Small-Fiber Neuropathy: An Etiology-Oriented Review
Abstract
1. Introduction
2. Research Methods
3. Diagnosis
3.1. History and Clinical Examination
3.2. Diagnostic Techniques
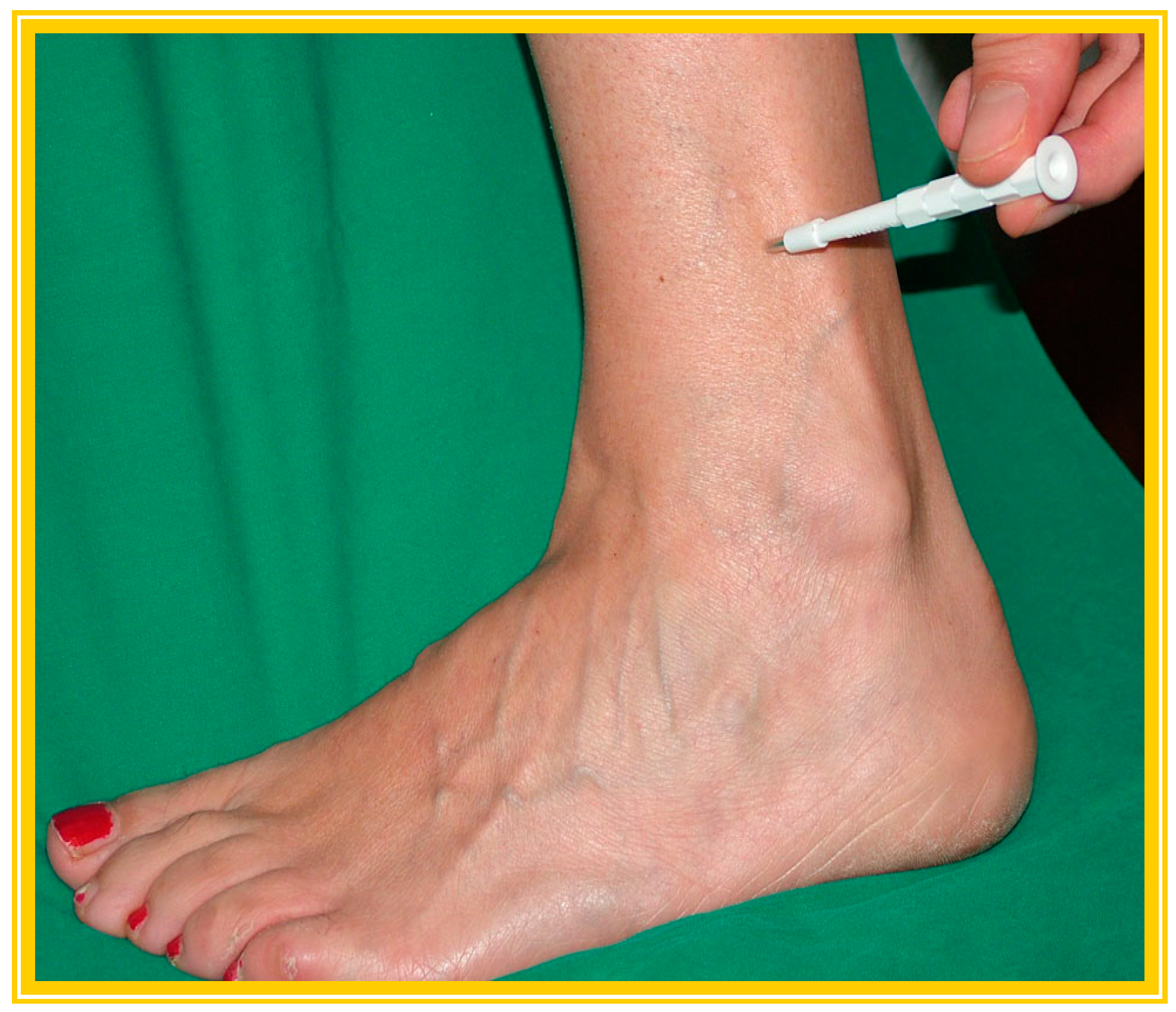
3.2.1. Skin Biopsy
3.2.2. Quantitative Sensory Testing (QST)
3.2.3. Laser-Evoked Potentials
3.2.4. Autonomic Testing
3.2.5. Corneal Confocal Microscopy
3.2.6. Microneurography
4. Etiology
4.1. Metabolic
4.2. Infectious
4.3. Immune-Mediated
4.3.1. Autoimmune Systemic Disorders
4.3.2. Antibodies in SFN
4.4. Drug-Induced and Toxic
4.5. Genetic
4.6. Idiopathic
5. Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kandel, E.R.; Koester, J.D.; Mack, S.H.; Siegelbaum, S.A. Principles of Neural Science, 6th ed.; McGraw Hill: New York, NY, USA, 2021. [Google Scholar]
- Devigili, G.; Tugnoli, V.; Penza, P.; Camozzi, F.; Lombardi, R.; Melli, G.; Broglio, L.; Granieri, E.; Lauria, G. The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain J. Neurol. 2008, 131, 1912–1925. [Google Scholar] [CrossRef]
- Misery, L.; Brenaut, E.; Le Garrec, R.; Abasq, C.; Genestet, S.; Marcorelles, P.; Zagnoli, F. Neuropathic pruritus. Nat. Rev. Neurol. 2014, 10, 408–416. [Google Scholar] [CrossRef]
- Gorson, K.C.; Herrmann, D.N.; Thiagarajan, R.; Brannagan, T.H.; Chin, R.L.; Kinsella, L.J.; Ropper, A.H. Non-length dependent small fibre neuropathy/ganglionopathy. J. Neurol. Neurosurg. Psychiatry 2008, 79, 163–169. [Google Scholar] [CrossRef]
- Gemignani, F.; Giovanelli, M.; Vitetta, F.; Santilli, D.; Bellanova, M.F.; Brindani, F.; Marbini, A. Non-length dependent small fiber neuropathy. a prospective case series. J. Peripher. Nerv. Syst. 2010, 15, 57–62. [Google Scholar] [CrossRef]
- Lauria, G.; Majorana, A.; Borgna, M.; Lombardi, R.; Penza, P.; Padovani, A.; Sapelli, P. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain 2005, 115, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Devigili, G.; Rinaldo, S.; Lombardi, R.; Cazzato, D.; Marchi, M.; Salvi, E.; Eleopra, R.; Lauria, G. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain 2019, 142, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, W.R.; Wendelschafer-Crabb, G. The innervation of human epidermis. J. Neurol. Sci. 1993, 115, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Terkelsen, A.J.; Karlsson, P.; Lauria, G.; Freeman, R.; Finnerup, N.B.; Jensen, T.S. The diagnostic challenge of small fibre neuropathy: Clinical presentations, evaluations, and causes. Lancet Neurol. 2017, 16, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Fabry, V.; Gerdelat, A.; Acket, B.; Cintas, P.; Rousseau, V.; Uro-Coste, E.; Evrard, S.M.; Pavy-Le Traon, A. Which Method for Diagnosing Small Fiber Neuropathy? Front. Neurol. 2020, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Boulton, A.J.M.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- Cazzato, D.; Lauria, G. Small fibre neuropathy. Curr. Opin. Neurol. 2017, 30, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef]
