Effects of Navigated rTMS on Post-Stroke Upper-Limb Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Search Strategy
2.4. Data Extraction
2.5. Quality Assessment
2.6. Strategy for Data Synthesis
2.6.1. Sensitivity Analyses
2.6.2. Computation of Change Scores and Variances
3. Results
3.1. Literature Search and Characteristics of the Included Randomized Clinical Trials
3.2. Methodological Quality Assessment
3.3. Navigated rTMS for Patients with Stroke
3.4. Effect of Navigated rTMS on Upper Limb Function
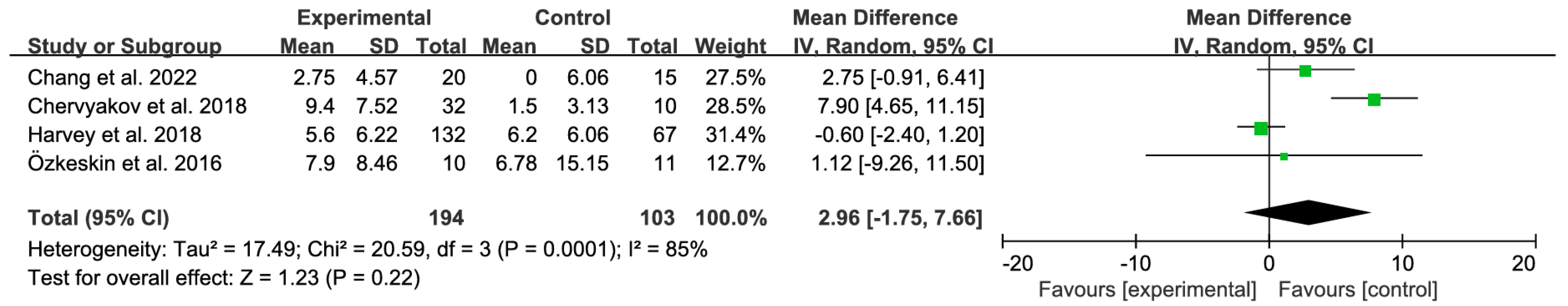
3.4.1. Primary Analysis
3.4.2. Sensitivity Analyses
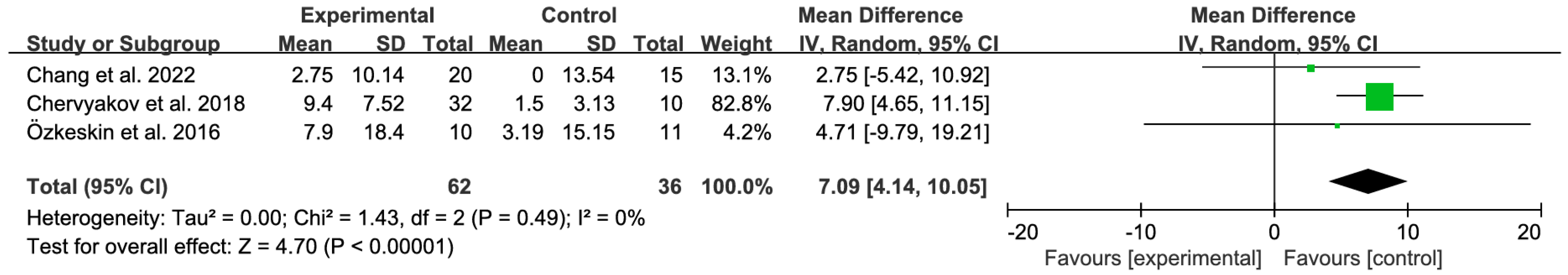
3.4.3. Subgroup Analysis by Treatment Duration (2-Week Protocols)
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwakkel, G.; Kollen, B.J.; van der Grond, J.; Prevo, A.J. Probability of regaining dexterity in the flaccid upper limb: Impact of severity of paresis and time since onset in acute stroke. Stroke 2003, 34, 2181–2186. [Google Scholar] [CrossRef]
- Norman, S.L.; Wolpaw, J.R.; Reinkensmeyer, D.J. Targeting neuroplasticity to improve motor recovery after stroke. bioRxiv 2020. [Google Scholar] [CrossRef]
- D’Arcy, R.C.N.; Greene, T.; Greene, D.; Frehlick, Z.; Fickling, S.D.; Campbell, N.; Etheridge, T.; Smith, C.; Bollinger, F.; Danilov, Y.; et al. Portable neuromodulation induces neuroplasticity to re-activate motor function recovery from brain injury: A high-density MEG case study. J. Neuroeng. Rehabil. 2020, 17, 158. [Google Scholar] [CrossRef]
- Duan, X.; Huang, D.; Zhong, H.; Wu, J.; Xiao, Z.; Yang, P.; Han, Y.; Jiang, H.; Zhou, P.; Liu, X. Efficacy of rTMS in treating functional impairment in post-stroke patients: A systematic review and meta-analysis. Neurol. Sci. 2024, 45, 3887–3899. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Valle, A.C.; Rocha, R.R.; Duarte, J.; Ferreira, M.J.; Wagner, T.; Fecteau, S.; Rigonatti, S.P.; Riberto, M.; et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 2006, 37, 2115–2122. [Google Scholar] [CrossRef]
- Takeuchi, N.; Chuma, T.; Matsuo, Y.; Watanabe, I.; Ikoma, K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke 2005, 36, 2681–2686. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, W.; Zhang, H.; Fang, L.; Chen, J.; Li, X.; Yu, H.; Song, J.; Chen, S.; Zheng, B. Effects of robot-assisted upper limb training combined with intermittent theta burst stimulation (iTBS) on cortical activation in stroke patients: A functional near-infrared spectroscopy study. NeuroRehabilitation 2024, 54, 421–434. [Google Scholar] [CrossRef]
