CT Morphometric Analysis of Ossification Centres in the Fetal Th12 Vertebra
Abstract
1. Introduction
- examine potential sex-related and side-related differences in all analyzed parameters;
- conduct a quantitative analysis of Th12 ossification centers including linear, surface, and volumetric parameters in order to establish normative reference values for successive gestational weeks;
- determine the growth dynamics of the examined parameters and develop mathematical models that best describe the observed relationships.
2. Materials and Methods
2.1. Examined Sample
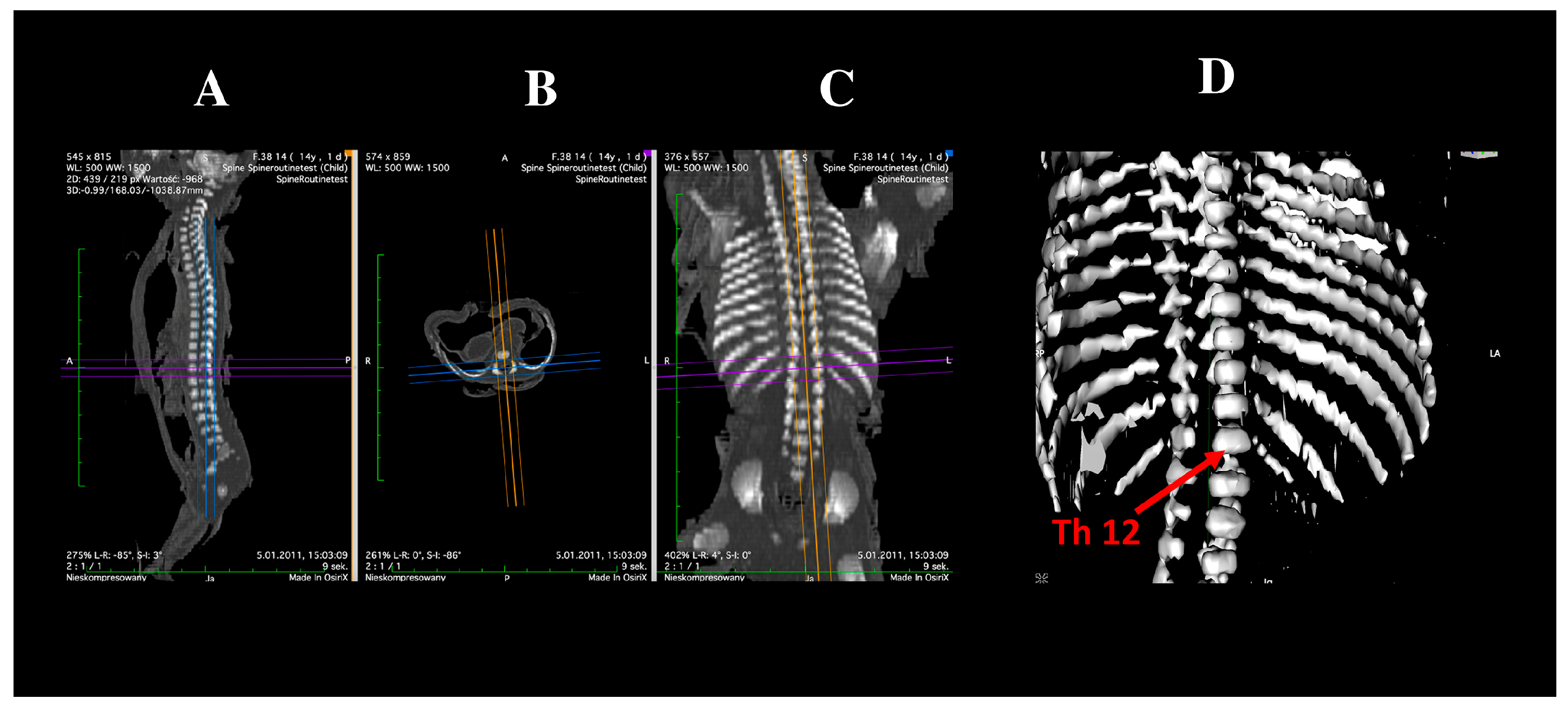
2.2. Morphometric Measurements and Assessment of Ossification Centers