- Galosi, E.; Falco, P.; Di Pietro, G.; Leone, C.; Esposito, N.; De Stefano, G.; Di Stefano, G.; Truini, A. The diagnostic accuracy of the small fiber neuropathy symptoms inventory questionnaire (SFN-SIQ) for identifying pure small fiber neuropathy. J. Peripher. Nerv. Syst. 2022, 27, 283–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devigili, G.; Lombardi, R.; Lauria, G.; Cazzato, D. The Evolving Landscape of Small Fiber Neuropathy. In Seminars in Neurology; Thieme Medical Publishers, Inc.: New York, NY, USA, 2024. [Google Scholar] [CrossRef]
- Raicher, I.; Ravagnani, L.H.C.; Correa, S.G.; Dobo, C.; Mangueira, C.L.P.; Macarenco, R.S.e.S. Investigation of nerve fibers in the skin by biopsy: Technical aspects, indications, and contribution to diagnosis of small-fiber neuropathy. Einstein 2022, 20, eMD8044. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Cornblath, D.R.; Johansson, O.; McArthur, J.C.; Mellgren, S.I.; Nolano, M.; Rosenberg, N.; Sommer, C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur. J. Neurol. 2005, 12, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Bakkers, M.; Schmitz, C.; Lombardi, R.; Penza, P.; Devigili, G.; Smith, A.G.; Hsieh, S.; Mellgren, S.I.; Umapathi, T.; et al. Intraepidermal nerve fiber density at the distal leg: A worldwide normative reference study. J. Peripher. Nerv. Syst. 2010, 15, 202–207. [Google Scholar] [CrossRef]
- Provitera, V.; Gibbons, C.H.; Wendelschafer-Crabb, G.; Donadio, V.; Vitale, D.F.; Stancanelli, A.; Caporaso, G.; Liguori, R.; Wang, N.; Santoro, L.; et al. A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur. J. Neurol. 2016, 23, 333–338. [Google Scholar] [CrossRef]
- Kennedy, W.; Wendelschafer-Crabb, G. Utility of Skin Biopsy in Diabetic Neuropathy. Semin. Neurol. 1996, 16, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Holland, N.; Hauer, P.; Cornblath, D.R.; Griffin, J.W.; McArthur, J.C. Epidermal innervation: Changes with aging, topographic location, and in sensory neuropathy. J. Neurol. Sci. 1999, 164, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Donadio, V.; Incensi, A.; Vacchiano, V.; Infante, R.; Magnani, M.; Liguori, R. The autonomic innervation of hairy skin in humans: An in vivo confocal study. Sci. Rep. 2019, 9, 16982. [Google Scholar] [CrossRef] [PubMed]
- Krøigård, T.; Svendsen, T.K.; Wirenfeldt, M.; Schrøder, H.D.; Qvortrup, C.; Pfeiffer, P.; Gaist, D.; Sindrup, S.H. Oxaliplatin Neuropathy: Predictive Values of Skin Biopsy, QST and Nerve Conduction. J. Neuromuscul. Dis. 2021, 8, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Cuhls, H.; Radbruch, L.; Baron, R.; Maier, C.; Tölle, T.; Treede, R.-D.; Rolke, R. Quantitative sensory testing (QST). English version. Schmerz 2021, 35, 153–160. [Google Scholar] [CrossRef]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, D.R.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Sveen, K.A.; Karimé, B.; Jørum, E.; Mellgren, S.I.; Fagerland, M.W.; Monnier, V.M.; Dahl-Jørgensen, K.; Hanssen, K.F. Small- and Large-Fiber Neuropathy After 40 Years of Type 1 Diabetes. Diabetes Care 2013, 36, 3712–3717. [Google Scholar] [CrossRef]
