Type 2 Diabetes Mellitus Exacerbates Brain Injury After Status Epilepticus in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Type 2 Diabetes Mellitus Model
2.3. Status Epilepticus Model
2.4. Experimental Groups for Histological Analysis
2.5. Tissue Processing for Histological Analysis
2.6. Fluoro-Jade B Staining
2.7. Hematoxylin and Eosin Staining (H&E)
2.8. Immunohistochemical Detection of Glia and Microglia
2.9. Cell Counting and Tissue Loss Analysis
2.10. Statistical Analysis
3. Results
3.1. Blood Glucose Levels
3.2. Status Epilepticus Severity
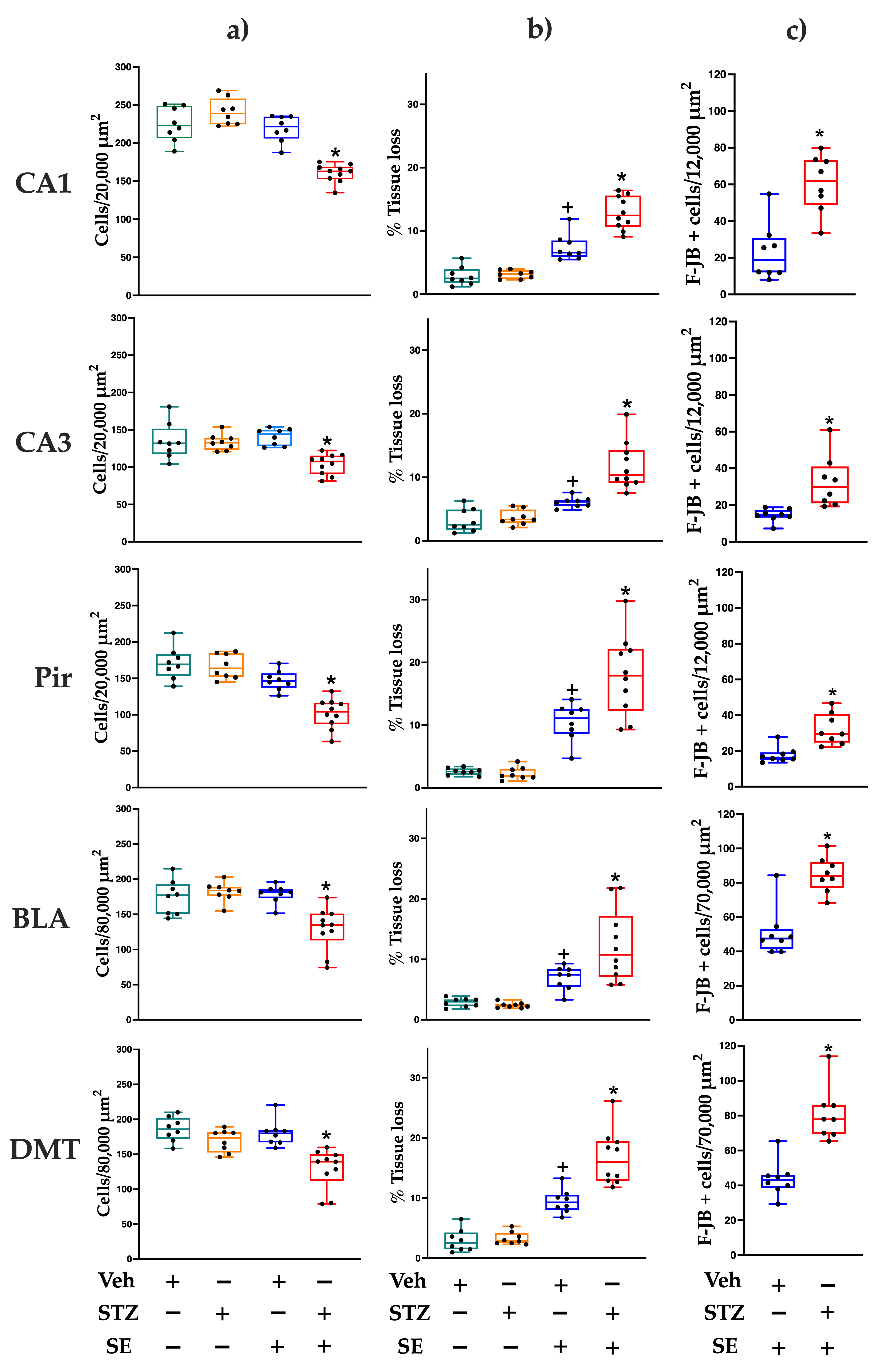
3.3. Neurodegeneration
3.4. Cell Counting and Tissue Loss
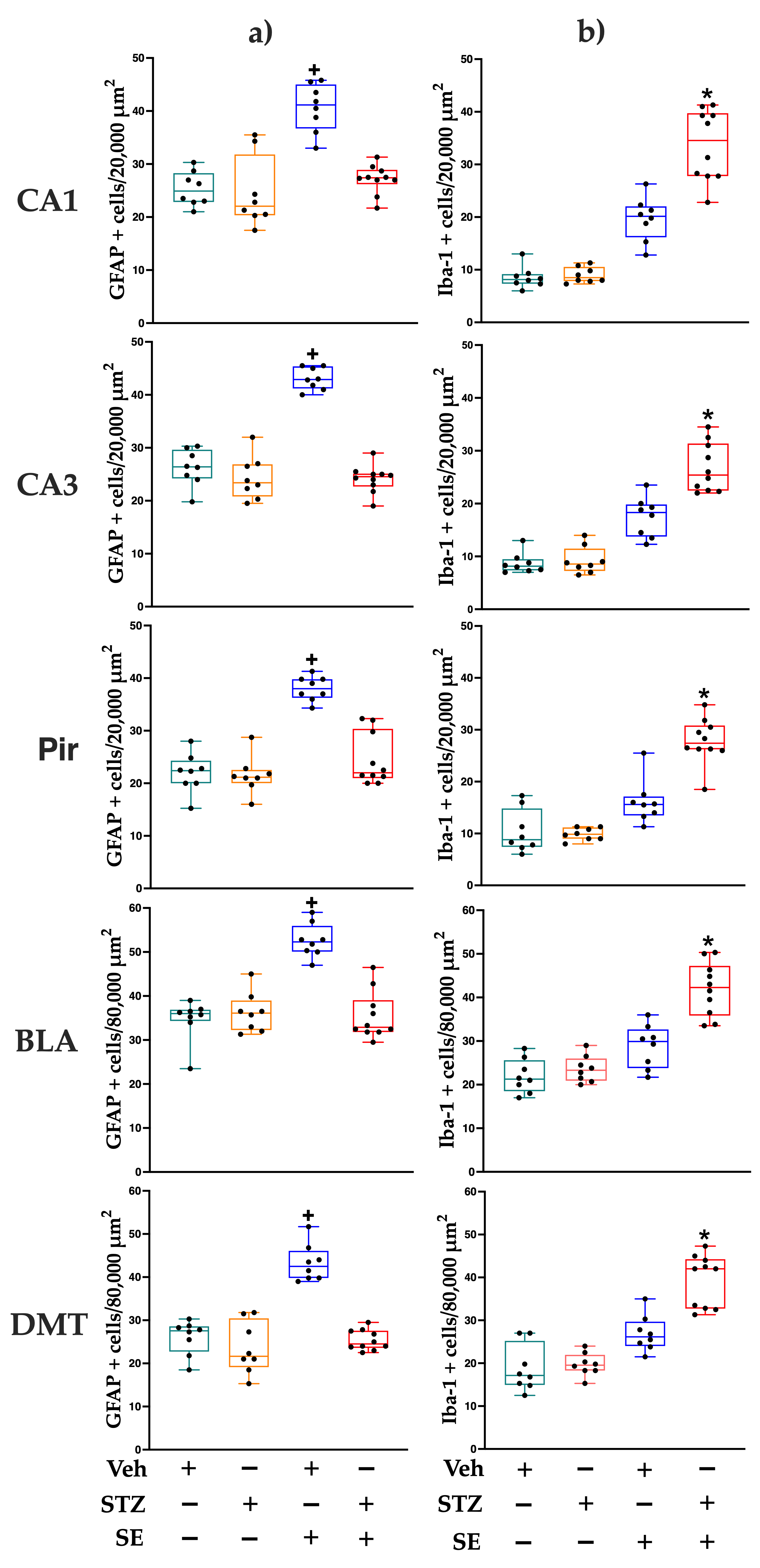
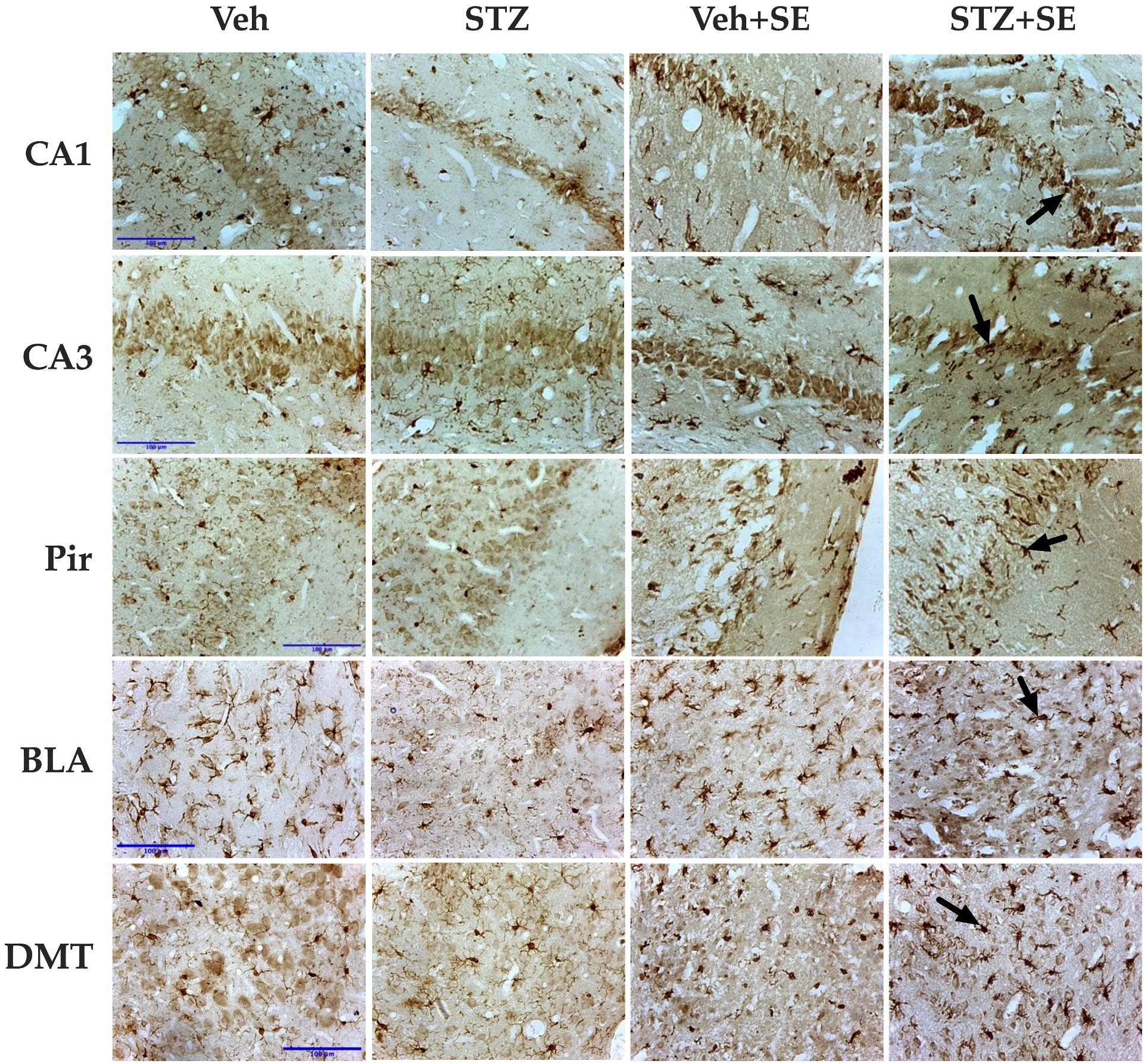
3.5. Glia and Microglia Counting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SE | Status epilepticus |
| T2DM | Type 2 diabetes mellitus |
| STZ | Streptozocin |
| P | Postnatal day |
| i.p. | Intraperitoneal |
| s.c. | Subcutaneous |
| DM | Diabetes mellitus |
| T1DM | Type 1 diabetes mellitus |
| Veh | Vehicle |
| PB | Phosphate buffer |
| F-JB | Fluoro-Jade B |
| H&E | Hematoxylin and eosin staining |
| PBT | Phosphate-buffer containing 1% triton |
| GFAP | Glial fibrillary acidic protein |
| Iba-1 | Ionized calcium-binding adapter molecule 1 |
| CA1 | CA1 hippocampal field |
| CA3 | CA3 hippocampal field |
| Pir | Piriform cortex |
| BLA | Basolateral amygdala nucleus |
| DMT | Dorsomedial thalamic nucleus |
| ROI | Region of interest |
| ANOVA | Analysis of variance |
References
- Yun, C.; Wang, X. Association between seizures and diabetes mellitus: A comprehensive review of literature. Curr. Diabetes Rev. 2013, 9, 350–354. [Google Scholar] [CrossRef]
- Shlobin, N.A.; Sander, J.W. Drivers for the comorbidity of type 2 diabetes mellitus and epilepsy: A scoping review. Epilepsy Behav. 2020, 106, 107043. [Google Scholar] [CrossRef]
- Wu, S.; Ding, Y. Type 1 diabetes and the risk of epilepsy: A meta-analysis. J. Diabetes Investig. 2024, 15, 364–373. [Google Scholar] [CrossRef]