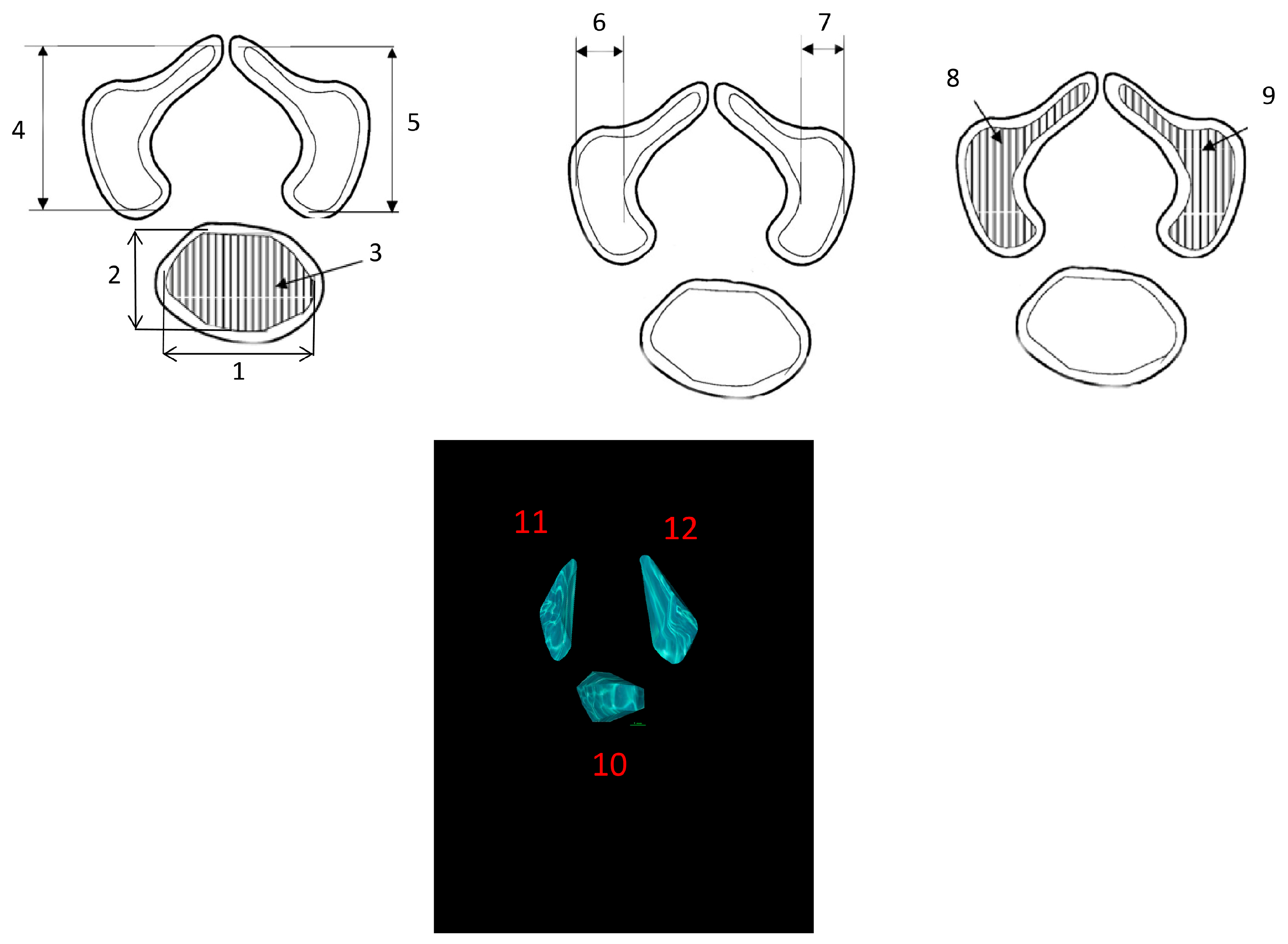
- Transverse diameter of the vertebral body ossification center—defined as distance between the left and right borders of the center in the transverse plane (Figure 2);
- Sagittal diameter of the vertebral body ossification center—measured as the distance between the anterior and posterior borders of the center in the transverse plane (Figure 2);
- Cross-sectional area of the vertebral body ossification center—calculated by tracing its outline in the transverse plane (Figure 2);
- Length of the right and left neural process ossification centers—defined as distance between the proximal and distal borders of each center in the transverse plan (Figure 2);
