The Effect of 5G Mobile Phone Electromagnetic Exposure on Corticospinal and Intracortical Excitability in Healthy Adults: A Randomized Controlled Pilot Study
Abstract
1. Introduction
1.1. Background
1.1.1. Evolution of Mobile Phone Technology and Electromagnetic Radiation
1.1.2. Neurophysiological Implications of Mobile Phone EM Exposure
1.1.3. Gaps in Current Research
1.1.4. Significance of Current Research
1.1.5. Study Aims
- To determine whether a 5 min exposure to 5G mobile phone EM exposure significantly affects CSE and associated inhibitory and facilitatory mechanisms (ICF, SICI, and LICI) compared to a sham condition.
- To assess whether a more prolonged exposure duration (20 min) differentially influences CSE and these mechanisms, compared to both 5 min exposure and sham conditions.
1.1.6. Hypotheses
- Both 5 min and 20 min exposures to 5G mobile phone EM radiation will cause significant changes in CSE and intracortical excitability (ICF, SICI, and LICI) compared to a sham exposure.
- A 20 min 5G EM exposure will produce greater modulation of CSE and intracortical excitability (ICF, SICI, LICI) compared with both the 5 min and sham conditions, consistent with a duration-dependent effect.
2. Materials and Methods
2.1. Participants
2.2. Sample Size Justification
2.3. Inclusion-Exclusion Criteria
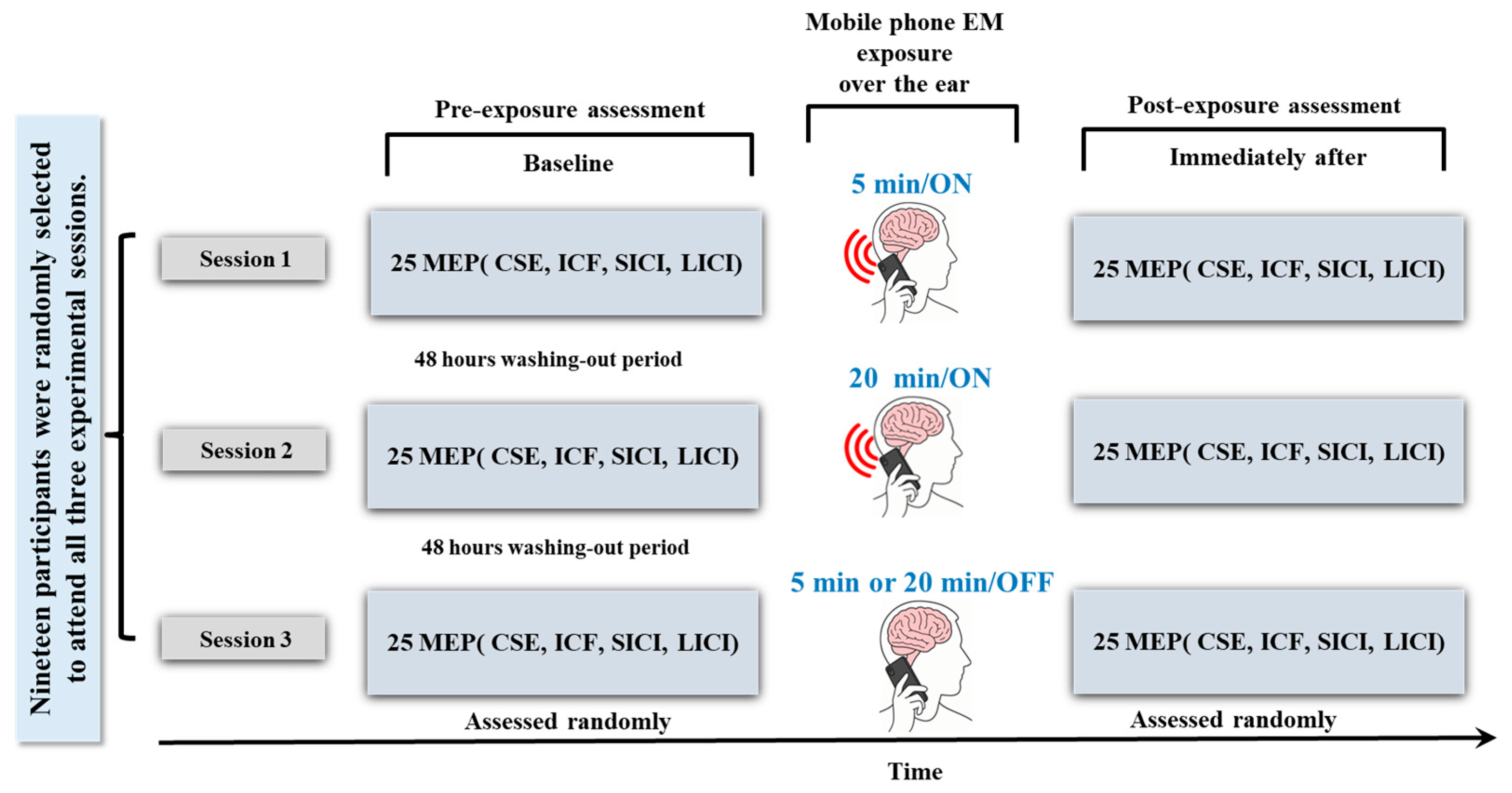
