Resting-State EEG Power and Aperiodic Activity in Individuals with Mild Cognitive Impairment and Cognitively Healthy Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. EEG Acquisition and Processing
2.3. Cognitive Measures
2.4. Statistical Analyses
3. Results
3.1. Demographics and Cognitive Measures
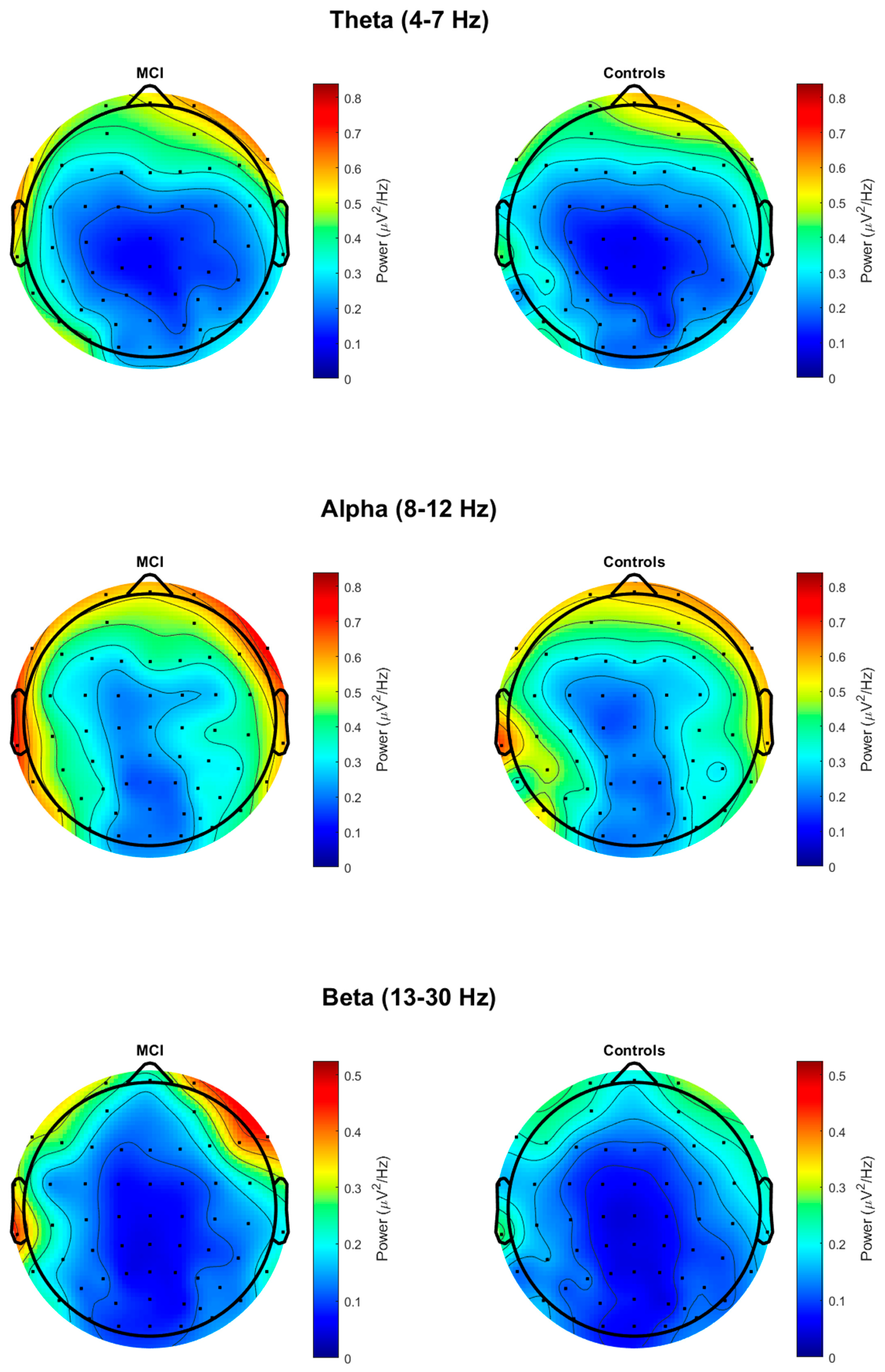
3.2. Resting-State EEG Group Differences
3.3. Associations Between Resting-State EEG Measures and Cognitive Measures
3.3.1. Correlations with 1/f-Adjusted Power and Cognitive Measures
3.3.2. Correlations with 1/f Slope and Cognitive Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCI | Mild cognitive impairment |
| AD | Alzheimer’s disease |
| EEG | Electroencephalography |
| MMSE | Mini-Mental Status Examination |
| MoCA | Montreal Cognitive Assessment |
| TMT-A | Trail Making Test-A |
| TMT-B | Trail Making Test-B |
| BNT | Boston Naming Test |
| COWAT | Controlled Oral Word Association Test |
| LM | Logical memory |
References
- Petersen, R.C. Mild Cognitive Impairment. N. Engl. J. Med. 2011, 364, 2227–2234. [Google Scholar] [CrossRef]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R., Jr. Mild Cognitive Impairment: Ten Years Later. Arch. Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S. Practice Guideline Update Summary: Mild Cognitive Impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef]
- Salari, N.; Lotfi, F.; Abdolmaleki, A.; Heidarian, P.; Rasoulpoor, S.; Fazeli, J.; Najafi, H.; Mohammadi, M. The Global Prevalence of Mild Cognitive Impairment in Geriatric Population with Emphasis on Influential Factors: A Systematic Review and Meta-Analysis. BMC Geriatr. 2025, 25, 313. [Google Scholar] [CrossRef]
- Gu, D.; Andreev, K.; Dupre, M.E. Major Trends in Population Growth Around the World. China CDC Wkly. 2021, 3, 604–613. [Google Scholar] [CrossRef]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Update on Hypothetical Model of Alzheimer’s Disease Biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, Z. Exploring Potential Electrophysiological Biomarkers in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis of Event-Related Potential Studies. J. Alzheimer’s Dis. 2017, 58, 1283–1292. [Google Scholar] [CrossRef]
- Morrison, C.; Rabipour, S.; Taler, V.; Sheppard, C.; Knoefel, F. Visual Event-Related Potentials in Mild Cognitive Impairment and Alzheimer’s Disease: A Literature Review. Curr. Alzheimer Res. 2019, 16, 67–89. [Google Scholar] [CrossRef]
- Paitel, E.R.; Samii, M.R.; Nielson, K.A. A Systematic Review of Cognitive Event-Related Potentials in Mild Cognitive Impairment and Alzheimer’s Disease. Behav. Brain Res. 2021, 396, 112904. [Google Scholar] [CrossRef]
- Tarawneh, H.Y.; Mulders, W.H.A.M.; Sohrabi, H.R.; Martins, R.N.; Jayakody, D.M.P. Investigating Auditory Electrophysiological Measures of Participants with Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Event-Related Potential Studies. J. Alzheimer’s Dis. 2021, 84, 419–448. [Google Scholar] [CrossRef]
- Yang, S.; Bornot, J.M.S.; Wong-Lin, K.; Prasad, G. M/EEG-Based Bio-Markers to Predict the MCI and Alzheimer’s Disease: A Review from the ML Perspective. IEEE Trans. Biomed. Eng. 2019, 66, 2924–2935. [Google Scholar] [CrossRef]