- Width of the right and left neural process ossification centers—measured in the transverse plane (Figure 2);
- Cross-sectional area of the right and left neural process ossification centers—determined by tracing their outlines in the transverse plane (Figure 2);
- Volume of each ossification center—computed using advanced diagnostic imaging software that enables three-dimensional reconstruction based on spatial position and tissue X-ray attenuation (Figure 2).
2.3. Statistical Analysis
3. Results
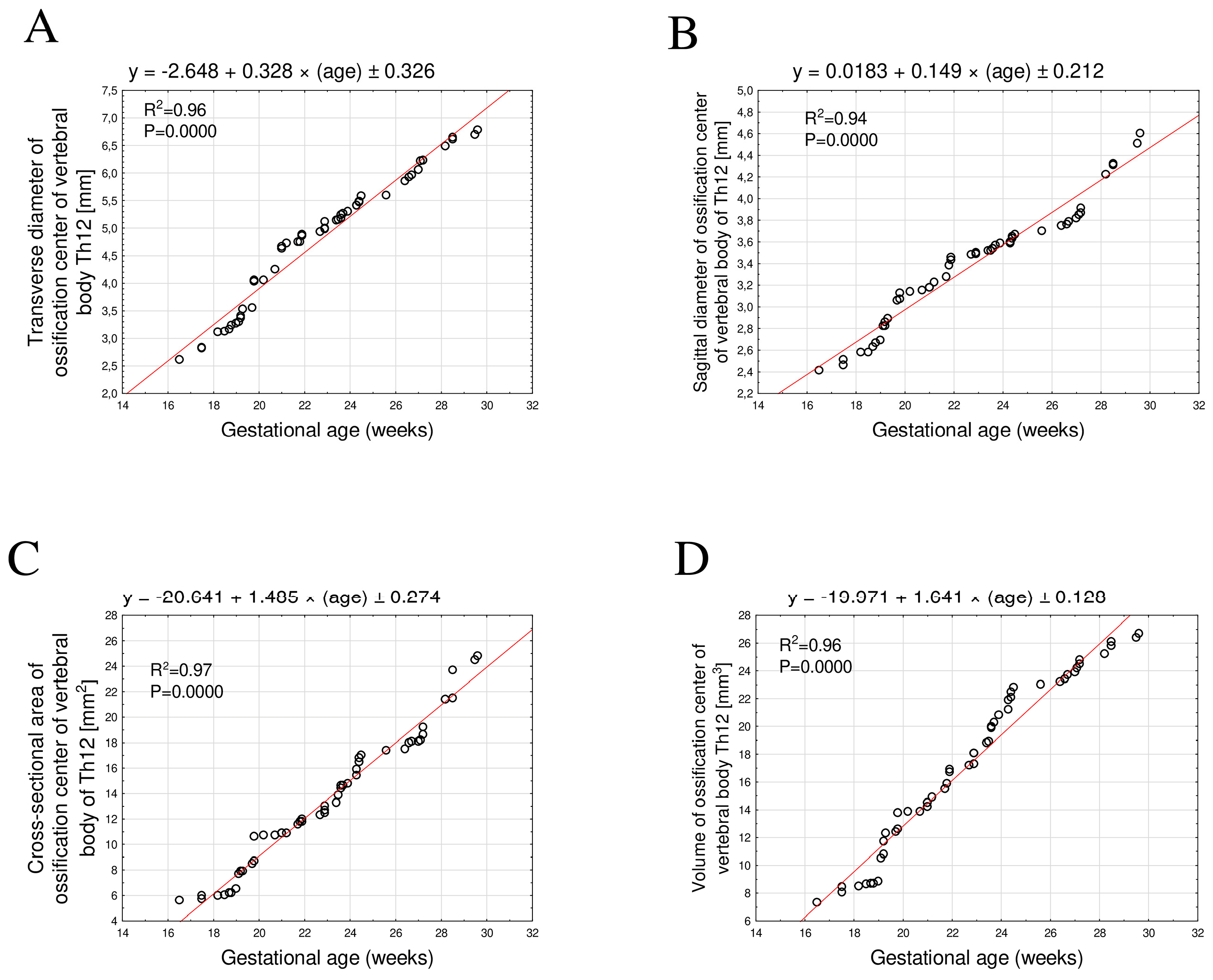
3.1. Morphometric Parameters of the Ossification Center of the Th12 Vertebral Body
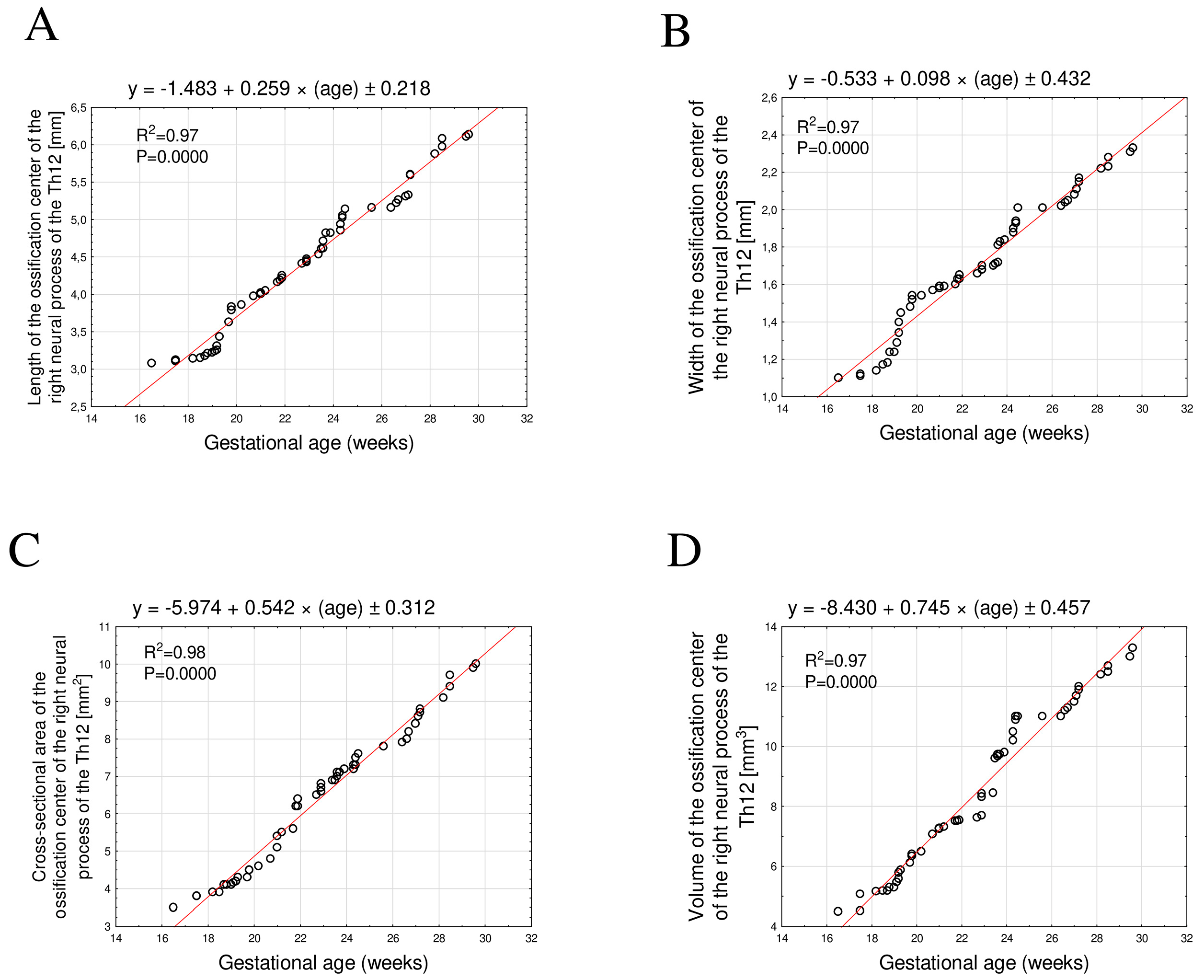
