Effects of Competition on Left Prefrontal and Temporal Cortex During Conceptual Comparison of Brand-Name Product Pictures: Analysis of fNIRS Using Tensor Decomposition †
Abstract
1. Introduction
1.1. Cognitive Neuropsychology Investigations of Semantic Cognition
1.2. Cognitive Neuroscience Investigations of Selection Demands During Semantic Retrieval
Manipulating Selection Demands During Semantic Retrieval
1.3. The Current Experiment
Prior Switch Results and Predictions for the Current Experiment
1.4. Other Novel Aspects of the Current Experiment
1.4.1. Pictures of Brand-Name Products
1.4.2. Rationale for fNIRS Imaging
1.4.3. Rationale for the Use of Tensor Decomposition Instead of Grand Averaging
2. Materials and Methods
2.1. Participants
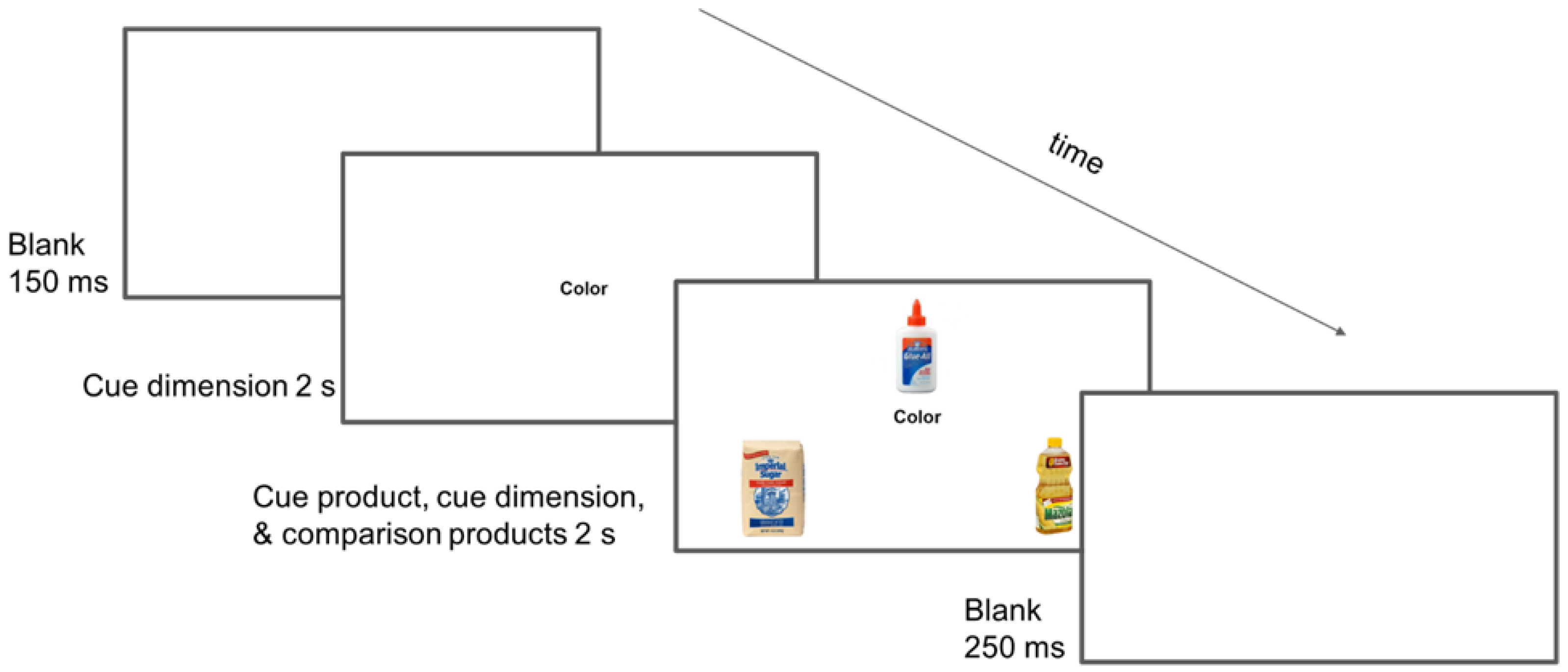
2.2. Experimental Design and Procedures
2.3. Materials
2.4. FNIRS Instrumentation, Configuration, and Preprocessing
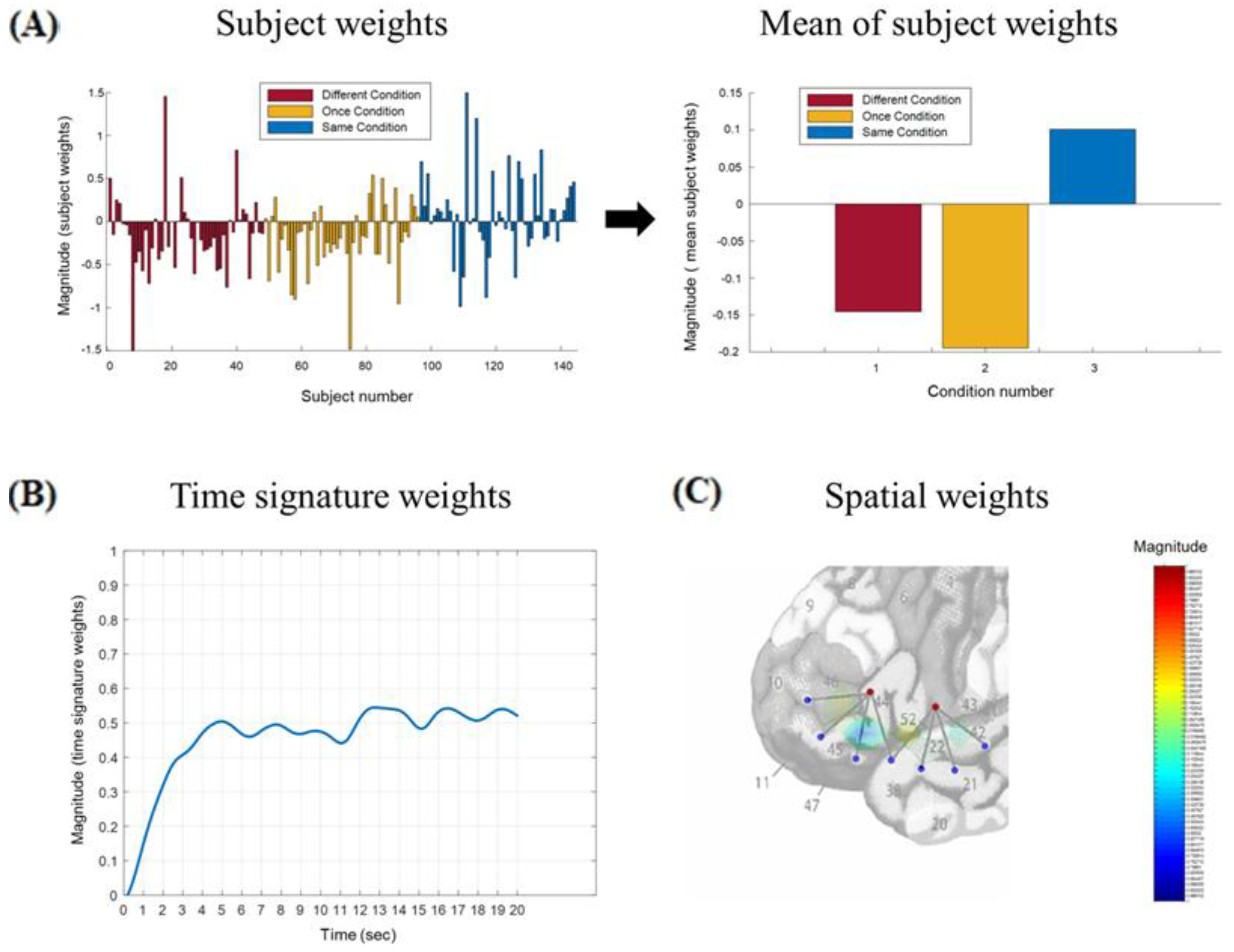
2.5. Tensor Decomposition
3. Results
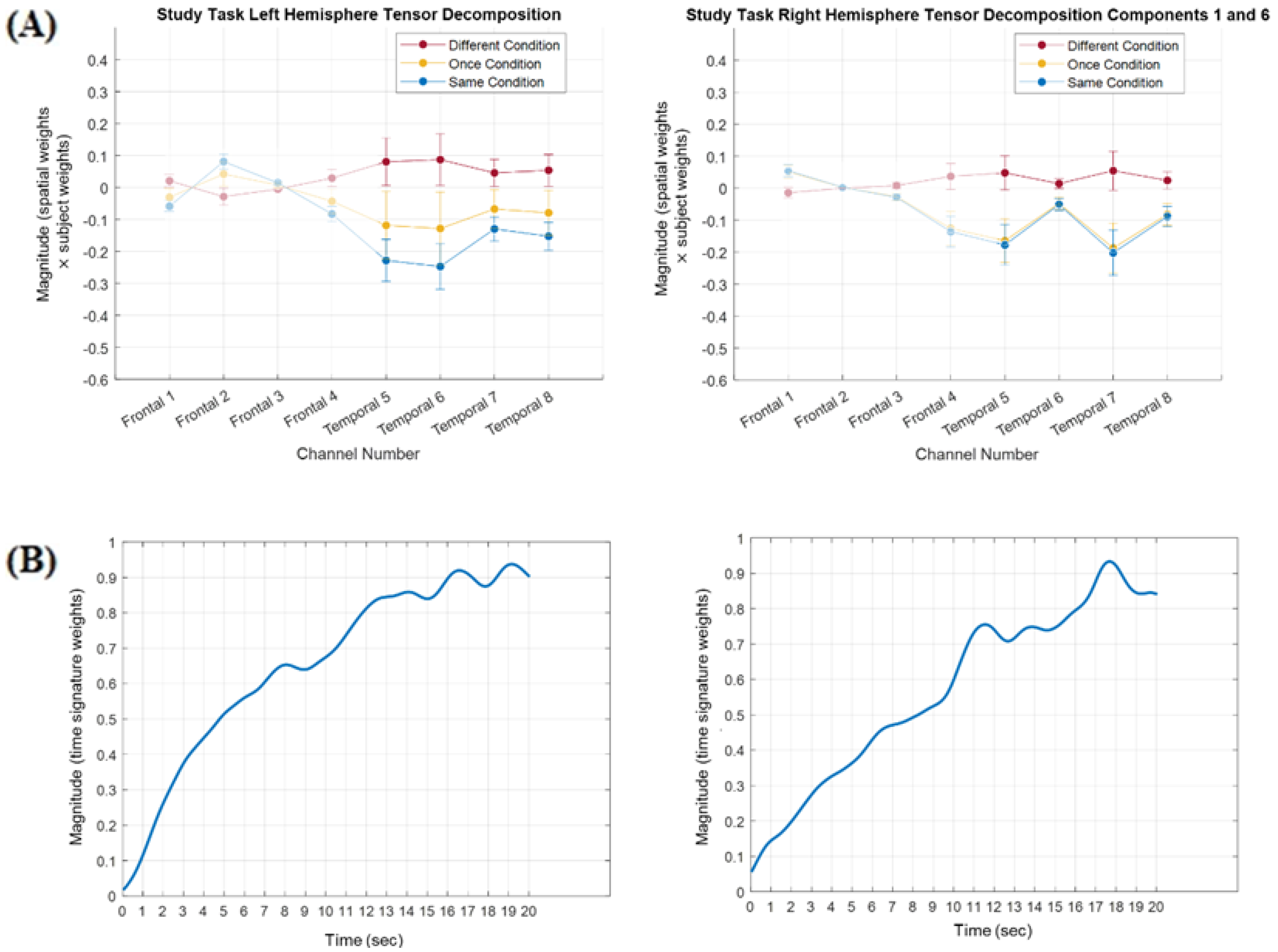
3.1. Tensor Decomposition Results
3.1.1. Frontal Cortex Components Analysis
3.1.2. Temporal Cortex Components Analysis
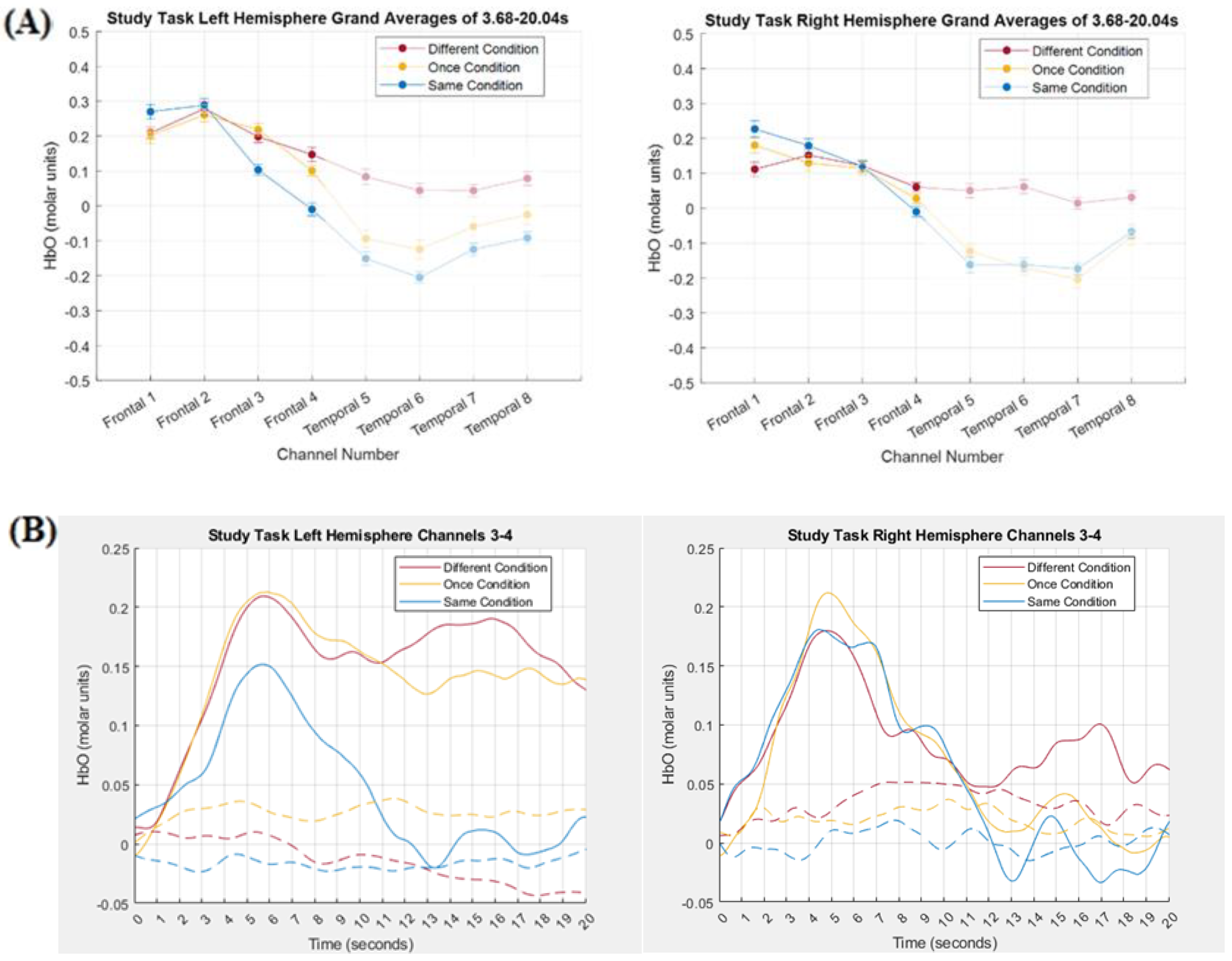