- Schleicher, E.; Gerdes, C.; Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Freckmann, G.; Heinemann, L.; Nauck, M. Landgraf, R. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2022, 130, S1–S8. [Google Scholar] [CrossRef]
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S. Redefining epilepsy. Curr. Opin. Neurol. 2015, 28, 130–135. [Google Scholar] [CrossRef]
- Klimek, P.; Kautzky-Willer, A.; Chmiel, A.; Schiller-Frühwirth, I.; Thurner, S. Quantification of Diabetes Comorbidity Risks across Life Using Nation-Wide Big Claims Data. PLoS Comput. Biol. 2015, 11, e1004125. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, E.; Berkovic, S.; Capovilla, G.; Connolly, M.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.; Moshe, S.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Maccario, M.; Messis, C.P.; Vastola, E.F. Focal seizures as a manifesta- tion of hyperglycemia without ketoacidosis. A report of seven cases with review of the literature. Neurology 1965, 15, 195–206. [Google Scholar] [CrossRef]
- Whiting, S.; Camfield, P.; Arab, D.; Salisbury, S. Insulin-dependent diabetes mellitus presenting in children as frequent, medically unresponsive, partial seizures. J. Child. Neurol. 1997, 12, 178–180. [Google Scholar] [CrossRef]
- Huang, C.W.; Tsai, J.J.; Ou, H.Y.; Wang, S.T.; Cheng, J.T.; Wu, S.N.; Huang, C.C. Diabetic hyperglycemia is associated with the severity of epileptic seizures in adults. Epilepsy Res. 2008, 79, 71–77. [Google Scholar] [CrossRef]
- Chiewthanakul, P.; Noppaklao, P.; Sawanyawisuth, K.; Tiamkao, S. Hyperglycem ia associated with seizure control in status epilepticus. Epilepsy Behav. 2015, 49, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Cokar, O.; Aydin, B.; Ozer, F. Non-ketotic hyperglycaemia presenting as epilepsia partialis continua. Seizure 2004, 13, 264–269. [Google Scholar] [CrossRef]
