Short-Term Practice Modulates ERP Components Without Behavioral Change in a Short-ISI Go/NoGo Task
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
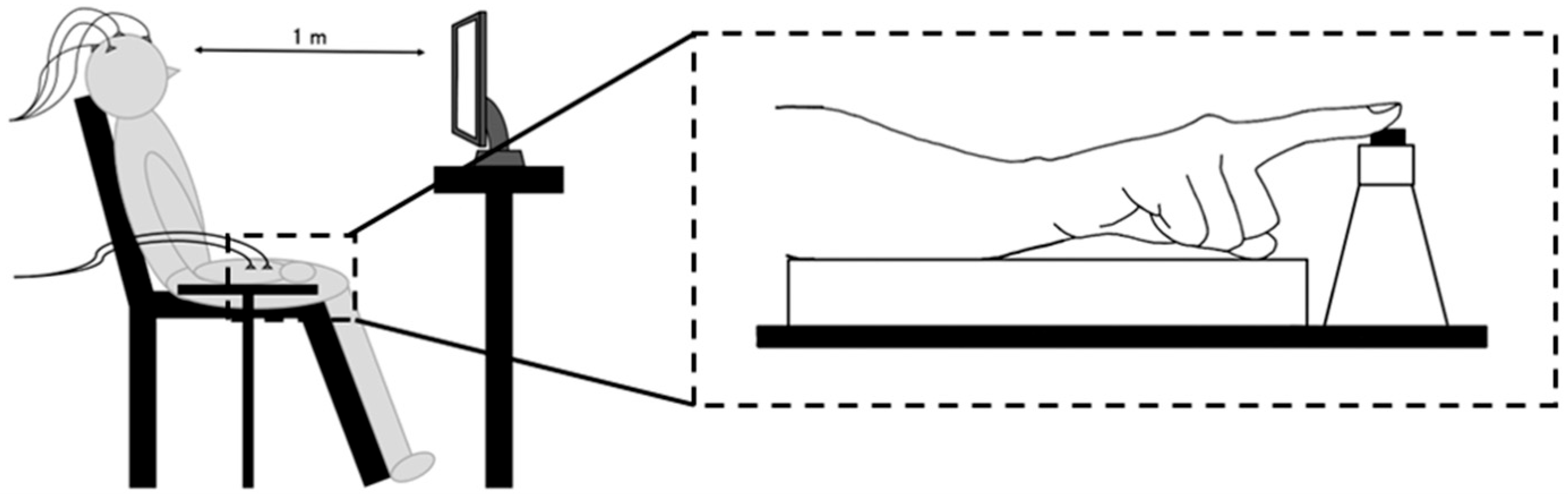
2.2. Experimental Procedures
2.3. Data Recordings
2.3.1. EEG Data
2.3.2. Go/NoGo Stimuli and Button Responses
2.4. Data Analysis
2.4.1. EEG Data
2.4.2. Behavioral Data
2.5. Statistical Analysis
3. Results
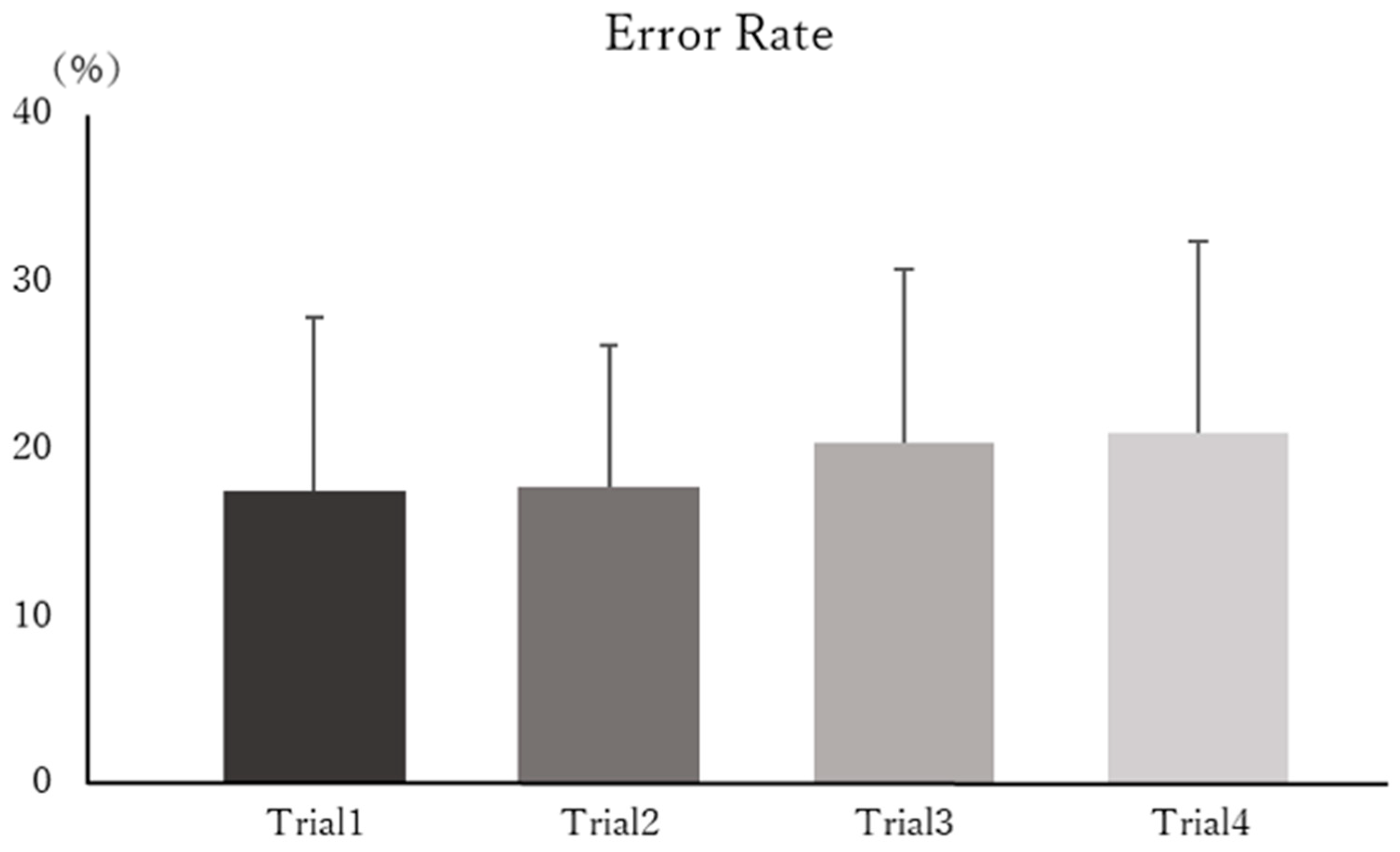
3.1. Task Performance
3.2. EEG Data
4. Discussion
4.1. Task Performance
4.2. EEG Data
4.3. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aron, A.R. The neural basis of inhibition in cognitive control. Neuroscientist 2007, 13, 214–228. [Google Scholar] [CrossRef]
- Aron, A.R. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef]
- Heilbronner, U.; Munte, T.F. Rapid event-related near-infrared spectroscopy detects age-related qualitative changes in the neural correlates of response inhibition. Neuroimage 2013, 65, 408–415. [Google Scholar] [CrossRef]
- Hong, X.; Sun, J.; Bengson, J.J.; Tong, S. Age-related spatiotemporal reorganization during response inhibition. Int. J. Psychophysiol. 2014, 93, 371–380. [Google Scholar] [CrossRef]
- Langenecker, S.A.; Nielson, K.A. Frontal recruitment during response inhibition in older adults replicated with fMRI. Neuroimage 2003, 20, 1384–1392. [Google Scholar] [CrossRef]
- Cooper, J.A.; Sagar, H.J.; Tidswell, P.; Jordan, N. Slowed central processing in simple and go/no-go reaction time tasks in Parkinson’s disease. Brain 1994, 117, 517–529. [Google Scholar] [CrossRef]
- Wu, H.M.; Hsiao, F.J.; Chen, R.S.; Shan, D.E.; Hsu, W.Y.; Chiang, M.C.; Lin, Y.Y. Attenuated NoGo-related beta desynchronisation and synchronisation in Parkinson’s disease revealed by magnetoencephalographic recording. Sci. Rep. 2019, 9, 7235. [Google Scholar] [CrossRef]
- Donders, F.C. On the speed of mental processes. Acta Psychol. 1969, 30, 412–431. [Google Scholar] [CrossRef]
- Filipovic, S.R.; Jahanshahi, M.; Rothwell, J.C. Uncoupling of contingent negative variation and alpha band event-related desynchronization in a go/no-go task. Clin. Neurophysiol. 2001, 112, 1307–1315. [Google Scholar] [CrossRef]
- Smith, J.L.; Johnstone, S.J.; Barry, R.J. Effects of pre-stimulus processing on subsequent events in a warned Go/NoGo paradigm: Response preparation, execution and inhibition. Int. J. Psychophysiol. 2006, 61, 121–133. [Google Scholar] [CrossRef]
- Smith, J.L.; Smith, E.A.; Provost, A.L.; Heathcote, A. Sequence effects support the conflict theory of N2 and P3 in the Go/NoGo task. Int. J. Psychophysiol. 2010, 75, 217–226. [Google Scholar] [CrossRef]