- Babiloni, C.; Arakaki, X.; Azami, H.; Bennys, K.; Blinowska, K.; Bonanni, L.; Bujan, A.; Carrillo, M.C.; Cichocki, A.; de Frutos-Lucas, J. Measures of Resting State EEG Rhythms for Clinical Trials in Alzheimer’s Disease: Recommendations of an Expert Panel. Alzheimers Dement. 2021, 17, 1528–1553. [Google Scholar] [CrossRef]
- Babiloni, C.; Vecchio, F.; Lizio, R.; Ferri, R.; Rodriguez, G.; Marzano, N.; Frisoni, G.B.; Rossini, P.M. Resting State Cortical Rhythms in Mild Cognitive Impairment and Alzheimer’s Disease: Electroencephalographic Evidence. J. Alzheimers Dis. 2011, 26, 201–214. [Google Scholar] [CrossRef]
- Babiloni, C.; Binetti, G.; Cassetta, E.; Cerboneschi, D.; Dal Forno, G.; Del Percio, C.; Ferreri, F.; Ferri, R.; Lanuzza, B.; Miniussi, C.; et al. Mapping Distributed Sources of Cortical Rhythms in Mild Alzheimer’s Disease. A Multicentric EEG Study. NeuroImage 2004, 22, 57–67. [Google Scholar] [CrossRef]
- Babiloni, C.; Lizio, R.; Vecchio, F.; Frisoni, G.B.; Pievani, M.; Geroldi, C.; Claudia, F.; Ferri, R.; Lanuzza, B.; Rossini, P.M. Reactivity of Cortical Alpha Rhythms to Eye Opening in Mild Cognitive Impairment and Alzheimer’s Disease: An EEG Study. J. Alzheimers Dis. 2010, 22, 1047–1064. [Google Scholar] [CrossRef]
- Huang, C.; Wahlund, L.-O.; Dierks, T.; Julin, P.; Winblad, B.; Jelic, V. Discrimination of Alzheimer’s Disease and Mild Cognitive Impairment by Equivalent EEG Sources: A Cross-Sectional and Longitudinal Study. Clin. Neurophysiol. 2000, 111, 1961–1967. [Google Scholar] [CrossRef]
- Jeong, J. EEG Dynamics in Patients with Alzheimer’s Disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef]
- Meghdadi, A.H.; Stevanović Karić, M.; McConnell, M.; Rupp, G.; Richard, C.; Hamilton, J.; Salat, D.; Berka, C. Resting State EEG Biomarkers of Cognitive Decline Associated with Alzheimer’s Disease and Mild Cognitive Impairment. PLoS ONE 2021, 16, e0244180. [Google Scholar] [CrossRef]
- Musaeus, C.S.; Engedal, K.; Høgh, P.; Jelic, V.; Mørup, M.; Naik, M.; Oeksengaard, A.-R.; Snaedal, J.; Wahlund, L.-O.; Waldemar, G.; et al. EEG Theta Power Is an Early Marker of Cognitive Decline in Dementia Due to Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, 1359–1371. [Google Scholar] [CrossRef]
- Ponomareva, N.V.; Selesneva, N.D.; Jarikov, G.A. EEG Alterations in Subjects at High Familial Risk for Alzheimer’s Disease. Neuropsychobiology 2003, 48, 152–159. [Google Scholar] [CrossRef]
- Stam, C.J.; Montez, T.; Jones, B.; Rombouts, S.; Van Der Made, Y.; Pijnenburg, Y.A.; Scheltens, P. Disturbed Fluctuations of Resting State EEG Synchronization in Alzheimer’s Disease. Clin. Neurophysiol. 2005, 116, 708–715. [Google Scholar] [CrossRef]
- Del Percio, C.; Lopez, S.; Noce, G.; Lizio, R.; Tucci, F.; Soricelli, A.; Ferri, R.; Nobili, F.; Arnaldi, D.; Famà, F.; et al. What a Single Electroencephalographic (EEG) Channel Can Tell Us About Alzheimer’s Disease Patients with Mild Cognitive Impairment. Clin. EEG Neurosci. 2023, 54, 21–35. [Google Scholar] [CrossRef]
- Meghdadi, A.H.; Salat, D.; Hamilton, J.; Hong, Y.; Boeve, B.F.; Louis, E.K.S.; Verma, A.; Berka, C. EEG and ERP Biosignatures of Mild Cognitive Impairment for Longitudinal Monitoring of Early Cognitive Decline in Alzheimer’s Disease. PLoS ONE 2024, 19, e0308137. [Google Scholar] [CrossRef]