- Hennis, A.; Corbin, D.; Fraser, H. Focal seizures and nonketotic hyperglycaemia. J. Neurol. Neurosurg. Psychiatry 1992, 55, 195–197. [Google Scholar] [CrossRef]
- Hwang, K.J.; Yoon, S.; Park, K.-C. Non-ketotic hyperglycaemia presenting as epilepsia partialis continua. Epileptic Disord. 2016, 18, 201–203. [Google Scholar] [CrossRef]
- Baltyde, D.; De Toffol, B.; Nacher, M.; Sabbah, N. Epileptic seizures during Non-Ketotic Hyperglycemia (NKH) in French Guiana: A retrospective study. Front. Endocrinol. 2022, 13, 946642. [Google Scholar] [CrossRef]
- Kowkabi, S.; Nemati, R.; Mohammadi, M.; Kaboodkhani, R.; Omrani, A.; Ghavipisheh, M. Temporal subcortical T2 hypointensity on MRI in epilepsia partialis continua, a non ketotic hyperglycemia rather than herpes encephalitis. Int. J. Neurosci. 2022, 132, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Tjälve, H.; Wilander, E.; Johansson, E.B. Distribution of labelled streptozotocin in mice: Uptake and retention in pancreatic islets. J. Endocrinol. 1976, 69, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan and streptozotocin induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Huang, C.W.; Cheng, J.T.; Tsai, J.J.; Wu, S.N.; Huang, C.C. Diabetic hyperglycemia aggravates seizures and status epilepticus-induced hippocampal damage. Neurotox. Res. 2009, 15, 71–81. [Google Scholar] [CrossRef]
- Schauwecker, P.E. The effects of glycemic control on seizures and seizures-induced excitotoxic cell death. BMC Neurosci. 2012, 13, 94. [Google Scholar] [CrossRef]
- Ramos-Riera, K.P.; Beltrán-Parrazal, L.; Morgado-Valle, C.; Pérez-Severiano, F.; Martínez-Gopar, P.E.; López-Meraz, M.L. Type 2 diabetes mellitus facilitates status epilepticus in adult rats: Seizure severity, neurodegeneration, and oxidative stress. Epilepsia Open 2024, 9, 665–678. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Wasterlain, C.G.; Chen, J. Definition and Classification of Status Epilepticus. In Status Epilepticus, Mechanisms and Management, 1st ed.; Wasterlain, C.G., Treiman, D.M., Eds.; The MIT Press: Cambridge, MA, USA, 2006; pp. 11–16. [Google Scholar]