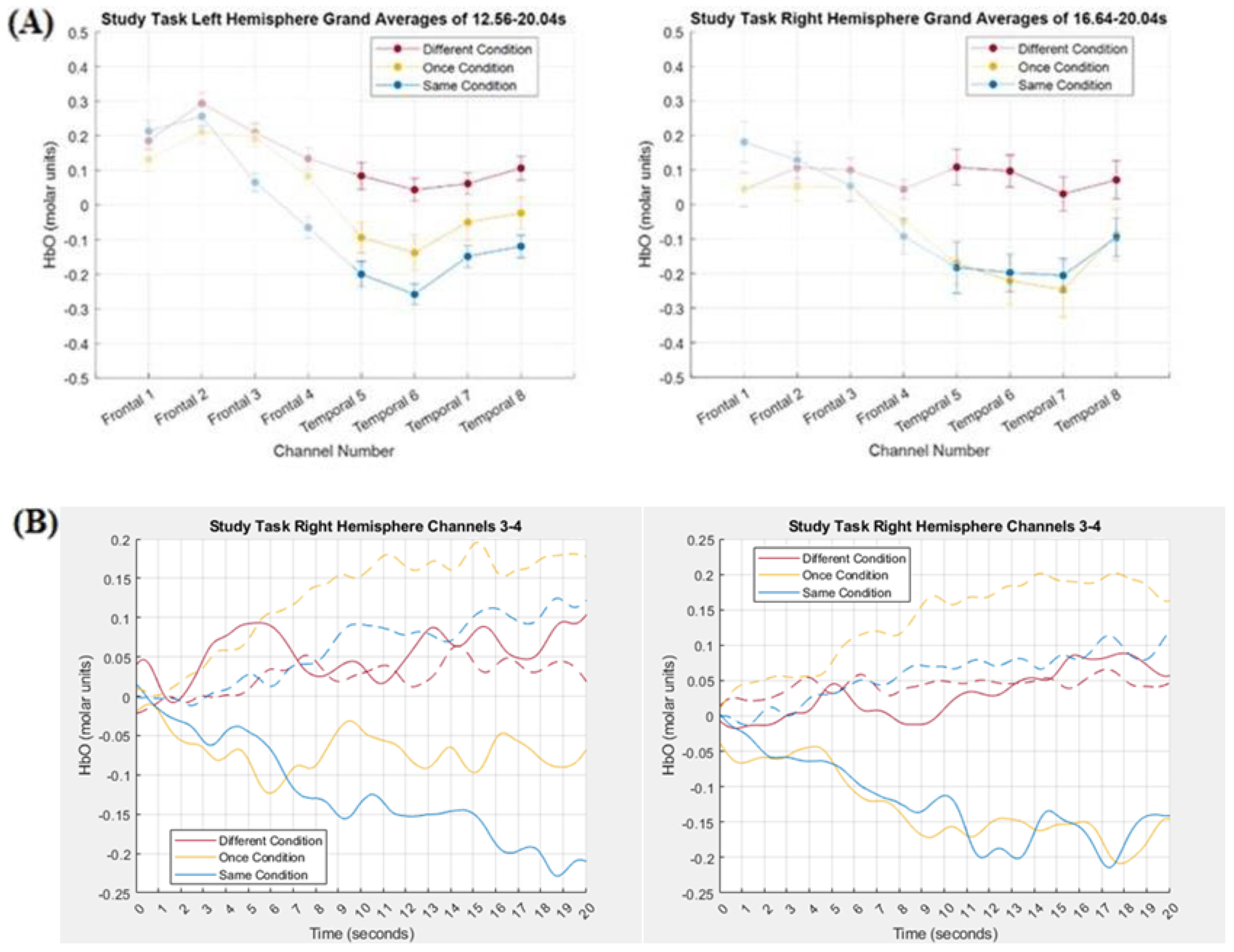
3.2. Comparing Tensor Decomposition and Grand Averaging
3.3. Behavioral Results
4. Discussion
4.1. Synopsis
4.2. Potential Reasons for Unusual Findings
4.2.1. Lack of Selection Demands?
4.2.2. Stimulus Characteristics?
4.2.3. Alternative Conceptualizations of the High-Selection Semantic Comparison Task?
4.3. Evaluation of the Tensor Decomposition Approach to fNIRS Data Analysis
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Tensor Construction
Appendix A.2. Tensor Decomposition
Appendix B
Determination of TOI and ROI
References
- Tulving, E. Episodic and semantic memory. In Organization and Memory; Tulving, E., Donaldson, W., Eds.; Academic Press: New York, NY, USA, 1972; pp. 381–403. [Google Scholar]
- Jackson, R.L.; Hoffman, P.; Pobric, G.; Lambon Ralph, M.A. The nature and neural correlates of semantic association versus conceptual similarity. Cereb. Cortex 2015, 25, 4319–4333. [Google Scholar] [CrossRef] [PubMed]
- Ralph, M.A.L.; Jefferies, E.; Patterson, K.; Rogers, T.T. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 2017, 18, 42–55. [Google Scholar] [CrossRef]
