Dendritic Inhibition Effects in Memory Retrieval of a Neuromorphic Microcircuit Model of the Rat Hippocampus
Abstract
1. Introduction
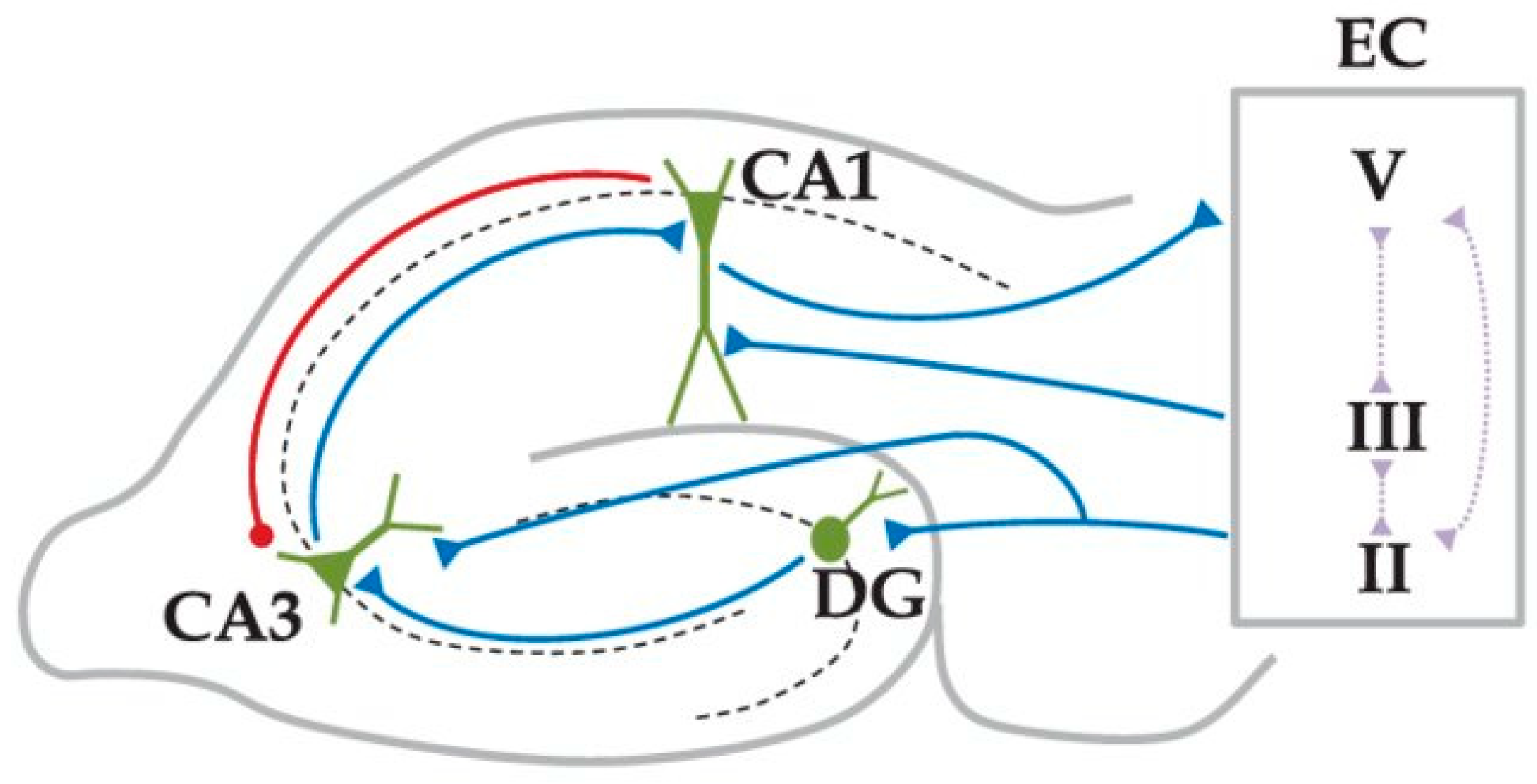
2. Background
3. Materials and Methods
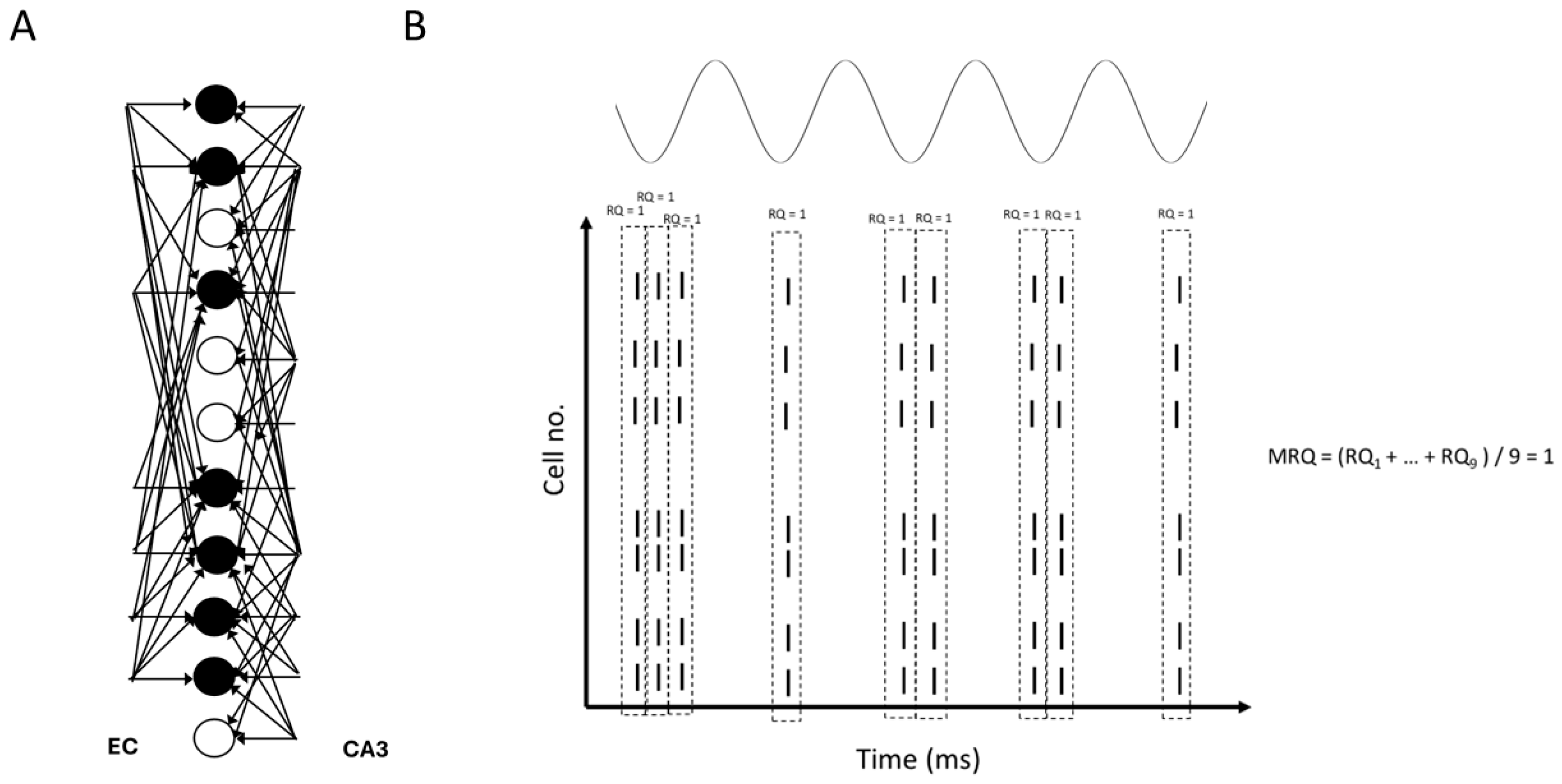
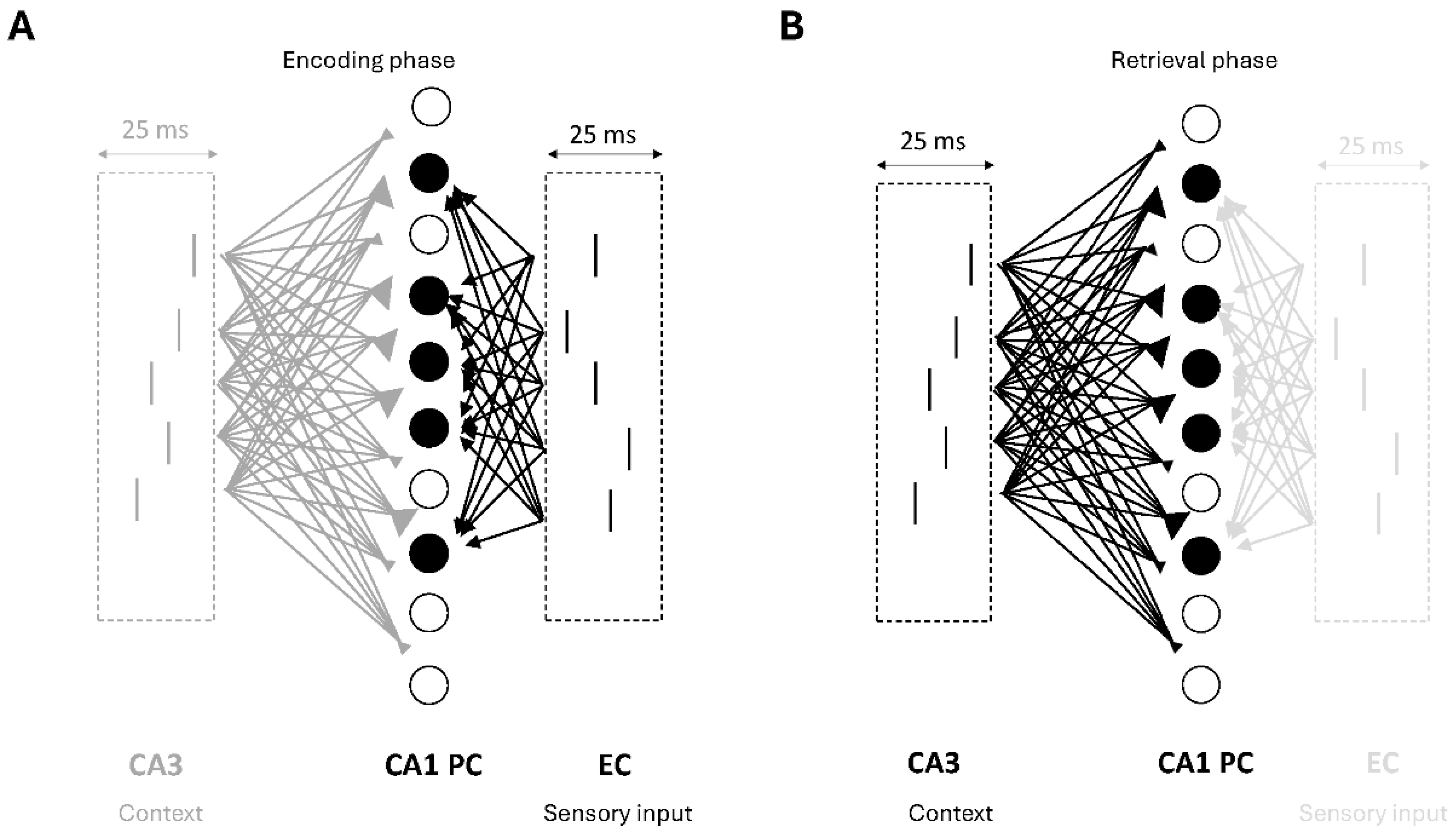
3.1. The Model
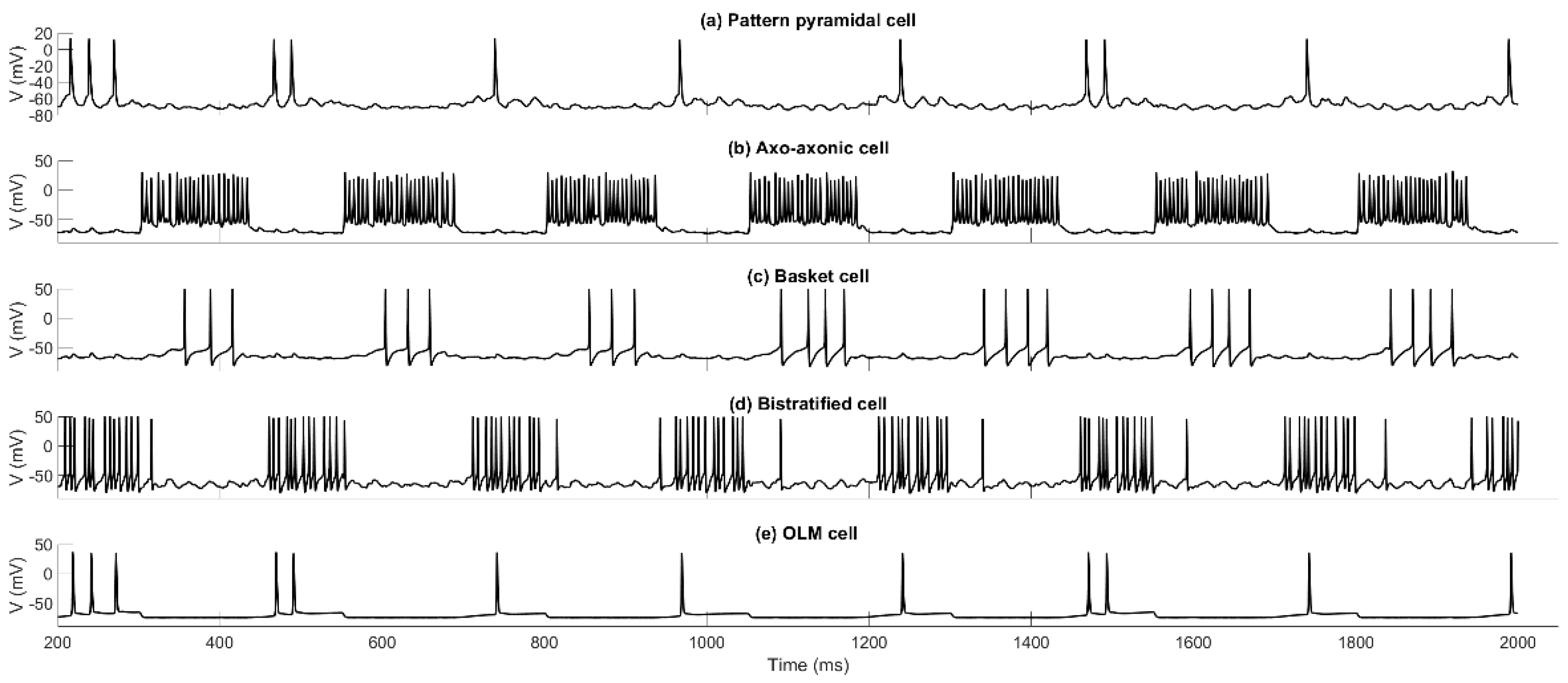
3.2. Cell Firings During the Encoding and Retrieval Theta Cycles
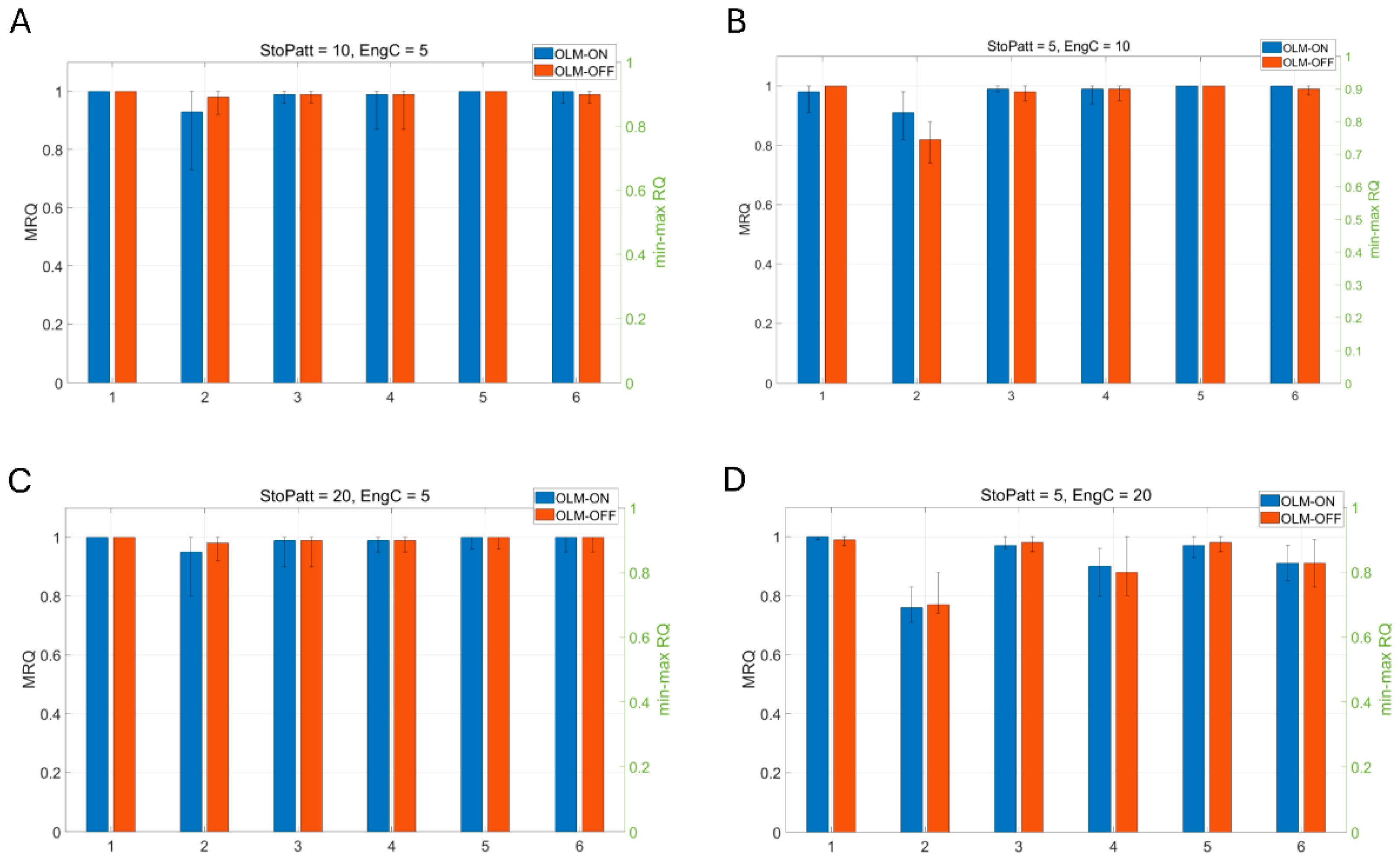
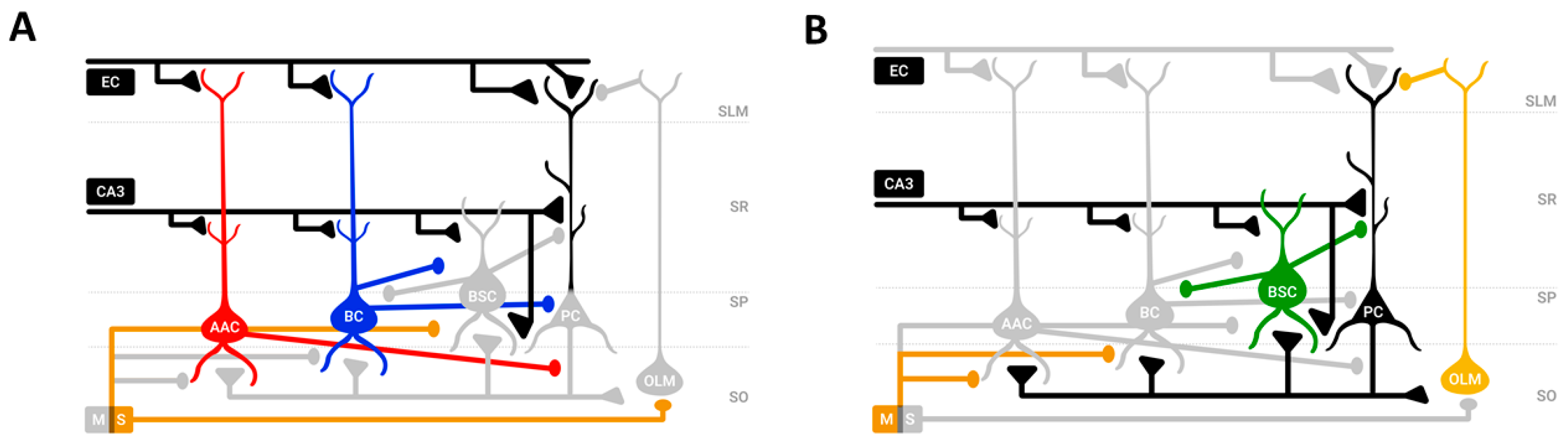
3.3. CA1 Model Variations
- Model 1: Scaling of excitatory synapses in the proximal to the soma BSC dendrites. These synapses were excited by feedforward excitatory Schaffer collateral CA3 input (Figure 5A).
- Model 2: Scaling of inhibitory synapses from the medial to the soma PC dendrites. The synapses are excited by feedforward excitatory Schaffer collateral CA3 input and inhibited by the BSC inhibition (Figure 5B).
- Model 3: Scaling of excitatory synapses in the BSC basal dendrites. These synapses were excited by PC feedback excitation (Figure 5C).
- Model 4: Model 1 and Model 2 (Figure 5D).
- Model 5: Model 1 and Model 3 (Figure 5E).
- Model 6: Model 2 and Model 3 (Figure 5F).
3.4. Memory Patterns
3.5. Weight Matrix
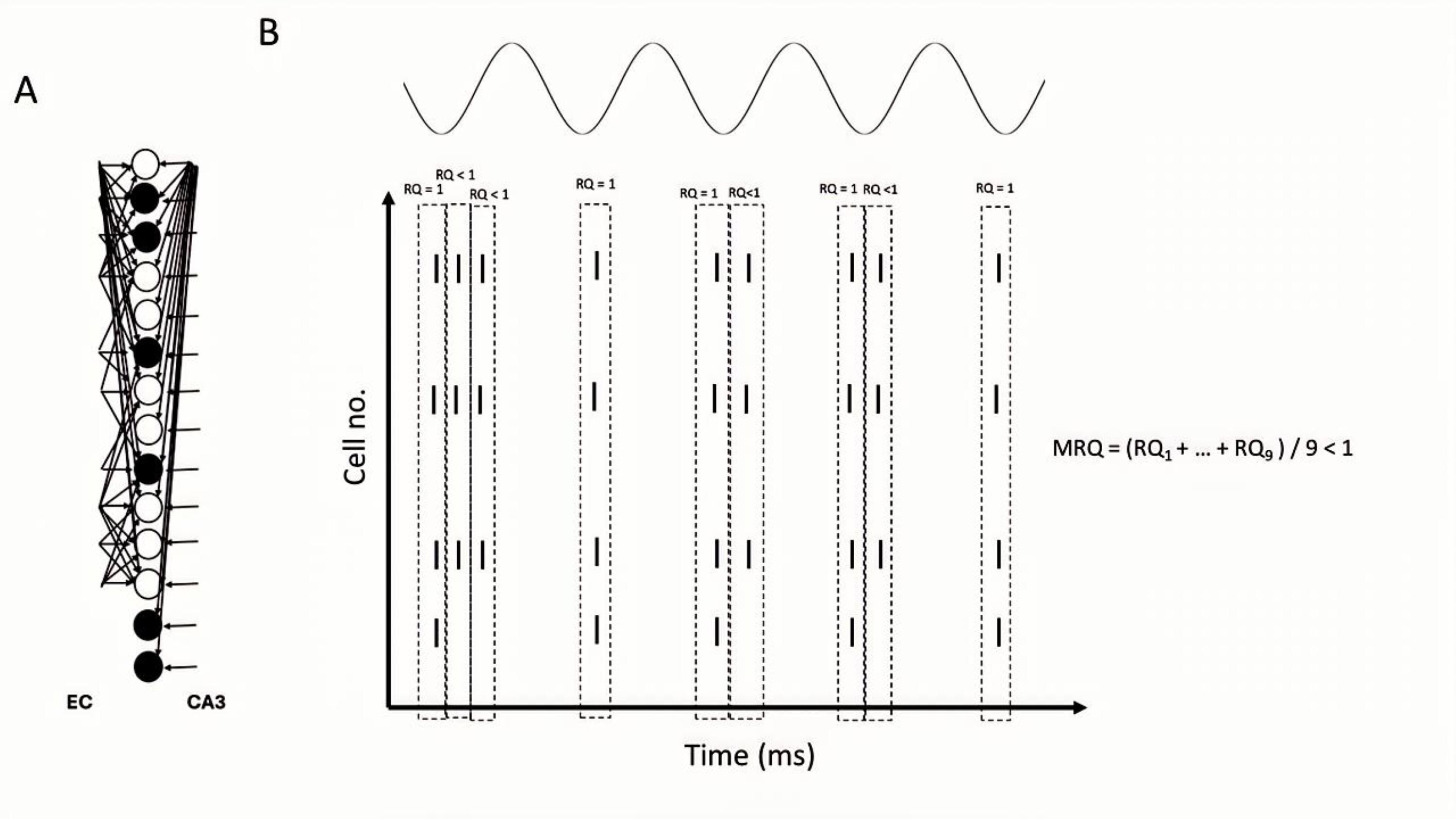
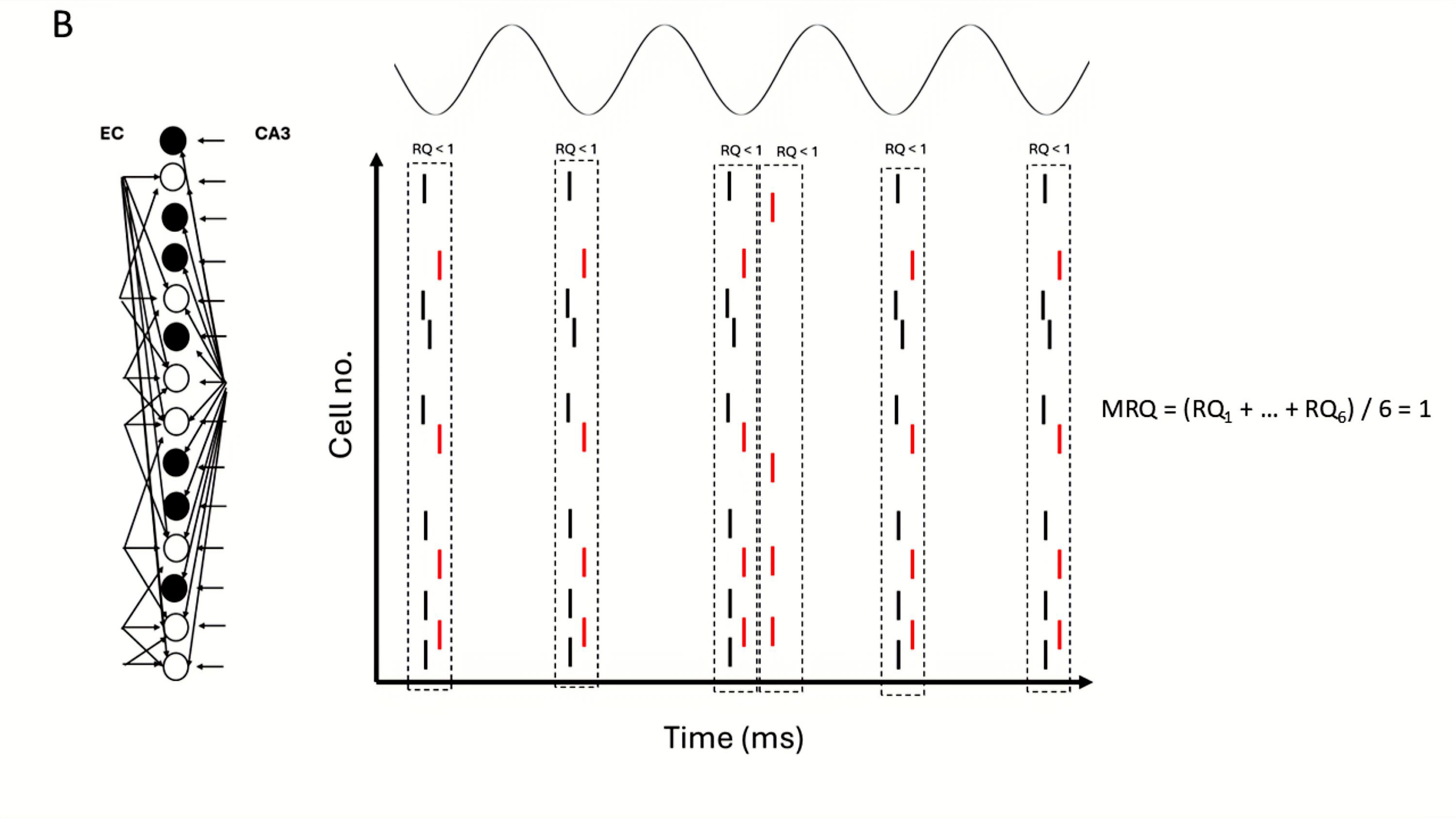
3.6. Recall Quality and Mean Recall Quality
3.7. Simulation Environment
4. Results
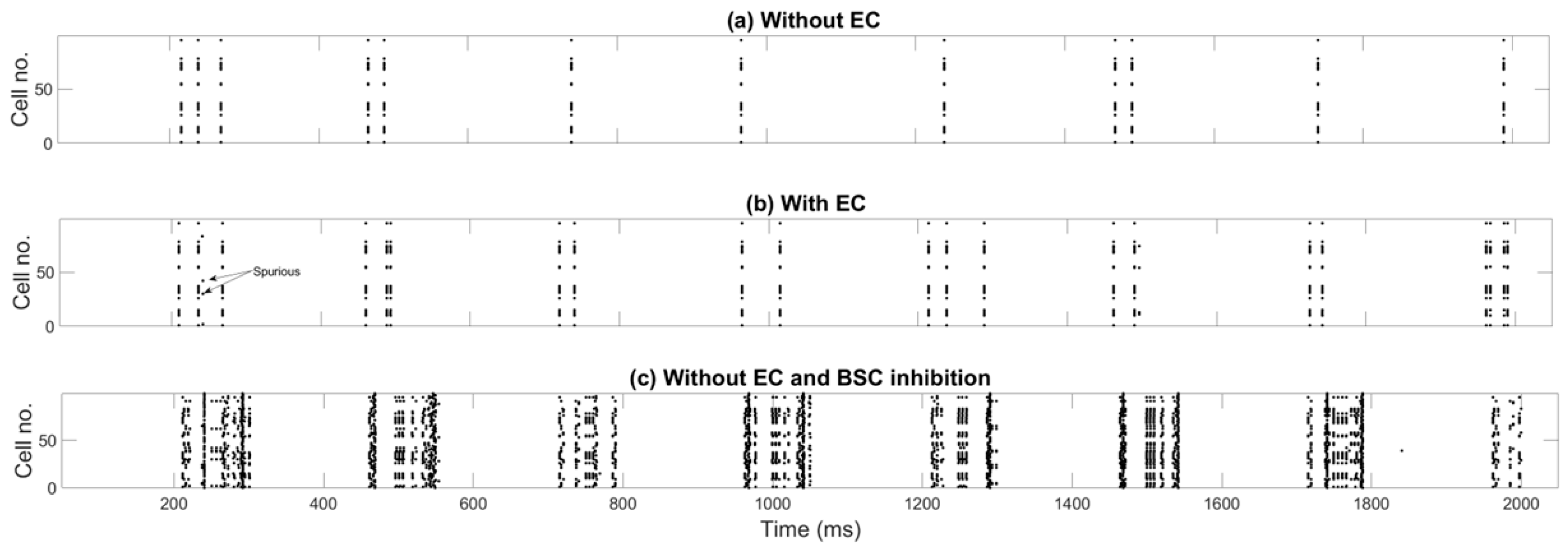
4.1. Pure Recall of Previously Stored Patterns
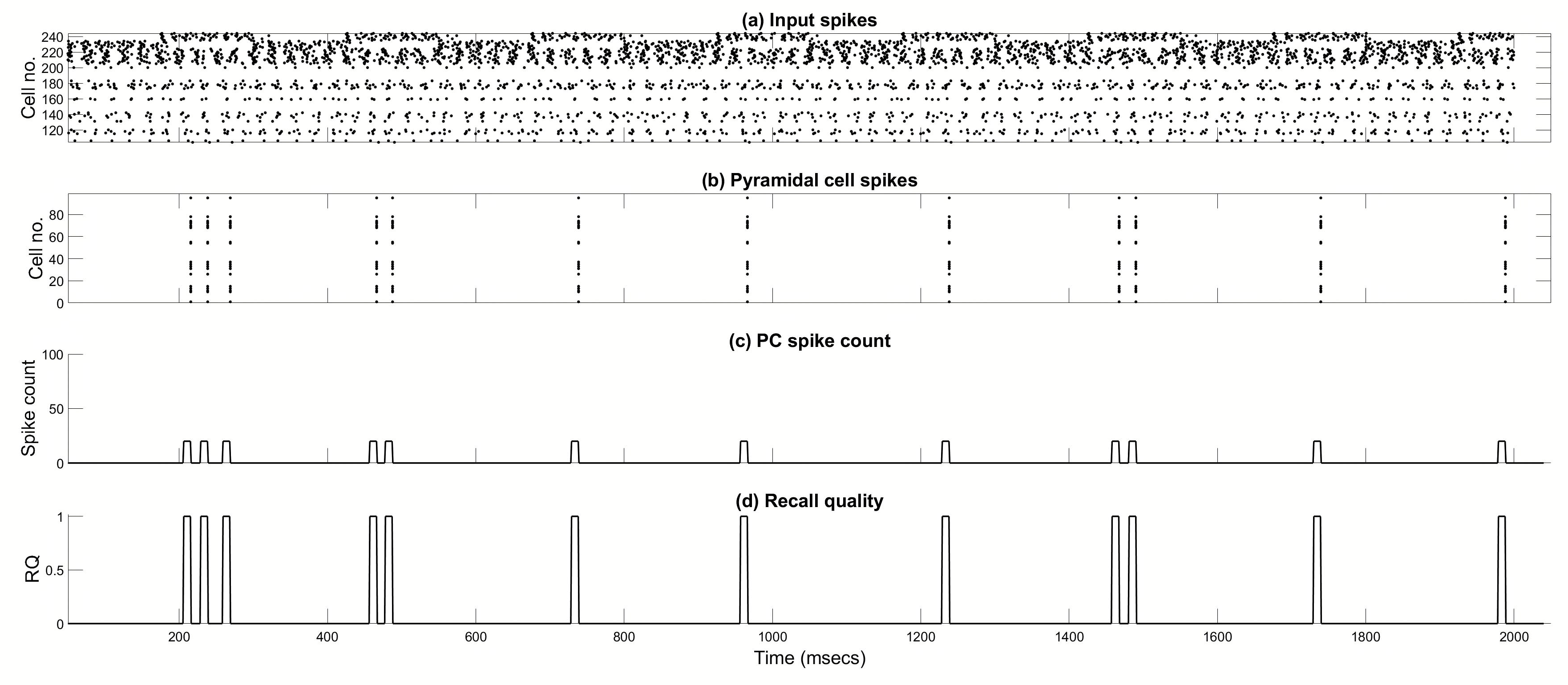
