Sex-Dependent Effects of Prenatal Stress on Seizure Susceptibility and Neurodegeneration in Neonatal Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Movement Restraint Stress
2.3. Experimental Groups
- Control pregnant rats (control): These rats were maintained under standard housing conditions throughout gestation.
- Pregnant rats in a stressful environment (stress): This group consisted of rats subjected to stress conditions due to movement restriction during gestation from days 12 to 20.
- Offspring of control pregnant rats (control): These offspring were born to mothers that were kept in standard housing conditions during gestation.
- Offspring of stressed pregnant rats (stress): This group included offspring whose mothers experienced stress from movement restriction during gestation days 12 to 20.
2.4. Litter Characterization and Somatometric Evaluation
2.5. Blood Sample Collection and Determination of Serum Corticosterone
2.6. Induction of Status Epilepticus
2.7. Evaluation of Neurodegeneration
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Litters and Somatometric Evaluation
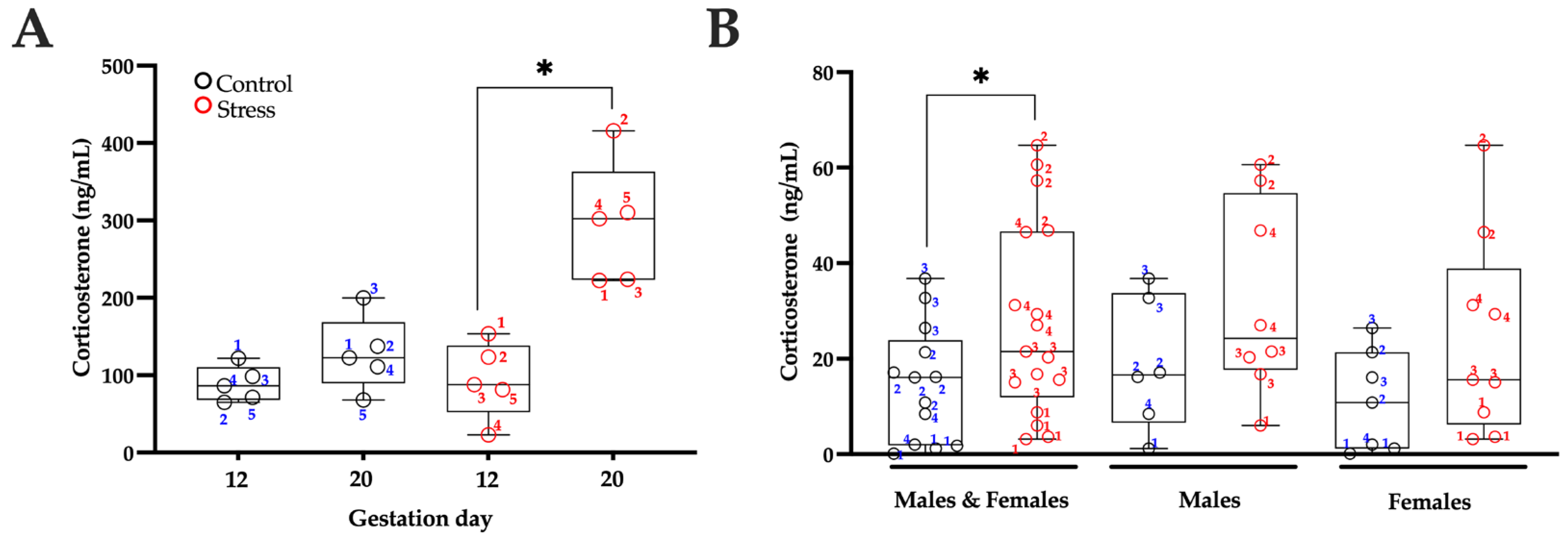
3.2. Serum Corticosterone Levels
3.3. Seizure Severity
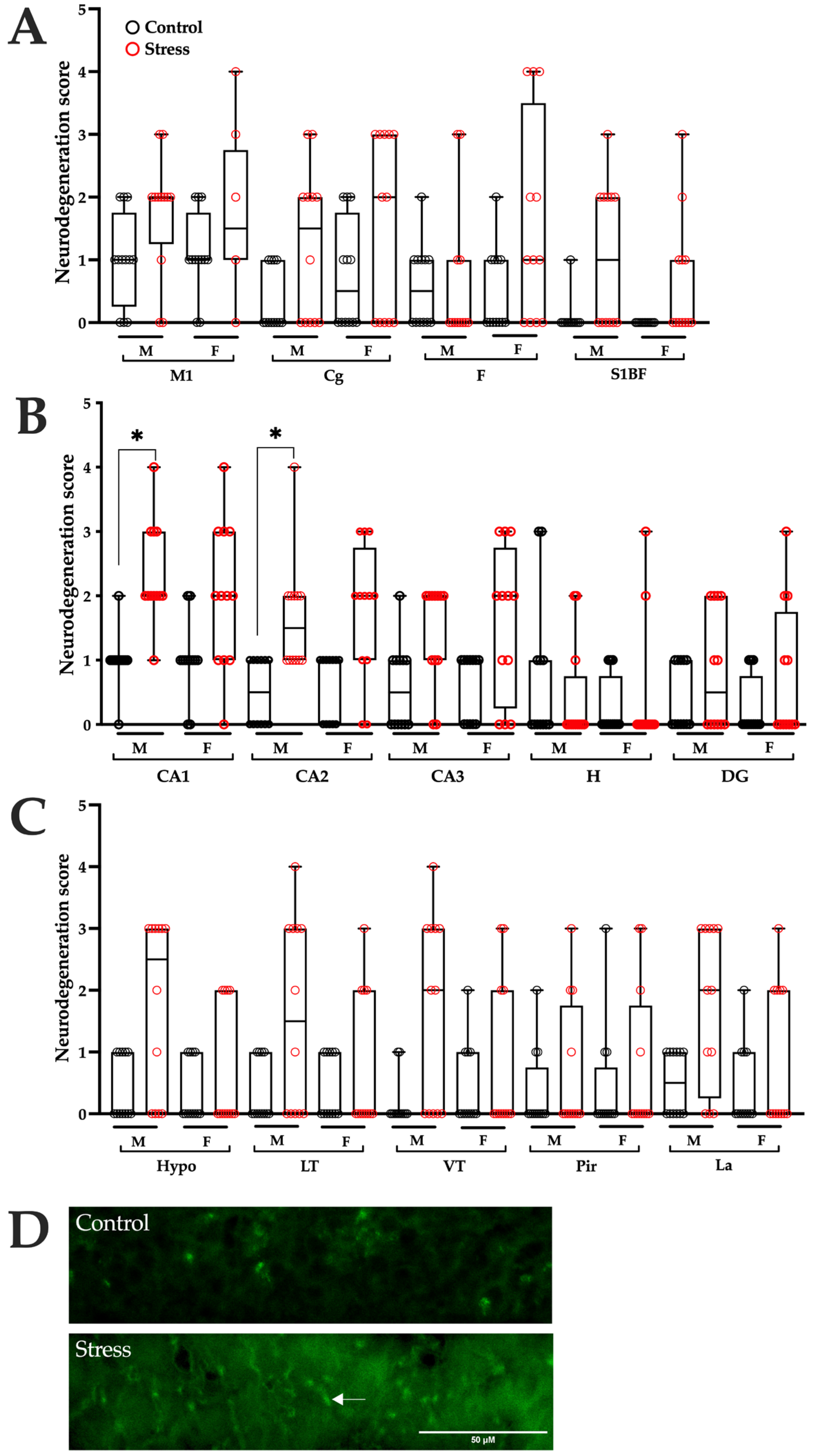
3.4. Neurodegeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SE | Status epilepticus |
| P | Postnatal day |
| i.p. | Intraperitoneal |
| s.c. | Subcutaneous |
| F-JB | Fluoro-Jade B |
| F | Frontal cortex |
| M1 | Primary motor cortex |
| Cg | Cingulate cortex |
| S1BF | Somatosensory cortex |
| CA1 | CA1 hippocampal field |
| CA2 | CA2 hippocampal field |
| CA3 | CA3 hippocampal field |
| H | Hilus |
| DG | Dentate gyrus |
| Hypo | Hypothalamus |
| LT | Lateral thalamus |
| VT | Ventral thalamus |
| Pir | Piriform cortex |
| La | Lateral amygdala nucleus |
| ANOVA | Analysis of variance |
References
- Pascal, R.; Casas, I.; Genero, M.; Nakaki, A.; Youssef, L.; Larroya, M.; Benitez, L.; Gomez, Y.; Martinez-Aran, A.; Morilla, I.; et al. Maternal Stress, Anxiety, Well-Being, and Sleep Quality in Pregnant Women throughout Gestation. J. Clin. Med. 2023, 12, 7333. [Google Scholar] [CrossRef]