2.4. Study Design
2.5. Sham Intervention
2.6. Exposure Setting
2.6.1. Device Specifications and EMR Compliance
2.6.2. Environmental EMR Control
2.7. Outcome Measures
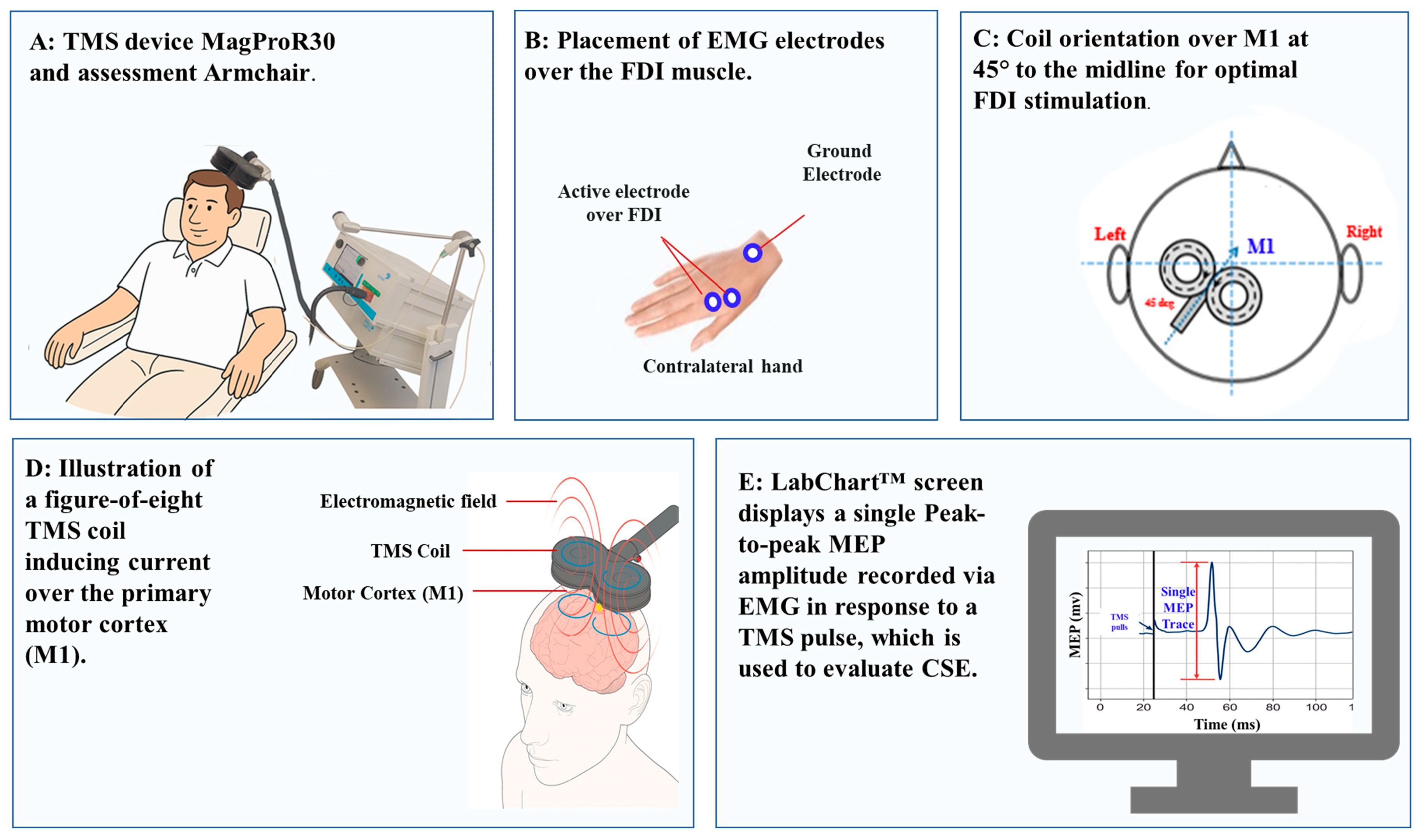
2.8. TMS Assessment of CSE
- Standardization of CSE Assessment: Our primary aim was to examine the effects of 5G EMR on CSE and intracortical excitability under baseline (resting) conditions. Resting MEPs are widely used in TMS protocols to evaluate CSE, ICF, SICI, and LICI, without the confounding influence of peripheral or voluntary motor drive. This approach ensures that changes in MEP amplitude or inhibition/facilitation reflect cortical modulation, not task-related motor activation.
- Avoidance of Variability from Contraction Effort: Sustaining even low levels of contraction (5–10% MVC) can introduce variability due to differences in attention, fatigue, or effort across time points and participants. Using the resting state minimizes such sources of variability and enhances reproducibility in pre–post comparisons.
- Safety and Comfort: Resting MEPs reduce the physical demands placed on participants, which is particularly relevant given the repeated-measures design involving multiple TMS protocols across several sessions. This choice optimized participant comfort and compliance without compromising data quality.