4.2. Effects of Spurious EC Input During Pure Recall of Previously Stored Patterns




5. Discussion
5.1. Model Predictions
5.2. Model Extensions and Alternatives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasselmo, M.E.; Eichenbaum, H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005, 18, 1172–1190. [Google Scholar]
- Cutsuridis, V.; Graham, B.P.; Cobb, S.; Vida, I. Hippocampal Microcircuits: A Computational Modeller’s Resource Book, 1st ed.; Springer: Cham, Switzerland, 2010. [Google Scholar]
- Amaral, D.G. Emerging principles of intrinsic hippocampal organization. Curr. Opin. Neurobiol. 1993, 3, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Remondes, M.; Schuman, E.M. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature 2002, 416, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Jarsky, T.; Roxin, A.; Kath, W.L.; Spruston, N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2005, 8, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Dudman, J.T.; Tsay, D.; Siegelbaum, S.A. A role for synaptic inputs at distal dendrites: Instructive signals for hippocampal long-term plasticity. Neuron 2007, 56, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.W.; Carlson, G.C.; Coulter, D.A. Hippocampal CA1 circuitry dynamically gates direct crtical inputs preferentially at theta frequencies. J. Neurosci. 2005, 25, 9567–9580. [Google Scholar]
- Dvorak-Carbone, H.; Schuman, E.M. Patterned activity in stratum lacunosum moleculare inhibits CA1 pyramidal neuron firing. J. Neurophysiol. 1999, 82, 3213–3222. [Google Scholar] [CrossRef]