- Dütsch, M.; Marthol, H.; Stemper, B.; Brys, M.; Haendl, T.; Hilz, M.J. Small Fiber Dysfunction Predominates in Fabry Neuropathy. J. Clin. Neurophysiol. 2002, 19, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Aleksovska, K.; Anderson, C.C.; Attal, N.; Baron, R.; Bennett, D.L.; Bouhassira, D.; Cruccu, G.; Eisenberg, E.; Enax-Krumova, E.; et al. Joint European Academy of Neurology–European Pain Federation–Neuropathic Pain Special Interest Group of the International Association for the Study of Pain guidelines on neuropathic pain assessment. Eur. J. Neurol. 2023, 30, 2177–2196. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, G.; La Cesa, S.; Leone, C.; Pepe, A.; Galosi, E.; Fiorelli, M.; Valeriani, M.; Lacerenza, M.; Pergolini, M.; Biasiotta, A.; et al. Diagnostic accuracy of laser-evoked potentials in diabetic neuropathy. Pain 2017, 158, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Moak, J.P.; Ramwell, C.B.; Gordish-Dressman, H.; Sule, S.D.; Bettini, E. Small fiber neuropathy in children, adolescents, and young adults with chronic orthostatic intolerance and postural orthostatic tachycardia syndrome: A retrospective study. Auton. Neurosci. Basic Clin. 2024, 253, 103163. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef]
- Rosenberg, M.E.; Tervo, T.M.T.; Immonen, I.J.; Muller, L.J.; Gronhagen, C.; Vesaluoma, M.H. Corneal Structure and Sensitivity in Type 1 Diabetes Mellitus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2915–2921. [Google Scholar]
- Petropoulos, I.N.; Ponirakis, G.; Khan, A.; Gad, H.; Almuhannadi, H.; Brines, M.; Cerami, A.; Malik, R.A. Corneal confocal microscopy: Ready for prime time. Clin. Exp. Optom. 2020, 103, 265–277. [Google Scholar] [CrossRef]
- Ferrari, G.; Grisan, E.; Scarpa, F.; Fazio, R.; Comola, M.; Quattrini, A.; Comi, G.; Rama, P.; Riva, N. Corneal confocal microscopy reveals trigeminal small sensory fiber neuropathy in amyotrophic lateral sclerosis. Front. Aging Neurosci. 2014, 6, 278. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Akhtar, N.; Kamran, S.; Ponirakis, G.; Petropoulos, I.N.; Tunio, N.A.; Dargham, S.R.; Imam, Y.; Sartaj, F.; Parray, A.; et al. Corneal Confocal Microscopy Detects Corneal Nerve Damage in Patients Admitted With Acute Ischemic Stroke. Stroke 2017, 48, 3012–3018. [Google Scholar] [CrossRef]
- Donadio, V.; Liguori, R. Microneurographic recording from unmyelinated nerve fibers in neurological disorders: An update. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015, 126, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Torebjörk, H.E.; Hallin, R.G. Identification of afferent C units in intact human skin nerves. Brain Res. 1974, 67, 387–403. [Google Scholar] [CrossRef]
- Serra, J.; Campero, M.; Bostock, H.; Ochoa, J. Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J. Neurophysiol. 2004, 91, 2770–2781. [Google Scholar] [CrossRef] [PubMed]
- Sumner, C.J.; Sheth, S.; Griffin, J.W.; Cornblath, D.R.; Polydefkis, M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003, 60, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Das, S.; Das, A.K.; Bhattacharjee, N.; Dihingia, A.; Dua, T.K.; Kalita, J.; Manna, P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 2018, 833, 472–523. [Google Scholar] [CrossRef]