2.9. TMS Assessment of Intracortical Excitability (ICF, SICI, LICI)
2.10. TMS Experimental Process
2.11. Statistical Analysis
3. Results
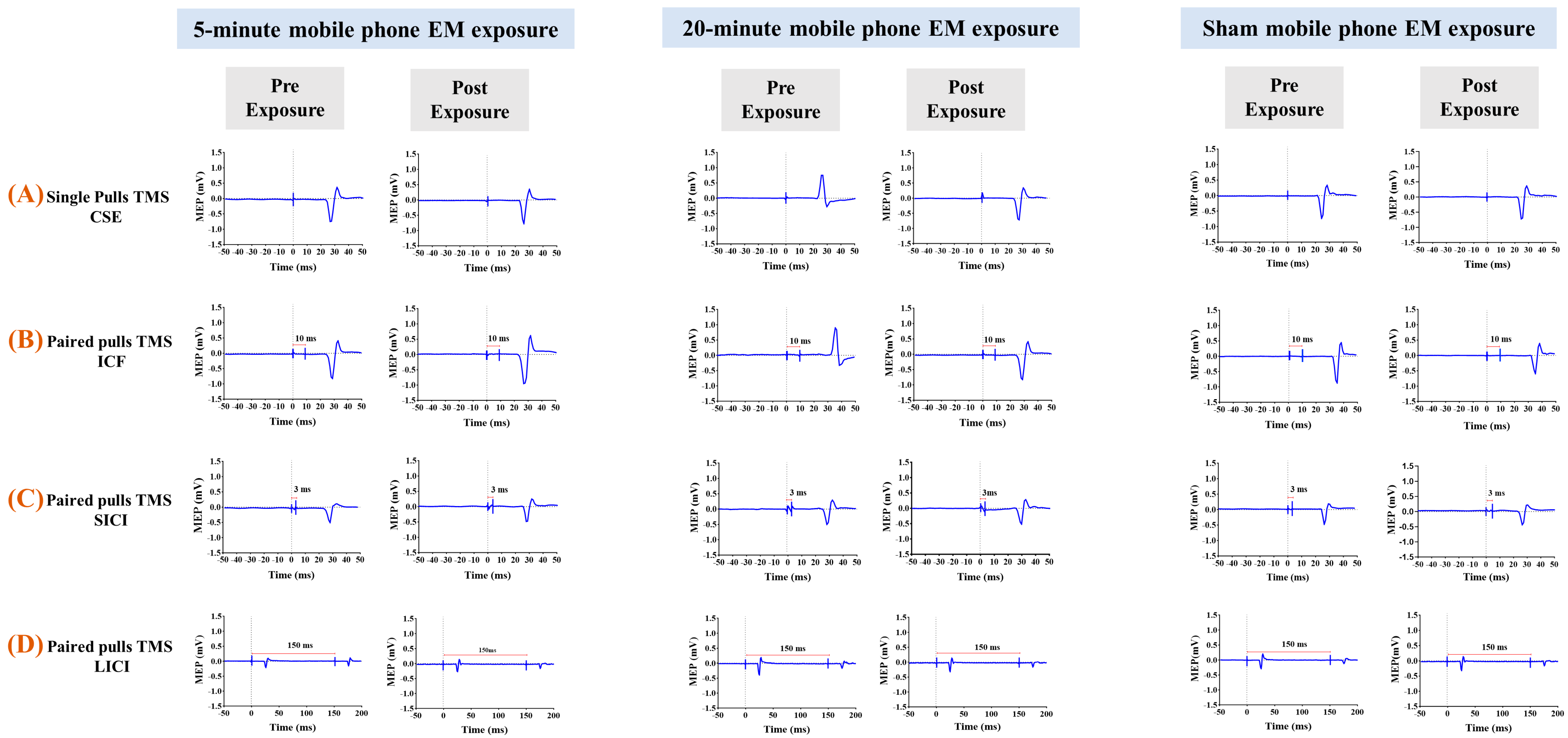
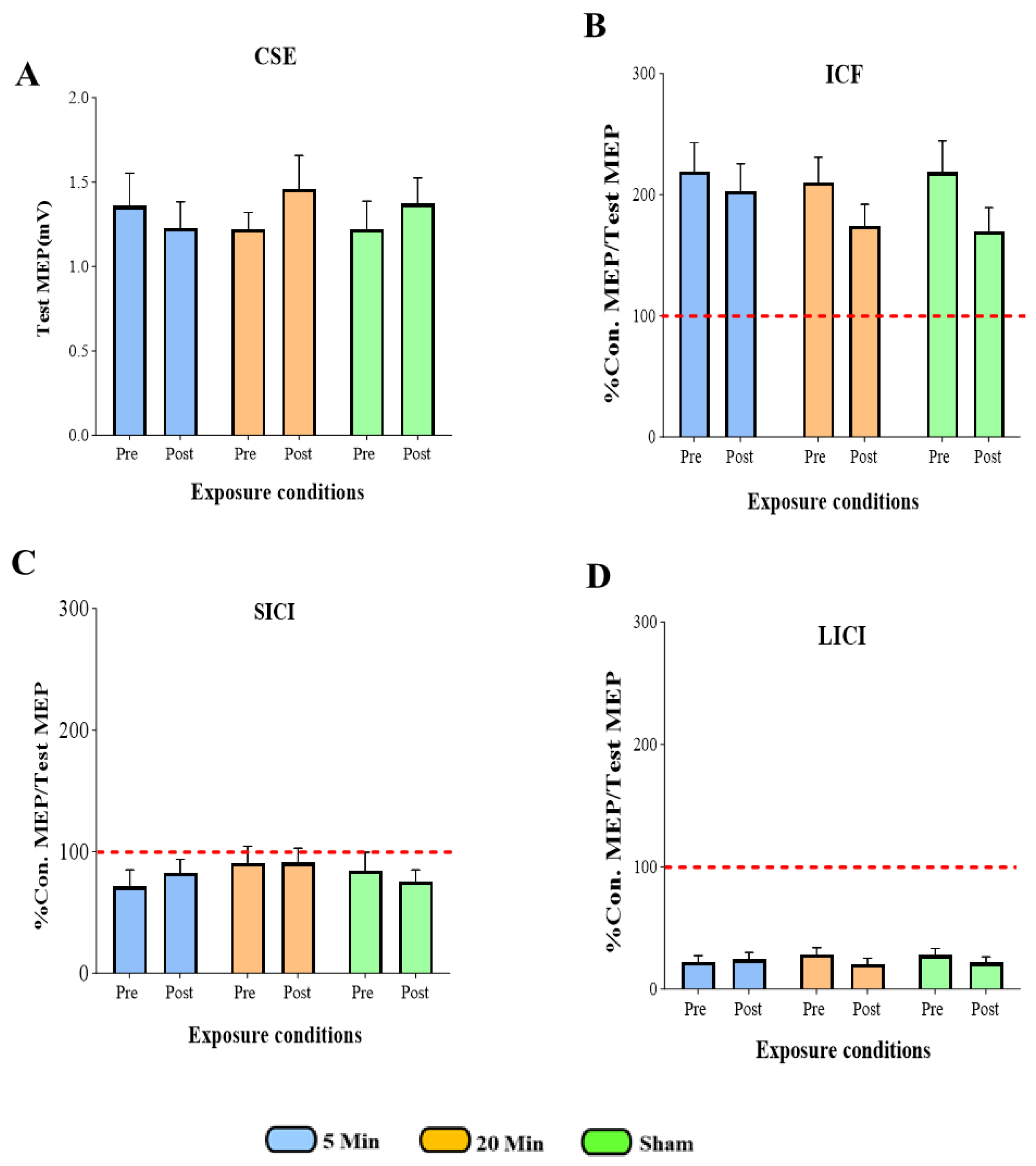
3.1. The Effects of 5G Mobile Phone EM Exposure on CSE, ICF, SICI, and LICI
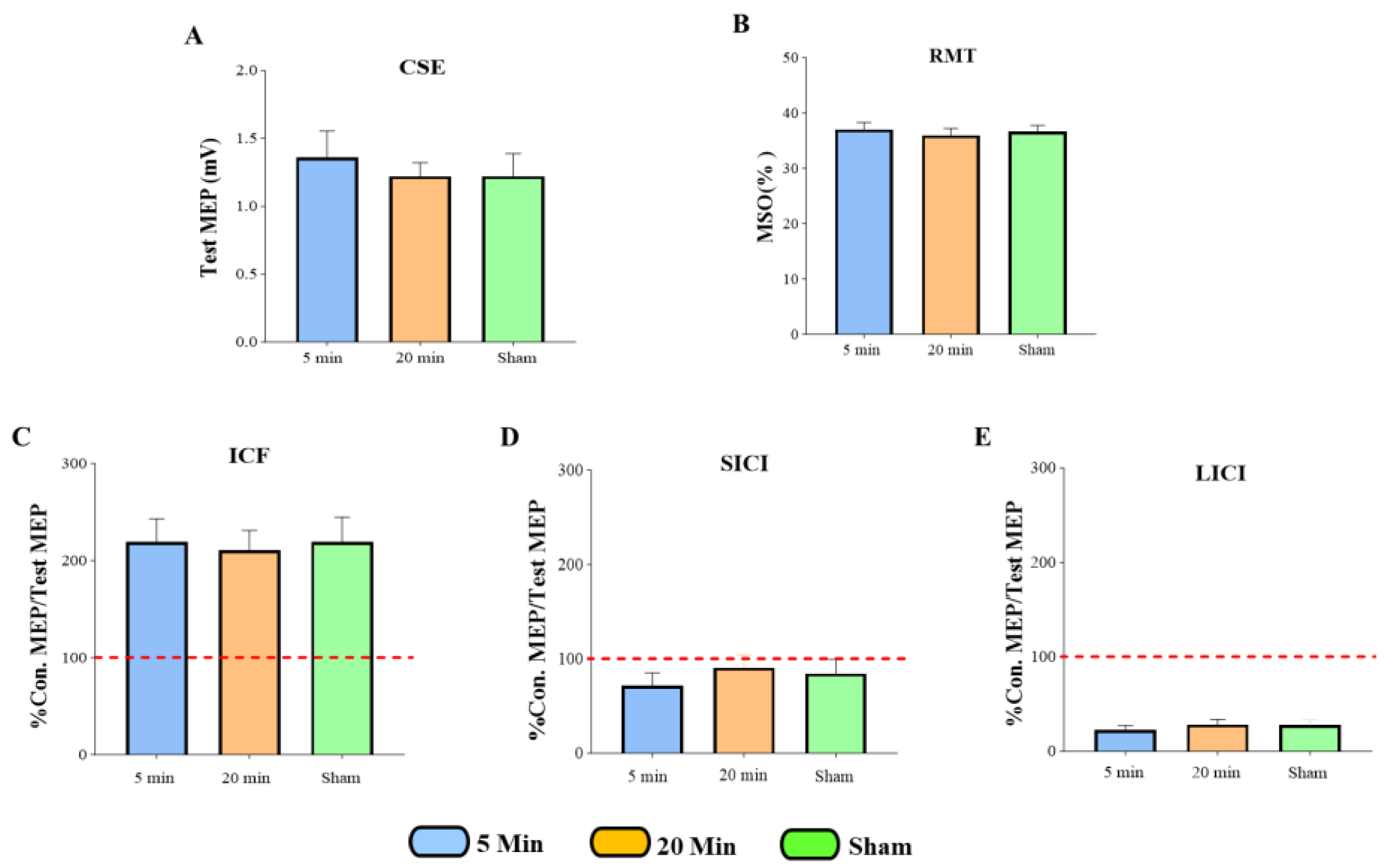
3.2. The Effects of 5G Mobile Phone EM Exposure on CSE
3.3. The Effects of 5G Mobile Phone EM Exposure on ICF
3.4. The Effects of 5G Mobile Phone EM Exposure on SICI
3.5. The Effects of 5G Mobile Phone EM Exposure on LICI
3.6. Summary of All TMS Measures
4. Discussion
4.1. Effect of 5G Mobile Phone EM Exposure on CSE
4.2. Effect of 5G Mobile Phone EM Exposure on ICF, SICI, LICI
4.3. Limitations and Suggestions for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joshi, A.W.; Kumar, A. What can we Learn from the Electromagnetic Spectrum? Resonance 2003, 8, 8–25. [Google Scholar] [CrossRef]
- Salih, A.A.; Zeebaree, S.R.; Abdulraheem, A.S.; Zebari, R.R.; Sadeeq, M.A.; Ahmed, O.M. Evolution of mobile wireless communication to 5G revolution. Technol. Rep. Kansai Univ. 2020, 62, 2139–2151. [Google Scholar]
- Omer, H. Radiobiological effects and medical applications of non-ionizing radiation. Saudi J. Biol. Sci. 2021, 28, 5585–5592. [Google Scholar] [CrossRef]
- Fields, E. Icnirp Guidelines. Health 2020, 17. [Google Scholar]
- Mehta, A. Introduction to the Electromagnetic Spectrum and Spectroscopy, Pharmaxchange. Available online: http://pharmaxchange.info/press/2011/08/introduction-to-the-electromagnetic-spectrum-and-spectroscopy/ (accessed on 16 October 2025).
- Horwitz, J. The definitive guide to 5G low, mid, and high band speeds. Ventur. Beat Online Mag. 2019. [Google Scholar]
- Hinrikus, H.; Koppel, T.; Lass, J.; Orru, H.; Roosipuu, P.; Bachmann, M. Possible health effects on the human brain by various generations of mobile telecommunication: A review-based estimation of 5G impact. Int. J. Radiat. Biol. 2022, 98, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Foroughimehr, N.; Wood, A.; McKenzie, R.; Karipidis, K.; Yavari, A. Design and implementation of a specialised millimetre-wave exposure system for investigating the radiation effects of 5g and future technologies. Sensors 2024, 24, 1516. [Google Scholar] [CrossRef] [PubMed]
- International Telecommunication Union. Detailed Specifications of the Terrestrial Radio Interfaces of International Mobile Telecommunications-2020 (IMT-2020); Recommendation ITU-R M. 2150-0, 2021.02; ITU: Geneva, Switzerland, 2021. [Google Scholar]
- ETSI TS 138 101-1; User Equipment (UE) Radio Transmission and Reception. ETSI: Paris, France, 2018.
- Han, S.; Bian, S. Energy-efficient 5G for a greener future. Nat. Electron. 2020, 3, 182–184. [Google Scholar] [CrossRef]
- Lemon, R.N. Descending pathways in motor control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef]