- Tomasello, L.; Carlucci, L.; Laganà, A.; Galletta, S.; Marinelli, C.V.; Raffaele, M.; Zoccolotti, P. Neuropsychological Evaluation and Quantitative EEG in Patients with Frontotemporal Dementia, Alzheimer’s Disease, and Mild Cognitive Impairment. Brain Sci. 2023, 13, 930. [Google Scholar] [CrossRef]
- Wang, J.; Sun, T.; Zhang, Y.; Yu, X.; Wang, H. Distinct Effects of the Apolipoprotein E Ε4 Genotype on Associations Between Delayed Recall Performance and Resting-State Electroencephalography Theta Power in Elderly People Without Dementia. Front. Aging Neurosci. 2022, 14, 830149. [Google Scholar] [CrossRef]
- Fröhlich, S.; Kutz, D.F.; Müller, K.; Voelcker-Rehage, C. Characteristics of Resting State EEG Power in 80+-Year-Olds of Different Cognitive Status. Front. Aging Neurosci. 2021, 13, 675689. [Google Scholar] [CrossRef]
- Kim, S.-E.; Shin, C.; Yim, J.; Seo, K.; Ryu, H.; Choi, H.; Park, J.; Min, B.-K. Resting-State Electroencephalographic Characteristics Related to Mild Cognitive Impairments. Front. Psychiatry 2023, 14, 1231861. [Google Scholar] [CrossRef]
- Walters, K.F.; Shukla, R.; Kumar, V.; Schueren, S.; Yadav, H.; Schilaty, N.D.; Jain, S. Resting-State EEG Power Spectral Density Analysis Between Healthy and Cognitively Impaired Subjects. Brain Sci. 2025, 15, 173. [Google Scholar] [CrossRef]
- Kałamała, P.; Gyurkovics, M.; Bowie, D.C.; Clements, G.M.; Low, K.A.; Dolcos, F.; Fabiani, M.; Gratton, G. Event-Induced Modulation of Aperiodic Background EEG: Attention-Dependent and Age-Related Shifts in E:I Balance, and Their Consequences for Behavior. Imaging Neurosci. 2024, 2, 1–18. [Google Scholar] [CrossRef]
- Voytek, B.; Kramer, M.A.; Case, J.; Lepage, K.Q.; Tempesta, Z.R.; Knight, R.T.; Gazzaley, A. Age-Related Changes in 1/f Neural Electrophysiological Noise. J. Neurosci. 2015, 35, 13257–13265. [Google Scholar] [CrossRef]
- He, B.J. Scale-Free Brain Activity: Past, Present, and Future. Trends Cogn. Sci. 2014, 18, 480–487. [Google Scholar] [CrossRef]
- Ouyang, G.; Hildebrandt, A.; Schmitz, F.; Herrmann, C.S. Decomposing Alpha and 1/f Brain Activities Reveals Their Differential Associations with Cognitive Processing Speed. NeuroImage 2020, 205, 116304. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Chen, Y.; Dubois, A.E.E.; Jia, G.; Wu, Q.; Bringas-Vega, M.L.; Dumas, G.; Valdes-Sosab, P.A. Aperiodic and Periodic EEG Component Lifespan Trajectories: Monotonic Decrease versus Growth-Then-Decline. bioRxiv 2025. [Google Scholar] [CrossRef]
- Cesnaite, E.; Steinfath, P.; Jamshidi Idaji, M.; Stephani, T.; Kumral, D.; Haufe, S.; Sander, C.; Hensch, T.; Hegerl, U.; Riedel-Heller, S.; et al. Alterations in Rhythmic and Non-Rhythmic Resting-State EEG Activity and Their Link to Cognition in Older Age. NeuroImage 2023, 268, 119810. [Google Scholar] [CrossRef]
- Clements, G.M.; Bowie, D.C.; Gyurkovics, M.; Low, K.A.; Fabiani, M.; Gratton, G. Spontaneous Alpha and Theta Oscillations Are Related to Complementary Aspects of Cognitive Control in Younger and Older Adults. Front. Hum. Neurosci. 2021, 15, 621620. [Google Scholar] [CrossRef]
- Dave, S.; Brothers, T.A.; Swaab, T.Y. 1/f Neural Noise and Electrophysiological Indices of Contextual Prediction in Aging. Brain Res. 2018, 1691, 34–43. [Google Scholar] [CrossRef]
