Adaptations in the Structure and Function of the Cerebellum in Basketball Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Physical Abilities Test
2.3. MRI Acquisition
2.4. VBM Analysis
2.5. Resting-State fMRI Analyses
2.6. Diffusion Kurtosis Image Analysis
2.7. Correlation Analysis
3. Results
3.1. Demographic and Physical Data
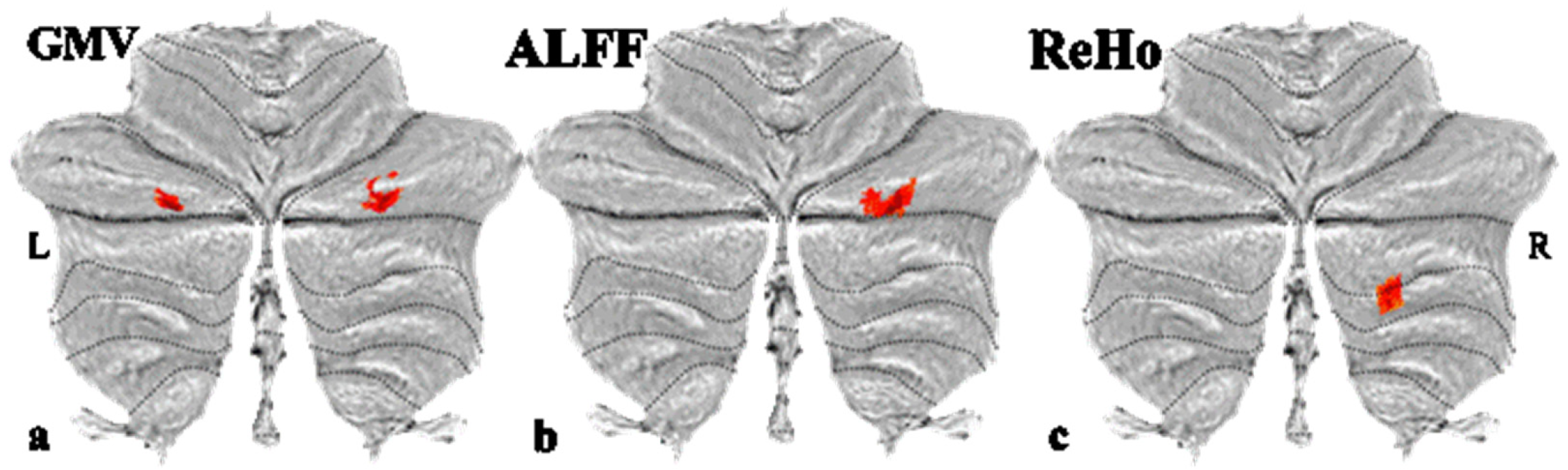
3.2. Grey Matter Volume
3.3. Resting-State Function Activity Alterations
3.4. Microstructure Alterations
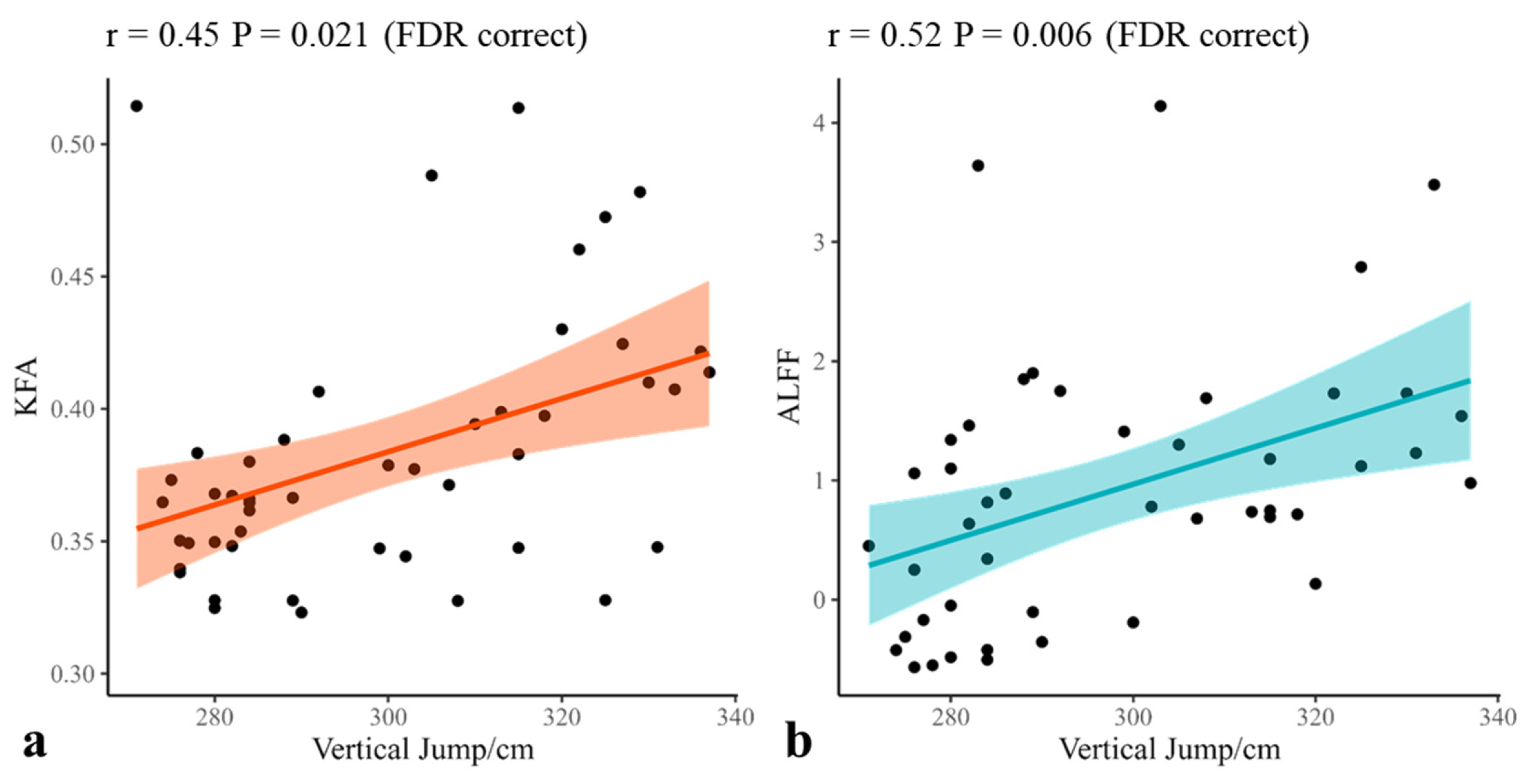
3.5. Correlation Between Training Experience and Physical Abilities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | axial diffusivity |
| AK | axial kurtosis |
| ALFF | Amplitude of Low-Frequency Fluctuations |
| DC | Degree Centrality |
| DKI | Diffusion Kurtosis Imaging |
| DTI | Diffusion Tensor Imaging |
| FA | fractional anisotropy |
| fALFF | fractional ALFF |
| GMV | gray matter volume |
| KFA | kurtosis fractional anisotropy |
| MD | mean diffusivity |
| MK | mean kurtosis |
| MRI | Magnetic Resonance Imaging |
| RD | radial diffusivity |
| ReHo | Regional Homogeneity |
| RK | radial kurtosis |
| TBSS | Tract-Based Spatial Statistics |
| TFCE | Threshold-Free Cluster Enhancement |
References
- King, M.; Hernandez-Castillo, C.R.; Poldrack, R.A.; Ivry, R.B.; Diedrichsen, J. Functional Boundaries in the Human Cerebellum Revealed by a Multi-Domain Task Battery. Nat. Neurosci. 2019, 22, 1371–1378. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, Y.; Li, X.; Fang, W.; Du, X. Characteristics of Positive and Negative Networks in Working Memory Task of Basketball Athletes. Psychol. Sport Exerc. 2025, 80, 102880. [Google Scholar] [CrossRef]
- Warthen, K.G.; Walker, N.C.; Wicklund, B.D.; Gonzalez, M.M.; Ramirez, N.; Gee, S.C.; Al-Dasouqi, H.; Madore, M.R. Neuromodulation of the Cerebellum for Motor Applications: A Systematic Review. J. Integr. Neurosci. 2024, 23, 195. [Google Scholar] [CrossRef]
- Ito, M. Control of Mental Activities by Internal Models in the Cerebellum. Nat. Rev. Neurosci. 2008, 9, 304–313. [Google Scholar] [CrossRef]
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus Paper: The Cerebellum’s Role in Movement and Cognition. Cerebellum 2014, 13, 151–177. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Guell, X.; Stoodley, C.J.; Halko, M.A. The Theory and Neuroscience of Cerebellar Cognition. Annu. Rev. Neurosci. 2019, 42, 337–364. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-F.; Fu, H.