- Pittenger, G.L.; Mehrabyan, A.; Simmons, K.; Amandarice; Dublin, C.; Barlow, P.; Vinik, A.I. Small fiber neuropathy is associated with the metabolic syndrome. Metab. Syndr. Relat. Disord. 2005, 3, 113–121. [Google Scholar] [CrossRef]
- Ørstavik, K.; Norheim, I.; Jørum, E. Pain and small-fiber neuropathy in patients with hypothyroidism. Neurology 2006, 67, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Güneş, H.N.; Bekircan-Kurt, C.E.; Tan, E.; Erdem-Özdamar, S. The histopathological evaluation of small fiber neuropathy in patients with vitamin B12 deficiency. Acta Neurol. Belg. 2018, 118, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, V.; Corse, A.M.; O’Brian, R.; Cornblath, D.R.; Klein, A.S.; Thuluvath, P.J. Autonomic and peripheral (sensorimotor) neuropathy in chronic liver disease: A clinical and electrophysiologic study. Hepatol. Baltim. Md 1999, 29, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Boger, M.S.; Hulgan, T.; Haas, D.W.; Mitchell, V.; Smith, A.G.; Singleton, J.R.; Peltier, A.C. Measures of small-fiber neuropathy in HIV infection. Auton. Neurosci. Basic Clin. 2012, 169, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Tharwa, E.-S.; Mohamed, A.; Elshazly, H.; Salama, M.; Youssef, M.I.; Bakeer, M.S.; Kamel, S.Y.; Abdelmageed, S.M.; Shabana, H.S.; Allam, M.A.; et al. Sudomotor Changes in Hepatitis C Virus Infection with or without Diabetes Mellitus: A Pilot Study in Egyptian Patients. Am. J. Trop. Med. Hyg. 2020, 104, 580–584. [Google Scholar] [CrossRef]
- Abravanel, F.; Pique, J.; Couturier, E.; Nicot, F.; Dimeglio, C.; Lhomme, S.; Chiabrando, J.; Saune, K.; Péron, J.-M.; Kamar, N.; et al. Acute hepatitis E in French patients and neurological manifestations. J. Infect. 2018, 77, 220–226. [Google Scholar] [CrossRef]
- Novak, P.; Felsenstein, D.; Mao, C.; Octavien, N.R.; Zubcevik, N. Association of small fiber neuropathy and post treatment Lyme disease syndrome. PLoS ONE 2019, 14, e0212222. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Okuzawa, M.; Yamamoto, M.; Okuno, S.; Satoh, T. Increased touch-evoked itch (punctate hyperknesis) in postherpetic itch: Implications of reduced intraepidermal nerve fibers representing small fiber neuropathy. J. Dermatol. 2023, 50, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L.; Mahalingam, R.; Wellish, M.C.; Gilden, D.H. Epstein-Barr virus--associated acute autonomic neuropathy. Ann. Neurol. 1996, 40, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, O.J.M. Leprosy neuropathy: Clinical presentations. Arq. Neuropsiquiatr. 2013, 71, 661–666. [Google Scholar] [CrossRef]
- Donadio, V.; Incensi, A.; Furia, A.; Parisini, S.; Colaci, F.; Giannoccaro, M.P.; Morelli, L.; Ricciardiello, F.; Di Stasi, V.; De Maria, A.; et al. Small fiber neuropathy associated with COVID-19 infection and vaccination: A prospective case-control study. Eur. J. Neurol. 2024, 32, e16538. [Google Scholar] [CrossRef]