- Tanzarella, S.; Muceli, S.; Santello, M.; Farina, D. Synergistic organization of neural inputs from spinal motor neurons to extrinsic and intrinsic hand muscles. J. Neurosci. 2021, 41, 6878–6891. [Google Scholar] [CrossRef]
- Brandt, I.M.; Lundbye-Jensen, J.; Grünbaum, T.; Christensen, M.S. Force, angle, and velocity parameters of finger movements are reflected in corticospinal excitability. bioRxiv 2024. [Google Scholar] [CrossRef]
- Betti, S.; Fedele, M.; Castiello, U.; Sartori, L.; Budisavljević, S. Corticospinal excitability and conductivity are related to the anatomy of the corticospinal tract. Brain Struct. Funct. 2022, 227, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Ziemann, U.; Tergau, F.; Wassermann, E.M.; Wischer, S.; Hildebrandt, J.; Paulus, W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J. Physiol. 1998, 511, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, E.M.; Samii, A.; Mercuri, B.; Ikoma, K.; Oddo, D.; Grill, S.E.; Hallett, M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp. Brain Res. 1996, 109, 158–163. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Mimura, Y.; Nishida, H.; Nakajima, S.; Tsugawa, S.; Morita, S.; Yoshida, K.; Tarumi, R.; Ogyu, K.; Wada, M.; Kurose, S.; et al. Neurophysiological biomarkers using transcranial magnetic stimulation in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 121, 47–59. [Google Scholar] [CrossRef]
- He, J.; Fuelscher, I.; Coxon, J.; Teo, W.; Barhoun, P.; Enticott, P.; Chowdhury, N.; Hyde, C. Interindividual differences in both resting-state intracortical and interhemispheric inhibition predicts individual differences in relevant motor performance. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 2019, 12, 477. [Google Scholar] [CrossRef]
- Maruyama, A. S14-4. Possibility of a useful intervention of exercise induced by muscle fatigue to change excitabilities of corticospinal and cortico-cortical tracts for functional recovery in neurorehabilitation. Clin. Neurophysiol. 2013, 124, e26. [Google Scholar] [CrossRef]
- Pedapati, E.V.; Mooney, L.N.; Wu, S.W.; Erickson, C.A.; Sweeney, J.A.; Shaffer, R.C.; Horn, P.S.; Wink, L.K.; Gilbert, D.L. Motor cortex facilitation: A marker of attention deficit hyperactivity disorder co-occurrence in autism spectrum disorder. Transl. Psychiatry 2019, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Bolden, L.B.; Griffis, J.C.; Pati, S.; Szaflarski, J.P. Cortical excitability and neuropsychological functioning in healthy adults. Neuropsychologia 2017, 102, 190–196. [Google Scholar] [CrossRef]
- Spampinato, D.; Celnik, P. Multiple motor learning processes in humans: Defining their neurophysiological bases. Neuroscientist 2021, 27, 246–267. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, C.A.; DeVries, W.; Dowdle, L.T.; West, J.A.; Siekman, B.; Li, X.; George, M.S. A comprehensive study of sensorimotor cortex excitability in chronic cocaine users: Integrating TMS and functional MRI data. Drug Alcohol Depend. 2015, 157, 28–35. [Google Scholar] [CrossRef]