-L.; Liang, C.-W.; Li, Y.-J.; Wang, C.-H. Electrophysiological Differences in Inhibitory Control Processing between Collegiate Level Soccer Players and Non-Athletes in the Absence of Performance Differences. Brain Cogn. 2024, 178, 106179. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.R. The Cerebellum. Curr. Biol. 2024, 34, R7–R11. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; LeBel, A.; D’Mello, A.M. Ignoring the Cerebellum Is Hindering Progress in Neuroscience. Trends Cogn. Sci. 2025, 29, 318–330. [Google Scholar] [CrossRef]
- Carrillo, J.; Cheng, S.-Y.; Ko, K.W.; Jones, T.A.; Nishiyama, H. The Long-Term Structural Plasticity of Cerebellar Parallel Fiber Axons and Its Modulation by Motor Learning. J. Neurosci. 2013, 33, 8301–8307. [Google Scholar] [CrossRef] [PubMed]
- Kang, N. Increased Cerebellar Gray Matter Volume in Athletes: A Voxel-Wise Coordinate-Based Meta-Analysis. Res. Q. Exerc. Sport 2022, 94, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Rhyu, I.J.; Pak, D.T.S. Synapses Need Coordination to Learn Motor Skills. Rev. Neurosci. 2014, 25, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jan, Y.-K.; Liu, Y.; Zhao, T.; Zhang, L.; Liu, R.; Liu, J.; Cao, C. Exercise Intensity and Brain Plasticity: What’s the Difference of Brain Structural and Functional Plasticity Characteristics Between Elite Aerobic and Anaerobic Athletes? Front. Hum. Neurosci. 2022, 16, 757522. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Y.; Huang, R.; Li, L.; Xia, F.; Zou, L.; Yu, Q.; Lin, J.; Herold, F.; Perrey, S.; et al. Structural and Functional Brain Signatures of Endurance Runners. Brain Struct. Funct. 2021, 226, 93–103. [Google Scholar] [CrossRef]
- Jang, D.C.; Shim, H.G.; Kim, S.J. Intrinsic Plasticity of Cerebellar Purkinje Cells Contributes to Motor Memory Consolidation. J. Neurosci. 2020, 40, 4145–4157. [Google Scholar] [CrossRef]
- Manto, M.; Huisman, T.A.G.M. The Cerebellum: Handbook of Clinical Neurology Series; Elsevier: San Diego, CA, USA, 2018; ISBN 978-0-444-63956-1. [Google Scholar]
- Li, W.; Kong, X.; Ma, J. Effects of Combat Sports on Cerebellar Function in Adolescents: A Resting-State fMRI Study. Br. J. Radiol. 2022, 95, 20210826. [Google Scholar] [CrossRef]
- Jalanko, P.; Säisänen, L.; Kallioniemi, E.; Könönen, M.; Lakka, T.A.; Määttä, S.; Haapala, E.A. Associations between Physical Fitness and Cerebellar Gray Matter Volume in Adolescents. Scand. J. Med. Sci. Sports 2024, 34, e14513. [Google Scholar] [CrossRef]