- Merkin, A.; Sghirripa, S.; Graetz, L.; Smith, A.E.; Hordacre, B.; Harris, R.; Pitcher, J.; Semmler, J.; Rogasch, N.C.; Goldsworthy, M. Do Age-Related Differences in Aperiodic Neural Activity Explain Differences in Resting EEG Alpha? Neurobiol. Aging 2023, 121, 78–87. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ray, S. Slope of the Power Spectral Density Flattens at Low Frequencies (<150 Hz) with Healthy Aging but Also Steepens at Higher Frequency (>200 Hz) in Human Electroencephalogram. Cereb. Cortex Commun. 2023, 4, tgad011. [Google Scholar] [CrossRef]
- Azami, H.; Zrenner, C.; Brooks, H.; Zomorrodi, R.; Blumberger, D.M.; Fischer, C.E.; Flint, A.; Herrmann, N.; Kumar, S.; Lanctôt, K.; et al. Beta to Theta Power Ratio in EEG Periodic Components as a Potential Biomarker in Mild Cognitive Impairment and Alzheimer’s Dementia. Alzheimers Res. Ther. 2023, 15, 133. [Google Scholar] [CrossRef]
- Rosenblum, Y.; Shiner, T.; Bregman, N.; Giladi, N.; Maidan, I.; Fahoum, F.; Mirelman, A. Decreased Aperiodic Neural Activity in Parkinson’s Disease and Dementia with Lewy Bodies. J. Neurol. 2023, 270, 3958–3969. [Google Scholar] [CrossRef]
- Wan, W.; Gao, Z.; Zhang, Q.; Gu, Z.; Chang, C.; Peng, C.-K.; Cui, X. Resting State EEG Complexity as a Predictor of Cognitive Performance. Phys. Stat. Mech. Its Appl. 2023, 624, 128952. [Google Scholar] [CrossRef]
- Stacey, J.E.; Crook-Rumsey, M.; Sumich, A.; Howard, C.J.; Crawford, T.; Livne, K.; Lenzoni, S.; Badham, S. Age Differences in Resting State EEG and Their Relation to Eye Movements and Cognitive Performance. Neuropsychologia 2021, 157, 107887. [Google Scholar] [CrossRef]
- Vlahou, E.L.; Thurm, F.; Kolassa, I.-T.; Schlee, W. Resting-State Slow Wave Power, Healthy Aging and Cognitive Performance. Sci. Rep. 2014, 4, 5101. [Google Scholar] [CrossRef]
- Finnigan, S.; Robertson, I.H. Resting EEG Theta Power Correlates with Cognitive Performance in Healthy Older Adults. Psychophysiology 2011, 48, 1083–1087. [Google Scholar] [CrossRef]
- Perez, V.; Garrido-Chaves, R.; Zapater-Fajarí, M.; Pulopulos, M.M.; Hidalgo, V.; Salvador, A. EEG Markers and Subjective Memory Complaints in Young and Older People. Int. J. Psychophysiol. 2022, 182, 23–31. [Google Scholar] [CrossRef]
- Babiloni, C.; Ferri, R.; Binetti, G.; Cassarino, A.; Dal Forno, G.; Ercolani, M.; Ferreri, F.; Frisoni, G.B.; Lanuzza, B.; Miniussi, C. Fronto-Parietal Coupling of Brain Rhythms in Mild Cognitive Impairment: A Multicentric EEG Study. Brain Res. Bull. 2006, 69, 63–73. [Google Scholar] [CrossRef]
- Babiloni, C.; Frisoni, G.B.; Vecchio, F.; Lizio, R.; Pievani, M.; Cristina, G.; Fracassi, C.; Vernieri, F.; Rodriguez, G.; Nobili, F. Stability of Clinical Condition in Mild Cognitive Impairment Is Related to Cortical Sources of Alpha Rhythms: An Electroencephalographic Study. Hum. Brain Mapp. 2011, 32, 1916–1931. [Google Scholar] [CrossRef]
- Kaiser, A.K.; Doppelmayr, M.; Iglseder, B. EEG Beta 2 Power as Surrogate Marker for Memory Impairment: A Pilot Study. Int. Psychogeriatr. 2017, 29, 1515–1523. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal Theta as a Mechanism for Cognitive Control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Cooper, P.S.; Wong, A.S.W.; Fulham, W.R.; Thienel, R.; Mansfield, E.; Michie, P.T.; Karayanidis, F. Theta Frontoparietal Connectivity Associated with Proactive and Reactive Cognitive Control Processes. NeuroImage 2015, 108, 354–363. [Google Scholar] [CrossRef]