- Cash, R.F.H.; Isayama, R.; Gunraj, C.A.; Ni, Z.; Chen, R. The influence of sensory afferent input on local motor cortical excitatory circuitry in humans. J. Physiol. 2015, 593, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, K.; Kirveskari, E.; Mäkelä, J.P.; Kaste, M.; Mustanoja, S.; Nummenmaa, L.; Tatlisumak, T.; Forss, N. Effect of afferent input on motor cortex excitability during stroke recovery. Clin. Neurophysiol. 2012, 123, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, P.G.; Térémetz, M.; Charron, S.; Kebir, O.; Saby, A.; Bendjemaa, N.; Lion, S.; Crépon, B.; Gaillard, R.; Oppenheim, C.; et al. Altered cortical processing of motor inhibition in schizophrenia. Cortex 2016, 85, 1–12. [Google Scholar] [CrossRef]
- Cerins, A.; Corp, D.; Opie, G.; Do, M.; Speranza, B.; He, J.; Barhoun, P.; Fuelscher, I.; Enticott, P.; Hyde, C. Assessment of cortical inhibition depends on inter individual differences in the excitatory neural populations activated by transcranial magnetic stimulation. Sci. Rep. 2022, 12, 9923. [Google Scholar] [CrossRef]
- Hummel, F.C.; Steven, B.; Hoppe, J.; Heise, K.; Thomalla, G.; Cohen, L.G.; Gerloff, C. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology 2009, 72, 1766–1772. [Google Scholar] [CrossRef]
- Tamás, G.; Simon, A.L.A.; Szabadics, J. Identified sources and targets of slow inhibition in the neocortex. Science 2003, 299, 1902–1905. [Google Scholar] [CrossRef]
- Brandt, I.M.; Grünbaum, T.; Christensen, M.S. Evidence of optimal control theory over active inference in corticospinal excitability modulations. bioRxiv 2024. [Google Scholar] [CrossRef]
- Classen, J.; Liepert, J.; Wise, S.P.; Hallett, M.; Cohen, L.G. RRapid plasticity of human cortical movement representation induced by practice. J. Neurophysiol. 1998, 79, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Iliać, T.V.; Pauli, C.; Meintzschel, F.; Ruge, D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J. Neurosci. 2004, 24, 1666–1672. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Orekhov, Y.; Ziemann, U. The role of GABA B receptors in intracortical inhibition in the human motor cortex. Experimental brain research. Exp. Brain Res. 2006, 173, 86–93. [Google Scholar] [CrossRef]
- McCormick, D.A.; Wang, Z.; Huguenard, J. Neurotransmitter control of neocortical neuronal activity and excitability. Cereb. Cortex 1993, 3, 387–398. [Google Scholar] [CrossRef]
- Jamal, L.; Yahia-Cherif, L.; Hugueville, L.; Mazet, P.; Lévêque, P.; Selmaoui, B. Assessment of electrical brain activity of healthy volunteers exposed to 3.5 GHz of 5G signals within environmental levels: A controlled–randomised study. Int. J. Environ. Res. Public Health 2023, 20, 6793. [Google Scholar] [CrossRef]