- Takahashi, H.; Magee, J.C. Pathway Interactions and Synaptic Plasticity in the Dendritic Tuft Regions of CA1 Pyramidal Neurons. Neuron 2009, 62, 102–111. [Google Scholar] [CrossRef]
- López-Madrona, V.J.; Matias, F.S.; Pereda, E.; Canals, S.; Mirasso, C.R. On the role of the entorhinal cortex in the effective connectivity of the hippocampal formation. Chaos 2017, 27, 047401. [Google Scholar] [CrossRef]
- Magee, J.C.; Carruth, M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J. Neurophysiol. 1999, 82, 1895–1901. [Google Scholar] [CrossRef]
- Larkum, M.E.; Zhu, J.J.; Sakmann, B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 1999, 398, 338–341. [Google Scholar] [CrossRef]
- Cutsuridis, V.; Graham, B.P.; Cobb, S.; Vida, I. Hippocampal Microcircuits: A Computational Modeller’s Resource Book, 2nd ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Boivin, J.R.; Nedivi, E. Functional implications of inhibitory synapse placement on signal processing in pyramidal neuron dendrites. Curr. Opin. Neurobiol. 2018, 51, 16–22. [Google Scholar] [CrossRef]
- Müllner, F.E.; Wierenga, C.J.; Bonhoeffer, T. Precision of Inhibition: Dendritic Inhibition by Individual GABAergic Synapses on Hippocampal Pyramidal Cells Is Confined in Space and Time. Neuron 2015, 87, 576–589. [Google Scholar] [CrossRef]