- Cooper, P.S.; Karayanidis, F.; McKewen, M.; McLellan-Hall, S.; Wong, A.S.W.; Skippen, P.; Cavanagh, J.F. Frontal Theta Predicts Specific Cognitive Control-Induced Behavioural Changes beyond General Reaction Time Slowing. NeuroImage 2019, 189, 130–140. [Google Scholar] [CrossRef]
- Eisma, J.; Rawls, E.; Long, S.; Mach, R.; Lamm, C. Frontal Midline Theta Differentiates Separate Cognitive Control Strategies While Still Generalizing the Need for Cognitive Control. Sci. Rep. 2021, 11, 14641. [Google Scholar] [CrossRef]
- Klimesch, W.; Schimke, H.; Schwaiger, J. Episodic and Semantic Memory: An Analysis in the EEG Theta and Alpha Band. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 428–441. [Google Scholar] [CrossRef]
- Klimesch, W.; Doppelmayr, M.; Pachinger, T.; Russegger, H. Event-Related Desynchronization in the Alpha Band and the Processing of Semantic Information. Cogn. Brain Res. 1997, 6, 83–94. [Google Scholar] [CrossRef]
- Maguire, M.J.; Brier, M.R.; Ferree, T.C. EEG Theta and Alpha Responses Reveal Qualitative Differences in Processing Taxonomic versus Thematic Semantic Relationships. Brain Lang. 2010, 114, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zioga, I.; Kenett, Y.N.; Giannopoulos, A.; Luft, C.D.B. The Role of Alpha Oscillations in Free- and Goal-Directed Semantic Associations. Hum. Brain Mapp. 2024, 45, e26770. [Google Scholar] [CrossRef] [PubMed]
- Fleck, J.I.; Kuti, J.; Brown, J.; Mahon, J.R.; Gayda-Chelder, C. Frontal-Posterior Coherence and Cognitive Function in Older Adults. Int. J. Psychophysiol. 2016, 110, 217–230. [Google Scholar] [CrossRef]
- Hanouneh, S.; Amin, H.U.; Saad, N.M.; Malik, A.S. EEG Power and Functional Connectivity Correlates with Semantic Long-Term Memory Retrieval. IEEE Access 2018, 6, 8695–8703. [Google Scholar] [CrossRef]
- Nyhus, E. Brain Networks Related to Beta Oscillatory Activity during Episodic Memory Retrieval. J. Cogn. Neurosci. 2018, 30, 174–187. [Google Scholar] [CrossRef]
- Almeida, O.P.; Almeida, S.A. Short Versions of the Geriatric Depression Scale: A Study of Their Validity for the Diagnosis of a Major Depressive Episode According to ICD-10 and DSM-IV. Int. J. Geriatr. Psychiatry 1999, 14, 858–865. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II. Psychol. Assess. 1996, 1, 210. [Google Scholar]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current Concepts in Mild Cognitive Impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D., III. WMS-III Administration and Scoring Manual; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current Version and Scoring Rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Semlitsch, H.V.; Anderer, P.; Schuster, P.; Presslich, O. A Solution for Reliable and Valid Reduction of Ocular Artifacts, Applied to the P300 ERP. Psychophysiology 1986, 23, 695–703. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2010, 2011, e156869. [Google Scholar] [CrossRef]