- Jefferies, E.; Thompson, H.; Cornelissen, P.; Smallwood, J. The neurocognitive basis of knowledge about object identity and events: Dissociations reflect opposing effects of semantic coherence and control. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190300. [Google Scholar] [CrossRef]
- Thompson-Schill, S.L. Neuroimaging studies of semantic memory: Inferring “how” from “where”. Neuropsychologia 2003, 41, 280–292. [Google Scholar] [CrossRef]
- Hodges, J.R.; Graham, N.; Patterson, K. Charting the progression in semantic dementia: Implications for the organisation of semantic memory. Memory 1995, 3, 463–495. [Google Scholar] [CrossRef]
- Jefferies, E.; Lambon Ralph, M.A. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain 2006, 129, 2132–2147. [Google Scholar] [CrossRef]
- Jefferies, E.; Patterson, K.; Ralph, M.A.L. Deficits of knowledge versus executive control in semantic cognition: Insights from cued naming. Neuropsychologia 2008, 46, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Fox, P.T.; Posner, M.I.; Mintun, M.; Raichle, M.E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature 1988, 331, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Demb, J.B.; Desmond, J.E.; Wagner, A.D.; Vaidya, C.J.; Glover, G.H.; Gabrieli, J.D. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. J. Neurosci. 1995, 15, 5870–5878. [Google Scholar] [CrossRef]