- Falco, P.; Litewczuk, D.; Di Stefano, G.; Galosi, E.; Leone, C.; De Stefano, G.; Di Pietro, G.; Tramontana, L.; Ciardi, M.R.; Pasculli, P.; et al. Small fibre neuropathy frequently underlies the painful long-COVID syndrome. Pain 2024, 165, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Beecher, G.; Power, C.; Bridgland, L.; Zochodne, D.W. A neuropathic pain syndrome associated with hantavirus infection. J. Neurovirol. 2017, 23, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.; Devere, T.; Sakurai-Burton, S.; Santi, B.; McAllister, C.; Frank, K. Case Report: Angiostrongylus cantonensis Infection Presenting as Small Fiber Neuropathy. Am. J. Trop. Med. Hyg. 2022, 107, 367–369. [Google Scholar] [CrossRef]
- Oomatia, A.; Fang, H.; Petri, M.; Birnbaum, J. Peripheral neuropathies in systemic lupus erythematosus: Clinical features, disease associations, and immunologic characteristics evaluated over a twenty-five-year study period. Arthritis Rheumatol. 2014, 66, 1000–1009. [Google Scholar] [CrossRef]
- Liampas, A.; Parperis, K.; Erotocritou, M.F.; Nteveros, A.; Papadopoulou, M.; Moschovos, C.; Akil, M.; Coaccioli, S.; Hadjigeorgiou, G.M.; Hadjivassiliou, M.; et al. Primary Sjögren syndrome-related peripheral neuropathy: A systematic review and meta-analysis. Eur. J. Neurol. 2023, 30, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, N.; Starshinova, A.; Zinchenko, Y.; Kudlay, D.; Shapkina, V.; Malkova, A.; Belyaeva, E.; Pavlova, M.; Yablonskiy, P.; Shoenfeld, Y. Small Fiber Neuropathy in Sarcoidosis. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2021, 28, 544–550. [Google Scholar] [CrossRef]
- Morelli, L.; Serra, L.; Ricciardiello, F.; Gligora, I.; Donadio, V.; Caprini, M.; Liguori, R.; Giannoccaro, M.P. The role of antibodies in small fiber neuropathy: A review of currently available evidence. Rev. Neurosci. 2024, 35, 877–893. [Google Scholar] [CrossRef]
- Shah, A.; Hoffman, E.M.; Mauermann, M.L.; Loprinzi, C.L.; Windebank, A.J.; Klein, C.J.; Staff, N.P. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 2018, 89, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Lin, C.S.-Y.; Krishnan, A.V.; Goldstein, D.; Friedlander, M.L.; Kiernan, M.C. Oxaliplatin-induced neurotoxicity: Changes in axonal excitability precede development of neuropathy. Brain 2009, 132, 2712–2723. [Google Scholar] [CrossRef]
- Giannoccaro, M.P.; Donadio, V.; Gomis Pèrez, C.; Borsini, W.; Di Stasi, V.; Liguori, R. Somatic and autonomic small fiber neuropathy induced by bortezomib therapy: An immunofluorescence study. Neurol. Sci. 2011, 32, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Krøigård, T.; Schrøder, H.D.; Qvortrup, C.; Eckhoff, L.; Pfeiffer, P.; Gaist, D.; Sindrup, S.H. Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies. Eur. J. Neurol. 2014, 21, 623–629. [Google Scholar] [CrossRef]
- Tan, C.-H.; Chen, Y.-F.; Chen, C.-C.; Chao, C.-C.; Liou, H.-H.; Hsieh, S.-T. Painful neuropathy due to skin denervation after metronidazole-induced neurotoxicity. J. Neurol. Neurosurg. Psychiatry 2011, 82, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Kokotis, P.; Papantoniou, M.; Schmelz, M.; Buntziouka, C.; Tzavellas, E.; Paparrigopoulos, T. Pure small fiber neuropathy in alcohol dependency detected by skin biopsy. Alcohol 2023, 111, 67–73. [Google Scholar] [CrossRef]