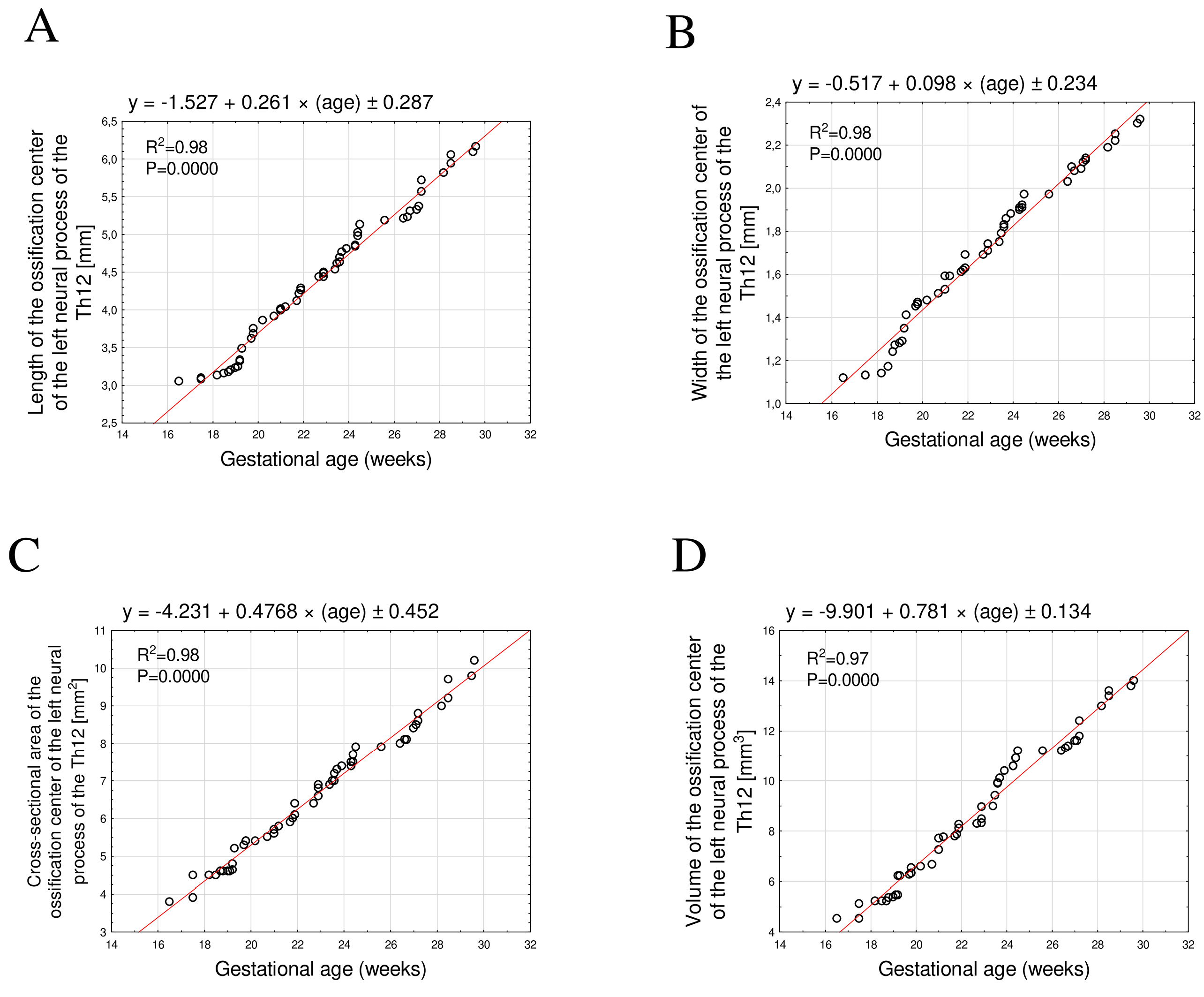
3.2. Morphometric Parameters of the Right and Left Th12 Neural Process Ossification Centers
4. Discussion
Limitations of the Study
5. Conclusions
- In the studied population of human fetuses aged 17–30 weeks, no significant sex-related or lateral differences were found in the morphometric parameters of the ossification centers of the Th12 vertebral body or neural processes.
- All analyzed linear, surface, and volumetric dimensions of the Th12 ossification centers exhibited directly proportional growth with fetal age. The growth dynamics followed a linear pattern with a very high degree of regression fit (R2 ≥ 0.94).
