Abstract
Background/Objectives: Accumulating evidence suggests that cognitive training can induce functional reorganization of intrinsic connectivity networks involved in higher-order cognitive processes. However, few interventions have specifically targeted language, an essential domain tightly interwoven with memory, attention, and executive functions. Given their foundational role in communication, reasoning, and knowledge acquisition, enhancing language-related abilities may yield widespread cognitive benefits. This study investigated the neural impact of a new structured, language-based cognitive training program on neurotypical older adults. Methods: Twenty Brazilian Portuguese-speaking women (aged 63–77 years; schooling 9–20 years; low-to-medium socioeconomic status) participated in linguistic activities designed to engage language and general cognitive processing. Behavioral testing and resting-state functional Magnetic Resonance Imaging (fMRI) were conducted before and after the intervention. Results: Functional connectivity analyses revealed significant post-intervention increases in connectivity within the frontoparietal network, critical for language processing, and the ventral attentional network, associated with attentional control. Conclusions: The observed neural enhancements indicate substantial plasticity in cognitive networks among older adults, highlighting the effectiveness of linguistic interventions in modulating critical cognitive functions. These findings provide a foundation for future research on targeted cognitive interventions to promote healthy aging and sustain cognitive vitality.
1. Introduction
Cognitive changes are well-documented aspects of both neurotypical and pathological aging. Changes in several cognitive domains are expected to occur with advancing age, including reductions in working memory capacity, processing speed, and attentional control. For example, older adults often exhibit slower reaction times and increased interference in tasks that require attentional control, such as the Stroop task [1,2]. Executive functioning is particularly relevant because its decline is a significant predictor of Alzheimer’s disease (AD) [3,4] together with episodic memory [5]. Preserving cognitive functions may help delay or mitigate functional decline or, at the very least, contribute to maintaining or improving cognition.
Over the past two decades, there has been growing interest in cognitive training interventions aimed at improving cognitive function in older adults. These interventions vary in modality (e.g., online vs. face-to-face), duration, frequency, and target domains (domain-specific vs. general cognition). Studies have demonstrated that cognitive training can enhance cognitive performance in older adults with cognitive impairment (e.g., [6,7,8,9,10]) and without (e.g., [11,12,13,14,15,16]). For instance, Corbett et al. [12] reported significant benefits from online cognitive training interventions targeting reasoning and general cognitive abilities, noting improvements in self-reported instrumental activities of daily living (IADL) among adults aged over 60 years. This study further indicated considerable positive effects on reasoning and verbal learning, as well as moderate gains in spatial working memory, compared with control participants. Similarly, Borella et al. [11] reported positive outcomes specifically related to working-memory-focused cognitive training programs. Moreover, cognitive training interventions have shown lasting benefits. Long-term evidence from the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study indicated that cognitive improvements persisted for up to 10 years post-intervention, notably in areas such as IADL, reasoning, and processing speed [14]. Earlier findings from the same program similarly showed sustained benefits in IADL and cognitive performance lasting up to 5 years [15]. From a neurophysiological perspective, numerous studies have shown that cognitive training interventions can affect brain activity. Consistent neural changes have been identified primarily within the frontoparietal network (FPN), including alterations in the medial frontal gyrus, precentral gyrus, inferior frontal gyrus, superior parietal cortex, supramarginal gyrus, and superior temporal gyrus [17,18,19,20].
Most cognitive training programs are delivered either online or face-to-face, primarily targeting specific cognitive domains such as executive functions [21] or episodic memory [22]. However, interventions that solely adopt linguistic stimuli are comparatively rare [23,24]. Moreover, studies examining the impact of language-based training on broader cognitive functions are scarce [25]. However, language processing fundamentally underpins cognitive performance. For instance, reading and writing habits in neurotypical older adults significantly mitigate age-related cognitive decline [26]. Additionally, semantic memory, as reflected by speech connectivity measures, is closely linked to cognitive functioning in Alzheimer’s disease [26]. Linguistically oriented interventions may be particularly beneficial for populations with lower socioeconomic status (SES), whose educational attainment and literacy levels are typically lower.
Despite the well-established relationship between language and other cognitive functions, interventions that specifically utilize language-based tasks remain limited. Language processing heavily depends on cognitive functions, such as executive control, which supports comprehension by resolving ambiguities in sentences and enabling efficient word retrieval by inhibiting competing lexical alternatives [27]. Similarly, working memory substantially contributes to language comprehension by allowing listeners to maintain earlier parts of sentences while processing subsequent segments [28]. Executive functions have also been demonstrated to influence everyday language use in older adults; higher executive abilities correlate positively with more analytical linguistic expressions such as using longer words and more frequent numerical references [29]. Furthermore, deficits in semantic short-term memory are associated with impaired word production and sentence comprehension, which reflects increased lexical competition due to inhibitory dysfunction [30]. Attention, together with other general cognitive skills, is intrinsically related to language processing at both the comprehension and production levels, providing insights into the relationship between language, cognition, and brain-related circuitry [31]. Understanding these cognitive–language interdependencies emphasizes the potential effectiveness of language-based cognitive training interventions in promoting cognitive health.
To better understand how cognitive training may shape the neural mechanisms supporting cognitive–linguistic interactions in aging, resting-state functional magnetic resonance imaging (fMRI) offers a powerful approach for examining the intrinsic organization and plasticity of large-scale brain networks that underlie cognitive and language functions. By assessing synchronized activity among spatially distinct brain regions, resting-state fMRI enables the identification of intrinsic connectivity networks that are fundamental to cognitive processes including language. Among these, the default mode network (DMN), frontoparietal network (FPN), dorsal attention network (DAN), ventral attention network (VAN), and salience network (SN) are particularly relevant because of their involvement in language and cognitive functions. The DMN primarily supports internally directed cognitive activities, such as autobiographical memory, planning, social cognition, and executive processes, such as working memory and cognitive flexibility. It is active at rest but deactivates during goal-directed tasks, with reduced connectivity linked to cognitive decline and memory impairment in aging and Alzheimer’s disease [32]. In contrast, the FPN, also commonly referred to as the central executive network (CEN) or executive control network (ECN), serves as a cognitive control hub crucial for executive functions, including cognitive flexibility, working memory, and semantic control [33,34]. The FPN dynamically interacts with the DMN and attention networks, facilitating shifts between internal and external cognitive states and modulating distractions [35,36,37].
Attention is regulated primarily by two complementary networks. The DAN directs attention toward relevant stimuli and is essential for working memory and memory encoding, influencing tasks such as reading by modulating attention to visual and spatial cues [38,39]. Meanwhile, VAN detects and reorients attention to unexpected stimuli, contributing to cognitive flexibility and reading fluency [40,41]. Central to orchestrating these interactions is the SN, which monitors and filters relevant stimuli, and facilitates cognitive flexibility by alternating between internally focused processes (DMN-driven) and externally directed tasks (FPN/DAN-driven). Key regions of the SN, including the anterior insula and anterior cingulate cortex, have significant implications for language functions, such as articulation, word retrieval, and phonological discrimination [42,43,44]. Moreover, SN connectivity patterns have been linked to age-related decline in cognitive functions, particularly executive control, working memory updating, and attention regulation [45].
Accumulating evidence suggests that cognitive training can induce functional reorganization in intrinsic connectivity networks implicated in higher-order cognitive processes. For instance, Cao et al. [46] reported maintained or increased resting-state functional connectivity within the DMN, FPN, and SN in healthy older adults following a one-year cognitive training intervention. Similarly, Chapman et al. [47] demonstrated increased functional connectivity within the DMN and PFN after a shorter (12-week) program, with connectivity increases correlating with cognitive gain. Consistent with these findings, De Marco et al. [48] also reported strengthened connectivity within the DMN after just one month of computerized cognitive training focused specifically on this network.
However, not all studies reported uniform findings across networks. For instance, Hardcastle et al. [49] identified significant increases solely within the FPN following a multi-domain cognitive intervention, attributing the absence of detectable DMN and DAN changes to potentially high cognitive reserves in their highly educated sample, suggesting that a longer duration might be necessary to reveal changes in these networks. Additionally, Strenziok et al. [50] proposed that DAN connectivity changes specifically underpin the mechanisms of far transfer, and their results indicated reduced connectivity between the superior parietal cortex (part of the DAN) and inferior temporal regions exclusively when far transfer occurred [51]. Furthermore, only a few studies have specifically examined the effects of cognitive training on VAN connectivity. Nevertheless, Hampstead et al. [52] reported an enhanced VAN connectivity around the temporoparietal junction following an explicit training program. Taken together, these findings highlight the capacity of cognitive training to reshape the architecture of large-scale brain networks and underscore the importance of the possibility that language-centered interventions might similarly modulate these networks, and consequently, cognitive functions.