- Chan, A.C.Y.; Kumar, S.; Tan, G.; Wong, H.Y.; Ong, J.J.Y.; Chandra, B.; Huang, H.; Sharma, V.K.; Lai, P.S. Expanding the genetic causes of small-fiber neuropathy: SCN genes and beyond. Muscle Nerve 2023, 67, 259–271. [Google Scholar] [CrossRef]
- McDermott, L.A.; Weir, G.A.; Themistocleous, A.C.; Segerdahl, A.R.; Blesneac, I.; Baskozos, G.; Clark, A.J.; Millar, V.; Peck, L.J.; Ebner, D.; et al. Defining the Functional Role of NaV1.7 in Human Nociception. Neuron 2019, 101, 905–919.e8. [Google Scholar] [CrossRef]
- Yang, Y. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J. Med. Genet. 2004, 41, 171–174. [Google Scholar] [CrossRef]
- Catterall, W.A.; Yu, F.H. Painful Channels. Neuron 2006, 52, 743–744. [Google Scholar] [CrossRef]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y.; et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006, 444, 894–898. [Google Scholar] [CrossRef]
- Faber, C.G.; Hoeijmakers, J.G.J.; Ahn, H.; Cheng, X.; Han, C.; Choi, J.; Estacion, M.; Lauria, G.; Vanhoutte, E.K.; Gerrits, M.M.; et al. Gain of function Nav 1.7 mutations in idiopathic small fiber neuropathy. Ann. Neurol. 2012, 71, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Blesneac, I.; Themistocleous, A.C.; Fratter, C.; Conrad, L.J.; Ramirez, J.D.; Cox, J.J.; Tesfaye, S.; Shillo, P.R.; Rice, A.S.C.; Tucker, S.J.; et al. Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain 2018, 159, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Dabby, R.; Sadeh, M.; Broitman, Y.; Yosovich, K.; Dickman, R.; Leshinsky-Silver, E. Painful small fiber neuropathy with gastroparesis: A new phenotype with a novel mutation in the SCN10A gene. J. Clin. Neurosci. 2016, 26, 84–88. [Google Scholar] [CrossRef]
- Leipold, E.; Liebmann, L.; Korenke, G.C.; Heinrich, T.; Gießelmann, S.; Baets, J.; Ebbinghaus, M.; Goral, R.O.; Stödberg, T.; Hennings, J.C.; et al. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat. Genet. 2013, 45, 1399–1404. [Google Scholar] [CrossRef]
- Eijkenboom, I.; Sopacua, M.; Hoeijmakers, J.G.J.; De Greef, B.T.A.; Lindsey, P.; Almomani, R.; Marchi, M.; Vanoevelen, J.; Smeets, H.J.M.; Waxman, S.G.; et al. Yield of peripheral sodium channels gene screening in pure small fibre neuropathy. J. Neurol. Neurosurg. Psychiatry 2019, 90, 342–352. [Google Scholar] [CrossRef]
- Kummer, K.K.; Kalpachidou, T.; Kress, M.; Langeslag, M. Signatures of Altered Gene Expression in Dorsal Root Ganglia of a Fabry Disease Mouse Model. Front. Mol. Neurosci. 2017, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Burand, A.J.; Stucky, C.L. Fabry disease pain: Patient and preclinical parallels. Pain 2021, 162, 1305–1321. [Google Scholar] [CrossRef]