- Wenzel, U.; Taubert, M.; Ragert, P.; Krug, J.; Villringer, A. Functional and Structural Correlates of Motor Speed in the Cerebellar Anterior Lobe. PLoS ONE 2014, 9, e96871. [Google Scholar] [CrossRef]
- Terner, Z.; Franks, A. Modeling Player and Team Performance in Basketball. Annu. Rev. Stat. Its Appl. 2021, 8, 1–23. [Google Scholar] [CrossRef]
- Ishihara, T.; Miyazaki, A.; Tanaka, H.; Matsuda, T. Identification of the Brain Networks That Contribute to the Interaction between Physical Function and Working Memory: An fMRI Investigation with over 1,000 Healthy Adults. NeuroImage 2020, 221, 117152. [Google Scholar] [CrossRef] [PubMed]
- Reuben, D.B.; Magasi, S.; McCreath, H.E.; Bohannon, R.W.; Wang, Y.-C.; Bubela, D.J.; Rymer, W.Z.; Beaumont, J.; Rine, R.M.; Lai, J.-S.; et al. Motor Assessment Using the NIH Toolbox. Neurology 2013, 80, S65–S75. [Google Scholar] [CrossRef]
- Wen, N.; Dalbo, V.J.; Burgos, B.; Pyne, D.B.; Scanlan, A.T. Power Testing in Basketball: Current Practice and Future Recommendations. J. Strength Cond. Res. 2018, 32, 2677–2691. [Google Scholar] [CrossRef]
- Essentials of Strength Training and Conditioning, 4th ed.; Haff, G., Triplett, N.T., National Strength & Conditioning Association, Eds.; Human Kinetics: Champaign, IL, USA; Windsor, ON, Canada; Leeds, UK, 2015; ISBN 978-1-4925-0162-6. [Google Scholar]
- Diedrichsen, J.; Balsters, J.H.; Flavell, J.; Cussans, E.; Ramnani, N. A Probabilistic MR Atlas of the Human Cerebellum. NeuroImage 2009, 46, 39–46. [Google Scholar] [CrossRef]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef]
- Zou, Q.H.; Zhu, C.Z.; Yang, Y.; Zuo, X.N.; Long, X.Y.; Cao, Q.J.; Wang, Y.F.; Zang, Y.F. An Improved Approach to Detection of Amplitude of Low-Frequency Fluctuation (ALFF) for Resting-State fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Sepulcre, J.; Talukdar, T.; Krienen, F.M.; Liu, H.; Hedden, T.; Andrews-Hanna, J.R.; Sperling, R.A.; Johnson, K.A. Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. J. Neurosci. 2009, 29, 1860–1873. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.-D.; Smith, R.; Raffelt, D.; Tabbara, R.; Dhollander, T.; Pietsch, M.; Christiaens, D.; Jeurissen, B.; Yeh, C.-H.; Connelly, A. MRtrix3: A Fast, Flexible and Open Software Framework for Medical Image Processing and Visualisation. NeuroImage 2019, 202, 116137. [Google Scholar] [CrossRef]