- The preliminary normative values obtained for the body and neural processes of the Th12 vertebra represent the first detailed reference data for this anatomical level within the analyzed gestational age range. These data may aid in fetal age estimation and serve as valuable support for ultrasonographic and tomographic evaluation of congenital spinal anomalies, particularly within the thoracolumbar junction. Further studies involving larger and more diverse populations, including both earlier and later prenatal stages, are required to fully validate these observations and to determine their clinical applicability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heiskanen, S.; Syvänen, J.; Helenius, I.; Kemppainen, T.; Löyttyniemi, E.; Gissler, E.; Raitio, A. Increasing prevalence and high risk of associated anomalies in congenital vertebral defects: A population-based study. J. Pediatr. Orthop. 2022, 17, e538–e543. [Google Scholar] [CrossRef]
- Heiskanen, S.; Helenius, I.; Raitio, A. Maternal risk factors for congenital vertebral formation and mixed defects: A population-based case–control study. J. Child. Orthop. 2024, 18, 340–345. [Google Scholar] [CrossRef]
- Nemec, U.; Nemec, S.F.; Krakow, D.; Brugger, P.C.; Malinger, G.; Graham, J.M., Jr.; Rimoin, D.L.; Prayer, D. The skeleton and musculature on foetal MRI. Insights Imaging 2011, 2, 309–318. [Google Scholar] [CrossRef][Green Version]
- Duczkowska, A.; Bekiesińska-Figatowska, M.; Herman-Sucharska, I.; Duczkowski, M.; Romaniuk-Doroszewska, A.; Jurkiewicz, E.; Dubis, A.; Urbanik, A.; Furmanek, M.; Walecki, J. Magnetic resonance imaging in the evaluation of the fetal spinal canal contents. Brain Dev. 2011, 33, 10–20. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Klionsky, N.B.; Nadarajah, U.; Chaturvedi, A.; Meyers, S.P. Malformed vertebrae: A clinical and imaging review. Insights Imaging 2018, 3, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Hedequist, D.; Emans, J. Congenital scoliosis. J. Am. Acad. Orthop. Surg. 2004, 12, 266–275. [Google Scholar] [CrossRef]
- Katsuura, Y.; Kim, H.J. Butterfly Vertebrae: A systematic review of the literature and analysis. Glob. Spine J. 2018, 18, 666–679. [Google Scholar] [CrossRef]
- Ruf, M.; Harms, J. Hemivertebra resection in children: Early correction and stable results. Spine 2002, 15, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Tortori-Donati, P.; Rossi, A.; Cama, A. Spinal dysraphism: A review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology 2000, 42, 471–491. [Google Scholar] [CrossRef]
- Victoria, T.; Epelman, M.; Coleman, B.G.; Horii, S.C.; Oliver, E.R.; Mahboubi, S.; Khalek, N.; Kasperski, S.; Edgar, J.C.; Jaramillo, D. Low-dose fetal CT in the prenatal evaluation of skeletal dysplasias and other severe skeletal abnormalities. AJR Am. J. Roentgenol. 2013, 200, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Dawood, Y.; Strijkers, G.Y.; Limpens, J.; Oostra, R.J.; de Bakker, B.S. Novel imaging techniques to study postmortem human fetal anatomy: A systematic review on microfocus-CT and ultra-high-field MRI. Eur. Radiol. 2020, 30, 2280–2292. [Google Scholar] [CrossRef]
- Prayer, D.; Malinger, G.; De Catte, L.; De Keersmaecker, B.; Gonçalves, L.F.; Kasprian, G.; Laifer-Narin, S.; Lee, W.; Millischer, A.-E.; Platt, L.; et al. ISUOG Practice Guidelines (updated): Performance of fetal magnetic resonance imaging. Ultrasound Obstet. Gynecol. 2023, 61, 278–287. [Google Scholar] [CrossRef]
- Macé, G.; Sonigo, P.; Cormier-Daire, V.; Aubry, M.C.; Martinovic, J.; Elie, C.; Gonzales, M.; Carbonne, B.; Dumez, Y.; Le Merrer, M.; et al. Three-dimensional helical computed tomography in prenatal diagnosis of fetal skeletal dysplasia. Ultrasound Obstet. Gynecol. 2013, 42, 161–168. [Google Scholar] [CrossRef]