Finally, further evidence on the impact of language-based training, cognition, and brain circuitry has been provided by studies on bilingualism, second language training, and functional illiteracy. Research on bilingualism has demonstrated enhanced executive function in multilingual individuals, including improved monitoring, inhibition, attention, and delayed cognitive decline [53,54,55]. Furthermore, a few studies have explored the direct impact of second-language-based interventions on cognitive function. For instance, a four-month second-language learning intervention in older adults resulted in improved global cognition and increased connectivity within language and executive networks, suggesting a promising avenue for cognitive interventions focusing on language [56]. Complementing these findings, Mohammadi et al. [57] reported that literacy training in functional illiterates reduced hyperconnectivity within the left FPN, suggesting a compensatory mechanism by which executive resources initially support cognitive deficits [56]. These findings collectively underscore the interconnected roles of language, cognitive functions, and their underlying neural networks, suggesting that targeted cognitive interventions leveraging linguistic training may effectively enhance cognitive function in older adults.
Building upon prior evidence, the present study aimed to determine whether language-based cognitive intervention can induce changes in resting-state functional connectivity in healthy older adults. Specifically, we examined whether a two-month linguistic training program modulated connectivity within large-scale brain networks associated with language processing and cognitive functions. At the behavioral level, we hypothesized improvements in episodic memory [25]. At the neurobiological level, we predicted increased functional connectivity in the DMN [46,47,48] and FPN [47,49,57], both of which have been implicated in cognitive training-related plasticity. We also anticipate subtle changes in the SN [46] and either preserved or reduced connectivity in the DAN, consistent with findings suggesting compensatory reallocation [49,50,51]. Although the ventral attention network (VAN) has received limited attention in this context, we explored the possibility of enhanced connectivity based on previous work [52]. By elucidating these neurofunctional adaptations, our study sought to establish the efficacy of language-based cognitive training as a targeted approach to support brain health in aging.
2. Materials and Methods
2.1. Participants
Twenty neurologically healthy individuals were recruited from a metropolitan area in southern Brazil. Three participants were excluded from fMRI data analysis due to excessive head motion or global signal fluctuations during fMRI acquisition. Only women were included in this study due to difficulties recruiting men. The demographic and clinical characteristics of the participants are summarized in Table 1. Their ages ranged from 63 to 77 years (mean = 69.3, SD = 4.7), and their years of schooling ranged from 9 to 20 years (mean = 14.85, SD = 3.3). To be included, participants were required to be native Brazilian Portuguese speakers, right-handed, literate, and eligible for fMRI. Exclusion criteria included a history of major psychiatric disorders, learning disabilities, severe or uncorrected self-reported perceptual deficits, and any additional neurological diagnoses. Handedness was assessed using the Edinburgh Handedness Inventory [58]. All participants provided written informed consent before enrollment. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS)-protocol number 53696221.4.1001.5336, approved on 18 January 2022)

Table 1.
Sociodemographic Profile and Mental Health, Cognitive, and Functional Assessment Results.
2.2. Materials and Procedures
Participants underwent a complete assessment before and after cognitive training, which included fMRI as well as language, cognitive, and neuropsychological assessments.
2.2.1. Sociodemographic, Emotional, Functional and Cognitive Assessment
Questionnaires were administered to gather information about the participants’ language profiles, socialization habits, physical training habits, and general health. Socioeconomic status (SES) was determined using Critério Brasil 2024 [59]. SES scoring is based on household characteristics, the schooling level of the household head, and the availability of consumer goods and amenities. The scores are categorized into six socioeconomic groups, from highest to lowest: A (45–100 points), B1 (38–44 points), B2 (29–37 points), C1 (23–28 points), C2 (17–22 points), and DE (0–16 points). Reading and writing habits (RWH) were assessed using a questionnaire developed in Brazil [60]. This instrument includes 30 questions assessing the frequency of engaging in various reading activities (e.g., magazines, newspapers, books, social media) and writing tasks (e.g., notes, text messages, literary and non-literary texts) as well as the participants’ history of literacy development. Scores range from 0 to 100, with lower scores indicating more frequent engagement in reading and writing.
Participants were assessed for depressive symptoms using the Brazilian version of the Geriatric Depression Scale (GDS) [61] and for functional impairments indicative of cognitive decline or dementia using the Functional Activities Questionnaire (FAQ) [62]. Anxiety symptoms were evaluated using the Geriatric Anxiety Inventory (GAI) [63]. Furthermore, participants underwent a comprehensive neuropsychological assessment that included the Brazilian version of Addenbrooke’s Cognitive Examination–Revised (ACE-R, [64,65], the Mini-Mental State Examination (MMSE) [66], the Rey Auditory Verbal Learning Test (RAVLT) [67], the Digit Span of the WAIS-III [68], the Victoria Stroop Test [69], the Camel and Cactus Test (CCT) [70], the the standardized Brazilian version [71] of the Colors Trails Test (CTT), phonemic verbal fluency (“p”), semantic verbal fluency (animals) and a naming task (subtest of the Battery for Language Assessment in Aging (BALE)) [72].
2.2.2. MRI Protocol
All MRI data were acquired using a Siemens Magnetom Vida 3.0T HDxt scanner. For structural imaging, a high-resolution three-dimensional (3D) T1-weighted scan was obtained using a magnetization-prepared rapid gradient echo (MP-RAGE) sequence. The imaging parameters were as follows: the phase encoding direction was anterior–posterior, the repetition time (TR) was 2300 ms, the echo time (TE) was 2.98 ms, and the inversion time (TI) was 900 ms. The field of view (FOV) was 256 × 256 mm, with a voxel size of 1 × 1 × 1 mm3 and a slice thickness of 1 mm. A multiband acceleration factor of three was used with a matrix size of 256 × 256 mm, yielding a total of 176 slices. The data were collected using a 16-channel skull coil.
For functional imaging, a gradient-recalled echo planar imaging (GRE-EPI) sequence was used to acquire a total of 462 scans. The imaging parameters included anterior–posterior phase-encoding direction and interleaved slice acquisition. The TR was 1280 ms, and the TE was 35 ms. The FOV was 212 × 212 × 212 mm, with a voxel size of 2.4 × 2.4 × 2.4 mm3 and a slice thickness of 2.4 mm. A multiband acceleration factor of four was applied, with a matrix size of 256 × 256 mm and 60 slices acquired per volume. Functional data were collected by using a 16-channel skull coil.
2.2.3. Cognitive Training
The linguistic–cognitive training intervention lasted 16 weeks, consisting of one weekly 90 min in-person group session and four individual sets of activities for home-practice. Each session included supplementary pen-and-paper activities distributed to participants, who were instructed to complete them individually at home between meetings. These home activities consisted of five tasks per day, conducted four days per week (excluding weekends and session days), amounting to approximately 30–40 min of daily individual practice.
Activities encompassed reading and comprehension of short texts from different genres, tasks requiring inference and analysis of text structure, and logical reasoning exercises based on short text analyses. Participants also engaged in short writing activities, including personal narratives, news summaries, notes, messages, and collaborative story writing. Vocabulary-focused tasks emphasized word generation, exploration of word meanings, and syntactic exercises such as sentence reordering and the analysis of active versus passive constructions. Importantly, the activities were not organized in a linear progression of difficulty; instead, they integrated analyses at word, sentence, and text levels to sustain participants’ engagement.
The group sessions emphasized dynamic interactions, combining individual and collaborative activities with collective feedback and corrections. Sessions utilized pre-prepared materials presented via a projector, incorporating engaging formats such as board games, music-based language activities, oral narrative exercises, and speech tasks. The session structure was as follows: (1) approximately 5 min dedicated to welcoming participants and conducting a warm-up; (2) approximately 15–20 min for group correction and discussion of home activities; (3) 50–60 min allocated to core language-based cognitive training tasks performed collaboratively; and (4) 5 min to express appreciation to participants, summarizing session achievements, and distributing materials and instructions for the upcoming week’s individual tasks.
2.3. Analysis
2.3.1. Statistical Analysis of the Behavioral Data
Descriptive statistics (mean, standard deviation, minimum, and maximum) were computed for the test variables at the baseline. To examine the changes from pre-to post-intervention, paired comparisons were conducted for each cognitive and self-report measure. Shapiro–Wilk tests were conducted to assess the normality of the difference scores. Due to violations of normality in several variables (p < 0.05), the non-parametric Wilcoxon Signed-Rank Test was employed. To correct for multiple comparisons, p-values were adjusted using the Benjamini (BH) procedure [73]. A significance threshold of p < 0.05 (adjusted) was adopted for identifying statistically significant changes. The data were processed using R (version 4.3.1) [74] in the R Studio environment [75], version 2025.05.0+496.pro5.