- Cao, Z.; Lin, H.; Gao, F.; Shen, X.; Zhang, H.; Zhang, J.; Du, L.; Lai, C.; Ma, X.; Wu, D. Microstructural Alterations in Projection and Association Fibers in Neonatal Hypoxia–Ischemia. J. Magn. Reson. Imaging 2023, 57, 1131–1142. [Google Scholar] [CrossRef]
- Veraart, J.; Fieremans, E.; Novikov, D.S. Diffusion MRI Noise Mapping Using Random Matrix Theory. Magn. Reson. Med. 2016, 76, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Kellner, E.; Dhital, B.; Kiselev, V.G.; Reisert, M. Gibbs-ringing Artifact Removal Based on Local Subvoxel-shifts. Magn. Reson. Med. 2016, 76, 1574–1581. [Google Scholar] [CrossRef]
- Tabesh, A.; Jensen, J.H.; Ardekani, B.A.; Helpern, J.A. Estimation of Tensors and Tensor-derived Measures in Diffusional Kurtosis Imaging. Magn. Reson. Med. 2011, 65, 823–836. [Google Scholar] [CrossRef]
- Van Baarsen, K.M.; Kleinnijenhuis, M.; Jbabdi, S.; Sotiropoulos, S.N.; Grotenhuis, J.A.; Van Cappellen Van Walsum, A.M. A Probabilistic Atlas of the Cerebellar White Matter. NeuroImage 2016, 124, 724–732. [Google Scholar] [CrossRef]
- Boonstra, J.T. The Cerebellar Connectome. Behav. Brain Res. 2025, 482, 115457. [Google Scholar] [CrossRef] [PubMed]
- Habas, C. Functional Connectivity of the Cognitive Cerebellum. Front. Syst. Neurosci. 2021, 15, 642225. [Google Scholar] [CrossRef]
- Henschke, J.U.; Pakan, J.M. Disynaptic Cerebrocerebellar Pathways Originating from Multiple Functionally Distinct Cortical Areas. eLife 2020, 9, e59148. [Google Scholar] [CrossRef]
- Guell, X.; Gabrieli, J.D.E.; Schmahmann, J.D. Triple Representation of Language, Working Memory, Social and Emotion Processing in the Cerebellum: Convergent Evidence from Task and Seed-Based Resting-State fMRI Analyses in a Single Large Cohort. NeuroImage 2018, 172, 437–449. [Google Scholar] [CrossRef]
- Clark, S.V.; Semmel, E.S.; Aleksonis, H.A.; Steinberg, S.N.; King, T.Z. Cerebellar-Subcortical-Cortical Systems as Modulators of Cognitive Functions. Neuropsychol. Rev. 2021, 31, 422–446. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The Cerebellum and Cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B. An Introduction to Kurtosis Fractional Anisotropy. Am. J. Neuroradiol. 2019, 40, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Glenn, G.R.; Helpern, J.A.; Tabesh, A.; Jensen, J.H. Quantitative Assessment of Diffusional Kurtosis Anisotropy: KURTOSIS ANISOTROPY. NMR Biomed. 2015, 28, 448–459. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional Kurtosis Imaging: The Quantification of Non-gaussian Water Diffusion by Means of Magnetic Resonance Imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, A.; Rossi-Espagnet, M.C.; De Palma, L.; Carai, A.; Marras, C.E. Networking of the Human Cerebellum: From Anatomo-Functional Development to Neurosurgical Implications. Front. Neurol. 2022, 13, 806298. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Qi, Y.; Wang, Y.; Li, X.; Du, X.; Shao, B. Long-Term Basketball Training Shapes Cerebellar-Frontal Integration for Enhanced Cognitive Control. BMC Sports Sci. Med. Rehabil. 2025, 17, 253. [Google Scholar] [CrossRef]
- Meijer, A.; Königs, M.; Vermeulen, G.T.; Visscher, C.; Bosker, R.J.; Hartman, E.; Oosterlaan, J. The Effects of Physical Activity on Brain Structure and Neurophysiological Functioning in Children: A Systematic Review and Meta-Analysis. Dev. Cogn. Neurosci. 2020, 45, 100828. [Google Scholar] [CrossRef] [PubMed]

| Basketball Athletes | Non-Athletes | p | |
|---|---|---|---|
| Gender (F, M) | 25, 30 | 26, 29 | 0.847 |
| Age (M ± SD) | 20.75 ± 1.83 | 20.87 ± 1.98 | 0.727 |
| Educated Years | 14.75 ± 1.82 | 14.80 ± 1.67 | 0.870 |
| Years of Sport Training | 9.25 ± 3.22 | - | - |
| Years of Basketball Training | 8.44 ± 2.69 | - | - |
| Training time (hours/week) | 7.21 ± 4.50 | - | - |
| Vertical Jump (cm) | 299.29 ± 23.36 | - | - |
| Dribbling (s) | 39.93 ± 2.19 | - | - |
| Shooting percentage (%) | 0.68 ± 0.14 | - | - |
| Agility (s) | 16.74 ± 2.47 | 24.77 ± 5.92 | <0.001 |
| Gait Speed (m/s) | 1.52 ± 0.44 | 1.51 ± 0.12 | 0.182 |
| Explosive power (cm) | 33.79 ± 7.39 | 33.04 ± 8.43 | 0.704 |
| Imaging Methods | Geometry Parameters | Contrast Parameters |
|---|---|---|
| T1-weighted imaging | FOV = 256 mm × 256 mm Slice number = 192 Voxel size = 1 × 1 × 1 mm3 | TR = 2530 ms TE = 2.98 ms TI = 1100 ms flip angle = 7° |
| Diffusion-weighted imaging (DWI) | FOV = 224 mm × 224 mm Slice number = 74 Voxel size = 2 × 2 × 2 mm3 | TR = 5000 ms TE = 95 ms b = 0, 1000, 2000 s/mm2 directions = 30 flip angle = 90° |
| BOLD weighted imaging | FOV = 192 mm × 192 mm Slice number = 58 interslice gap = 20% Voxel size = 2 × 2 × 2 mm3 | TR = 2000 ms, TE = 30 ms, flip angle = 90° |
| Field map for DWI | FOV = 224 mm × 224 mm Slice number = 74 Voxel size = 2 × 2 × 2 mm3 | TR = 735 ms, TE = 4.92 & 7.38 ms flip angle = 90° |
| Field map for fMRI | FOV = 192 mm × 192 mm Slice number = 58 interslice gap = 20% Voxel size = 2 × 2 × 2 mm3 | TR = 571 ms, TE = 4.92 & 7.38 ms flip angle = 90° |