- Willadt, S.; Nenniger, M.; Vogt, K.E. Hippocampal feedforward inhibition focuses excitatory synaptic signals into distinct dendritic compartments. PLoS ONE 2013, 8, e80984. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Garcıa-Austt, E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. I. Laminar distribution of theta field potentials. Exp. Brain Res. 1997, 67, 493–501. [Google Scholar] [CrossRef]
- Grastyan, E.; Lissak, K.; Madarasz, I.; Donhoffer, H. Hippocampal electrical activity during the development of conditioned reflexes. Electroencephalogr. Clin. Neurophysiol. 1959, 11, 409–430. [Google Scholar] [CrossRef]
- Vanderwolf, C.H. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 1969, 26, 407–418. [Google Scholar] [CrossRef]
- Hasselmo, M.; Bodelon, C.; Wyble, B. A proposed function of the hippocampal theta rhythm: Separate phases of encoding and retrieval of prior learning. Neural Comput. 2002, 14, 793–817. [Google Scholar] [CrossRef]
- Jensen, O.; Lisman, J.E. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005, 28, 67–72. [Google Scholar] [CrossRef]
- Kragel, J.E.; VanHaerents, S.; Templer, J.W.; Schuele, S.; Rosenow, J.M.; Nilakantan, A.S.; Bridge, D.J. Hippocampal theta coordinates memory processing during visual exploration. eLife 2020, 9, e52108. [Google Scholar] [CrossRef]
- Winson, J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 1978, 210, 160–163. [Google Scholar] [CrossRef]
- Stewart, M.; Fox, S. Do septal neurons pace the hippocampal theta rhythm? TINS 1990, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G. Theta oscillations in the hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.M.; Betancur, M.I.; Buzsaki, G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J. Neurosci. 2009, 29, 1381–1394. [Google Scholar] [CrossRef]