- Zentai, N.; Csathó, Á.; Trunk, A.; Fiocchi, S.; Parazzini, M.; Ravazzani, P.; Thuróczy, G.; Hernádi, I. No effects of acute exposure to Wi-Fi electromagnetic fields on spontaneous EEG activity and psychomotor vigilance in healthy human volunteers. Radiat. Res. 2015, 184, 568–577. [Google Scholar] [CrossRef]
- Wallace, J.; Yahia-Cherif, L.; Gitton, C.; Hugueville, L.; Lemaréchal, J.-D.; Selmaoui, B. Human resting-state EEG and radiofrequency GSM mobile phone exposure: The impact of the individual alpha frequency. Int. J. Radiat. Biol. 2022, 98, 986–995. [Google Scholar] [CrossRef]
- Torkan, A.; Zoghi, M.; Foroughimehr, N.; Yavari, A.; Jaberzadeh, S. Effects of Mobile Electromagnetic Exposure on Brain Oscillations and Cortical Excitability: Scoping Review. Sensors 2025, 25, 2749. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.; Minarik, T.; Liuzzi, G.; Hummel, F.C.; Sauseng, P. EEG oscillatory phase-dependent markers of corticospinal excitability in the resting brain. BioMed Res. Int. 2014, 2014, 936096. [Google Scholar] [CrossRef]
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Kitajo, K.; Okazaki, Y.O. TMS-EEG for probing distinct modes of neural dynamics in the human brain. In Advances in Cognitive Neurodynamics (V), Proceedings of the Fifth International Conference on Cognitive Neurodynamics-2015, Virtual, 30 January 2016; Springer: Singapore, 2016. [Google Scholar]
- Lepage, J.-F.; Saint-Amour, D.; Théoret, H. EEG and neuronavigated single-pulse TMS in the study of the observation/execution matching system: Are both techniques measuring the same process? J. Neurosci. Methods 2008, 175, 17–24. [Google Scholar] [CrossRef] [PubMed]
- IEEE Std C95. 1-2019; IEEE Standard for Safety Levels with Respect to Human Exposure to Electric, Magnetic, and Electromagnetic Fields, 0 Hz to 300 GHz. Revision of IEEE Std C95. 1-2005/Incorporates IEEE Std C95. 1-2019/Cor 1-2019. IEEE: New York, NY, USA, 2019; pp. 1–312. [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Ferreri, F.; Curcio, G.; Pasqualetti, P.; De Gennaro, L.; Fini, R.; Rossini, P.M. Mobile phone emissions and human brain excitability. Ann. Neurol. 2006, 60, 188–196. [Google Scholar] [CrossRef]
- Inomata-Terada, S.; Okabe, S.; Arai, N.; Hanajima, R.; Terao, Y.; Frubayashi, T.; Ugawa, Y. Effects of high frequency electromagnetic field (EMF) emitted by mobile phones on the human motor cortex. Bioelectromagnetics 2007, 28, 553–561. [Google Scholar] [CrossRef] [PubMed]
- García-Larrea, L.; Perchet, C.; Perrin, F.; Amenedo, E. Interference of cellular phone conversations with visuomotor tasks: An ERP study. J. Psychophysiol. 2001, 15, 14–21. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Rouintan, M.S.; Taeb, S.; Dehghan, N.; Ghaffarpanah, A.A.; Sadeghi, Z.; Ghafouri, F. Human short-term exposure to electromagnetic fields emitted by mobile phones decreases computer-assisted visual reaction time. Acta Neurol. Belg. 2012, 112, 171–175. [Google Scholar] [CrossRef]