- Schmued, L.C.; Albertson, C.; Slikker, W., Jr. Fluoro-Jade: A novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997, 751, 37–46. [Google Scholar] [CrossRef]
- Sankar, R.; Shin, D.H.; Liu, H.; Mazarati, A.; Pereida de Vasconcelos, A.; Wasterlain, C.G. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J. Neurosci. 1998, 18, 8382–8393. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2007; pp. 60–61. [Google Scholar]
- Turski, W.A.; Cavalheiro, E.A.; Schwarz, M.; Czuczwar, S.J.; Kleinrok, Z.; Turski, L. Limbic seizures produced by pilocarpine in rats: Behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983, 9, 315–335. [Google Scholar] [CrossRef]
- Fujikawa, D.G.; Shinmei, S.S.; Cai, B. Lithium-pilocarpine-induced status epilepticus produces necrotic neurons with internucleosomal DNA fragmentation in adult rats. Eur. J. Neurosci. 1999, 11, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Kamat, P.K.; Tyagi, N. Diabetic Stroke Severity: Epigenetic Remodeling and Neuronal, Glial, and Vascular Dysfunction. Diabetes 2015, 64, 4260–4271. [Google Scholar] [CrossRef]
- Fujikawa, D.G.; Shinmei, S.S.; Cai, B. Kainic acid-induced seizures produce necrotic, not apoptotic, neurons with internucleosomal DNA cleavage: Implications for programmed cell death mechanisms. Neuroscience 2000, 98, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Huang, C.C.; Cheng, J.T.; Tsai, J.J.; Wu, S.N. Glucose and hippocampal neuronal excitability: Role of ATP sensitive potassium channels. J. Neurosci. Res. 2007, 86, 368–377. [Google Scholar] [CrossRef]