- Amieva, H.; Phillips, L.H.; Della Sala, S.; Henry, J.D. Inhibitory functioning in Alzheimer’s disease. Brain 2004, 127, 949–964. [Google Scholar] [CrossRef]
- Kok, A. Varieties of inhibition: Manifestations in cognition, event-related potentials and aging. Acta Psychol. 1999, 101, 129–158. [Google Scholar] [CrossRef] [PubMed]
- Bekker, E.M.; Kenemans, J.L.; Verbaten, M.N. Electrophysiological correlates of attention, inhibition, sensitivity and bias in a continuous performance task. Clin. Neurophysiol. 2004, 115, 2001–2013. [Google Scholar] [CrossRef]
- Smith, J.L.; Johnstone, S.J.; Barry, R.J. Movement-related potentials in the Go/NoGo task: The P3 reflects both cognitive and motor inhibition. Clin. Neurophysiol. 2008, 119, 704–714. [Google Scholar] [CrossRef]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 1999, 101, 267–291. [Google Scholar] [CrossRef]
- Randall, W.M.; Smith, J.L. Conflict and inhibition in the cued-Go/NoGo task. Clin. Neurophysiol. 2011, 122, 2400–2407. [Google Scholar] [CrossRef]
- Klingberg, T. Training and plasticity of working memory. Trends Cogn. Sci. 2010, 14, 317–324. [Google Scholar] [CrossRef]
- Li, W.; Shang, Y.; Zhuang, W.; Mai, W.; Cheng, W.; Chen, Z. Effectiveness of Response Inhibition Training and Its Long-Term Effects in Healthy Adults: A Systematic Review and Meta-Analysis. Front. Neurosci. 2022, 16, 813975. [Google Scholar] [CrossRef]
- Spierer, L.; Chavan, C.F.; Manuel, A.L. Training-induced behavioral and brain plasticity in inhibitory control. Front. Hum. Neurosci. 2013, 7, 427. [Google Scholar] [CrossRef]
- Kida, N.; Oda, S.; Matsumura, M. Intensive baseball practice improves the Go/Nogo reaction time, but not the simple reaction time. Brain Res. Cogn. Brain Res. 2005, 22, 257–264. [Google Scholar] [CrossRef]
- Yamashiro, K.; Sato, D.; Onishi, H.; Sugawara, K.; Nakazawa, S.; Shimojo, H.; Akatsuka, K.; Nakata, H.; Maruyama, A. Skill-Specific Changes in Somatosensory Nogo Potentials in Baseball Players. PLoS ONE 2015, 10, e0142581. [Google Scholar] [CrossRef]
- Nakamoto, H.; Mori, S. Effects of stimulus-response compatibility in mediating expert performance in baseball players. Brain Res. 2008, 1189, 179–188. [Google Scholar] [CrossRef]
- Simonet, M.; Ruggeri, P.; Sallard, E.; Barral, J. The field of expertise modulates the time course of neural processes associated with inhibitory control in a sport decision-making task. Sci. Rep. 2022, 12, 7657. [Google Scholar] [CrossRef]
- Manuel, A.L.; Grivel, J.; Bernasconi, F.; Murray, M.M.; Spierer, L. Brain dynamics underlying training-induced improvement in suppressing inappropriate action. J. Neurosci. 2010, 30, 13670–13678. [Google Scholar] [CrossRef]