| Cluster | Area | Size | MNI (mm) | Peak T | p | ||
|---|---|---|---|---|---|---|---|
| GMV 1 | Left Crus I | 79 | −23 | −73 | −35 | 4.21 | 0.023 |
| GMV 2 | Right Crus I | 113 | 28 | −73 | −33 | 3.99 | 0.012 |
| Cluster | Area | Size | MNI (mm) | Peak T | p | ||
|---|---|---|---|---|---|---|---|
| ALFF | Right Crus I | 48 | 36 | −80 | −31 | 3.87 | 0.002 |
| ReHo | Right Crus II and right VII b | 58 | 22 | −68 | −41 | 3.65 | 0.038 |
| Cluster | Area | Size | MNI (mm) | Peak T | p | ||
|---|---|---|---|---|---|---|---|
| RK | 4758 | 12 | −53 | −25 | 5.09 | 0.023 | |
| White matter (CWMA) | |||||||
| Right Middle Cerebellar Peduncle | 592 | ||||||
| Right Inferior Cerebellar Peduncle | 512 | ||||||
| Left Inferior Cerebellar Peduncle | 424 | ||||||
| Left Middle Cerebellar Peduncle | 320 | ||||||
| Gray matter (SUIT) | |||||||
| Right Crus I | 746 | ||||||
| Right VI | 452 | ||||||
| Left VI | 419 | ||||||
| Left Crus I | 301 | ||||||
| Right Crus II | 174 | ||||||
| Right V | 127 | ||||||
| Right IX | 94 | ||||||
| Vermis VI | 86 | ||||||
| Vermis IX | 53 | ||||||
| KFA | 1378 | 33 | −59 | −43 | 3.86 | 0.031 | |
| White matter (CWMA) | |||||||
| Right Middle Cerebellar Peduncle | 389 | ||||||
| Right Inferior Cerebellar Peduncle | 154 | ||||||
| Gray matter (SUIT) | |||||||
| Right Crus I | 249 | ||||||
| Right VI | 151 | ||||||
| Left VI | 140 | ||||||
| Left Crus I | 101 | ||||||
| Right Crus II | 58 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wang, Y.; Fang, W.; Li, X.; Du, J.; Zhou, Q.; Ning, J.; Zhang, B.; Du, X. Adaptations in the Structure and Function of the Cerebellum in Basketball Athletes. Brain Sci. 2025, 15, 1221. https://doi.org/10.3390/brainsci15111221
Qi Y, Wang Y, Fang W, Li X, Du J, Zhou Q, Ning J, Zhang B, Du X. Adaptations in the Structure and Function of the Cerebellum in Basketball Athletes. Brain Sciences. 2025; 15(11):1221. https://doi.org/10.3390/brainsci15111221
Chicago/Turabian StyleQi, Yapeng, Yihan Wang, Wenxuan Fang, Xinwei Li, Jiaxin Du, Qichen Zhou, Jilan Ning, Bin Zhang, and Xiaoxia Du. 2025. "Adaptations in the Structure and Function of the Cerebellum in Basketball Athletes" Brain Sciences 15, no. 11: 1221. https://doi.org/10.3390/brainsci15111221
APA StyleQi, Y., Wang, Y., Fang, W., Li, X., Du, J., Zhou, Q., Ning, J., Zhang, B., & Du, X. (2025). Adaptations in the Structure and Function of the Cerebellum in Basketball Athletes. Brain Sciences, 15(11), 1221. https://doi.org/10.3390/brainsci15111221