- Bach, P.; Cassart, M.; Chami, M.; Garel, C.; Panuel, M. Exploration of the fetal skeleton by ultra-low-dose computed tomography: Guidelines from the fetal imaging task force of the european society of paediatric radiology. Pediatr. Radiol. 2023, 53, 621–631. [Google Scholar] [CrossRef]
- Simcock, I.C.; Shelmerdine, S.C.; Langan, D.; Anna, G.; Sebire, N.J.; Arthurs, O.J. Micro-CT yields high image quality in human fetal post-mortem imaging despite maceration. BMC Med. Imaging 2021, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Simcock, I.C.; Arthurs, O.J.; Hutchinson, J.C.; Sebire, N.J.; Jacques, T.S.; Sekar, T.; Shelmerdine, S.C. Impact of non-invasive post-mortem micro-CT imaging on a fetal autopsy service: A single centre retrospective study. Clin. Radiol. 2024, 79, 791–798. [Google Scholar] [CrossRef]
- Dawood, Y.; Honhoff, C.; van der Post, A.-S.; Roosendaal, S.D.; Coolen, B.F.; Strijkers, G.Y.; Pajkrt, E.; de Bakker, B.S. Comparison of postmortem whole-body contrast-enhanced microfocus computed tomography and high-field magnetic resonance imaging of human fetuses. Ultrasound Obstet. Gynecol. 2022, 60, 109–117. [Google Scholar] [CrossRef]
- Vignolo, M.; Ginocchio, G.; Parodi, A.; Torrisi, C.; Pistorio, A.; Venturini, P.L.; Aicardi, G.; De Biasio, P. Fetal spine ossification: The gender and individual differences illustrated by ultrasonography. Ultrasound Med. Biol. 2005, 31, 733–738. [Google Scholar] [CrossRef]
- Skórzewska, A.; Grzymisławska, M.; Bruska, M.; Łupicka, J.; Woźniak, W. Ossification of the vertebral column in human foetuses: Histological and computed tomography studies. Folia Morphol. 2013, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- De Biasio, P.; Ginocchio, G.; Aicardi, G.; Ravera, G.; Venturini, P.L.; Vignolo, M. Ossification timing of sacra vertebrae by ultrasound in the mid-second trimester of pregnancy. Prenat. Diag. 2003, 23, 1056–1059. [Google Scholar]
- Grzonkowska, M.; Kułakowski, M.; Elster, K.; Hankiewicz, B.; Janiak, M.; Rogalska, A.; Świtońska, M.; Żytkowski, A.; Baumgart, M. CT-Based Quantitative Analysis of Ossification Centres in the C7 Vertebra of Human Fetuses. Brain Sci. 2025, 15, 1018. [Google Scholar] [CrossRef]
- Grzonkowska, M.; Bogacz, K.; Żytkowski, A.; Szkultecka-Dębek, M.; Kułakowski, M.; Janiak, M.; Rogalska, A.; Baumgart, M. Digital Image Analysis of Vertebral Body S1 and Its Ossification Center in the Human Fetus. Brain Sci. 2025, 15, 74. [Google Scholar] [CrossRef]
- Chano, T.; Masumoto, K.; Ishizawa, M.; Morimoto, S.; Hukuda, S.; Okabe, H.; Kato, H.; Fujino, S. Analysis of the presence of osteocalcin, S-100 protein, and proliferating cell nuclear antigen in cells of various types of osteosarcomas. Eur. J. Histochem. 1996, 40, 189–198. [Google Scholar]
- Duarte, W.R.; Shibata, T.; Takenaga, K.; Takahashi, E.; Kubota, K.; Ohya, K.; Ishikawa, I.; Yamauchi, M.; Kasugai, S. S100A4: A novel negative regulator of mineralization and osteoblast differentiation. J. Bone Miner. Res. 2003, 18, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Szpinda, M.; Baumgart, M.; Szpinda, A.; Woźniak, A.; Mila-Kierzenkowska, C. Cross-sectional study of the neural ossification centers of vertebrae C1–S5 in the human fetus. Surg. Radiol. Anat. 2013, 35, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Szpinda, M.; Baumgart, M.; Szpinda, A.; Woźniak, A.; Małkowski, B.; Wiśniewski, M.; Mila-Kierzenkowska, C.; Króliczewski, D. Cross-sectional study of the ossification center of the C1–S5 vertebral bodies. Surg. Radiol. Anat. 2013, 35, 395–402. [Google Scholar] [CrossRef][Green Version]
- Costas, T.; de la O Rodríguez, M.; Esquilas, M.M.; Alarcón, V.; Goenaga, F.J.; Cabrero, M.Á.; Cubo, A.M. Long-Term Outcomes of Prenatally Diagnosed Fetal Hemivertebra: A 15-Year Single-Center Review. Children 2025, 12, 1236. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. The Developing Human: Clinically Oriented Embryology, 11th ed.; Elsevier: Philadelphia, PA, USA, 2019. [Google Scholar]