- Thompson-Schill, S.L.; D’Esposito, M.; Aguirre, G.K.; Farah, M.J. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc. Natl. Acad. Sci. USA 1997, 94, 14792–14797. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.; Patterson, K. Pyramids and Palm Trees: A Test of Semantic Access from Pictures and Words; Thames Valley Test Company: Bury St. Edmunds, Suffolk, UK, 1992. [Google Scholar]
- Bozeat, S.; Ralph, M.A.L.; Patterson, K.; Garrard, P.; Hodges, J.R. Non-verbal semantic impairment in semantic dementia. Neuropsychologia 2000, 38, 1207–1215. [Google Scholar] [CrossRef]
- Thompson-Schill, S.L.; D’Esposito, M.; Kan, I.P. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron 1999, 23, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Moss, H.E.; Abdallah, S.; Fletcher, P.; Bright, P.; Pilgrim, L.; Acres, K.; Tyler, L.K. Selecting among competing alternatives: Selection and retrieval in the left inferior frontal gyrus. Cereb. Cortex 2005, 15, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Bedny, M.; McGill, M.; Thompson-Schill, S.L. Semantic adaptation and competition during word comprehension. Cereb. Cortex 2008, 18, 2574–2585. [Google Scholar] [CrossRef]
- Hindy, N.C.; Altmann, G.T.; Kalenik, E.; Thompson-Schill, S.L. The effect of object state-changes on event processing: Do objects compete with themselves? J. Neurosci. 2012, 32, 5795–5803. [Google Scholar] [CrossRef] [PubMed]
- Badre, D.; Poldrack, R.A.; Paré-Blagoev, E.J.; Insler, R.Z.; Wagner, A.D. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 2005, 47, 907–918. [Google Scholar] [CrossRef]
- Musz, E.; Thompson-Schill, S.L. Tracking competition and cognitive control during language comprehension with multi-voxel pattern analysis. Brain Lang 2017, 165, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Loken, B.; Barsalou, L.W.; Joiner, C. Categorization theory and research in consumer psychology: Category representation and category-based inference. In Marketing and Consumer Psychology Series: Vol. 4. Handbook of Consumer Psychology; Haugtvedt, C.P., Herr, P.M., Kardes, F.R., Eds.; Taylor & Francis Group: New York, NY, USA, 2008; pp. 133–163. [Google Scholar]
- Alvino, L.; Pavone, L.; Abhishta, A.; Robben, H. Picking your brains: Where and how neuroscience tools can enhance marketing research. Front. Neurosci. 2020, 14, 577666. [Google Scholar] [CrossRef]