- Karam, F.; Sheehy, O.; Huneau, M.C.; Chambers, C.; Fraser, W.D.; Johnson, D.; Kao, K.; Martin, B.; Riordan, S.H.; Roth, M.; et al. Impact of maternal prenatal and parental postnatal stress on 1-year-old child development: Results from the OTIS antidepressants in pregnancy study. Arch. Womens Ment. Health 2016, 19, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Scheinost, D.; Sinha, R.; Cross, S.N.; Kwon, S.H.; Sze, G.; Constable, R.T.; Ment, L.R. Does prenatal stress alter the developing connectome? Pediatr. Res. 2017, 81, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Crovetto, F.; Nakaki, A.; Arranz, A.; Borras, R.; Vellvé, K.; Paules, C.; Boutet, M.L.; Castro-Barquero, S.; Freitas, T.; Casas, R.; et al. Effect of a Mediterranean Diet or Mindfulness-Based Stress Reduction During Pregnancy on Child Neurodevelopment: A Prespecified Analysis of the IMPACT BCN Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2330255. [Google Scholar] [CrossRef] [PubMed]
- Korgan, A.C.; Green, A.D.; Perrot, T.S.; Esser, M.J. Limbic system activation is affected by prenatal predator exposure and postnatal environmental enrichment and further moderated by dam and sex. Behav. Brain Res. 2014, 259, 106–118. [Google Scholar] [CrossRef]
- Fatima, M.; Srivastav, S.; Mondal, A.C. Prenatal stress and depression associated neuronal development in neonates. Int. J. Dev. Neurosci. 2017, 60, 1–7. [Google Scholar] [CrossRef]
- Chachua, T.; Yum, M.S.; Velíšková, J.; Velíšek, L. Validation of the rat model of cryptogenic infantile spasms. Epilepsia 2011, 52, 1666–1677. [Google Scholar] [CrossRef]
- Yum, M.S.; Chachua, T.; Velíšková, J.; Velíšek, L. Prenatal stress promotes development of spasms in infant rats. Epilepsia 2012, 53, e46–e49. [Google Scholar] [CrossRef]
- Baek, H.; Yi, M.H.; Pandit, S.; Park, J.B.; Kwon, H.H.; Zhang, E.; Kim, S.; Shin, N.; Kim, E.; Lee, Y.H.; et al. Altered expression of KCC2 in GABAergic interneuron contributes prenatal stress-induced epileptic spasms in infant rat. Neurochem. Int. 2016, 97, 57–64. [Google Scholar] [CrossRef]
- Kwon, H.H.; Lee, T.; Hong, J.; Kim, D.W.; Kang, J.W. Long-term prenatal stress increases susceptibility of N-methyl-D-aspartic acid-induced spasms in infant rats. Korean J. Pediatr. 2018, 61, 150–155. [Google Scholar] [CrossRef]
- Bagheri, M.; Saboory, E.; Nejatbakhsh, M.; Roshan-Milani, S.; Derafshpour, L.; Sayyadi, H.; Rasmi, Y. Prenatal stress increased γ2 GABAA receptor subunit gene expression in hippocampus and potentiated pentylenetetrazol-induced seizure in rats. Iran. J. Basic. Med. Sci. 2020, 23, 724–729. [Google Scholar] [CrossRef]
- Edwards, H.E.; Dortok, D.; Tam, J.; Won, D.; Burnham, W.M. Prenatal stress alters seizure thresholds and the development of kindled seizures in infant and adult rats. Horm. Behav. 2002, 42, 437–447. [Google Scholar] [CrossRef]