- Brankack, J.; Stewart, M.; Fox, S. Current source density analysis of the hippocampal theta rhythm: Associated sustained potentials and candidate synaptic generators. Brain Res. 1993, 615, 310–327. [Google Scholar] [CrossRef]
- Klausberger, T.; Somogyi, P. Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science 2008, 321, 53–57. [Google Scholar] [CrossRef]
- Freund, T.F.; Buzsaki, G. Interneurons of the hippocampus. Hippocampus 1996, 6, 347–470. [Google Scholar] [CrossRef]
- Klausberger, T.; Magill, P.J.; Marton, L.F.; David, J.; Roberts, B.; Cobden, P.M.; Buzsaki, G.; Somogyi, P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 2003, 421, 844–848. [Google Scholar] [CrossRef]
- Klausberger, T.; Marton, L.F.; Baude, A.; Roberts, J.D.B.; Magill, P.J.; Somogyi, P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat. Neurosci. 2024, 7, 41–47. [Google Scholar] [CrossRef]
- Mizuseki, K.; Sirota, A.; Pastalkova, E.; Buzsaki, G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron 2009, 64, 267–280. [Google Scholar] [CrossRef]
- Borhegyi, Z.; Varga, V.; Szilagyi, N.; Fabo, D.; Freund, T.F. Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J. Neurosci. 2004, 24, 8470–8479. [Google Scholar] [CrossRef]
- Dragoi, G.; Carpi, D.; Recce, M.; Csicsvari, J.; Buzsaki, G. Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. J. Neurosci. 1999, 19, 6191–6199. [Google Scholar] [CrossRef]