- Szpinda, M.; Baumgart, M.; Szpinda, A.; Woźniak, A.; Mila-Kierzenkowska, C.; Dombek, M.; Kosiński, A.; Grzybiak, M. Morphometric study of the T6 vertebra and its three ossification centers in the human fetus. Surg. Radiol. Anat. 2013, 30, 901–916. [Google Scholar] [CrossRef]
- Waratani, M.; Ito, F.; Tanaka, Y.; Mabuchi, A.; Mori, T.; Kitawaki, J. Prenatal diagnosis of fetal skeletal dysplasia using 3-dimensional computed tomography: A prospective study. BMC Musculoskelet. Disord. 2020, 8, 662. [Google Scholar] [CrossRef]
- Nabizadeh, N.; Dimar, J.R. Congenital spine deformities: Timing of insult during development of the spine in utero. Spine Deform. 2022, 10, 31–44. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, L.; Wang, T.; Yi, M.; Song, M. Prenatal diagnosis and pregnancy outcomes in fetuses with vertebral abnormalities. J. Matern. Fetal Neonatal. Med. 2025, 38, 2468000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yuan, X.; Peng, Z.; Jian, N.; Tian, M.; Feng, X.; Lin, X.; Wang, X. Normal fetal development of the cervical, thoracic, and lumbar spine: A postmortem study based on magnetic resonance imaging. Prenat. Diagn. 2021, 41, 989–997. [Google Scholar] [CrossRef] [PubMed]
| Month | GA (Weeks) | Crown-Rump Length (mm) | N | Sex | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min. | Max. | ♂ | ♀ | |||
| V | 17 | 115 | – | 115 | 115 | 1 | 0 | 1 |
| 18 | 133.3 | 5.77 | 130 | 140 | 3 | 1 | 2 | |
| 19 | 149.5 | 3.82 | 143 | 154 | 8 | 3 | 5 | |
| 20 | 161 | 2.71 | 159 | 165 | 4 | 2 | 2 | |
| VI | 21 | 174.8 | 2.87 | 171 | 178 | 4 | 3 | 1 |
| 22 | 185 | 1.41 | 183 | 186 | 4 | 1 | 3 | |
| 23 | 197.6 | 2.61 | 195 | 202 | 5 | 2 | 3 | |
| 24 | 208.7 | 3.81 | 204 | 213 | 9 | 5 | 4 | |
| VII | 25 | 214 | – | 214 | 214 | 1 | 0 | 1 |
| 26 | 229 | 5.66 | 225 | 233 | 2 | 1 | 1 | |
| 27 | 237.5 | 3.33 | 233 | 241 | 6 | 6 | 0 | |
| 28 | 249.5 | 0.71 | 249 | 250 | 2 | 0 | 2 | |
| VIII | 29 | 253 | 0 | 253 | 253 | 2 | 0 | 2 |
| 30 | 263.3 | 1.26 | 262 | 265 | 4 | 3 | 1 | |
| Total | 55 | 27 | 28 | |||||
| Parameter of the Body Ossification Center of Vertebra Th12 | ICC |
|---|---|
| Transverse diameter | 0.995 * |
| Sagittal diameter | 0.996 * |
| Cross-sectional area | 0.998 * |
| Volume | 0.996 * |
| Parameter of the Right Ossification Center of the Neural Process of Th12 | |
| Length | 0.996 * |
| Width | 0.996 * |
| Cross-sectional area | 0.997 * |
| Volume | 0.996 * |
| Parameter of the Left Ossification Center of the Neural Process of Th12 | |
| Length | 0.997 * |
| Width | 0.997 * |
| Cross-sectional area | 0.997 * |
| Volume | 0.996 * |
| Month | GA (Weeks) | N | Ossification Center of the Vertebral Body Th12 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Transverse Diameter (mm) | Sagittal Diameter (mm) | Cross-Sectional Area (mm2) | Volume (mm3) | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| V | 17 | 1 | 2.61 | – | 2.41 | – | 5.6 | – | 7.32 | – |
| 18 | 3 | 2.92 | 0.16 | 2.52 | 0.06 | 5.9 | 0.17 | 8.33 | 0.25 | |
| 19 | 8 | 3.3 | 0.13 | 2.75 | 0.12 | 7.04 | 0.87 | 10.02 | 1.5 | |
| 20 | 4 | 3.92 | 0.25 | 3.1 | 0.04 | 9.63 | 1.19 | 13.18 | 0.78 | |
| VI | 21 | 4 | 4.57 | 0.22 | 3.19 | 0.03 | 10.85 | 0.1 | 14.38 | 0.43 |
| 22 | 5 | 4.81 | 0.07 | 3.39 | 0.08 | 11.8 | 0.16 | 16.25 | 0.66 | |
| 23 | 5 | 5.03 | 0.09 | 3.5 | 0.02 | 12.76 | 0.4 | 17.9 | 0.66 | |
| 24 | 9 | 5.32 | 0.13 | 3.58 | 0.04 | 15.21 | 1 | 20.84 | 1.19 | |
| VII | 25 | 1 | 5.58 | – | 3.67 | – | 17 | – | 22.8 | – |
| 26 | 2 | 5.72 | 0.18 | 3.73 | 0.04 | 17.45 | 0.07 | 23.1 | 0.14 | |
| 27 | 5 | 6.11 | 0.14 | 3.83 | 0.05 | 18.37 | 0.46 | 24.08 | 0.52 | |