2.3.2. Functional Connectivity Preprocessing
MRI data pre-processing and statistical analyses were conducted using the CONN toolbox (RRID:SCR_009550, version 22.v2407) [76] and SPM12 (Wellcome Department of Imaging Neuroscience, London, UK) [77] implemented in MATLAB R2024a (MathWorks, Natick, MA, USA) [78]. Preprocessing followed CONN’s standardized pipeline of the CONN [79], which included realignment with susceptibility distortion correction [80], slice-timing correction [81,82] outlier detection [83], segmentation, normalization to MNI space, and spatial smoothing with an 8 mm FWHM Gaussian kernel.
Functional images were realigned to the first scan of the first session using 6-parameter rigid-body transformation [84] and B-spline interpolation to correct motion and susceptibility interactions. Temporal misalignment due to interleaved slice acquisition was corrected using sinc interpolation to a mid-acquisition reference time. Prior to connectivity analysis, data quality was assessed for all participants. Three individuals were excluded because of excessive head motion or global signal fluctuations during fMRI acquisition, which rendered their data unsuitable for reliable connectivity analysis. Specifically, two participants exceeded motion thresholds (framewise displacement > 0.5 mm), and one participant exhibited excessive global signal changes (>3 SD), as identified by Artifact Detection Tools [85].
Anatomical and functional images were segmented into gray matter, white matter, and CSF and normalized to a 2 mm isotropic MNI space using SPM’s unified segmentation and normalization algorithm [86,87] with the IXI-549 tissue probability maps [88]. Denoising included the regression of five white matter and five principal components from white matter and CSF (CompCor) [89,90] and their first-order derivatives, outlier scans, session effects, and linear trends. CompCor components were extracted from the eroded tissue masks and orthogonalized with respect to the average BOLD signal, motion, and outliers. Following the nuisance regression, a bandpass filter (0.008–0.09 Hz) was applied to the residual BOLD signal [91]. The effective degrees of freedom after denoising ranged from 138.5 to 183.1 (mean = 179.7) across the participants [92].
2.3.3. Functional Connectivity Analyses
Seed-based connectivity (SBC) maps and region of interest (ROI-to-ROI) connectivity matrices were computed to characterize functional connectivity patterns. These analyses were conducted using 17 HPC-ICA networks [76] and regions defined by the Harvard Center for Morphometric Analysis [93]. Functional connectivity strength was quantified using Fisher-transformed bivariate correlation coefficients derived from a weighted General Linear Model (GLM) [94]. Connectivity estimates were computed separately for each seed-target pair by modeling the association between their respective BOLD signal time series. Statistical analyses were performed at the group level using GLM [94]. A separate GLM was estimated for each voxel, with first-level connectivity measures at the corresponding voxel serving as the dependent variables. Each subject contributed an independent sample at each time point (i.e., before and after cognitive training).
Seed-based functional connectivity analyses were performed using the CONN toolbox [76], version 22.v2407. Seeds were defined a priori within five intrinsic connectivity networks of interest: default mode network (DMN), dorsal attention network (DAN), ventral attention network (VAN), frontoparietal network (FPN), and salience network (SN). Specifically, the posterior cingulate cortex (PCC) and medial prefrontal cortex (MPFC) were selected as seeds for the DMN; the intraparietal sulcus (IPS) and frontal eye fields (FEF) for the DAN; the superior temporal gyrus (STG), inferior frontal gyrus (IFG), and medial frontal gyrus (MFG) for the VAN; the left prefrontal cortex (lPFC), superior frontal gyrus (SFG), and medial temporal gyrus (MTG) for the FPN; and the anterior cingulate cortex (ACC) and insula for the SN. These regions were defined using the Harvard-Oxford atlas (RRID:SCR_001476; Harvard Center for Morphometric Analysis [93]).
Seed-to-voxel connectivity maps were generated by computing Fisher-transformed bivariate correlation coefficients between the BOLD time series of each seed region and all other voxels in the brain using a weighted general linear model [94]. Connectivity estimates were computed for each participant at two time points (pre- and post-intervention) and entered into the second level, within-subject analyses. Group-level effects were modeled using random-effects general linear models, with subject-level contrast maps (post > pre) serving as inputs.
Statistical inference was conducted using multivariate parametric statistics implemented in CONN, with voxel-level significance thresholds set at p < 0.001 (uncorrected) and cluster-level significance assessed using family-wise error (FWE) correction at p < 0.05, based on the Gaussian Random Field theory.
3. Results
3.1. Behavioral Results
The participants’ pre- and post-intervention scores are reported in Table 2. A significant improvement in episodic memory was observed following the intervention, as measured by the Rey Auditory Verbal Learning Test (RAVLT) (BH-corrected p = 0.048). Although increases were also found in the overall cognitive performance (ACE-R total score; p = 0.016) and semantic association abilities (Camel and Cactus Test; p = 0.057), these effects did not remain significant after adjusting for multiple comparisons.

Table 2.
Pre- to Post-Intervention Changes in Behavioral Performance Following Language-Based Cognitive Training.
3.2. Functional Connectivity Results
3.2.1. ROI-to-ROI Connectivity
No significant differences were observed in the ROI-to-ROI connectivity analysis between pre- and post-interventions. However, a visual inspection of the connectivity patterns suggests potential network-level changes, as illustrated in Figure 1.
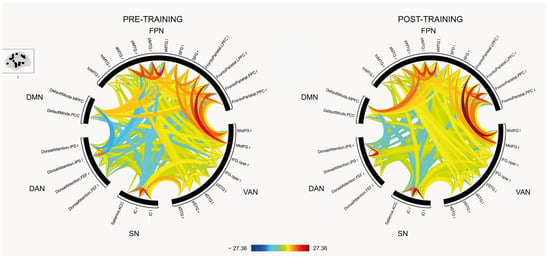
Figure 1.
Resting state connectivity between Regions-of-interest (ROIs) pre- and post-training. The colors corresponds to t-values, with red indicating increased connectivity and blue indicating decreased connectivity (see color bar at the bottom). DMN, default mode network; FPN = Frontoparietal network; VAN = Ventral attentional network; SN = Salience network; DAN = Dorsal attentional network.
3.2.2. Seed-to-Voxel Connectivity
The areas of differential changes in resting-state functional connectivity are reported in detail in Table 3. Figure 2 provides a visual representation of the seed locations and spatial extent of connectivity changes within the FPN and VAN, which exhibited the most prominent changes following the intervention program. The results for each network are summarized separately in the following section.

Table 3.
Seed-to-Voxel Functional Connectivity Changes Following Language-Based Cognitive Training.
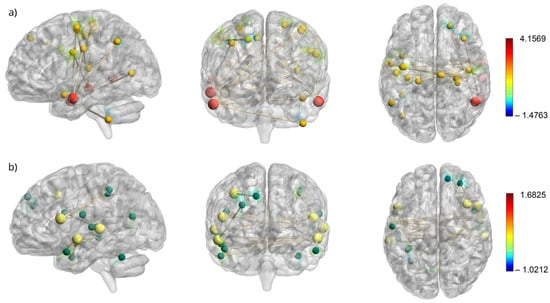
Figure 2.
Resting state connectivity maps of FPN seeds and VAN seeds and their corresponding activated voxels. (a) Resting-state connectivity map for the frontoparietal network (FPN) with seed regions shown as larger red nodes and their corresponding activated voxels as smaller yellow nodes. (b) Resting-state connectivity map for the ventral attention network (VAN) with seed regions shown as larger yellow nodes and their corresponding activated voxels as smaller green nodes. The colorbars corresponds to t-values, with red indicating increased connectivity and blue indicating decreased connectivity.
3.2.3. Default Mode Network (DMN)
A significant decrease in functional connectivity was observed between the medial prefrontal cortex (mPFC) and right superior lateral occipital cortex. No significant changes were detected for the posterior cingulate cortex (PCC).
3.2.4. The Dorsal Attention Network (DAN)
No significant changes in connectivity were found across any of the DAN seed regions.
3.2.5. The Ventral Attention Network (VAN)
The inferior frontal gyrus (IFG) exhibited increased connectivity with the precuneus and superior parietal lobule (SPL). The middle frontal gyrus (MFG) showed decreased connectivity with the frontal pole (FP). The superior temporal gyrus (STG) demonstrates increased and decreased connectivity in various regions, including the temporal pole (TP), middle temporal gyrus (MTG), and cerebellar areas.
3.2.6. Frontoparietal Network (FPN)
The right PCC showed decreased connectivity with the frontal pole. The MTG regions exhibited both increased and decreased connectivity with the motor and sensory cortices, as well as with the insular cortex (IC) and cerebellum.
3.2.7. Salience Network (SN)
The left insular cortex (IC) showed increased connectivity with the right anterior parahippocampal gyrus and the amygdala. No significant changes were observed in the right IC or anterior cingulate gyrus.