- Sankar, R.; Shin, D.H.; Liu, H.; Mazarati, A.; Pereira de Vasconcelos, A.; Wasterlain, C.G. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J. Neurosci. 1998, 18, 8382–8393. [Google Scholar] [CrossRef]
- Torolira, D.; Suchomelova, L.; Wasterlain, C.G.; Niquet, J. Widespread neuronal injury in a model of cholinergic status epilepticus in postnatal day 7 rat pups. Epilepsy Res. 2016, 120, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Saboory, E.; Ahmadzadeh, R.; Roshan-Milani, S. Prenatal exposure to restraint or predator stresses attenuates field excitatory postsynaptic potentials in infant rats. Int. J. Dev. Neurosci. 2011, 29, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Sadaghiani, M.M.; Saboory, E. Prenatal stress potentiates pilocarpine-induced epileptic behaviors in infant rats both time and sex dependently. Epilepsy Behav. 2010, 18, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Khazipov, R.; Zaynutdinova, D.; Ogievetsky, E.; Valeeva, G.; Mitrukhina, O.; Manent, J.B.; Represa, A. Atlas of the Postnatal Rat Brain in Stereotaxic Coordinates. Front. Neuroanat. 2015, 9, 161. [Google Scholar] [CrossRef]
- Schmued, L.C.; Albertson, C.; Slikker, W. Fluoro-Jade: A novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997, 751, 37–46. [Google Scholar] [CrossRef]
- Govindaraj, S.; Shanmuganathan, A.; Rajan, R. Maternal psychological stress-induced developmental disability, neonatal mortality and stillbirth in the offspring of Wistar albino rats. PLoS ONE 2017, 12, e0171089. [Google Scholar] [CrossRef]
- Pardo, G.V.; Goularte, J.F.; Hoefel, A.L.; de Castro, A.L.; Kucharski, L.C.; da Rosa Araujo, A.S.; Lucion, A.B. Effects of sleep restriction during pregnancy on the mother and fetuses in rats. Physiol. Behav. 2016, 155, 66–76. [Google Scholar] [CrossRef]
- Iturra-Mena, A.M.; Arriagada-Solimano, M.; Luttecke-Anders, A.; Dagnino-Subiabre, A. Effects of prenatal stress on anxiety- and depressive-like behaviours are sex-specific in prepubertal rats. J. Neuroendocr. 2018, 30, e12609. [Google Scholar] [CrossRef]
- Soti, M.; Ranjbar, H.; Kohlmeier, K.A.; Shabani, M. Sex differences in the vulnerability of the hippocampus to prenatal stress. Dev. Psychobiol. 2022, 64, e22305. [Google Scholar] [CrossRef] [PubMed]
- Mejías-Aponte, C.A.; Jiménez-Rivera, C.A.; Segarra, A.C. Sex differences in models of temporal lobe epilepsy: Role of testosterone. Brain Res. 2002, 944, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Pollo, M.L.M.; Gimenes, C.; Covolan, L. Male rats are more vulnerable to pentylenetetrazole-kindling model but females have more spatial memory-related deficits. Epilepsy Behav. 2022, 129, 108632. [Google Scholar] [CrossRef] [PubMed]