- Khedr, E.M.; Etraby, A.E.; Hemeda, M.; Nasef, A.M.; Razek, A.A. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol. Scand. 2010, 121, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Lee, S. Effects of high-frequency repetitive transcranial magnetic stimulation combined with motor learning on motor function and grip force of the upper limbs and activities of daily living in patients with a subacute stroke. Int. J. Environ. Res. Public Health 2023, 20, 6093. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, D.; Watanabe, H.; Dan, I.; Taga, G. MinR 10/20 system: Quantitative and reproducible cranial landmark setting method for MRI based on minimum initial reference points. J. Neurosci. Methods 2016, 264, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Montemurro, N.; Cattari, N.; Autelitano, M.; Cutolo, F.; Ferrari, V.; Cigna, E.; Condino, S. Targeting accuracy of neuronavigation: A comparative evaluation of an innovative wearable AR platform vs. traditional EM navigation. Front. Digit. Health 2025, 6, 1500677. [Google Scholar] [CrossRef]
- Caulfield, K.A.; Fleischmann, H.H.; Cox, C.E.; Wolf, J.P.; George, M.S.; McTeague, L.M. Neuronavigation maximizes accuracy and precision in TMS positioning: Evidence from 11,230 distance, angle, and electric field modeling measurements. Brain Stimul. 2022, 15, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Pellegrino, G.; Lina, J.-M.; Benali, H.; Grova, C. Hierarchical Bayesian modeling of the relationship between task-related hemodynamic responses and neuronal excitability: A simultaneous fNIRS/TMS study. bioRxiv 2021. [Google Scholar] [CrossRef]
- Harvey, R.L.; Edwards, D.; Dunning, K.; Fregni, F.; Stein, J.; Laine, J.; Rogers, L.M.; Vox, F.; Durand-Sanchez, A.; Bockbrader, M. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke 2018, 49, 2138–2146. [Google Scholar] [CrossRef]
- Gentner, R.; Wankerl, K.; Reinsberger, C.; Zeller, D.; Classen, J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: Evidence of rapid polarity-reversing metaplasticity. Cereb. Cortex 2008, 18, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Narushima, K.; McCormick, L.M.; Yamada, T.; Thatcher, R.W.; Robinson, R.G. Subgenual cingulate theta activity predicts treatment response of repetitive transcranial magnetic stimulation in participants with vascular depression. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 75–84. [Google Scholar] [CrossRef]
- Willems, R.M.; Labruna, L.; D’Esposito, M.; Ivry, R.; Casasanto, D. A functional role for the motor system in language understanding: Evidence from theta-burst transcranial magnetic stimulation. Psychol. Sci. 2011, 22, 849–854. [Google Scholar] [CrossRef]