- Yamada, K.; Ji, J.; Yuan, H.; Miki, T.; Sato, S.; Horimoto, N.; Shimizu, T.; Susumu, S.; Inagaki, N. Protective Role of ATP-Sensitive Potassium Channels in Hypoxia-Induced Generalized Seizure. Science 2001, 292, 1543–1546. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Yim, H.S.; Jung, H.Y.; Nam, S.M.; Kim, J.W.; Choi, J.H.; Seong, J.K.; Yoon, Y.S.; Kim, D.W.; Hwang, I.K. Chronic type 2 diabetes reduces the integrity of the blood-brain barrier by reducing tight junction proteins in the hippocampus. J. Vet. Med. Sci. 2016, 78, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Riera, K.P.; Pérez-Severiano, F.; López-Meraz, M.L. Oxidative stress: A common imbalance in diabetes and epilepsy. Metab. Brain Dis. 2023, 38, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Alatawi, F.S.; Faridi, U.A.; Alatawi, M.S. Effect of treatment with vitamin D plus calcium on oxidative stress in streptozotocin-induced diabetic rats. Saudi Pharm. J. 2018, 26, 1208–1213. [Google Scholar] [CrossRef]
- Elahi, M.; Hasan, Z.; Motoi, Y.; Matsumoto, S.E.; Ishiguro, K.; Hattori, N. Region-Specific Vulnerability to Oxidative Stress, Neuroinflammation, and Tau Hyperphosphorylation in Experimental Diabetes Mellitus Mice. J. Alzheimers Dis. 2016, 51, 1209–1224. [Google Scholar] [CrossRef]
- Zhang, B.; Song, C.; Tang, X.; Tian, M.; Liu, Y.; Yan, Z.; Duan, R.; Liu, Y. Type 2 diabetes microenvironment promotes the development of Parkinson’s disease by activating microglial cell inflammation. Front. Cell Dev. Biol. 2024, 12, 1422746. [Google Scholar] [CrossRef]
- Garcia-Serrano, A.M.; Duarte, J.M.N. Brain Metabolism Alterations in Type 2 Diabetes: What Did We Learn From Diet-Induced Diabetes Models? Front. Neurosci. 2020, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.; Judd, R.; Hoe, L.; Dennis, J.; Posner, P. Effects of diabetes mellitus on astrocyte GFAP and glutamate transporters in the CNS. Glia 2004, 48, 166–178. [Google Scholar] [CrossRef]
- Li, W.; Roy Choudhury, G.; Winters, A.; Prah, J.; Lin, W.; Liu, R.; Yang, S.H. Hyperglycemia Alters Astrocyte Metabolism and Inhibits Astrocyte Proliferation. Aging Dis. 2018, 9, 674–684. [Google Scholar] [CrossRef]