- Kałamała, P.; Gyurkovics, M.; Clements, G.; Low, K.; Gratton, G.; Fabiani, M.; Chen, T. How to Improve the Reliability of Aperiodic Parameter Estimates in M/EEG: A Method Comparison and Recommendations for Best Practices. bioRxiv 2025. [Google Scholar] [CrossRef]
- Miller, K.J.; Sorensen, L.B.; Ojemann, J.G.; Nijs, M. den. Power-Law Scaling in the Brain Surface Electric Potential. PLoS Comput. Biol. 2009, 5, e1000609. [Google Scholar] [CrossRef] [PubMed]
- Pertermann, M.; Mückschel, M.; Adelhöfer, N.; Ziemssen, T.; Beste, C. On the Interrelation of 1/f Neural Noise and Norepinephrine System Activity during Motor Response Inhibition. J. Neurophysiol. 2019, 121, 1633–1643. [Google Scholar] [CrossRef]
- Haegens, S.; Cousijn, H.; Wallis, G.; Harrison, P.J.; Nobre, A.C. Inter- and Intra-Individual Variability in Alpha Peak Frequency. NeuroImage 2014, 92, 46–55. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”: A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Fasnacht, J.S.; Wueest, A.S.; Berres, M.; Thomann, A.E.; Krumm, S.; Gutbrod, K.; Steiner, L.A.; Goettel, N.; Monsch, A.U. Conversion between the Montreal Cognitive Assessment and the Mini-Mental Status Examination. J. Am. Geriatr. Soc. 2023, 71, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Skills 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Kaplan, E.; Goodglass, H.; Weintraub, S. Boston Naming Test; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Benton, A.L.; Hamsher, d.S.K.; Sivan, A.B. Controlled Oral Word Association Test; American Psychological Association: Washington, DC, USA, 1983. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG Differences between Eyes-Closed and Eyes-Open Resting Conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; De Blasio, F.M. EEG Differences between Eyes-Closed and Eyes-Open Resting Remain in Healthy Ageing. Biol. Psychol. 2017, 129, 293–304. [Google Scholar] [CrossRef]
- Polich, J. On the Relationship between EEG and P300: Individual Differences, Aging, and Ultradian Rhythms. Int. J. Psychophysiol. 1997, 26, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Kavcic, V.; Zalar, B.; Giordani, B. The Relationship between Baseline EEG Spectra Power and Memory Performance in Older African Americans Endorsing Cognitive Concerns in a Community Setting. Int. J. Psychophysiol. 2016, 109, 116–123. [Google Scholar] [CrossRef]
- Požar, R.; Kero, K.; Martin, T.; Giordani, B.; Kavcic, V. Task Aftereffect Reorganization of Resting State Functional Brain Networks in Healthy Aging and Mild Cognitive Impairment. Front. Aging Neurosci. 2023, 14, 1061254. [Google Scholar] [CrossRef]
- Požar, R.; Giordani, B.; Kavcic, V. Pre vs Post-Task Modulation of Resting State EEG Functional Connectivity and Network Topology. Alzheimers Dement. 2022, 18, e066926. [Google Scholar] [CrossRef]
- Lydon, E.A.; Nguyen, L.T.; Shende, S.A.; Chiang, H.-S.; Spence, J.S.; Mudar, R.A. EEG Theta and Alpha Oscillations in Early versus Late Mild Cognitive Impairment during a Semantic Go/NoGo Task. Behav. Brain Res. 2022, 416, 113539. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Mudar, R.A.; Chiang, H.-S.; Schneider, J.M.; Maguire, M.J.; Kraut, M.A.; Hart, J. Theta and Alpha Alterations in Amnestic Mild Cognitive Impairment in Semantic Go/NoGo Tasks. Front. Aging Neurosci. 2017, 9, 160. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Marini, F.; Zacharczuk, L.; Llano, D.A.; Mudar, R.A. Theta and Alpha Band Oscillations during Value-Directed Strategic Processing. Behav. Brain Res. 2019, 367, 210–214. [Google Scholar] [CrossRef]