- Cazzato, D.; Castori, M.; Lombardi, R.; Caravello, F.; Bella, E.D.; Petrucci, A.; Grammatico, P.; Dordoni, C.; Colombi, M.; Lauria, G. Small fiber neuropathy is a common feature of Ehlers-Danlos syndromes. Neurology 2016, 87, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Giannoccaro, M.P.; Donadio, V.; Incensi, A.; Avoni, P.; Liguori, R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve 2014, 49, 757–759. [Google Scholar] [CrossRef]
- Moisset, X.; Bouhassira, D.; Avez Couturier, J.; Alchaar, H.; Conradi, S.; Delmotte, M.H.; Lanteri-Minet, M.; Lefaucheur, J.P.; Mick, G.; Piano, V.; et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev. Neurol. 2020, 176, 325–352. [Google Scholar] [CrossRef]
- Moisset, X.; Bouhassira, D.; Attal, N. French guidelines for neuropathic pain: An update and commentary. Rev. Neurol. 2021, 177, 834–837. [Google Scholar] [CrossRef]
- Emery, E.C.; Luiz, A.P.; Wood, J.N. Nav 1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin. Ther. Targets 2016, 20, 975–983. [Google Scholar] [CrossRef] [PubMed]
| Fiber Type | Conduction Velocity | Function | Receptors |
|---|---|---|---|
| Aδ: thinly myelinated | 4–36 m/s | Cold sensation (<25 °C) | Transient Receptor Potential Cation Channel, Subfamily M, Member 8 (TRPM8) |
| Heat sensation (>45 °C) and nociception | Transient Receptor Potential Cation Channel, Subfamily V, Member 1 and 2 (TRPV1 and TRPV2) | ||
| Mechanical nociception | |||
| C: unmyelinated | 0.4–2 m/s | Warm sensation (>35 °C) | Transient Receptor Potential Cation Channel, Subfamily V, Member 3 (TRPV3) |
| Cold sensation (<5 °C) and nociception | Transient Receptor Potential Cation Channel, Subfamily A, Member 1 (TRPA1), Subfamily V, Member 1 (TRPV1), and Subfamily M, Member 8 (TRPM8) | ||
| Polymodal nociception | Transient Receptor Potential Cation Channel, Subfamily V, Member 1 (TRPV1) and Subfamily A, Member 1 (TRPA1) | ||
| Postganglionic autonomic | Acetylcholine (parasympathetic)/Adrenergic receptors (sympathetic) |
| Besta Criteria ≥2 of: | NEURODIAB Criteria | |
|---|---|---|
| (1) Clinical signs of small nerve fiber disease (2) Altered foot thermal threshold at quantitative sensory testing (3) Reduced intraepidermal nerve fiber density at distal leg as assessed by skin biopsy | (1) Length-dependent symptoms of small nerve fiber disease (2) Length-dependent signs of small nerve fiber disease (3) Normal sural nerve conduction studies (4) Reduced intraepidermal nerve fiber density at distal leg as assessed by skin biopsy (5) Altered foot thermal threshold at quantitative sensory testing | |
| Possible SFN | 1 and/or 2 | |
| Probable SFN | 1 and 2 and 3 | |
| Definite SFN | 1 and 2 and 3 and 4 and/or 5 | |
| Paper | Summary |
|---|---|
| Metabolic | |
| Sumner et al., 2003 [40] | Explores the wide spectrum of diabetes-associated neuropathies |
| Pittenger et al., 2005 [42] | Patients with metabolic syndrome and pain may present reduced leg IENFD |
| Ørstavik et al., 2006 [43] | Altered QST in patients with hypothyroidism and painful symptoms |
| Güneş et al., 2018 [44] | Reduced IENFD in patients with vitamin B12 deficiency and pain |
| Chaudhry et al., 1999 [45] | 28/58 (48%) of chronic liver disease patients presented with autonomic neuropathy |
