecci, M.; Rita Bentivoglio, A.; Provinciali, L.; et al. Prevalence of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2011, 26, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lin, J.W.; Liu, Y.C.; Chang, C.H.; Wu, R.M. Risk of Parkinson’s disease following severe constipation: A nationwide population-based cohort study. Park. Relat. Disord. 2014, 20, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Nikitin, V.; Coward, T.; Davis, D.M.; Fiske, J. The potential benefits of dental implants on the oral health quality of life of people with Parkinson’s disease. Gerodontology 2009, 26, 11–18. [Google Scholar] [CrossRef] [PubMed]
- South, A.R.; Somers, S.M.; Jog, M.S. Gum chewing improves swallow frequency and latency in Parkinson patients: A preliminary study. Neurology 2010, 74, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Martin-Harris, B.; Jones, B. The videofluorographic swallowing study. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 769–785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheard, J.M.; Ash, S.; Mellick, G.D.; Silburn, P.A.; Kerr, G.K. Markers of disease severity are associated with malnutrition in Parkinson’s disease. PLoS ONE 2013, 8, e57986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alster, P.; Madetko-Alster, N. Significance of dysautonomia in Parkinson’s Disease and atypical parkinsonisms. Neurol. Neurochir. Pol. 2024, 58, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhou, X.; Wang, C.; Chen, Z.; Peng, H.; Hou, X.; Peng, Y.; Wang, P.; Li, T.; Yuan, H.; et al. Alterations of the Gut Microbiota in Multiple System Atrophy Patients. Front. Neurosci. 2019, 13, 1102, Erratum in Front. Neurosci. 2020, 14, 19. https://doi.org/10.3389/fnins.2020.00019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, R.; Sun, H.; Cao, Y.; Zhang, Z.; Chen, Y.; Wang, X.; Liu, L.; Wu, J.; Xu, H.; Wu, D.; et al. Glucosylceramide accumulation in microglia triggers STING-dependent neuroinflammation and neurodegeneration in mice. Sci. Signal. 2024, 17, eadk8249. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.D.; Radford, R.A.; Chung, R.S.; Guillemin, G.J.; Pountney, D.L. Neuroinflammation in Multiple System Atrophy: Response to and Cause of α-Synuclein Aggregation. Front. Cell Neurosci. 2015, 9, 437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Refolo, V.; Stefanova, N. Neuroinflammation and Glial Phenotypic Changes in Alpha-Synucleinopathies. Front. Cell Neurosci. 2019, 13, 263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rydbirk, R.; Elfving, B.; Andersen, M.D.; Langbøl, M.A.; Folke, J.; Winge, K.; Pakkenberg, B.; Brudek, T.; Aznar, S. Cytokine profiling in the prefrontal cortex of Parkinson’s Disease and Multiple System Atrophy patients. Neurobiol. Dis. 2017, 106, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Sidoroff, V.; Bower, P.; Stefanova, N.; Fanciulli, A.; Stankovic, I.; Poewe, W.; Seppi, K.; Wenning, G.K.; Krismer, F. Disease-Modifying Therapies for Multiple System Atrophy: Where Are We in 2022? J. Parkinsons Dis. 2022, 12, 1369–1387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tseng, F.S.; Foo, J.Q.X.; Mai, A.S.; Tan, E.K. The genetic basis of multiple system atrophy. J. Transl. Med. 2023, 21, 104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, J.; Kim, H.J.; Jeon, B. Immunotherapy Targeting Neurodegenerative Proteinopathies: α-Synucleinopathies and Tauopathies. J. Mov. Disord. 2020, 13, 11–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Olst, L.; Verhaege, D.; Franssen, M.; Kamermans, A.; Roucourt, B.; Carmans, S.; Ytebrouck, E.; van der Pol, S.M.A.; Wever, D.; Popovic, M.; et al. Microglial activation arises after aggregation of phosphorylated-tau in a neuron-specific P301S tauopathy mouse model. Neurobiol. Aging 2020, 89, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, A.; Trender-Gerhard, I.; Turkheimer, F.; Quinn, N.P.; Bhatia, K.P.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in progressive supranuclear palsy. Mov. Disord. 2006, 21, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, E.; Ramirez, P.; Gonzalez, E.; De Mange, J.; Ray, W.J.; Bieniek, K.F.; Frost, B. Pathogenic tau-induced transposable element-derived dsRNA drives neuroinflammation. Sci. Adv. 2023, 9, eabq5423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benito-León, J.; Alvarez-Linera, J.; Louis, E.D. Neurosyphilis masquerading as corticobasal degeneration. Mov. Disord. 2004, 19, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Murialdo, A.; Marchese, R.; Abbruzzese, G.; Tabaton, M.; Michelozzi, G.; Schiavoni, S. Neurosyphilis presenting as progressive supranuclear palsy. Mov. Disord. 2000, 15, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Leńska-Mieciek, M.; Madetko-Alster, N.; Alster, P.; Królicki, L.; Fiszer, U.; Koziorowski, D. Inflammation in multiple system atrophy. Front. Immunol. 2023, 14, 1214677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, H.; Wang, J.; Feng, R.; Zhang, R.; Liu, H.; Qin, C.; Meng, L.; Chen, Y.; Fu, Y.; Liang, D.; et al. Efficacy of faecal microbiota transplantation in patients with progressive supranuclear palsy-Richardson’s syndrome: A phase 2, single centre, randomised clinical trial. eClinicalMedicine 2023, 58, 101888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marras, C.; Cunningham, C.R.; Hou, J.; Proudfoot, J.; Standaert, D.G.; Juncos, J.; Riley, D.; Reich, S.G.; Hall, D.; Kluger, B.; et al. Anti-inflammatory drug use and progressive supranuclear palsy. Parkinsonism Relat. Disord. 2018, 48, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Agid, Y.; Calne, D.; Campbell, G.; Dubois, B.; Duvoisin, R.C.; Goetz, C.G.; Golbe, L.I.; Grafman, J.; Growdon, J.H.; et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, A.; Lv, J.; Su, Y. The Inflammatory Mechanism of Parkinson’s Disease: Gut Microbiota Metabolites Affect the Development of the Disease Through the Gut–Brain Axis. Brain Sci. 2025, 15, 159. https://doi.org/10.3390/brainsci15020159
Gao A, Lv J, Su Y. The Inflammatory Mechanism of Parkinson’s Disease: Gut Microbiota Metabolites Affect the Development of the Disease Through the Gut–Brain Axis. Brain Sciences. 2025; 15(2):159. https://doi.org/10.3390/brainsci15020159
Chicago/Turabian StyleGao, Ai, Jiaqi Lv, and Yanwei Su. 2025. "The Inflammatory Mechanism of Parkinson’s Disease: Gut Microbiota Metabolites Affect the Development of the Disease Through the Gut–Brain Axis" Brain Sciences 15, no. 2: 159. https://doi.org/10.3390/brainsci15020159
APA StyleGao, A., Lv, J., & Su, Y. (2025). The Inflammatory Mechanism of Parkinson’s Disease: Gut Microbiota Metabolites Affect the Development of the Disease Through the Gut–Brain Axis. Brain Sciences, 15(2), 159. https://doi.org/10.3390/brainsci15020159