- Benikos, N.; Johnstone, S.J.; Roodenrys, S.J. Short-term training in the Go/Nogo task: Behavioural and neural changes depend on task demands. Int. J. Psychophysiol. 2013, 87, 301–312. [Google Scholar] [CrossRef]
- Young, M.E.; Sutherland, S.C.; McCoy, A.W. Optimal go/no-go ratios to maximize false alarms. Behav. Res. Methods 2018, 50, 1020–1029. [Google Scholar] [CrossRef]
- Nakata, H.; Inui, K.; Wasaka, T.; Tamura, Y.; Kida, T.; Kakigi, R. Effects of ISI and stimulus probability on event-related go/nogo potentials after somatosensory stimulation. Exp. Brain Res. 2005, 162, 293–299. [Google Scholar] [CrossRef]
- Zamorano, F.; Billeke, P.; Hurtado, J.M.; Lopez, V.; Carrasco, X.; Ossandon, T.; Aboitiz, F. Temporal constraints of behavioral inhibition: Relevance of inter-stimulus interval in a Go-Nogo task. PLoS ONE 2014, 9, e87232. [Google Scholar] [CrossRef]
- Houben, K.; Havermans, R.C.; Nederkoorn, C.; Jansen, A. Beer a no-go: Learning to stop responding to alcohol cues reduces alcohol intake via reduced affective associations rather than increased response inhibition. Addiction 2012, 107, 1280–1287. [Google Scholar] [CrossRef]
- Jodo, E.; Inoue, K. Effects of practice on the P300 in a Go/NoGo task. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 249–257. [Google Scholar] [CrossRef]
- Johnstone, S.J.; Roodenrys, S.; Blackman, R.; Johnston, E.; Loveday, K.; Mantz, S.; Barratt, M.F. Neurocognitive training for children with and without AD/HD. Atten. Defic. Hyperact. Disord. 2012, 4, 11–23. [Google Scholar] [CrossRef]
- Houben, K.; Jansen, A. Training inhibitory control. A recipe for resisting sweet temptations. Appetite 2011, 56, 345–349. [Google Scholar] [CrossRef]
- Jones, A.; Di Lemma, L.C.; Robinson, E.; Christiansen, P.; Nolan, S.; Tudur-Smith, C.; Field, M. Inhibitory control training for appetitive behaviour change: A meta-analytic investigation of mechanisms of action and moderators of effectiveness. Appetite 2016, 97, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.S.; Verbruggen, F.; Morrison, S.; Adams, R.C.; Chambers, C.D. Stopping to food can reduce intake. Effects of stimulus-specificity and individual differences in dietary restraint. Appetite 2015, 85, 91–103. [Google Scholar] [CrossRef]
- Chavan, C.F.; Mouthon, M.; Draganski, B.; van der Zwaag, W.; Spierer, L. Differential patterns of functional and structural plasticity within and between inferior frontal gyri support training-induced improvements in inhibitory control proficiency. Hum. Brain Mapp. 2015, 36, 2527–2543. [Google Scholar] [CrossRef]