- Bystritsky, A.; Kaplan, J.T.; Feusner, J.D.; Kerwin, L.E.; Wadekar, M.; Burock, M.; Wu, A.D.; Iacoboni, M. A preliminary study of fMRI-guided rTMS in the treatment of generalized anxiety disorder. J. Clin. Psychiatry 2008, 69, 1092–1098. [Google Scholar] [CrossRef]
- Hirayama, A.; Saitoh, Y.; Kishima, H.; Shimokawa, T.; Oshino, S.; Hirata, M.; Kato, A.; Yoshimine, T. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain 2006, 122, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Plewnia, C.; Reimold, M.; Najib, A.; Reischl, G.; Plontke, S.K.; Gerloff, C. Moderate therapeutic efficacy of positron emission tomography-navigated repetitive transcranial magnetic stimulation for chronic tinnitus: A randomised, controlled pilot study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 152–156. [Google Scholar] [CrossRef]
- Chervyakov, A.V.; Poydasheva, A.G.; Lyukmanov, R.H.; Suponeva, N.A.; Chernikova, L.A.; Piradov, M.A.; Ustinova, K.I. Effects of navigated repetitive transcranial magnetic stimulation after stroke. J. Clin. Neurophysiol. 2018, 35, 166–172. [Google Scholar] [CrossRef]
- Özkeskin, M.; Öztürk, V.; Cakmur, R.; Kara, B. Navigated repetitive transcranial magnetic stimulation or Brunnstrom hand manipulation: Which treatment is more effective in stroke cases? J. Neurol. Sci. 2016, 33, 361. [Google Scholar]
- Chang, P.-W.; Lu, C.-F.; Chang, S.-T.; Tsai, P.-Y. Functional near-infrared spectroscopy as a target navigator for rTMS modulation in patients with hemiplegia: A randomized control study. Neurol. Ther. 2022, 11, 103–121. [Google Scholar] [CrossRef]
- Yu, G.H.; Park, C.; Jeong, M.G.; Jung, G.S.; Kim, K.T. Clinical implementation, barriers, and unmet needs of rTMS and neuro-navigation systems in stroke rehabilitation: A nationwide survey in South Korea. Front. Neurol. 2024, 15, 1423013. [Google Scholar] [CrossRef] [PubMed]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.-H.; Di Bella, P.; Johnson, G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [CrossRef]
- Berlim, M.; Van den Eynde, F.; Daskalakis, Z.J. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychol. Med. 2013, 43, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.W.; Shuster, J.J.; Chung, J.W.; Vaillancourt, D.E.; Patten, C.; Ostrem, J.; Okun, M.S. Repetitive transcranial magnetic stimulation (rTMS) therapy in Parkinson disease: A meta-analysis. PM&R 2016, 8, 356–366. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- National Institute for Health Research (NIHR). PROSPERO: International Prospective Register of Systematic Reviews; Centre for Reviews and Dissemination, University of York: York, UK, 2012; Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 20 October 2025).