- Shende, S.A.; Jones, S.E.; Mudar, R.A. Alpha and Theta Oscillations on a Visual Strategic Processing Task in Age-Related Hearing Loss. Front. Neurosci. 2024, 18, 1382613. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Paul, R.H.; Cohen, R.A.; Williams, L.M.; MacGregor, K.L.; Jefferson, A.L.; Tate, D.F.; Gunstad, J.; Gordon, E. Category and Letter Verbal Fluency across the Adult Lifespan: Relationship to EEG Theta Power. Arch. Clin. Neuropsychol. 2005, 20, 561–573. [Google Scholar] [CrossRef]
- Hatta, K.; Kishi, Y.; Wada, K.; Takeuchi, T.; Taira, T.; Uemura, K.; Ogawa, A.; Takahashi, K.; Sato, A.; Shirakawa, M.; et al. Suvorexant for Reduction of Delirium in Older Adults After Hospitalization: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2427691. [Google Scholar] [CrossRef] [PubMed]
- McCarrey, A.C.; An, Y.; Kitner-Triolo, M.H.; Ferrucci, L.; Resnick, S.M. Sex Differences in Cognitive Trajectories in Clinically Normal Older Adults. Psychol. Aging 2016, 31, 166–175. [Google Scholar] [CrossRef]
- McKeown, D.J.; Roberts, E.; Finley, A.J.; Kelley, N.J.; Keage, H.A.D.; Schinazi, V.R.; Baumann, O.; Moustafa, A.A.; Angus, D.J. Lower Aperiodic EEG Activity Is Associated with Reduced Verbal Fluency Performance across Adulthood. Neurobiol. Aging 2025, 151, 29–41. [Google Scholar] [CrossRef]
- Gao, R.; Peterson, E.J.; Voytek, B. Inferring Synaptic Excitation/Inhibition Balance from Field Potentials. NeuroImage 2017, 158, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Scaduto, P.; Lauterborn, J.C.; Cox, C.D.; Fracassi, A.; Zeppillo, T.; Gutierrez, B.A.; Keene, C.D.; Crane, P.K.; Mukherjee, S.; Russell, W.K.; et al. Functional Excitatory to Inhibitory Synaptic Imbalance and Loss of Cognitive Performance in People with Alzheimer’s Disease Neuropathologic Change. Acta Neuropathol. 2023, 145, 303–324. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, Y.; Sato, K.; Fujimoto, A. Inhibitory and Excitatory Imbalance in Neurodegenerative Diseases. Neurol. Lett. 2025, 4, 86–91. [Google Scholar] [CrossRef]
- van Nifterick, A.M.; Mulder, D.; Duineveld, D.J.; Diachenko, M.; Scheltens, P.; Stam, C.J.; van Kesteren, R.E.; Linkenkaer-Hansen, K.; Hillebrand, A.; Gouw, A.A. Resting-State Oscillations Reveal Disturbed Excitation–Inhibition Ratio in Alzheimer’s Disease Patients. Sci. Rep. 2023, 13, 7419. [Google Scholar] [CrossRef] [PubMed]
| MCI | Controls | p-Value | |
|---|---|---|---|
| Demographics | |||
| Total N | 19 | 19 | -- |
| Age (yrs) | 68.84 (7.28) | 69.58 (6.75) | 0.748 |
| Education (yrs) | 17.21 (1.51) | 16.58 (2.57) | 0.363 |
| Sex | 8 F/11 M | 16 F/3 M | 0.019 * |
| Cognitive Measures | |||
| MMSE | 28.05 (1.47) | -- | -- |
| MoCA | 23.53 (2.99) | 28.11 (1.29) | <0.001 * |
| LM: Immediate | 10.53 (1.84) | 17.14 (1.69) | <0.001 * |
| LM: Delayed | 8.63 (2.19) | 16.14 (1.91) | <0.001 * |
| TMT-A (S)s.) | 29.74 (9.25) | 26.32 (7.14) | 0.211 |
| TMT-B (S)s.) | 76.37 (27.24) | 65.79 (14.60) | 0.147 |
| BNT | 27.24 (2.37) | 28.47(1.12) | 0.050 * |