- Manuel, A.L.; Bernasconi, F.; Spierer, L. Plastic modifications within inhibitory control networks induced by practicing a stop-signal task: An electrical neuroimaging study. Cortex 2013, 49, 1141–1147. [Google Scholar] [CrossRef]
- He, Y.; Yang, T.; He, C.; Sun, K.; Guo, Y.; Wang, X.; Bai, L.; Xue, T.; Xu, T.; Guo, Q.; et al. Effects of audiovisual interactions on working memory: Use of the combined N-back + Go/NoGo paradigm. Front. Psychol. 2023, 14, 1080788. [Google Scholar] [CrossRef]
- Kida, T.; Nishihira, Y.; Hatta, A.; Wasaka, T. Somatosensory N250 and P300 during discrimination tasks. Int. J. Psychophysiol. 2003, 48, 275–283. [Google Scholar] [CrossRef]
- Kato, Y.; Endo, H.; Kizuka, T. Mental fatigue and impaired response processes: Event-related brain potentials in a Go/NoGo task. Int. J. Psychophysiol. 2009, 72, 204–211. [Google Scholar] [CrossRef]
- Ikarashi, K.; Sato, D.; Ochi, G.; Fujimoto, T.; Yamashiro, K. Action Postponing and Restraint Varies among Sensory Modalities. Brain Sci. 2022, 12, 1530. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Akaiwa, M.; Matsuda, Y.; Shibata, E.; Saito, H.; Sasaki, T. Movement of the stimulated finger in a Go/NoGo task enhances attention directed to that finger as evidenced by P300 amplitude modulation. Front. Hum. Neurosci. 2023, 17, 1178509. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Yuan, J.; Fu, A.; Meng, X.; Li, H. The impact of emotion valence on brain processing of behavioral inhibitory control: Spatiotemporal dynamics. Neurosci. Lett. 2011, 502, 112–116. [Google Scholar] [CrossRef]
- Boisgontier, M.P.; Wittenberg, G.F.; Fujiyama, H.; Levin, O.; Swinnen, S.P. Complexity of central processing in simple and choice multilimb reaction-time tasks. PLoS ONE 2014, 9, e90457. [Google Scholar] [CrossRef]
- Wong, A.L.; Haith, A.M.; Krakauer, J.W. Motor Planning. Neuroscientist 2015, 21, 385–398. [Google Scholar] [CrossRef]
- De Pretto, M.; Hartmann, L.; Garcia-Burgos, D.; Sallard, E.; Spierer, L. Stimulus Reward Value Interacts with Training-induced Plasticity in Inhibitory Control. Neuroscience 2019, 421, 82–94. [Google Scholar] [CrossRef]
- Hartmann, L.; Sallard, E.; Spierer, L. Enhancing frontal top-down inhibitory control with Go/NoGo training. Brain Struct. Funct. 2016, 221, 3835–3842. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, P.; Huang, X. The Practice Effect on Time-Based Prospective Memory: The Influences of Ongoing Task Difficulty and Delay. Front. Psychol. 2019, 10, 2002. [Google Scholar] [CrossRef]