- Stumpf, C.; Petsche, H.; Gogolak, G. The significance of the rabbit’s septum as a relay station between the midbrain and the hippocampus. II. The differential influence of drugs upon both the septal cell firing pattern and the hippocampus theta activity. Electroencephalogr. Clin. Neurophysiol. 1962, 14, 212–219. [Google Scholar] [CrossRef]
- Andreakos, N.; Yue, S.; Cutsuridis, V. Systematic Evaluation of Memory Retrieval in a Neuromorphic Model of the Hippocampus. Cogn. Comput. 2025, 17, 123. [Google Scholar] [CrossRef]
- Cutsuridis, V.; Cobb, S.; Graham, B.P. Encoding and retrieval in the hippocampal CA1 microcircuit model. Hippocampus 2010, 20, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Cutsuridis, V. Improving the recall performance of a brain mimetic microcircuit model. Cogn. Comput. 2019, 11, 644–655. [Google Scholar] [CrossRef]
- Andreakos, N.; Yue, S.; Cutsuridis, V. Quantitative investigation of memory recall performance of a computational microcircuit model of the hippocampus. Brain Inform. 2021, 8, 9. [Google Scholar] [CrossRef]
- Unal, G.; Crump, M.C.; Viney, T.J.; Eltes, T.; Katona, L.; Klausberger, T.; Somogyi, P. Spatio-temporal specialization of GABAergic septo-hippocampal neurons for rhythmic network activity. Brain Struct. Funct. 2018, 223, 2409–2432. [Google Scholar] [CrossRef]
- Petersen, C.C.H.; Malenka, R.C.; Nicoll, R.A.; Hopfield, J.J. All-or none potentiation at CA3-CA1 synapses. Proc. Natl. Acad. Sci. USA 1998, 95, 4732–4737. [Google Scholar] [CrossRef]