- Meyerding, S.G.; Risius, A. Reading minds: Mobile functional near-infrared spectroscopy as a new neuroimaging method for economic and marketing research—A feasibility study. J. Neurosci. Psychol. Econ. 2018, 11, 197–212. [Google Scholar] [CrossRef]
- Cong, F.; Lin, Q.-H.; Kuang, L.-D.; Gong, X.-F.; Astikainen, P.; Ristaniemi, T. Tensor decomposition of EEG signals: A brief review. J. Neurosci. Methods 2015, 248, 59–69. [Google Scholar] [CrossRef]
- Hssayeni, M.D.; Wilcox, T.; Ghoraani, B. Tensor decomposition of functional near-infrared spectroscopy (fNIRS) signals for pattern discovery of cognitive response in infants. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 394–397. [Google Scholar] [CrossRef]
- Chan, J.Y.; Hssayeni, M.D.; Wilcox, T.; Ghoraani, B. Exploring the feasibility of tensor decomposition for analysis of fNIRS signals: A comparative study with grand averaging method. Front. Neurosci. 2023, 17, 1180293. [Google Scholar] [CrossRef] [PubMed]
- Psychology Software Tools, Inc. [E-Prime 3.0]. 2016. Available online: https://support.pstnet.com/ (accessed on 8 August 2017).