4. Discussion
This study investigated the effects of a language-based cognitive intervention on brain functional connectivity in a group of older native Brazilian Portuguese-speaking women with medium to high socioeconomic status. At the behavioral level, a significant improvement in verbal episodic memory was observed, suggesting that the intervention may have contributed to enhancing the participants’ verbal memory abilities, potentially through improved encoding, storage, or retrieval processes. As hypothesized, these gains were accompanied by increased functional connectivity within the FPN, SN, and VAN networks associated with cognitive control, attentional reorientation, and salience detection. In contrast, decreased connectivity was observed within the DMN, a finding that diverges from previous cognitive training studies in older adults where increased DMN connectivity has sometimes been interpreted as compensatory.
4.1. Cognitive Gains Post-Intervention
The intervention focusing on linguistic activities had a more pronounced impact on episodic memory among all the cognitive functions assessed in the pre- versus post-intervention comparison. This finding aligns with our hypotheses. In accordance with a previous study from our group, delivered online during the COVID-19 pandemic to a group of participants with similar SES, schooling, and RWH profiles, significant differences were found in episodic memory following the online intervention [25]. Considering that episodic memory decline is one of the most prominent signs of cognitive impairment in dementia [95], preventive interventions such as those reported in this study seem to be valuable for mitigating the individual, social, and governmental burdens of the increase in dementia worldwide.
In addition to the observed gains, the present results suggest that linguistic cognitive training can promote improvements across various cognitive constructs, even in individuals without established cognitive impairments or subjective memory complaints. More specifically, it was observed that even among participants with medium to high levels of schooling, there remains room for the enhancement of cognitive abilities such as memory, attention, language, and executive functions. These findings reinforce the relevance of cognitive training strategies not only in rehabilitation contexts but also as preventive and health-promoting approaches, contributing to the expansion of cognitive reserve and supporting the maintenance of intellectual functioning throughout aging. Furthermore, linguistic interventions have been shown to effectively enhance cognitive functions, such as memory and attention, in healthy populations [25] as well as in individuals with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) [24]. This shows the richness and complexity of language training, since language processing mobilizes several cognitive constructs to perform language computations at both the comprehension and production levels [31]. This finding has important implications for further research on the intertwined relation between language, cognition, and the brain, as well as for schooling and clinical settings.
A systematic review by Basak et al. [96] concluded that both single-domain and multi-domain cognitive training can produce near- and far-transfer effects, with stronger evidence supporting the benefits of multi-domain approaches. However, among the included studies, none had provided specifically language-based training, which engages executive functions and relies on crystallized knowledge, while being closely tied to daily activities. Compared to episodic memory training, language-based training targets higher-order cognitive processes without relying on free or cued delayed recall tasks, which can be particularly challenging for older adults. This approach may improve adherence and facilitate greater transfer to everyday activities due to its higher ecological validity. Our findings highlight the need for further controlled studies on language-based training, focusing on adherence, enjoyability, and transfer effects compared to other training modalities.
Although overall cognitive performance (ACE-R) and semantic association ability (CCT) improved, these results did not remain statistically significant after adjustments for multiple comparisons. These trends should be considered and further explored in future interventions with longer durations and/or greater intensities and larger groups. Moreover, the absence of a significant reduction in subjective memory complaints may reflect the frequently observed dissociation between subjective perception and objective performance in cognitive assessment.
4.2. Functional Connectivity Changes in Response to Cognitive Training
Consistent with previous research on general cognitive training [47], the present study found that FPN showed predominantly increased functional connectivity following language-based intervention. The FPN is frequently emphasized in cognitive training literature because of its role as a higher-order cognitive hub, supporting executive control, cognitive flexibility, semantic regulation, and working memory [33,34]. In the present study, the observed increase in FPN connectivity was accompanied by significant improvements in verbal episodic memory performance. This convergence aligns with prior studies linking the FPN to successful episodic memory retrieval [97], and suggests that enhanced connectivity within this network may contribute to the observed cognitive benefits. In a 24-month longitudinal study, Hsu et al. [98] observed that older adults who maintained their gait speed exhibited greater functional connectivity within the frontoparietal network (FPN), specifically between the ventral visual cortex and supramarginal gyrus. This enhanced connectivity was positively correlated with performance on verbal episodic memory tasks, suggesting a functional link between preserved network integrity and memory outcomes during aging. Together with the current findings, these results reinforce the notion that targeted cognitive training can effectively modulate large-scale brain networks to support domain-specific cognitive functions.
Building on this broader perspective, we now turn to the specific connectivity changes within the FPN observed following linguistic-based cognitive training, although most language-specific behavioral measures did not show significant improvement, in addition to verbal episodic memory, which, while verbal, is not uniquely linguistic [31]. The primary connectivity changes within the FPN were driven by seeds in the right MTG. The MTG is a well-established semantic hub [99], that plays a central role in language comprehension and conceptual integration [100]. The observed increase in connectivity between the right MTG and precentral gyrus is particularly noteworthy given the linguistic focus of the intervention. While the phonological loop is typically supported by left-lateralized regions, such as Broca’s area and the left precentral gyrus [101], aging and cognitive training have been associated with more bilateral or compensatory recruitment of frontotemporal networks [102]. In this context, the right MTG may contribute to verbal encoding and rehearsal through enhanced interactions with motor-planning regions, especially under increased cognitive demands [103,104]. In addition to phonological processing, the precentral gyrus has been implicated in higher-order semantic operations. We hypothesized that the observed enhancement in FPN connectivity may support improvements in working memory by facilitating more efficient encoding processes. Working memory performance showed only a non-significant trend toward improvement (p = 0.076). The neural findings are consistent with prior work indicating that semantic control and integration can recruit bilateral middle temporal regions, particularly under conditions of greater task complexity (e.g., [105,106]). Although these studies did not examine cognitive training, they suggest that increased semantic demands can be associated with broader bilateral engagement of the semantic network. The bilateral recruitment may also represent an age-related shift toward more bilateral language processing, consistent with compensatory mechanisms described in older adults [102,107].
In addition to the changes observed within the FPN, increased connectivity was also identified in the ventral attentional network (VAN), consistent with its proposed role in language processing and cognitive control. Several studies have highlighted the role of VAN in language processing. For instance, Freedman et al. [40] demonstrated that the ability to reorient attention to salient stimuli correlates with reading fluency, suggesting that VAN may facilitate shifts in attention to internal processes during the retrieval of meaning from written stimuli. This attentional flexibility is also critical for efficiently transitioning from one word to the next, thereby supporting reading speed and comprehension. In our study, the most significant increases in VAN connectivity were observed with seeds located in the left anterior STG and posterior STG bilaterally. Notably, these seeds showed increased connectivity with the temporal regions in the contralateral hemisphere, suggesting enhanced interhemispheric coordination. Given the established role of the STG in language processing (e.g., [108,109]), these findings may reflect a strengthened capacity to dynamically reorient attention during linguistic tasks, particularly those involving semantic access and word-to-word transitions. As with the frontoparietal network (FPN), this cross-hemispheric engagement of the VAN aligns with evidence that bilateral recruitment supports compensatory processing during aging, as proposed by Cabeza et al., [102].
The salience network (SN) also exhibited enhanced connectivity following the intervention, which is consistent with Cao et al. [46] reported multi-domain cognitive training. This network plays a critical role in detecting, monitoring, and filtering salient stimuli to guide attention and cognitive control toward the most relevant information [110]. Notably, key regions of the SN, such as the anterior insula, have also been implicated in language functions including articulation, word retrieval, and phonological discrimination, suggesting a broader role in supporting language-related cognitive processes [42]. More specifically, increased connectivity was observed between the left insular cortex (core SN hub) and right parahippocampal gyrus and amygdala. While previous studies have more commonly reported SN-related increases in the right anterior insula [43,46], our findings suggest that left-lateralized SN engagement may also play a role in modulating memory-related processes, particularly in language-based cognitive training. This pattern may reflect an improved coordination between salience detection and memory-related regions, potentially enhancing the ability to filter out irrelevant stimuli and prioritize emotionally or contextually significant information during encoding. The involvement of the parahippocampal gyrus and amygdala further suggests enhanced integration of attentional and affective cues, which is especially relevant in aging populations, where attentional control often declines.
In contrast to previous studies [46,47,48] the present findings revealed a decrease in connectivity within the default mode network (DMN), specifically between the medial prefrontal cortex and the lateral occipital cortex, following language-based cognitive training. Given the linguistic and externally oriented nature of the intervention, this reduction in DMN connectivity may reflect an adaptive disengagement from internally directed processes, such as mind-wandering and self-referential thought. Such disengagement could facilitate a more efficient recruitment of task-positive networks involved in attention and goal-directed behavior. This interpretation aligns with prior research indicating that the suppression of DMN activity is associated with improved performance during externally focused cognitive tasks [37,111,112]. Moreover, increased anterior DMN connectivity has been observed in the early stages of Alzheimer’s disease, often interpreted as a loss of network specificity or compensatory overactivation [113]. In this context, the observed decrease in connectivity may represent beneficial modulation, potentially contributing to more efficient neural processing and cognitive resilience.