- Matovu, D.; Cavalheiro, E.A. Differences in Evolution of Epileptic Seizures and Topographical Distribution of Tissue Damage in Selected Limbic Structures Between Male and Female Rats Submitted to the Pilocarpine Model. Front. Neurol. 2022, 13, 802587. [Google Scholar] [CrossRef]
- Wolf, D.C.; Desgent, S.; Sanon, N.T.; Chen, J.-S.; Elkaim, L.M.; Bosoi, C.M.; Awad, P.N.; Simard, A.; Salam, M.T.; Bilodeau, G.-A.; et al. Sex differences in the developing brain impact stress-induced epileptogenicity following hyperthermia-induced seizures. Neurobiol. Dis. 2021, 161, 105546. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Mairesse, J.; Lesage, J.; Breton, C.; Bréant, B.; Hahn, T.; Darnaudéry, M.; Dickson, S.L.; Seckl, J.; Blondeau, B.; Vieau, D.; et al. Maternal stress alters endocrine function of the feto-placental unit in rats. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1526–E1533. [Google Scholar] [CrossRef]
- Welberg, L.A.; Thrivikraman, K.V.; Plotsky, P.M. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J. Endocrinol. 2005, 186, R7–R12. [Google Scholar] [CrossRef]
- Cottrell, E.C.; Seckl, J.R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009, 3, 19. [Google Scholar] [CrossRef]
- Jafari, Z.; Mehla, J.; Kolb, B.; Mohajerani, M.H. Prenatal noise stress impairs HPA axis and cognitive performance in mice. Sci. Rep. 2017, 7, 10560. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, D.E.; Neigh, G.N.; Bourke, C.H.; Nemeth, C.L.; Hazra, R.; Ryan, S.J.; Rowson, S.; Jairam, N.; Sholar, C.A.; Rainnie, D.G.; et al. Prenatal stress, regardless of concurrent escitalopram treatment, alters behavior and amygdala gene expression of adolescent female rats. Neuropharmacology 2015, 97, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Iacobas, S.; Chachua, T.; Goletiani, C.; Sidyelyeva, G.; Velíšková, J.; Velíšek, L. Prenatal corticosteroids modify glutamatergic and GABAergic synapse genomic fabric: Insights from a novel animal model of infantile spasms. J. Neuroendocr. 2013, 25, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.; Sapolsky, R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991, 12, 118–134. [Google Scholar] [CrossRef]