| Infectious | |
| Boger et al., 2012 [46] | QSART may identify SFN in patients with HIV infection |
| Hashimoto et al., 2023 [50] | Reduced IENFD in patients with post-herpetic itch |
| Donadio et al., 2024 [53] | Somatic SFN in patients with SFN following COVID infection or vaccination |
| Falco et al., 2024 [54] | 50% of patients with painful long COVID syndrome meet criteria for SFN |
| Autoimmune | |
| Oomatia et al., 2014 [57] | 14/82 (17%) of SLE patients presented SFN, at times in non-length-dependent form |
| Liampas et al., 2023 [58] | Systematic review and meta-analysis of SFN in primary Sjögren syndrome |
| Gavrilova et al., 2021 [59] | Up to 60% of sarcoidosis patients present with a clinical picture congruent with SFN |
| Drug-induced/Toxic | |
| Giannoccaro et al., 2011 [63] | Bortezomib-associated SFN in three patients, as demonstrated by immunofluorescence |
| Krøigård et al., 2014 [64] | QST and skin biopsy disclose SFN in patients treated with oxaliplatin or docetaxel |
| Kokotis et al., 2023 [66] | 8/16 (50%) of alcohol-dependent subjects presented with SFN (QST and skin biopsy) |
| Inherited | |
| Faber et al., 2012 [72] | Gain-of-function mutations of SCN9A can cause SFN |
| Dabby et al., 2016 [74] | Gain-of-function mutations of SCN10A can cause SFN |
| Eijkenboom et al., 2019 [76] | Potentially pathogenic mutations of voltage-gated sodium channels in 11.6% of SFN |
| Burand et al., 2021 [78] | Many different phenomena, including SFN, cause neuropathic pain in Fabry disease |
| Cazzato et al., 2016 [79] | SFN described in Ehlers–Danlos patients |
| Other/Idiopathic | |
| Giannoccaro et al., 2014 [80] | Skin biopsy in patients with fibromyalgia |
| Therapy | Line | Notes |
|---|---|---|
| SNRIs (duloxetine, venlafaxine) | First | |
| Tricyclic antidepressants | First | |
| Gabapentinoids (gabapentin, pregabalin) | First (gabapentin), Second (pregabalin) | |
| Tramadol | Second | |
| Combination therapy (antidepressants + gabapentinoids) | Second | |
| Lidocaine plasters | First | Focal pain |
| TENS | First | Focal pain |
| Capsaicin patch | Second | Focal pain |
| Botulinum toxin A | Second | Focal pain |
| High-frequency repetitive transcranial magnetic stimulation | Third | |
| Spinal cord stimulation | Third | |
| Strong opioids | Third |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furia, A.; Liguori, R.; Donadio, V. Small-Fiber Neuropathy: An Etiology-Oriented Review. Brain Sci. 2025, 15, 158. https://doi.org/10.3390/brainsci15020158
Furia A, Liguori R, Donadio V. Small-Fiber Neuropathy: An Etiology-Oriented Review. Brain Sciences. 2025; 15(2):158. https://doi.org/10.3390/brainsci15020158
Chicago/Turabian StyleFuria, Alessandro, Rocco Liguori, and Vincenzo Donadio. 2025. "Small-Fiber Neuropathy: An Etiology-Oriented Review" Brain Sciences 15, no. 2: 158. https://doi.org/10.3390/brainsci15020158
APA StyleFuria, A., Liguori, R., & Donadio, V. (2025). Small-Fiber Neuropathy: An Etiology-Oriented Review. Brain Sciences, 15(2), 158. https://doi.org/10.3390/brainsci15020158