Unsurprisingly, no significant changes in connectivity were observed in the dorsal attentional network (DAN), which is consistent with previous studies involving cognitively healthy older adults [49,50]. The DAN is primarily engaged during top-down, goal-directed visuospatial attention tasks such as visual search, spatial orienting, and attentional tracking, which are not directly targeted by the present intervention. As such, the absence of a change may reflect the specificity of other networks. Greenwood and Parasuraman [51] further suggested that the lack of DAN modulation in older adults following cognitive interventions may stem from high levels of schooling or cognitive reserve, which could buffer training-related neural changes in this network. Alternatively, it may reflect the network’s limited involvement in far-transfer mechanisms, particularly when trained tasks do not overlap with the attentional demands typically mediated by the DAN.
Although the results of the seed-to-voxel analysis are promising and suggest localized changes in functional connectivity following the intervention, they should be interpreted with caution. Seed-to-voxel methods are inherently more exploratory and sensitive to spatial focal effects, which may not be captured by the more constrained ROI-to-ROI approach. The lack of significant findings in the ROI-to-ROI analysis may reflect the limited statistical power, as only 17 participants were included in the connectivity analysis. Given the small sample size, it is possible that subtle but meaningful effects were detected at the voxel level but were not robust enough to emerge when averaged across broader anatomical ROIs. These findings highlight the need for replication in larger samples and suggest that seed-to-voxel results can offer valuable preliminary insights into training-induced brain plasticity. Because only women were included, the findings cannot be generalized to men. In addition, the sample consisted of highly educated participants, which is not representative of the broader Brazilian population. The absence of an active control group further limits causal inferences regarding the intervention’s specific effects. Future studies should therefore include larger and more balanced samples of both sexes, a wider range of educational backgrounds, and appropriate control groups.
Another limitation of the present study should be acknowledged. First, part of the cognitive training program consisted of home-based individual activities designed to last approximately 30–40 min per day over four days each week, in addition to weekly face-to-face sessions. While these home activities likely contributed to maintaining participants’ engagement and participants reported adhering to the schedule as instructed, researchers had limited ability to monitor or control this component of the intervention. Finally, the relatively brief duration of the intervention and the absence of a follow-up assessment prevent us from determining the durability of the observed effects. The long-term impact of cognitive training programs, both in terms of behavioral outcomes and brain connectivity changes, remains unknown and should be explored in future studies.
5. Conclusions
This study provides compelling evidence that a language-based cognitive training intervention can yield measurable cognitive and neurofunctional benefits in cognitively healthy older adults from middle-to-high socioeconomic backgrounds. These findings underscore the capacity for cognitive enhancement, even among individuals with higher schooling levels, highlighting the relevance of preventive strategies beyond traditional clinical rehabilitation.
Importantly, the results support the hypothesis that targeted language-based cognitive training can strengthen neural and cognitive mechanisms, particularly those involved in verbal episodic memory, thereby contributing to the enhancement of cognitive reserve. These results have significant implications for aging populations, suggesting that such interventions may serve as vital components in strategies aimed at promoting healthy cognitive aging.
Our findings open promising avenues for future research on scalable, non-pharmacological interventions. Further investigation of their long-term efficacy and broader applicability could provide essential tools for addressing the increasing societal burden of age-related cognitive decline and dementia.
Author Contributions
Conceptualization, L.C.H., K.M., M.T.C.-G., E.d.S.R., L.P.S., A.P.B. and A.-S.B.; methodology L.C.H. and K.M.; resources E.d.S.R., L.C.H., M.T.C.-G., L.P.S., K.M.; formal analysis A.P.B., A.-S.B., B.M., L.C.H. and K.M.; investigation, A.P.B., A.-S.B., B.R.d.R., V.B. and F.S.E.B.; writing—original draft preparation, A.P.B. and A.-S.B.; writing—review and editing, E.d.S.R., L.C.H., M.T.C.-G. and K.M.; supervision, L.C.H. and K.M.; project administration, L.C.H.; funding acquisition L.C.H.; E.d.S.R.; M.T.C.-G.; K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) N° 4/2021-Research Productivity (Grant, protocol # 309715/2021-0) to LCH; call 07/2021 PqG # 21/2551-0002204-3 to LCH; and financial support from CAPES (Coordination for the Improvement of Higher Education Personnel) to LCH. MTCG acknowledges the support of CNPq (407022/2021-0) and CAPES (001). K.M. holds a Career Award from the “Fonds de Recherche du Québec–Santé” (https://doi.org/10.69777/330547).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS) (protocol number 53696221.4.1001.5336, approved on 18 January 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
Acknowledgments
The authors thank Bárbara Friederich and Maurício Anes for their assistance in data collection and neuroimaging protocol setup. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE-R | Addenbrooke’s Cognitive Examination–Revised |
| ACTIVE | Advanced Training for Independent and Vital Elderly (study) |
| AD | Alzheimer’s disease |
| BALE | Battery for Language Assessment in Aging |
| BH | Benjamini–Hochberg (correction) |
| BOLD | Blood-Oxygen-Level-Dependent |
| CCT | Camel and Cactus Test |
| CEN | Central executive network |
| CSF | Cerebrospinal Fluid |
| CTT | Colors Trail Test |
| DAN | Dorsal attention network |
| DMN | Default mode network |
| ECN | Executive control network |
| FAQ | Functional Activities Questionnaire |
| FOV | Field of view |
| FP | Frontal pole |
| FPN | Frontoparietal network |
| fMRI | Functional magnetic resonance imaging |
| FWE | Family-wise error |
| GAI | Geriatric Anxiety Inventory |
| GDS | Geriatric Depression Scale |
| GLM | General Linear Model |
| GRE-EPI | Gradient-recalled echo planar imaging (sequence) |
| IC | Insular cortex |
| IADL | Instrumental activities of daily living |
| IFG | Inferior frontal gyrus |
| MFG | Middle frontal gyrus |
| MNI | Montreal Neurological Institute |
| MMSE | Mini-Mental State Examination |
| MP-RAGE | Magnetization-prepared rapid gradient echo (sequence) |
| mPFC | Medial prefrontal cortex |
| MTG | Middle temporal gyrus |
| PCC | Posterior cingulate cortex |
| RAVLT | Rey Auditory Verbal Learning Test |
| ROI | Region of interest |
| RWH | Reading and writing habits |
| SBC | Seed-based connectivity |
| SD | Standard deviation |
| SES | Socioeconomic status |
| SN | Salience network |
| SPL | Superior parietal lobule |
| STG | Superior temporal gyrus |
| TE | Echo time |
| TI | Inversion time |
| TP | Temporal pole |
| TR | Repetition time |
| VAN | Ventral attention network |
References
- Jackson, J.D.; Balota, D.A. Age-Related Changes in Attentional Selection: Quality of Task Set or Degradation of Task Set across Time? Psychol. Aging 2013, 28, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, J.; Cohen-Shikora, E.R.; Balota, D.A. Re-Examining Age Differences in the Stroop Effect: The Importance of the Trees in the Forest (Plot). Psychol. Aging 2021, 36, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Bisiacchi, P.S.; Borella, E.; Bergamaschi, S.; Carretti, B.; Mondini, S. Interplay between Memory and Executive Functions in Normal and Pathological Aging. J. Clin. Exp. Neuropsychol. 2008, 30, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ficarro, S.; Duberstein, P.; Chapman, B.P.; Dubovsky, S.; Paroski, M.; Szigeti, K.; Benedict, R.H.B. Executive Function and Personality Predict Instrumental Activities of Daily Living in Alzheimer Disease. Am. J. Geriatr. Psychiatry 2016, 24, 1074–1083. [Google Scholar] [CrossRef]