- Knox-Concepcion, K.R.; Figueroa, J.D.; Hartman, R.E.; Li, Y.; Zhang, L. Repression of the Glucocorticoid Receptor Increases Hypoxic-Ischemic Brain Injury in the Male Neonatal Rat. Int. J. Mol. Sci. 2019, 20, 3493. [Google Scholar] [CrossRef]
- Lévy, F.; Keller, M.; Poindron, P. Olfactory regulation of maternal behavior in mammals. Horm. Behav. 2004, 46, 284–302. [Google Scholar] [CrossRef]
- Del Campo, C.H.; Ginther, O.J. Vascular anatomy of the uterus and ovaries and the unilateral luteolytic effect of the uterus: Guinea pigs, rats, hamsters and rabbits. Am. J. Vet. Res. 1972, 33, 2561–2578. [Google Scholar] [CrossRef]
- Liu, G.; Dong, Y.; Wang, Z.; Cao, J.; Chen, Y. Restraint stress alters immune parameters and induces oxidative stress in the mouse uterus during embryo implantation. Stress 2014, 17, 494–503. [Google Scholar] [CrossRef]


| Variable | MWU/t Test | p Value | p Value Bonferroni Correction | Significant |
|---|---|---|---|---|
| Seizure behavior | ||||
| Latency to stage 1 seizure | t = 4.524, df = 6 | 0.004 | 0.0056 | Yes |
| Latency to stage 2 seizure | t = 2.896, df = 6 | 0.0275 | No | |
| Latency to stage 3 seizure | U = 0 | 0.0286 | No | |
| Frequency of stage 1 seizure | U = 5 | 0.4857 | No | |
| Frequency of stage 2 seizure | U = 3 | 0.2 | No | |
| Frequency of stage 3 seizure | U = 1 | 0.0571 | No | |
| Duration of stage 1 seizure | t = 0.2386, df = 6 | 0.8193 | No | |
| Duration of stage 2 seizure | t = 1.582, df = 6 | 0.1646 | No | |
| Duration of stage 3 seizure | t = 3.269, df = 6 | 0.017 | No | |
| Neurodegeneration | ||||
| Frontal cortex (F) | U = 65 | 0.7179 | 0.0036 | No |
| Primary motor cortex (M1) | U = 37.50 | 0.037 | No | |
| Cingulate cortex (Cg) | U = 42 | 0.0699 | No | |
| Somatosensory cortex (S1BF) | U = 39 | 0.0137 | No | |
| CA1 | U = 9.500 | <0.0001 | Yes | |
| CA2 | U = 18 | 0.0007 | Yes | |
| CA3 | U = 32 | 0.0244 | No | |
| Hilus (H) | U = 60 | 0.4638 | No | |
| Dentate gyrus (DG) | U = 56 | 0.2928 | No | |
| Hypothalamus (Hypo) | U = 36.5 | 0.0312 | No | |
| Ventral thalamus (VT) | U = 35 | 0.0116 | No | |
| Lateral thalamus (LT) | U = 42 | 0.0646 | No | |
| Piriform cortex (Pir) | U = 63 | 0.5505 | No | |
| Lateral amygdala (La) | U = 33 | 0.0236 | No | |
| Variable | MWU/t Test | p Value | p Value Bonferroni Correction | Significant |
|---|---|---|---|---|
| Seizure behavior | ||||
| Latency to stage 1 seizure | U = 5 | 0.4857 | 0.0056 | No |
| Latency to stage 2 seizure | t = 2.428, df = 6 | 0.0513 | No | |
| Latency to stage 3 seizure | t = 1.684, df = 6 | 0.1432 | No | |
| Frequency of stage 1 seizure | U = 7 | 0.8857 | No | |