- Haque, M.M.; Washington, S. Effects of mobile phone distraction on drivers’ reaction times. J. Australas. Coll. Road Saf. 2013, 24, 20–29. [Google Scholar]
- Lu, Z.; Zhang, X.; Mao, C.; Liu, T.; Li, X.; Zhu, W.; Wang, C.; Sun, Y. Effects of Mobile Phone Use on Gait and Balance Control in Young Adults: A Hip–Ankle Strategy. Bioengineering 2023, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.-E.; Chia, H.-P.; Tan, J.-S. Prevalence of headache among handheld cellular telephone users in Singapore: A community study. Environ. Health Perspect. 2000, 108, 1059. [Google Scholar] [CrossRef]
- Sandstrom, M.; Wilén, J.; Mild, K.H.; Oftedal, G. Mobile phone use and subjective symptoms. Comparison of symptoms experienced by users of analogue and digital mobile phones. Occup. Med. 2001, 51, 25–35. [Google Scholar] [CrossRef]
- Cinel, C.; Russo, R.; Boldini, A.; Fox, E. Exposure to mobile phone electromagnetic fields and subjective symptoms: A double-blind study. Psychosom. Med. 2008, 70, 345–348. [Google Scholar] [CrossRef]
- Mat, D.A.A.; Kho, F.; Joseph, A.; Kipli, K.; Sahrani, S.; Lias, K.; Marzuki, A.S.W. Electromagnetic radiation from mobile phone near ear-skull region. In Proceedings of the International Conference on Computer and Communication Engineering (ICCCE’10), Kuala Lumpur, Malaysia, 11–13 May 2010. [Google Scholar]
- Szyjkowska, A.; Gadzicka, E.; Szymczak, W.; Bortkiewicz, A. The risk of subjective symptoms in mobile phone users in Poland—An epidemiological study. Int. J. Occup. Med. Environ. Health 2014, 27, 293–303. [Google Scholar] [CrossRef]
- Arain, M.; Campbell, M.J.; Cooper, C.L.; Lancaster, G.A. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med. Res. Methodol. 2010, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Thabane, L.; Ma, J.; Chu, R.; Cheng, J.; Ismaila, A.; Rios, L.P.; Robson, R.; Thabane, M.; Giangregorio, L.; Goldsmith, C.H. A tutorial on pilot studies: The what, why and how. BMC Med. Res. Methodol. 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. J. Appl. Stat. Pharm. Ind. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Lancaster, G.A.; Dodd, S.; Williamson, P.R. Design and analysis of pilot studies: Recommendations for good practice. J. Eval. Clin. Pract. 2004, 10, 307–312. [Google Scholar] [CrossRef]
- Keel, J.C.; Smith, M.J.; Wassermann, E.M. A safety screening questionnaire for transcranial magnetic stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2001, 112, 720. [Google Scholar] [CrossRef]
- Australian Radiation Protection and Nuclear Safety Agency (ARPANSA). Environmental Electromagnetic Energy Reports; Australian Radiation Protection and Nuclear Safety Agency (ARPANSA) Source; ARPANSA: Yallambie, VIC, Australia, 2024. Available online: https://www.arpansa.gov.au/research/surveys/environmental-electromagnetic-energy-reports (accessed on 23 June 2025).