- Malinovitch, T.; Jakoby, H.; Ahissar, M. Training-induced improvement in working memory tasks results from switching to efficient strategies. Psychon. Bull. Rev. 2021, 28, 526–536. [Google Scholar] [CrossRef]
- Benikos, N.; Johnstone, S.J.; Roodenrys, S.J. Varying task difficulty in the Go/Nogo task: The effects of inhibitory control, arousal, and perceived effort on ERP components. Int. J. Psychophysiol. 2013, 87, 262–272. [Google Scholar] [CrossRef]
- Eimer, M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol. Psychol. 1993, 35, 123–138. [Google Scholar] [CrossRef]
- Nakata, H.; Inui, K.; Wasaka, T.; Tamura, Y.; Kida, T.; Kakigi, R. The characteristics of the nogo-N140 component in somatosensory go/nogo tasks. Neurosci. Lett. 2006, 397, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Sakamoto, K.; Kakigi, R. Effects of task repetition on event-related potentials in somatosensory Go/No-go paradigm. Neurosci. Lett. 2015, 594, 82–86. [Google Scholar] [CrossRef]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef]
- Jodo, E.; Kayama, Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalogr. Clin. Neurophysiol. 1992, 82, 477–482. [Google Scholar] [CrossRef]
- Kok, A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biol. Psychol. 1986, 23, 21–38. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Ford, J.M.; Weller, B.J.; Kopell, B.S. ERPs to response production and inhibition. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 423–434. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Yeung, N.; van den Wildenberg, W.; Ridderinkhof, K.R. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cogn. Affect. Behav. Neurosci. 2003, 3, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Donkers, F.C.; van Boxtel, G.J. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004, 56, 165–176. [Google Scholar] [CrossRef]
- Ravden, D.; Polich, J. Habituation of P300 from visual stimuli. Int. J. Psychophysiol. 1998, 30, 359–365. [Google Scholar] [CrossRef]
- Schapkin, S.A.; Falkenstein, M.; Marks, A.; Griefahn, B. Practice-related effects in a Go-Nogo task. Percept. Mot. Skills 2007, 105, 1275–1288. [Google Scholar] [CrossRef]
- Bianco, V.; Di Russo, F.; Perri, R.L.; Berchicci, M. Different proactive and reactive action control in fencers’ and boxers’ brain. Neuroscience 2017, 343, 260–268. [Google Scholar] [CrossRef]
- Grill-Spector, K.; Henson, R.; Martin, A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn. Sci. 2006, 10, 14–23. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, H.; Wang, X.; Qi, C.; Luo, Y. Enhanced response inhibition in experienced fencers. Sci. Rep. 2015, 5, 16282. [Google Scholar] [CrossRef]