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, 280–298. [Google Scholar] [CrossRef]
- MATLAB, version 9.0.0.341360 (R2016a); The MathWorks Inc.: Natick, MA, USA, 2016.
- Kolda, T.G.; Bader, B.W. Tensor decompositions and applications. SIAM Rev. 2009, 51, 455–500. [Google Scholar] [CrossRef]
- Sidiropoulos, N.D.; De Lathauwer, L.; Fu, X.; Huang, K.; Papalexakis, E.E.; Faloutsos, C. Tensor decomposition for signal processing and machine learning. IEEE Trans. Signal Process. 2017, 65, 3551–3582. [Google Scholar] [CrossRef]
- Mørup, M. Applications of tensor (multiway array) factorizations and decompositions in data mining. Wires Data Min. Knowl. 2011, 1, 24–40. [Google Scholar] [CrossRef]
- MATLAB, version 9.13.0.2105380 (R2022b); The MathWorks Inc.: Natick, MA, USA, 2022.
- Tensorlab 3.0 [Computer Software]. 2016. Available online: https://www.tensorlab.net/ (accessed on 11 May 2020).
- Phan, A.H.; Cichocki, A. Tensor decompositions for feature extraction and classification of high dimensional datasets. Nonlinear Theory Appl. 2010, 1, 37–68. [Google Scholar] [CrossRef]
- Gotts, S.J.; Chow, C.C.; Martin, A. Repetition priming and repetition suppression: Multiple mechanisms in need of testing. Cogn. Neurosci. 2012, 3, 250–259. [Google Scholar] [CrossRef]
- Kan, I.P.; Thompson-Schill, S.L. Effect of name agreement on prefrontal activity during overt and covert picture naming. Cogn. Affect. Behav. Neurosci. 2004, 4, 43–57. [Google Scholar] [CrossRef]
- Jackson, R.L. The neural correlates of semantic control revisited. NeuroImage 2021, 224, 117444. [Google Scholar] [CrossRef]
- Tippett, L.J.; Gendall, A.; Farah, M.J.; Thompson-Schill, S.L. Selection ability in Alzheimer’s disease: Investigation of a component of semantic processing. Neuropsychology 2004, 18, 163–173. [Google Scholar] [CrossRef]
- Lyons, F.; Hanley, J.R.; Kay, J. Anomia for common names and geographical names with preserved retrieval of names of people: A semantic memory disorder. Cortex 2002, 38, 23–35. [Google Scholar] [CrossRef]
- Crutch, S.J.; Warrington, E.K. The semantic organisation of proper nouns: The case of people and brand names. Neuropsychologia 2004, 42, 584–596. [Google Scholar] [CrossRef]
- Gontijo, P.F.; Zhang, S. The mental representation of brand names: Are brand names a class by themselves? In Psycholinguistic Phenomena in Marketing Communications, 1st ed.; Lowrey, T.M., Ed.; Psychology Press: New York, NY, USA, 2007; pp. 23–38. [Google Scholar] [CrossRef]
- Damasio, H.; Tranel, D.; Grabowski, T.; Adolphs, R.; Damasio, A. Neural systems behind word and concept retrieval. Cognition 2004, 92, 179–229. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Heskje, J.; Bruss, J.; Tranel, D.; Belfi, A.M. The left temporal pole is a convergence region mediating the relation between names and semantic knowledge for unique entities: Further evidence from a “recognition-from-name” study in neurological patients. Cortex 2018, 109, 14–24. [Google Scholar] [CrossRef]
- Thompson-Schill, S.L.; Bedny, M.; Goldberg, R.F. The frontal lobes and the regulation of mental activity. Curr. Opin. Neurobiol. 2005, 15, 219–224. [Google Scholar] [CrossRef]