- Raposo Pereira, F.; George, N.; Dalla Barba, G.; Dubois, B.; La Corte, V. The Memory Binding Test Can Anticipate Alzheimer’s Disease Diagnosis at an Early Preclinical Stage: A Longitudinal Study in the INSIGHTpreAD Cohort. Front. Aging Neurosci. 2024, 16, 1414419. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Webb, S.; Bartsch, L.; Clare, L.; Rebok, G.; Cherbuin, N.; Anstey, K.J. Tailored and Adaptive Computerized Cognitive Training in Older Adults at Risk for Dementia: A Randomized Controlled Trial. J. Alzheimer’s Dis. 2017, 60, 889–911. [Google Scholar] [CrossRef]
- Brum, P.S.; Forlenza, O.V.; Yassuda, M.S. Cognitive Training in Older Adults with Mild Cognitive Impairment: Impact on Cognitive and Functional Performance. Dement. Neuropsychol. 2009, 3, 124–131. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Domellöf, M.E.; Leung, I.; Neely, A.S.; Launder, N.H.; Nategh, L.; Finke, C.; Lampit, A. Computerized Cognitive Training in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2022, 80, 101671. [Google Scholar] [CrossRef]
- Hyer, L.; Scott, C.; Atkinson, M.M.; Mullen, C.M.; Lee, A.; Johnson, A.; Mckenzie, L.C. Cognitive Training Program to Improve Working Memory in Older Adults with MCI. Clin. Gerontol. 2016, 39, 410–427. [Google Scholar] [CrossRef]
- Yang, Y.; Kwak, Y.T. Improvement of Cognitive Function after Computer-Based Cognitive Training in Early Stage of Alzheimer’s Dementia. Dement. Neurocogn Disord. 2017, 16, 7–11. [Google Scholar] [CrossRef]
- Borella, E.; Cantarella, A.; Carretti, B.; De Lucia, A.; De Beni, R. Improving Everyday Functioning in the Old-Old with Working Memory Training. Am. J. Geriatr. Psychiatry 2019, 27, 975–983. [Google Scholar] [CrossRef]
- Corbett, A.; Owen, A.; Hampshire, A.; Grahn, J.; Stenton, R.; Dajani, S.; Burns, A.; Howard, R.; Williams, N.; Williams, G.; et al. The Effect of an Online Cognitive Training Package in Healthy Older Adults: An Online Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2015, 16, 990–997. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Lampit, A.; Hallock, H.; Sabatés, J.; Bahar-Fuchs, A. Cognition-Oriented Treatments for Older Adults: A Systematic Overview of Systematic Reviews. Neuropsychol. Rev. 2020, 30, 167–193. [Google Scholar] [CrossRef]
- Rebok, G.W.; Ball, K.; Guey, L.T.; Jones, R.N.; Kim, H.; King, J.W.; Marsiske, M.; Morris, J.N.; Tennstedt, S.L.; Unverzagt, F.W.; et al. Ten-Year Effects of the Advanced Cognitive Training for Independent and Vital Elderly Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. J. Am. Geriatr. Soc. 2014, 62, 16–24. [Google Scholar] [CrossRef]
- Willis, S.L.; Tennstedt, S.L.; Marsiske, M.; Ball, K.; Elias, J.; Koepke, K.M.; Morris, J.N.; Rebok, G.W.; Unverzagt, F.W.; Stoddard, A.M.; et al. Long-Term Effects of Cognitive Training on Everyday Functional Outcomes in Older Adults. JAMA 2006, 296, 2805. [Google Scholar] [CrossRef]
- Zhang, H.; Huntley, J.; Bhome, R.; Holmes, B.; Cahill, J.; Gould, R.L.; Wang, H.; Yu, X.; Howard, R. Effect of Computerised Cognitive Training on Cognitive Outcomes in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e027062. [Google Scholar] [CrossRef]
- Duda, B.M.; Sweet, L.H. Functional Brain Changes Associated with Cognitive Training in Healthy Older Adults: A Preliminary ALE Meta-Analysis. Brain Imaging Behav. 2020, 14, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, D.; Tang, L.; Cheng, Y.; Wang, G.; Hu, G.; Gong, X.; Cao, X.; Jiang, L.; Li, C. Effects of Different Cognitive Trainings on Amnestic Mild Cognitive Impairment in the Elderly: A One-Year Longitudinal Functional Magnetic Resonance Imaging (MRI) Study. Med. Sci. Monit. 2018, 24, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Jolles, D.D.; van Buchem, M.A.; Crone, E.A.; Rombouts, S.A.R.B. Functional Brain Connectivity at Rest Changes after Working Memory Training. Hum. Brain Mapp. 2013, 34, 396–406. [Google Scholar] [CrossRef]
- Simon, S.S.; Hampstead, B.M.; Nucci, M.P.; Duran, F.L.S.; Fonseca, L.M.; Martin, M.d.G.M.; Ávila, R.; Porto, F.H.G.; Brucki, S.M.D.; Martins, C.B.; et al. Training Gains and Transfer Effects after Mnemonic Strategy Training in Mild Cognitive Impairment: A FMRI Study. Int. J. Psychophysiol. 2020, 154, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wollesen, B.; Wildbredt, A.; van Schooten, K.S.; Lim, M.L.; Delbaere, K. The Effects of Cognitive-Motor Training Interventions on Executive Functions in Older People: A Systematic Review and Meta-Analysis. Eur. Rev. Aging Phys. Act. 2020, 17, 9. [Google Scholar] [CrossRef]
- Mendonça, A.R.; Loureiro, L.M.; Nórte, C.E.; Landeira-Fernandez, J. Episodic Memory Training in Elderly: A Systematic Review. Front. Psychol. 2022, 13, 947519. [Google Scholar] [CrossRef]
- Kaspary, L.M.; Souza Espinosa Borges, F.; De Lima Amaral, M.; Silva da Rocha, G.; Hübner, L.C. Tarefas Linguísticas Para Treino Cognitivo No Envelhecimento Típico e No Declínio Cognitivo. Letrônica 2023, 16, e44274. [Google Scholar] [CrossRef]
- Poptsi, E.; Lazarou, I.; Markou, N.; Vassiloglou, M.; Nikolaidou, E.; Diamantidou, A.; Siatra, V.; Karathanassi, E.; Karakostas, A.; Zafeiropoulou, F.K.; et al. A Comparative Single-Blind Randomized Controlled Trial With Language Training in People With Mild Cognitive Impairment. Am. J. Alzheimers Dis. Other Demen. 2019, 34, 176–187. [Google Scholar] [CrossRef]
- Bisol, V.; Malcorra, B.L.C.; Rocha, B.R.d.; Peruzzo, A.L.L.; Zanatta, L.; Pacheco, L.P.; Rodrigues, E.d.S.; Carthery-Goulart, M.T.; Hübner, L.C. Episodic Memory Improvement in Community-Dwelling Women Following a Remote Language-Based Stimulation Program. Dement. Neuropsychol. 2025, 19, e20240248. [Google Scholar] [CrossRef]
- Malcorra, B.L.C.; Mota, N.B.; Weissheimer, J.; Schilling, L.P.; Wilson, M.A.; Hübner, L.C. Reading and Writing Habits Compensate for Aging Effects in Speech Connectedness. NPJ Sci. Learn. 2022, 7, 13. [Google Scholar] [CrossRef]
- Ye, Z.; Zhou, X. Executive Control in Language Processing. Neurosci. Biobehav. Rev. 2009, 33, 1168–1177. [Google Scholar] [CrossRef]
- Shokrkon, A.; Nicoladis, E. The Directionality of the Relationship Between Executive Functions and Language Skills: A Literature Review. Front. Psychol. 2022, 13, 848696. [Google Scholar] [CrossRef] [PubMed]
- Polsinelli, A.J.; Moseley, S.A.; Grilli, M.D.; Glisky, E.L.; Mehl, M.R. Natural, Everyday Language Use Provides a Window Into the Integrity of Older Adults’ Executive Functioning. J. Gerontol. Ser. B 2020, 75, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Allen, C. A Disorder of Executive Function and Its Role in Language Processing. Semin. Speech Lang. 2008, 29, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Federmeier, K.D.; Jongman, S.R.; Szewczyk, J.M. Examining the Role of General Cognitive Skills in Language Processing: A Window Into Complex Cognition. Curr. Dir. Psychol. Sci. 2020, 29, 575–582. [Google Scholar] [CrossRef]
- Faßbender, R.V.; Risius, O.J.; Dronse, J.; Richter, N.; Gramespacher, H.; Befahr, Q.; Fink, G.R.; Kukolja, J.; Onur, O.A. Decreased Efficiency of Between-Network Dynamics During Early Memory Consolidation With Aging. Front. Aging Neurosci. 2022, 14, 780630. [Google Scholar] [CrossRef] [PubMed]
- Chiou, R.; Humphreys, G.F.; Jung, J.; Lambon Ralph, M.A. Controlled Semantic Cognition Relies upon Dynamic and Flexible Interactions between the Executive ‘Semantic Control’ and Hub-and-Spoke ‘Semantic Representation’ Systems. Cortex 2018, 103, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Marek, S.; Dosenbach, N.U.F. The Frontoparietal Network: Function, Electrophysiology, and Importance of Individual Precision Mapping. Dialogues Clin. Neurosci. 2018, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Grady, C.L.; Ng, C.; Hasher, L. Age Differences in the Frontoparietal Cognitive Control Network: Implications for Distractibility. Neuropsychologia 2012, 50, 2212–2223. [Google Scholar] [CrossRef]