- Hines, M.L.; Carnevale, T. The NEURON simulation environment. Neural Comput. 1997, 9, 1179–1209. [Google Scholar] [CrossRef] [PubMed]
- Krook-Magnuson, E.; Luu, L.; Lee, S.H.; Varga, C.; Soltesz, I. Ivy and neurogliaform interneurons are a major target of mu-opioid receptor modulation. J. Neurosci. 2011, 31, 14861–14870. [Google Scholar] [CrossRef]
- Fuentealba, P.; Begum, R.; Capogna, M.; Jinno, S.; Márton, L.F.; Csicsvari, J.; Thomson, A.; Somogyi, P.; Klausberger, T. Ivy cells: A population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron 2008, 57, 917–929. [Google Scholar] [CrossRef]
- Lapray, D.; Lasztoczi, B.; Lagler, M.; Viney, T.J.; Katona, L.; Valenti, O.; Hartwich, K.; Borhegyi, Z.; Somogyi, P.; Klausberger, T. Behavior-dependent specialization of identified hippocampal interneurons. Nat. Neurosci. 2012, 15, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Tricoire, L.; Pelkey, K.A.; Daw, M.I.; Sousa, V.H.; Miyoshi, G.; Jeffries, B.; Cauli, B.; Fishell, G.; McBain, C.J. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J. Neurosci. 2010, 30, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Hasselmo, M.E. Theta rhythmic stimulation of stratum lacunosum-moleculare in rat hippocampus contributes to associative LTP at a phase offset in stratum radiatum. J. Neurophysiol. 2004, 92, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Capogna, M.; Castillo, P.E.; Maffei, A. The ins and outs of inhibitory synaptic plasticity: Neuron types, molecular mechanisms and functional roles. Eur. J. Neurosci. 2021, 54, 6882–6901. [Google Scholar] [CrossRef]