| Frequency of stage 2 seizure | U = 5 | 0.4857 | No | |
| Frequency of stage 3 seizure | U = 0.5 | 0.0571 | No | |
| Duration of stage 1 seizure | t = 0.5769, df = 6 | 0.585 | No | |
| Duration of stage 2 seizure | t = 2.596, df = 6 | 0.0409 | No | |
| Duration of stage 3 seizure | t = 2.980, df = 6 | 0.0246 | No | |
| Neurodegeneration | ||||
| Frontal cortex (F) | U = 44 | 0.1036 | 0.0036 | No |
| Primary motor cortex (M1) | U = 52.5 | 0.2493 | No | |
| Cingulate cortex (Cg) | U = 48 | 0.1512 | No | |
| Somatosensory cortex (S1BF) | U = 42 | 0.0373 | No | |
| CA1 | U = 33 | 0.0177 | No | |
| CA2 | U = 26 | 0.0057 | No | |
| CA3 | U = 35.5 | 0.0298 | No | |
| Hilus (H) | U = 69 | >0.9999 | No | |
| Dentate gyrus (DG) | U = 55.5 | 0.2776 | No | |
| Hypothalamus (Hypo) | U = 64 | 0.6425 | No | |
| Ventral thalamus (VT) | U = 65 | 0.6700 | No | |
| Lateral thalamus (LT) | U = 68 | 0.9048 | No | |
| Piriform cortex (Pir) | U = 64 | 0.6044 | No | |
| Lateral amygdala (La) | U = 58 | 0.3871 | No | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Rojas, D.A.; Beltrán-Parrazal, L.; Morgado-Valle, C.; Herrera-Meza, G.; Corona-Morales, A.A.; Martínez-Quiroz, J.; Martínez-Rojas, B.; López-Meraz, M.-L. Sex-Dependent Effects of Prenatal Stress on Seizure Susceptibility and Neurodegeneration in Neonatal Rats. Brain Sci. 2025, 15, 1220. https://doi.org/10.3390/brainsci15111220
Cruz-Rojas DA, Beltrán-Parrazal L, Morgado-Valle C, Herrera-Meza G, Corona-Morales AA, Martínez-Quiroz J, Martínez-Rojas B, López-Meraz M-L. Sex-Dependent Effects of Prenatal Stress on Seizure Susceptibility and Neurodegeneration in Neonatal Rats. Brain Sciences. 2025; 15(11):1220. https://doi.org/10.3390/brainsci15111220
Chicago/Turabian StyleCruz-Rojas, Daniel Antonio, Luis Beltrán-Parrazal, Consuelo Morgado-Valle, Grecia Herrera-Meza, Aleph A. Corona-Morales, Joel Martínez-Quiroz, Brenda Martínez-Rojas, and María-Leonor López-Meraz. 2025. "Sex-Dependent Effects of Prenatal Stress on Seizure Susceptibility and Neurodegeneration in Neonatal Rats" Brain Sciences 15, no. 11: 1220. https://doi.org/10.3390/brainsci15111220
APA StyleCruz-Rojas, D. A., Beltrán-Parrazal, L., Morgado-Valle, C., Herrera-Meza, G., Corona-Morales, A. A., Martínez-Quiroz, J., Martínez-Rojas, B., & López-Meraz, M.-L. (2025). Sex-Dependent Effects of Prenatal Stress on Seizure Susceptibility and Neurodegeneration in Neonatal Rats. Brain Sciences, 15(11), 1220. https://doi.org/10.3390/brainsci15111220