- Peelen, M.V.; Caramazza, A. Conceptual object representations in human anterior temporal cortex. J. Neurosci. 2012, 32, 15728–15736. [Google Scholar] [CrossRef] [PubMed]
- Folstein, J.R.; Dieciuc, M.A. The cognitive neuroscience of stable and flexible semantic typicality. Front. Psychol. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Bracci, S.; Daniels, N.; Op de Beeck, H. Task context overrules object-and category-related representational content in the human parietal cortex. Cereb. Cortex 2017, 27, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Soshi, T.; Fujimaki, N.; Ihara, A.S. Distinctive responses in anterior temporal lobe and ventrolateral prefrontal cortex during categorization of semantic information. Sci. Rep. 2021, 11, 13343. [Google Scholar] [CrossRef] [PubMed]
- Barsalou, L.W. Ad hoc categories. Mem. Cogn. 1983, 11, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Corbett, F.; Jefferies, E.; Lambon Ralph, M.A. Deregulated semantic cognition follows prefrontal and temporo-parietal damage: Evidence from the impact of task constraint on nonverbal object use. J. Cogn. Neurosci. 2011, 23, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Barsalou, L.W. Deriving categories to achieve goals. In Psychology of Learning and Motivation; Bower, G.A., Ed.; Academic Press: San Diego, CA, USA, 1991; Volume 27, pp. 1–64. [Google Scholar] [CrossRef]
- Chiou, R.; Ralph, M.A.L. The anterior temporal cortex is a primary semantic source of top-down influences on object recognition. Cortex 2016, 79, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wu, Y.C.; Poor, H.V. Probabilistic tensor canonical polyadic decomposition with orthogonal factors. IEEE Trans. Signal Process. 2016, 65, 663–676. [Google Scholar] [CrossRef]




| Type of Processing | |||
|---|---|---|---|
| Once | Same | Different | |
| Response time | 1554 (178) | 1328 (158) | 1460 (168) |
| Accuracy | 0.80 (0.15) | 0.85 (0.09) | 0.78 (0.12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnhardt, T.M.; Chan, J.Y.; Ghoraani, B.; Wilcox, T. Effects of Competition on Left Prefrontal and Temporal Cortex During Conceptual Comparison of Brand-Name Product Pictures: Analysis of fNIRS Using Tensor Decomposition. Brain Sci. 2025, 15, 127. https://doi.org/10.3390/brainsci15020127
Barnhardt TM, Chan JY, Ghoraani B, Wilcox T. Effects of Competition on Left Prefrontal and Temporal Cortex During Conceptual Comparison of Brand-Name Product Pictures: Analysis of fNIRS Using Tensor Decomposition. Brain Sciences. 2025; 15(2):127. https://doi.org/10.3390/brainsci15020127
Chicago/Turabian StyleBarnhardt, Terrence M., Jasmine Y. Chan, Behnaz Ghoraani, and Teresa Wilcox. 2025. "Effects of Competition on Left Prefrontal and Temporal Cortex During Conceptual Comparison of Brand-Name Product Pictures: Analysis of fNIRS Using Tensor Decomposition" Brain Sciences 15, no. 2: 127. https://doi.org/10.3390/brainsci15020127
APA StyleBarnhardt, T. M., Chan, J. Y., Ghoraani, B., & Wilcox, T. (2025). Effects of Competition on Left Prefrontal and Temporal Cortex During Conceptual Comparison of Brand-Name Product Pictures: Analysis of fNIRS Using Tensor Decomposition. Brain Sciences, 15(2), 127. https://doi.org/10.3390/brainsci15020127