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. BMJ 1995, 310, 1120–1123. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and Weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar] [CrossRef]
- Riley, R.D.; Higgins, J.P.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 241–284. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2021. [Google Scholar]
- Edwards, D.J.; Liu, C.Y.; Dunning, K.; Fregni, F.; Laine, J.; Leiby, B.E.; Rogers, L.M.; Harvey, R.L. Electric Field Navigated 1-Hz rTMS for Poststroke Motor Recovery: The E-FIT Randomized Controlled Trial. Stroke 2023, 54, 2254–2264. [Google Scholar] [CrossRef]
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 349–374. [Google Scholar] [CrossRef]
- Lioumis, P.; Rosanova, M. The role of neuronavigation in TMS–EEG studies: Current applications and future perspectives. J. Neurosci. Methods 2022, 380, 109677. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, R.; Luo, X.; Zhang, S.; Zhong, X.; Ning, Y.; Zhang, B. Repetitive transcranial magnetic stimulation target location methods for depression. Front. Neurosci. 2021, 15, 695423. [Google Scholar] [CrossRef]
- Chen, G.; Lin, T.; Wu, M.; Cai, G.; Ding, Q.; Xu, J.; Li, W.; Wu, C.; Chen, H.; Lan, Y. Effects of repetitive transcranial magnetic stimulation on upper-limb and finger function in stroke patients: A systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 2022, 13, 940467. [Google Scholar] [CrossRef]
- Sheng, R.; Chen, C.; Chen, H.; Yu, P. Repetitive transcranial magnetic stimulation for stroke rehabilitation: Insights into the molecular and cellular mechanisms of neuroinflammation. Front. Immunol. 2023, 14, 1197422. [Google Scholar] [CrossRef]
- Chung, C.; Mak, M. Effect of repetitive transcranial magnetic stimulation on physical function and motor signs in Parkinson’s disease: A systematic review and meta-analysis. Brain Stimul. 2016, 9, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Li, Y.; Gao, Y.; Cao, Q. Efficacy and safety of transcranial magnetic stimulation in the treatment of children, adolescents and young adults with depression: A meta-analysis of randomized controlled trials. J. Affect. Disord. 2025, 392, 120132. [Google Scholar] [CrossRef]