- Elfant, D.; Pál, B.Z.; Emptage, N.; Capogna, M. Specific inhibitory synapses shift the balance from feedforward to feedback inhibition of hippocampal CA1 pyramidal cells. Eur. J. Neurosci. 2008, 27, 104–113. [Google Scholar] [CrossRef]
- Kullmann, D.M.; Moreau, A.W.; Bakiri, Y.; Nicholson, E. Plasticity of inhibition. Neuron 2012, 75, 951–962. [Google Scholar] [CrossRef]
- Vogels, T.P.; Sprekeler, H.; Zenke, F.; Clopath, C.; Gerstner, W. Inhibitory Plasticity Balances Excitation and Inhibition in Sensory Pathways and Memory Networks. Science 2008, 334, 1569–1573. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreakos, N.; Cutsuridis, V. Dendritic Inhibition Effects in Memory Retrieval of a Neuromorphic Microcircuit Model of the Rat Hippocampus. Brain Sci. 2025, 15, 1219. https://doi.org/10.3390/brainsci15111219
Andreakos N, Cutsuridis V. Dendritic Inhibition Effects in Memory Retrieval of a Neuromorphic Microcircuit Model of the Rat Hippocampus. Brain Sciences. 2025; 15(11):1219. https://doi.org/10.3390/brainsci15111219
Chicago/Turabian StyleAndreakos, Nikolaos, and Vassilis Cutsuridis. 2025. "Dendritic Inhibition Effects in Memory Retrieval of a Neuromorphic Microcircuit Model of the Rat Hippocampus" Brain Sciences 15, no. 11: 1219. https://doi.org/10.3390/brainsci15111219
APA StyleAndreakos, N., & Cutsuridis, V. (2025). Dendritic Inhibition Effects in Memory Retrieval of a Neuromorphic Microcircuit Model of the Rat Hippocampus. Brain Sciences, 15(11), 1219. https://doi.org/10.3390/brainsci15111219