| COWAT: Letter Fluency | 41.47 (8.10) | 52.00 (7.96) | <0.001 * |
| COWAT: Category Fluency | 20.16 (6.82) | 22.32 (5.15) | 0.279 |
| MCI | Controls | Main Effect of Group | |
|---|---|---|---|
| Absolute Power | |||
| Theta (fronto-central) | 0.2106 (0.2032) | 0.1882 (0.1770) | F(1, 36) = 0.12; p = 0.726 |
| Alpha (parietal) | 0.2136 (0.2641) | 0.2142 (0.2797) | F(1, 36) = 0.00; p = 0.995 |
| Beta (parietal) | 0.0601 (0.0513) | 0.0554 (0.0550) | F(1, 36) = 0.07; p = 0.793 |
| 1/f-Adjusted Power | |||
| Theta (fronto-central) | −0.0347 (0.0476) | −0.0552 (0.0844) | F(1, 36) = 0.81; p = 0.373 |
| Alpha (parietal) | 0.0135 (0.0533) | −0.0056 (0.0629) | F(1, 36) = 0.97; p = 0.332 |
| Beta (parietal) | 0.0003 (0.0009) | −0.0002 (0.0020) | F(1, 36) = 0.89; p = 0.353 |
| 1/f Slope | |||
| Fronto-central | −0.0464 (0.0405) | −0.0621 (0.0611) | F(1, 36) = 0.83; p = 0.368 |
| Parietal | −0.0384 (0.0408) | −0.0564 (0.0663) | F(1, 36) = 0.96; p = 0.333 |
| MCI | Controls | |
|---|---|---|
| 1/f-Adjusted Power: Theta (Fronto-Central) | ||
| TMT-B | r(17) = −0.22, p = 0.361 | r(17) = −0.17, p = 0.478 |
| COWAT: Letter Fluency | r(17) = 0.04, p = 0.864 | r(17) = −0.48, p = 0.036 * |
| 1/f-Adjusted Power: Alpha (Parietal) | ||
| BNT | r(17) = 0.06, p = 0.818 | r(17) = 0.11, p = 0.665 |
| COWAT: Category Fluency | r(17) = −0.17, p = 0.495 | r(17) = −0.11, p = 0.641 |
| 1/f-Adjusted Power: Beta (Parietal) | ||
| MMSE/MoCA 1 | r(17) = −0.09, p = 0.709 | r(17) = 0.46, p = 0.048 * |
| LM: Delayed | r(17) = 0.12, p = 0.620 | r(17) = 0.45, p = 0.051 |
| MCI | Controls | |
|---|---|---|
| 1/f Slope: Fronto-Central | ||
| TMT-B | r(17) = −0.08, p = 0.750 | r(17) = −0.02, p = 0.920 |
| COWAT: Letter Fluency | r(17) = 0.11, p = 0.650 | r(17) = −0.63, p = 0.004 * |
| 1/f Slope: Parietal | ||
| BNT | r(17) = 0.22, p = 0.375 | r(17) = −0.29, p = 0.236 |
| COWAT: Category Fluency | r(17) = −0.10, p = 0.687 | r(17) = −0.31, p = 0.189 |
| MMSE/MoCA 1 | r(17) = 0.17, p = 0.481 | r(17) = 0.21, p = 0.385 |
| LM: Delayed | r(17) = 0.20, p = 0.402 | r(17) = 0.16, p = 0.509 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warren, T.S.; Shende, S.A.; Ashrafi, J.; Clements, G.M.; Mudar, R.A. Resting-State EEG Power and Aperiodic Activity in Individuals with Mild Cognitive Impairment and Cognitively Healthy Controls. Brain Sci. 2025, 15, 1305. https://doi.org/10.3390/brainsci15121305
Warren TS, Shende SA, Ashrafi J, Clements GM, Mudar RA. Resting-State EEG Power and Aperiodic Activity in Individuals with Mild Cognitive Impairment and Cognitively Healthy Controls. Brain Sciences. 2025; 15(12):1305. https://doi.org/10.3390/brainsci15121305
Chicago/Turabian StyleWarren, Teresa S., Shraddha A. Shende, Jaya Ashrafi, Grace M. Clements, and Raksha A. Mudar. 2025. "Resting-State EEG Power and Aperiodic Activity in Individuals with Mild Cognitive Impairment and Cognitively Healthy Controls" Brain Sciences 15, no. 12: 1305. https://doi.org/10.3390/brainsci15121305
APA StyleWarren, T. S., Shende, S. A., Ashrafi, J., Clements, G. M., & Mudar, R. A. (2025). Resting-State EEG Power and Aperiodic Activity in Individuals with Mild Cognitive Impairment and Cognitively Healthy Controls. Brain Sciences, 15(12), 1305. https://doi.org/10.3390/brainsci15121305