- Peng, X.-M.; Gong, C.; Xiao, M.-X.; Chen, L.-S.; Li, Y.; Chen, J.; Wang, M.-Y.; Luo, Y. Effect of repetitive transcranial magnetic stimulation with different stimulation parameters on post-stroke dysphagia: A systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 2025, 16, 1586734. [Google Scholar] [CrossRef] [PubMed]
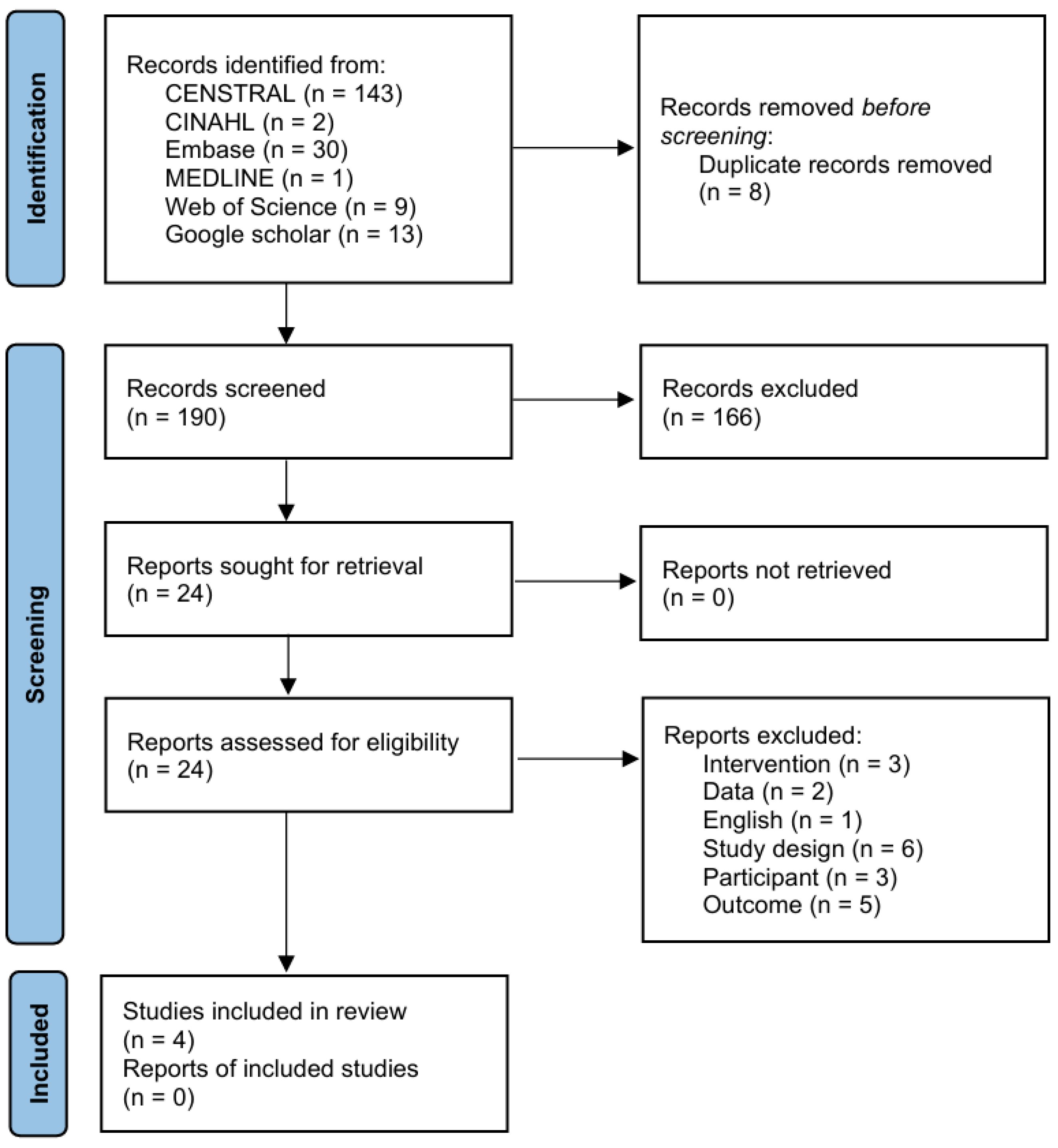
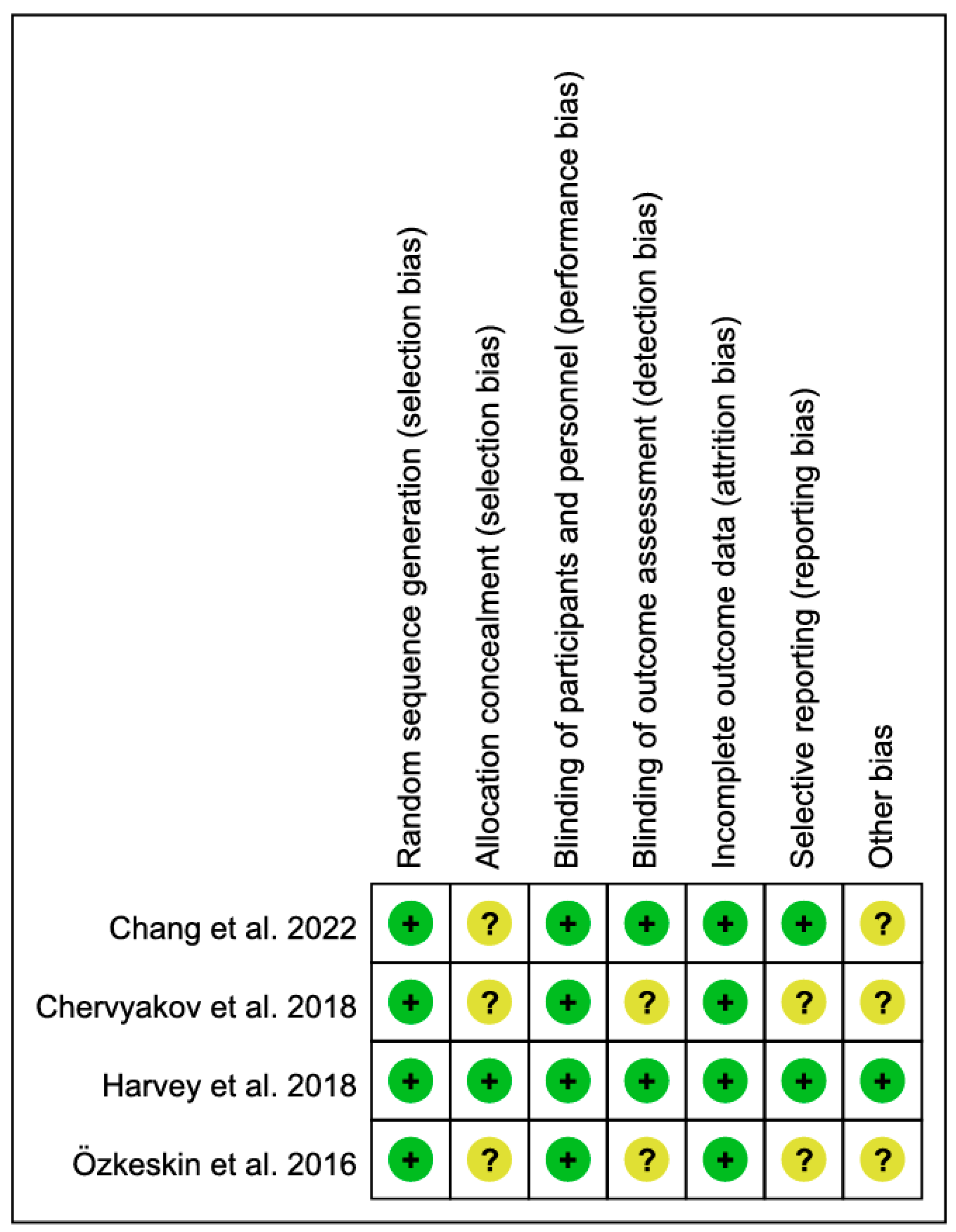
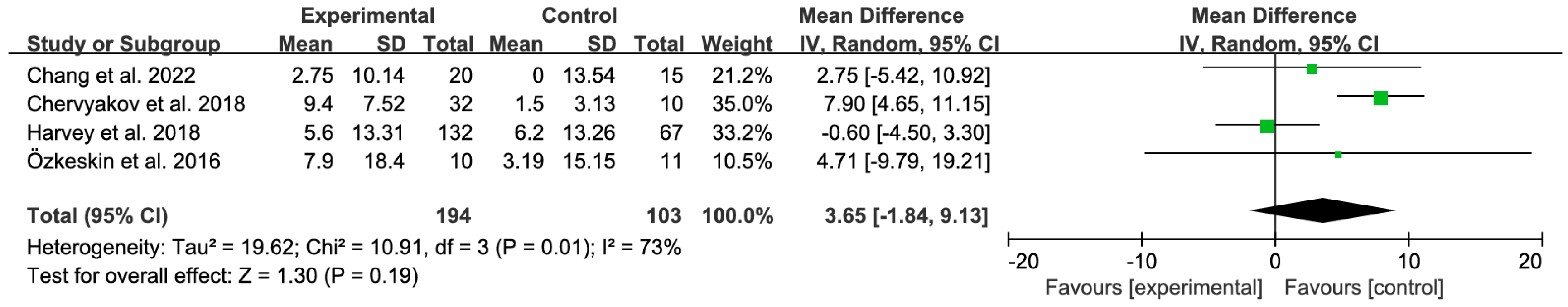
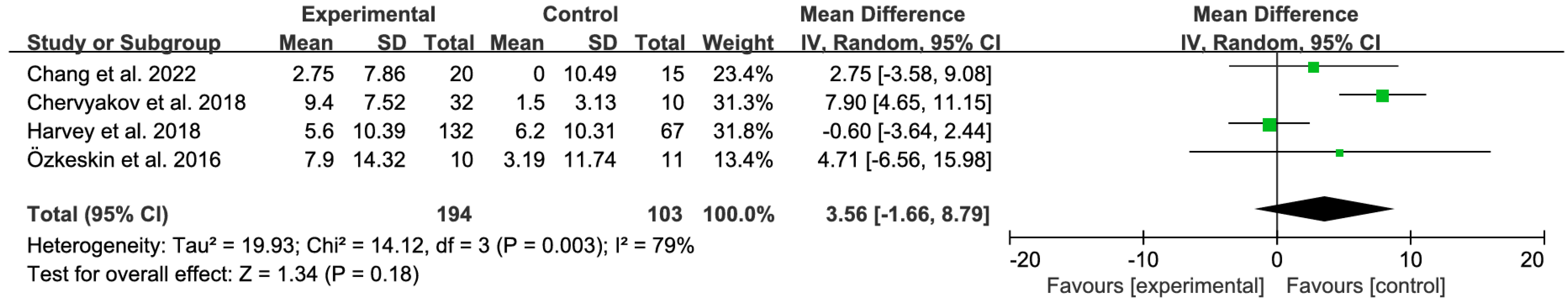
| Study | Participants | Therapeutic Intensity | Outcome |
|---|---|---|---|
| Chang et al., 2022 [23] | EG = 20 CG = 15 | 2 weeks, 5 sessions/week (10 sessions); fNIRS-guided iTBS over ipsilesional M1, 80% MT, 600 pulses/session. | Upper limb function: FMA-UE, WMFT |
| Chervyakov et al., 2018 [21] | EG = 32 CG = 10 | 2 weeks, 5 sessions/week (10 sessions); MRI-guided rTMS (Nextstim) over M1 at 90% RMT, 1 Hz (ipsilesional), 10 Hz (contralesional), or dual protocol (~1200–1500 pulses/session). | Upper limb function: FMA-UE |
| Harvey et al., 2018 [14] | EG = 132 CG = 67 | 6 weeks, 3 sessions/week (18 sessions); MRI-guided rTMS 1 Hz at 90% RMT, ~1200 pulses/session + task-oriented training. | Upper limb function: FMA-UE, WMFT, ARAT Quality of life: SIS-16, EQ-5D Spasticity: MAS |
| ÖZKESKİN et al., 2016 [22] | EG = 10 CG = 11 | 2 weeks, 5 sessions/week (10 sessions); MRI-guided 1 Hz rTMS at 90% RMT, 1500 pulses/session + Brunnstrom hand training. | Upper limb function: FMA-UE, JTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, J.; Kim, C. Effects of Navigated rTMS on Post-Stroke Upper-Limb Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Brain Sci. 2025, 15, 1247. https://doi.org/10.3390/brainsci15111247
Shim J, Kim C. Effects of Navigated rTMS on Post-Stroke Upper-Limb Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Brain Sciences. 2025; 15(11):1247. https://doi.org/10.3390/brainsci15111247
Chicago/Turabian StyleShim, Jungwoo, and Changju Kim. 2025. "Effects of Navigated rTMS on Post-Stroke Upper-Limb Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Brain Sciences 15, no. 11: 1247. https://doi.org/10.3390/brainsci15111247
APA StyleShim, J., & Kim, C. (2025). Effects of Navigated rTMS on Post-Stroke Upper-Limb Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Brain Sciences, 15(11), 1247. https://doi.org/10.3390/brainsci15111247