| 28 | 2 | 6.46 | 0.05 | 4.07 | 0.22 | 20.4 | 1.41 | 25.1 | 0.14 | |
| VIII | 29 | 2 | 6.63 | 0.03 | 4.32 | 0.01 | 22.6 | 1.56 | 25.95 | 0.21 |
| 30 | 4 | 6.82 | 0.11 | 4.67 | 0.14 | 25.08 | 0.53 | 26.78 | 0.3 | |
| Month | GA (Weeks) | N | Right Ossification Center of the Neural Process of Th12 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Cross-Sectional Area (mm2) | Volume (mm3) | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| V | 17 | 1 | 3.08 | – | 1.1 | – | 3.5 | – | 4.48 | – |
| 18 | 3 | 3.12 | 0.02 | 1.12 | 0.02 | 3.83 | 0.06 | 4.91 | 0.35 | |
| 19 | 8 | 3.25 | 0.09 | 1.29 | 0.1 | 4.13 | 0.12 | 5.45 | 0.26 | |
| 20 | 4 | 3.78 | 0.1 | 1.52 | 0.03 | 4.48 | 0.13 | 6.34 | 0.17 | |
| VI | 21 | 4 | 4.01 | 0.03 | 1.58 | 0.01 | 5.2 | 0.32 | 7.23 | 0.11 |
| 22 | 5 | 4.21 | 0.04 | 1.63 | 0.02 | 6.1 | 0.35 | 7.53 | 0.01 | |
| 23 | 5 | 4.46 | 0.05 | 1.68 | 0.02 | 6.7 | 0.16 | 8.1 | 0.41 | |
| 24 | 9 | 4.83 | 0.16 | 1.84 | 0.08 | 7.18 | 0.18 | 10.13 | 0.55 | |
| VII | 25 | 1 | 5.14 | – | 2.01 | – | 7.6 | – | 11 | – |
| 26 | 2 | 5.16 | 0 | 2.02 | 0.01 | 7.85 | 0.07 | 11 | 0 | |
| 27 | 5 | 5.39 | 0.17 | 2.1 | 0.05 | 8.45 | 0.31 | 11.6 | 0.32 | |
| 28 | 2 | 5.77 | 0.16 | 2.22 | 0.01 | 9.05 | 0.07 | 12.3 | 0.14 | |
| VIII | 29 | 2 | 6.03 | 0.07 | 2.26 | 0.04 | 9.55 | 0.21 | 12.6 | 0.14 |
| 30 | 4 | 6.16 | 0.04 | 2.34 | 0.03 | 10.3 | 0.41 | 13.4 | 0.34 | |
| Month | GA (Weeks) | N | Left Ossification Center of the Neural Arches of Th12 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Cross-Sectional Area (mm2) | Volume (mm3) | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| V | 17 | 1 | 3.05 | – | 1.12 | – | 3.8 | – | 4.53 | – |
| 18 | 3 | 3.1 | 0.03 | 1.13 | 0.01 | 4.3 | 0.35 | 4.96 | 0.37 | |
| 19 | 8 | 3.27 | 0.11 | 1.3 | 0.07 | 4.69 | 0.22 | 5.57 | 0.41 | |
| 20 | 4 | 3.73 | 0.1 | 1.47 | 0.01 | 5.38 | 0.05 | 6.42 | 0.16 | |
| VI | 21 | 4 | 3.99 | 0.06 | 1.56 | 0.04 | 5.65 | 0.13 | 7.35 | 0.51 |
| 22 | 5 | 4.22 | 0.07 | 1.64 | 0.04 | 6.1 | 0.22 | 8.01 | 0.22 | |
| 23 | 5 | 4.48 | 0.04 | 1.73 | 0.03 | 6.72 | 0.22 | 8.61 | 0.34 | |
| 24 | 9 | 4.8 | 0.14 | 1.87 | 0.05 | 7.33 | 0.23 | 10.3 | 0.5 | |
| VII | 25 | 1 | 5.13 | – | 1.97 | – | 7.9 | – | 11.2 | – |
| 26 | 2 | 5.2 | 0.01 | 2 | 0.04 | 7.95 | 0.07 | 11.2 | 0 | |
| 27 | 5 | 5.42 | 0.19 | 2.11 | 0.02 | 8.42 | 0.28 | 11.68 | 0.39 | |
| 28 | 2 | 5.77 | 0.08 | 2.18 | 0.02 | 8.95 | 0.07 | 12.8 | 0.28 | |
| VIII | 29 | 2 | 6 | 0.08 | 2.24 | 0.02 | 9.45 | 0.35 | 13.5 | 0.14 |
| 30 | 4 | 6.17 | 0.06 | 2.32 | 0.02 | 10.18 | 0.29 | 14.1 | 0.26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzonkowska, M.; Kułakowski, M.; Dzięcioł-Anikiej, Z.; Rogalska, A.; Zwierko, B.; Kierońska-Siwak, S.; Elster, K.; Orkisz, S.; Baumgart, M. CT Morphometric Analysis of Ossification Centres in the Fetal Th12 Vertebra. Brain Sci. 2025, 15, 1138. https://doi.org/10.3390/brainsci15111138
Grzonkowska M, Kułakowski M, Dzięcioł-Anikiej Z, Rogalska A, Zwierko B, Kierońska-Siwak S, Elster K, Orkisz S, Baumgart M. CT Morphometric Analysis of Ossification Centres in the Fetal Th12 Vertebra. Brain Sciences. 2025; 15(11):1138. https://doi.org/10.3390/brainsci15111138
Chicago/Turabian StyleGrzonkowska, Magdalena, Michał Kułakowski, Zofia Dzięcioł-Anikiej, Agnieszka Rogalska, Beata Zwierko, Sara Kierońska-Siwak, Karol Elster, Stanisław Orkisz, and Mariusz Baumgart. 2025. "CT Morphometric Analysis of Ossification Centres in the Fetal Th12 Vertebra" Brain Sciences 15, no. 11: 1138. https://doi.org/10.3390/brainsci15111138
APA StyleGrzonkowska, M., Kułakowski, M., Dzięcioł-Anikiej, Z., Rogalska, A., Zwierko, B., Kierońska-Siwak, S., Elster, K., Orkisz, S., & Baumgart, M. (2025). CT Morphometric Analysis of Ossification Centres in the Fetal Th12 Vertebra. Brain Sciences, 15(11), 1138. https://doi.org/10.3390/brainsci15111138