- Huster, R.J.; Enriquez-Geppert, S.; Lavallee, C.F.; Falkenstein, M.; Herrmann, C.S. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int. J. Psychophysiol. 2013, 87, 217–233. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Polich, J.; Bondurant, T. P300 sequence effects, probability, and interstimulus interval. Physiol. Behav. 1997, 61, 843–849. [Google Scholar] [CrossRef]
- Verleger, R.; Grauhan, N.; Smigasiewicz, K. Go and no-go P3 with rare and frequent stimuli in oddball tasks: A study comparing key-pressing with counting. Int. J. Psychophysiol. 2016, 110, 128–136. [Google Scholar] [CrossRef]
- Geisler, M.W.; Polich, J. P300 habituation from visual stimuli? Physiol. Behav. 1994, 56, 511–516. [Google Scholar] [CrossRef]
- Kok, A. Event-related-potential (ERP) reflections of mental resources: A review and synthesis. Biol. Psychol. 1997, 45, 19–56. [Google Scholar] [CrossRef]
- Maguire, M.J.; Brier, M.R.; Moore, P.S.; Ferree, T.C.; Ray, D.; Mostofsky, S.; Hart, J., Jr.; Kraut, M.A. The influence of perceptual and semantic categorization on inhibitory processing as measured by the N2-P3 response. Brain Cogn. 2009, 71, 196–203. [Google Scholar] [CrossRef]
- Chikazoe, J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr. Opin. Psychiatry 2010, 23, 267–272. [Google Scholar] [CrossRef]
- Frank, M.J.; Loughry, B.; O’Reilly, R.C. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cogn. Affect. Behav. Neurosci. 2001, 1, 137–160. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Munakata, Y.; Herd, S.A.; Chatham, C.H.; Depue, B.E.; Banich, M.T.; O’Reilly, R.C. A unified framework for inhibitory control. Trends Cogn. Sci. 2011, 15, 453–459. [Google Scholar] [CrossRef]
- Barry, R.J.; Rushby, J.A. An orienting reflex perspective on anteriorisation of the P3 of the event-related potential. Exp. Brain Res. 2006, 173, 539–545. [Google Scholar] [CrossRef]
- Gonzalez-Rosa, J.J.; Inuggi, A.; Blasi, V.; Cursi, M.; Annovazzi, P.; Comi, G.; Falini, A.; Leocani, L. Response competition and response inhibition during different choice-discrimination tasks: Evidence from ERP measured inside MRI scanner. Int. J. Psychophysiol. 2013, 89, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Huster, R.J.; Wolters, C.; Wollbrink, A.; Schweiger, E.; Wittling, W.; Pantev, C.; Junghofer, M. Effects of anterior cingulate fissurization on cognitive control during stroop interference. Hum. Brain Mapp. 2009, 30, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Atienza, M.; Cantero, J.L.; Dominguez-Marin, E. The time course of neural changes underlying auditory perceptual learning. Learn. Mem. 2002, 9, 138–150. [Google Scholar] [CrossRef]