- Muranyi, M.; Ding, C.; He, Q.; Lin, Y.; Li, P.A. Streptozotocin-induced diabetes causes astrocyte death after ischemia and reperfusion injury. Diabetes 2006, 55, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Vasile, F.; Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018, 136, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Nagayach, A.; Patro, N.; Patro, I. Astrocytic and microglial response in experimentally induced diabetic rat brain. Metab. Brain Dis. 2014, 29, 747–761. [Google Scholar] [CrossRef]
- Piani, D.; Frei, K.; Do, K.Q.; Cuenod, M.; Fontana, A. Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci. Lett. 1991, 133, 159–162. [Google Scholar] [CrossRef]
- Belarbi, K.; Rosi, S. Modulation of adult-born neurons in the inflamed hippocampus. Front. Cell Neurosci. 2013, 7, 145. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mérida-Portilla, C.-V.; Puig-Lagunes, Á.A.; Morgado-Valle, C.; Martínez-Quiroz, J.; Beltrán-Parrazal, L.; López-Meraz, M.-L. Type 2 Diabetes Mellitus Exacerbates Brain Injury After Status Epilepticus in Rats. Brain Sci. 2025, 15, 1227. https://doi.org/10.3390/brainsci15111227
Mérida-Portilla C-V, Puig-Lagunes ÁA, Morgado-Valle C, Martínez-Quiroz J, Beltrán-Parrazal L, López-Meraz M-L. Type 2 Diabetes Mellitus Exacerbates Brain Injury After Status Epilepticus in Rats. Brain Sciences. 2025; 15(11):1227. https://doi.org/10.3390/brainsci15111227
Chicago/Turabian StyleMérida-Portilla, Carol-Victoria, Ángel Alberto Puig-Lagunes, Consuelo Morgado-Valle, Joel Martínez-Quiroz, Luis Beltrán-Parrazal, and María-Leonor López-Meraz. 2025. "Type 2 Diabetes Mellitus Exacerbates Brain Injury After Status Epilepticus in Rats" Brain Sciences 15, no. 11: 1227. https://doi.org/10.3390/brainsci15111227
APA StyleMérida-Portilla, C.-V., Puig-Lagunes, Á. A., Morgado-Valle, C., Martínez-Quiroz, J., Beltrán-Parrazal, L., & López-Meraz, M.-L. (2025). Type 2 Diabetes Mellitus Exacerbates Brain Injury After Status Epilepticus in Rats. Brain Sciences, 15(11), 1227. https://doi.org/10.3390/brainsci15111227