- Dixon, M.L.; De La Vega, A.; Mills, C.; Andrews-Hanna, J.; Spreng, R.N.; Cole, M.W.; Christoff, K. Heterogeneity within the Frontoparietal Control Network and Its Relationship to the Default and Dorsal Attention Networks. Proc. Natl. Acad. Sci. USA 2018, 115, E1598–E1607. [Google Scholar] [CrossRef] [PubMed]
- Spreng, R.N.; Stevens, W.D.; Chamberlain, J.P.; Gilmore, A.W.; Schacter, D.L. Default Network Activity, Coupled with the Frontoparietal Control Network, Supports Goal-Directed Cognition. Neuroimage 2010, 53, 303–317. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Vogel, A.C.; Miezin, F.M.; Petersen, S.E.; Schlaggar, B.L. The Putative Visual Word Form Area Is Functionally Connected to the Dorsal Attention Network. Cereb. Cortex 2012, 22, 537–549. [Google Scholar] [CrossRef]
- Freedman, L.; Zivan, M.; Farah, R.; Horowitz-Kraus, T. Greater Functional Connectivity within the Cingulo-Opercular and Ventral Attention Networks Is Related to Better Fluent Reading: A Resting-State Functional Connectivity Study. Neuroimage Clin. 2020, 26, 102214. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, H.; Ma, L.; Luo, J.; Chu, C.; Hu, M.; Zhao, G.; Men, W.; Tan, S.; Gao, J.-H.; et al. Learning to Read May Help Promote Attention by Increasing the Volume of the Left Middle Frontal Gyrus and Enhancing Its Connectivity to the Ventral Attention Network. Cereb. Cortex 2023, 33, 2260–2272. [Google Scholar] [CrossRef]
- Ardila, A.; Bernal, B.; Rosselli, M. Participation of the Insula in Language Revisited: A Meta-Analytic Connectivity Study. J. Neurolinguistics 2014, 29, 31–41. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Oh, A.; Duerden, E.G.; Pang, E.W. The Role of the Insula in Speech and Language Processing. Brain Lang. 2014, 135, 96–103. [Google Scholar] [CrossRef]
- La Corte, V.; Sperduti, M.; Malherbe, C.; Vialatte, F.; Lion, S.; Gallarda, T.; Oppenheim, C.; Piolino, P. Cognitive Decline and Reorganization of Functional Connectivity in Healthy Aging: The Pivotal Role of the Salience Network in the Prediction of Age and Cognitive Performances. Front. Aging Neurosci. 2016, 8, 204. [Google Scholar] [CrossRef]
- Cao, W.; Cao, X.; Hou, C.; Li, T.; Cheng, Y.; Jiang, L.; Luo, C.; Li, C.; Yao, D. Effects of Cognitive Training on Resting-State Functional Connectivity of Default Mode, Salience, and Central Executive Networks. Front. Aging Neurosci. 2016, 8, 70. [Google Scholar] [CrossRef]
- Chapman, S.B.; Aslan, S.; Spence, J.S.; Hart, J.J.; Bartz, E.K.; Didehbani, N.; Keebler, M.W.; Gardner, C.M.; Strain, J.F.; DeFina, L.F.; et al. Neural Mechanisms of Brain Plasticity with Complex Cognitive Training in Healthy Seniors. Cereb. Cortex 2015, 25, 396–405. [Google Scholar] [CrossRef]
- De Marco, M.; Meneghello, F.; Duzzi, D.; Rigon, J.; Pilosio, C.; Venneri, A. Cognitive Stimulation of the Default-Mode Network Modulates Functional Connectivity in Healthy Aging. Brain Res. Bull. 2016, 121, 26–41. [Google Scholar] [CrossRef]
- Hardcastle, C.; Hausman, H.K.; Kraft, J.N.; Albizu, A.; O’Shea, A.; Boutzoukas, E.M.; Evangelista, N.D.; Langer, K.; Van Etten, E.J.; Bharadwaj, P.K.; et al. Proximal Improvement and Higher-Order Resting State Network Change after Multidomain Cognitive Training Intervention in Healthy Older Adults. Geroscience 2022, 44, 1011–1027. [Google Scholar] [CrossRef]
- Strenziok, M.; Parasuraman, R.; Clarke, E.; Cisler, D.S.; Thompson, J.C.; Greenwood, P.M. Neurocognitive Enhancement in Older Adults: Comparison of Three Cognitive Training Tasks to Test a Hypothesis of Training Transfer in Brain Connectivity. Neuroimage 2014, 85, 1027–1039. [Google Scholar] [CrossRef]
- Greenwood, P.M.; Parasuraman, R. The Mechanisms of Far Transfer from Cognitive Training: Review and Hypothesis. Neuropsychology 2016, 30, 742–755. [Google Scholar] [CrossRef]
- Hampstead, B.M.; Stringer, A.Y.; Stilla, R.F.; Deshpande, G.; Hu, X.; Moore, A.B.; Sathian, K. Activation and Effective Connectivity Changes Following Explicit-Memory Training for Face–Name Pairs in Patients With Mild Cognitive Impairment. Neurorehabil. Neural Repair 2011, 25, 210–222. [Google Scholar] [CrossRef]
- Bialystok, E. Bilingualism and the Development of Executive Function: The Role of Attention. Child Dev. Perspect. 2015, 9, 117–121. [Google Scholar] [CrossRef]
- Grundy, J.G. The Effects of Bilingualism on Executive Functions: An Updated Quantitative Analysis. J. Cult. Cogn. Sci. 2020, 4, 177–199. [Google Scholar] [CrossRef]
- Bialystok, E. The Bilingual Adaptation: How Minds Accommodate Experience. Psychol. Bull. 2017, 143, 233–262. [Google Scholar] [CrossRef]
- Bubbico, G.; Chiacchiaretta, P.; Parenti, M.; di Marco, M.; Panara, V.; Sepede, G.; Ferretti, A.; Perrucci, M.G. Effects of Second Language Learning on the Plastic Aging Brain: Functional Connectivity, Cognitive Decline, and Reorganization. Front. Neurosci. 2019, 13, 423. [Google Scholar] [CrossRef]
- Mohammadi, B.; Münte, T.F.; Cole, D.M.; Sami, A.; Boltzmann, M.; Rüsseler, J. Changed Functional Connectivity at Rest in Functional Illiterates after Extensive Literacy Training. Neurol. Res. Pract. 2020, 2, 12. [Google Scholar] [CrossRef]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Associação Brasileira de Empresas de Pesquisa. Critério de Classificação Econômica Brasil [Website]. 2024. Available online: https://www.abep.org/criterio-brasil (accessed on 2 January 2025).
- Pacheco, L.P. Leitura de Palavras e Pseudopalavras em Português Brasileiro por Pessoas Adultas Jovens e Idosas. Doctoral Dissertation, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil, 2024. [Google Scholar]
- Almeida, O.; Almeida, S. Confiabilidade Da Versão Brasileira Da Escala de Depressão Em Geriatria (GDS) Versão Reduzida. Arq. Neuropsiquiatr. 1999, 57, 421–426. [Google Scholar] [CrossRef]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H.; Chance, J.M.; Filos, S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef]
- Massena, P.N.; de Araújo, N.B.; Pachana, N.A.; Laks, J.; de Pádua, A.C.; Oude Voshaar, R.C. Validation of the Brazilian Portuguese Version of Geriatric Anxiety Inventory–GAI-BR. Int. Psychogeriatr. 2015, 27, 1113–1119. [Google Scholar] [CrossRef]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A Brief Cognitive Test Battery for Dementia Screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.A.; Caramelli, P. Brazilian Adaptation of the Addenbrooke’s Cognitive Examination-Revised (ACE-R). Dement. Neuropsychol. 2007, 1, 212–216. [Google Scholar] [CrossRef]
- Brucki, S.M.D.; Nitrini, R.; Caramelli, P.; Bertolucci, P.H.F.; Okamoto, I.H. Sugestões Para o Uso Do Mini-Exame Do Estado Mental No Brasil. Arq. Neuropsiquiatr. 2003, 61, 777–781. [Google Scholar] [CrossRef]
- Malloy-Diniz, L.F.; Lasmar, V.A.P.; Gazinelli, L.d.S.R.; Fuentes, D.; Salgado, J.V. The Rey Auditory-Verbal Learning Test: Applicability for the Brazilian Elderly Population. Rev. Bras. Psiquiatr. 2007, 29, 324–329. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Bayard, S.; Erkes, J.; Moroni, C. Victoria Stroop Test: Normative Data in a Sample Group of Older People and the Study of Their Clinical Applications in the Assessment of Inhibition in Alzheimer’s Disease. Arch. Clin. Neuropsychol. 2011, 26, 653–661. [Google Scholar] [CrossRef]
- Bozeat, S.; Lambon Ralph, M.A.; Patterson, K.; Garrard, P.; Hodges, J.R. Non-Verbal Semantic Impairment in Semantic Dementia. Neuropsychologia 2000, 38, 1207–1215. [Google Scholar] [CrossRef]
- D’Elia, L.F.; Satz, P.; Uchiyama, C.L.; White, T. Color Trails Test: Professional Manual; Psychological Assessment Resources: Odessa, FL, USA, 1996. [Google Scholar]
- Hübner, L.C.; Loureiro, F.S.; Smidarle, A.D.; Tessaro, B.; Siqueira, E.C.G.; Jerônimo, G.M.; Quadros, T.D.; Garcia, V.R.M.; Kochhann, R. Bateria de Avaliação da Linguagem no Envelhecimento (BALE). In Tarefas Para Avaliação Neuropsicológica 3: Avaliação de Memória Episódica, Percepção, Linguagem e Componentes Executivos Para Adultos; Zimmermann, N., Delaere, F.J., Fonseca, R.P., Eds.; Memnon: Porto Alegre, Brazil, 2019; Volume 1, pp. 188–218. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 16 October 2025).