| F3 | F4 | Fz | C3 | C4 | Cz | P3 | P4 | Pz | |
|---|---|---|---|---|---|---|---|---|---|
| Trial 1 | −4.82 (4.01) | −5.43 (6.85) | −7.58 (5.18) | −6.10 (7.70) | −7.89 (10.57) | −11.51 (6.08) | −0.66 (5.29) | −3.15 (6.27) | −5.26 (6.57) |
| Trial 2 | −3.10 (4.55) | −4.27 (7.59) | −6.45 (5.65) | −5.70 (6.25) | −5.74 (8.08) | −9.03 (5.58) | −0.53 (5.41) | −3.51 (4.88) | −4.13 (3.25) |
| Trial 3 | −5.38 (3.71) | −5.52 (5.79) | −8.27 (3.21) | −6.39 (3.56) | −7.28 (5.73) | −10.55 (3.18) | −1.03 (3.62) | −2.94 (4.48) | −3.23 (3.48) |
| Trial 4 | −5.56 (6.43) | −6.05 (4.80) | −9.06 (3.82) | −6.18 (2.85) | −7.26 (5.09) | −10.90 (3.05) | 0.10 (4.58) | −1.81 (4.19) | −2.82 (4.79) |
| F3 | F4 | Fz | C3 | C4 | Cz | P3 | P4 | Pz | |
|---|---|---|---|---|---|---|---|---|---|
| Trial 1 | 9.05 (6.70) | 9.46 (8.59) | 16.35 (7.61) * | 15.48 (7.70) | 14.11 (10.57) | 19.53 (6.08) | 14.80 (5.29) | 13.18 (6.27) | 15.80 (6.57) |
| Trial 2 | 8.54 (5.80) | 7.41 (8.38) | 13.02 (5.87) | 13.49 (6.87) | 12.35 (9.33) | 16.99 (6.94) | 12.94 (5.55) | 9.59 (6.83) | 14.27 (6.39) |
| Trial 3 | 7.64 (5.67) | 7.74 (7.34) | 12.96 (5.53) * | 13.94 (6.22) | 12.80 (7.01) | 18.40 (4.62) | 14.91 (6.41) | 11.74 (6.51) | 16.75 (5.79) |
| Trial 4 | 6.43 (5.36) | 6.87 (7.07) | 11.81 (5.62) * | 13.14 (4.95) | 12.69 (6.16) | 17.19 (4.28) | 14.91 (6.28) | 12.58 (6.55) | 16.75 (5.52) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugawara, Y.; Matsuda, Y.; Kurokawa, R.; Kosuge, R.; Kudoh, S.; Akaiwa, M.; Saito, H.; Sasaki, T.; Sugawara, K. Short-Term Practice Modulates ERP Components Without Behavioral Change in a Short-ISI Go/NoGo Task. Brain Sci. 2025, 15, 1208. https://doi.org/10.3390/brainsci15111208
Sugawara Y, Matsuda Y, Kurokawa R, Kosuge R, Kudoh S, Akaiwa M, Saito H, Sasaki T, Sugawara K. Short-Term Practice Modulates ERP Components Without Behavioral Change in a Short-ISI Go/NoGo Task. Brain Sciences. 2025; 15(11):1208. https://doi.org/10.3390/brainsci15111208
Chicago/Turabian StyleSugawara, Yasushi, Yuya Matsuda, Ryo Kurokawa, Rin Kosuge, Satoshi Kudoh, Mayu Akaiwa, Hidekazu Saito, Takeshi Sasaki, and Kazuhiro Sugawara. 2025. "Short-Term Practice Modulates ERP Components Without Behavioral Change in a Short-ISI Go/NoGo Task" Brain Sciences 15, no. 11: 1208. https://doi.org/10.3390/brainsci15111208
APA StyleSugawara, Y., Matsuda, Y., Kurokawa, R., Kosuge, R., Kudoh, S., Akaiwa, M., Saito, H., Sasaki, T., & Sugawara, K. (2025). Short-Term Practice Modulates ERP Components Without Behavioral Change in a Short-ISI Go/NoGo Task. Brain Sciences, 15(11), 1208. https://doi.org/10.3390/brainsci15111208