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2025; Available online: http://www.posit.co/ (accessed on 16 October 2025).
- Nieto-Castanon, A.; Whitfield-Gabrieli, S. CONN Functional Connectivity Toolbox: RRID SCR_009550, Release 22; Hilbert Press: Boston, MA, USA, 2022. [Google Scholar]
- Wellcome Centre for Human Neuroimaging. SPM12 (Statistical Parametric Mapping); Wellcome Centre for Human Neuroimaging: London, UK, 2014. [Google Scholar]
- MathWorks MATLAB; Version R2024a; The MathWorks Inc.: Natick, MA, USA , 2024.
- Nieto-Castanon, A. FMRI Denoising Pipeline. In Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Amazon Digital Services LLC—Kdp: Seattle, WA, USA, 2020; pp. 17–25. [Google Scholar]
- Andersson, J.L.R.; Hutton, C.; Ashburner, J.; Turner, R.; Friston, K. Modeling Geometric Deformations in EPI Time Series. Neuroimage 2001, 13, 903–919. [Google Scholar] [CrossRef]
- Henson, R.N.A.; Buechel, C.; Josephs, O.; Friston, K.J. The Slice-Timing Problem in Event-Related FMRI. Neuroimage 1999, 9, 125. [Google Scholar]
- Sladky, R.; Friston, K.J.; Tröstl, J.; Cunnington, R.; Moser, E.; Windischberger, C. Slice-Timing Effects and Their Correction in Functional MRI. Neuroimage 2011, 58, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A.; Ghosh , S. Artifact Detection Tools (ART), Release Version 7:11; Artifact Detection Tools: Cambridge, MA, USA, 2011. [Google Scholar]
- Friston, K.J.; Ashburner, J.; Frith, C.D.; Poline, J.B.; Heather, J.D.; Frackowiak, R.S. Spatial Registration and Normalization of Images. Hum. Brain Mapp. 1994, 3, 165–189. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to Detect, Characterize, and Remove Motion Artifact in Resting State FMRI. Neuroimage 2014, 84, 320–341. [Google Scholar] [CrossRef]
- Ashburner, J. A Fast Diffeomorphic Image Registration Algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified Segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Wager, T.D.; Krishnan, A.; Rosch, K.S.; Seymour, K.E.; Nebel, M.B.; Mostofsky, S.H.; Nyalakanai, P.; Kiehl, K. The Impact of T1 versus EPI Spatial Normalization Templates for FMRI Data Analyses. Hum. Brain Mapp. 2017, 38, 5331–5342. [Google Scholar] [CrossRef]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based FMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef]
- Chai, X.J.; Castañón, A.N.; Öngür, D.; Whitfield-Gabrieli, S. Anticorrelations in Resting State Networks without Global Signal Regression. Neuroimage 2012, 59, 1420–1428. [Google Scholar] [CrossRef]
- Hallquist, M.N.; Hwang, K.; Luna, B. The Nuisance of Nuisance Regression: Spectral Misspecification in a Common Approach to Resting-State FMRI Preprocessing Reintroduces Noise and Obscures Functional Connectivity. Neuroimage 2013, 82, 208–225. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. Preparing FMRI Data for Statistical Analysis. arXiv 2022, arXiv:2210.13564. [Google Scholar] [CrossRef]
- Harvard-Oxford Cortical and Subcortical Structural Atlases; Center for Morphometric Analysis: Charlestown, MA, USA, 2014.
- Nieto-Castanon, A. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Amazon Digital Services LLC—Kdp: Seattle, WA, USA, 2020; pp. 26–82. [Google Scholar]
- Dubois, B. The Emergence of a New Conceptual Framework for Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1059–1066. [Google Scholar] [CrossRef]
- Basak, C.; Qin, S.; O’Connell, M.A. Differential Effects of Cognitive Training Modules in Healthy Aging and Mild Cognitive Impairment: A Comprehensive Meta-Analysis of Randomized Controlled Trials. Psychol. Aging 2020, 35, 220–249. [Google Scholar] [CrossRef]
- Iidaka, T.; Matsumoto, A.; Nogawa, J.; Yamamoto, Y.; Sadato, N. Frontoparietal Network Involved in Successful Retrieval from Episodic Memory. Spatial and Temporal Analyses Using FMRI and ERP. Cereb. Cortex 2006, 16, 1349–1360. [Google Scholar] [CrossRef]
- Hsu, C.L.; Manor, B.; Travison, T.; Pascual-Leone, A.; Lipsitz, L.A. Sensorimotor and Frontoparietal Network Connectivity Are Associated With Subsequent Maintenance of Gait Speed and Episodic Memory in Older Adults. J. Gerontol. Ser. A 2023, 78, 521–526. [Google Scholar] [CrossRef]
- Binder, J.R.; Desai, R.H.; Graves, W.W.; Conant, L.L. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cereb. Cortex 2009, 19, 2767–2796. [Google Scholar] [CrossRef]
- Humphries, C.; Binder, J.R.; Medler, D.A.; Liebenthal, E. Syntactic and Semantic Modulation of Neural Activity during Auditory Sentence Comprehension. J. Cogn. Neurosci. 2006, 18, 665–679. [Google Scholar] [CrossRef]
- Baddeley, A. Working Memory: Looking Back and Looking Forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef]
- Cabeza, R. Hemispheric Asymmetry Reduction in Older Adults: The HAROLD Model. Psychol. Aging 2002, 17, 85–100. [Google Scholar] [CrossRef]
- Davey, J.; Thompson, H.E.; Hallam, G.; Karapanagiotidis, T.; Murphy, C.; De Caso, I.; Krieger-Redwood, K.; Bernhardt, B.C.; Smallwood, J.; Jefferies, E. Exploring the Role of the Posterior Middle Temporal Gyrus in Semantic Cognition: Integration of Anterior Temporal Lobe with Executive Processes. Neuroimage 2016, 137, 165–177. [Google Scholar] [CrossRef]
- Humphreys, G.F.; Lambon Ralph, M.A. Fusion and Fission of Cognitive Functions in the Human Parietal Cortex. Cereb. Cortex 2015, 25, 3547–3560. [Google Scholar] [CrossRef]
- Graves, W.W.; Binder, J.R.; Desai, R.H.; Conant, L.L.; Seidenberg, M.S. Neural Correlates of Implicit and Explicit Combinatorial Semantic Processing. Neuroimage 2010, 53, 638–646. [Google Scholar] [CrossRef]
- Jackson, R.L.; Hoffman, P.; Pobric, G.; Lambon Ralph, M.A. The Semantic Network at Work and Rest: Differential Connectivity of Anterior Temporal Lobe Subregions. J. Neurosci. 2016, 36, 1490–1501. [Google Scholar] [CrossRef]
- Tyler, L.K.; Shafto, M.A.; Randall, B.; Wright, P.; Marslen-Wilson, W.D.; Stamatakis, E.A. Preserving Syntactic Processing across the Adult Life Span: The Modulation of the Frontotemporal Language System in the Context of Age-Related Atrophy. Cereb. Cortex 2010, 20, 352–364. [Google Scholar] [CrossRef]
- Friederici, A.D. The Brain Basis of Language Processing: From Structure to Function. Physiol. Rev. 2011, 91, 1357–1392. [Google Scholar] [CrossRef]
- Hickok, G.; Poeppel, D. The Cortical Organization of Speech Processing. Nat. Rev. Neurosci. 2007, 8, 393–402. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Baldassarre, A.; Lewis, C.M.; Committeri, G.; Snyder, A.Z.; Romani, G.L.; Corbetta, M. Individual Variability in Functional Connectivity Predicts Performance of a Perceptual Task. Proc. Natl. Acad. Sci. USA 2012, 109, 3516–3521. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The Human Brain Is Intrinsically Organized into Dynamic, Anticorrelated Functional Networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Jones, D.T.; Machulda, M.M.; Vemuri, P.; McDade, E.M.; Zeng, G.; Senjem, M.L.; Gunter, J.L.; Przybelski, S.A.; Avula, R.T.; Knopman, D.S.; et al. Age-Related Changes in the Default Mode Network Are More Advanced in Alzheimer Disease. Neurology 2011, 77, 1524–1531. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).