- Udupa, K. Transcranial magnetic stimulation in exploring neurophysiology of cortical circuits and potential clinical implications. Indian J. Physiol. Pharmacol. 2021, 64, 244–257. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Pilato, F.; Saturno, E.; Dileone, M.; Mazzone, P.; Insola, A.; Tonali, P.A.; Rothwell, J.C. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin. Neurophysiol. 2004, 115, 255–266. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-H.; Sundman, M.; That, V.T.; Green, J.; Trapani, C. Cortical excitability and plasticity in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis of transcranial magnetic stimulation studies. Ageing Res. Rev. 2022, 79, 101660. [Google Scholar] [CrossRef] [PubMed]
- Thut, G.; Pascual-Leone, A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010, 22, 219–232. [Google Scholar] [CrossRef]
- Vaseghi, B.; Zoghi, M.; Jaberzadeh, S. Inter-pulse interval affects the size of single-pulse TMS-induced motor evoked potentials: A reliability study. Basic Clin. Neurosci. 2015, 6, 44–51. [Google Scholar]
- Farzan, F. Single-pulse transcranial magnetic stimulation (TMS) protocols and outcome measures. In Transcranial Magnetic Stimulation; Springer: Berlin/Heidelberg, Germany, 2014; pp. 69–115. [Google Scholar]
- Bastani, A.; Jaberzadeh, S. A higher number of TMS-elicited MEP from a combined hotspot improves intra-and inter-session reliability of the upper limb muscles in healthy individuals. PLoS ONE 2012, 7, e47582. [Google Scholar] [CrossRef]
- Möller, C.; Arai, N.; Lücke, J.; Ziemann, U. Hysteresis effects on the input–output curve of motor evoked potentials. Clin. Neurophysiol. 2009, 120, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, P.; Säisänen, L.; Danner, N.; Niskanen, E.; Hukkanen, T.; Mervaala, E.; Könönen, M. Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. NeuroImage 2009, 44, 790–795. [Google Scholar] [CrossRef]
- Rawji, V.; Kaczmarczyk, I.; Rocchi, L.; Fong, P.-Y.; Rothwell, J.C.; Sharma, N. Preconditioning stimulus intensity alters paired-pulse TMS evoked potentials. Brain Sci. 2021, 11, 326. [Google Scholar] [CrossRef]
- Silbert, B.; Patterson, H.; Pevcic, D.; Windnagel, K.; Thickbroom, G. A comparison of relative-frequency and threshold-hunting methods to determine stimulus intensity in transcranial magnetic stimulation. Clin. Neurophysiol. 2013, 124, 708–712. [Google Scholar] [CrossRef]
- Awiszus, F. On relative frequency estimation of transcranial magnetic stimulation motor threshold. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2012, 123, 2319–2320. [Google Scholar] [CrossRef] [PubMed]
- Jewett, B.E.; Thapa, B. Physiology, NMDA Receptor. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2022. [Google Scholar]
- Cash, R.F.H.; Noda, Y.; Zomorrodi, R.; Radhu, N.; Farzan, F.; Rajji, T.K.; Fitzgerald, P.B.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Characterization of glutamatergic and GABAA-mediated neurotransmission in motor and dorsolateral prefrontal cortex using paired-pulse TMS–EEG. Neuropsychopharmacology 2017, 42, 502–511. [Google Scholar] [CrossRef]
- Melo, L.; Beaupain, M.C.; Ghanavati, E.; Kuo, M.-F.; Nitsche, M.A. Neurochemical mechanisms underlying serotonergic modulation of neuroplasticity in humans. Brain Stimul. 2024, 17, 421–430. [Google Scholar] [CrossRef]
- Bachtiar, V.; Stagg, C. The role of inhibition in human motor cortical plasticity. Neuroscience 2014, 278, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Mooney, R.A.; Cirillo, J.; Byblow, W.D. GABA and primary motor cortex inhibition in young and older adults: A multimodal reliability study. J. Neurophysiol. 2017, 118, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.J.; MacDonald, H.J.; Cirillo, J.; Byblow, W.D. Proactive modulation of long-interval intracortical inhibition during response inhibition. J. Neurophysiol. 2016, 116, 859–867. [Google Scholar] [CrossRef]
- Zoghi, M.; Hafezi, P.; Amatya, B.; Khan, F.; Galea, M.P. Intracortical circuits in the contralesional primary motor cortex in patients with chronic stroke after botulinum toxin type A injection: Case studies. Front. Hum. Neurosci. 2020, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Werhahn, K.J.; Kunesch, E.; Noachtar, S.; Benecke, R.; Classen, J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J. Physiol. 1999, 517, 591–597. [Google Scholar] [CrossRef]
- Sale, M.V.; Ridding, M.C.; Nordstrom, M.A. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp. Brain Res. 2007, 181, 615–626. [Google Scholar] [CrossRef]
- GraphPad. GraphPad Prism; Version 10.1.2; GraphPad Software: San Diego, CA, USA, 2024. [Google Scholar]
- van den Bergh, D.; van Doorn, J.; Marsman, M.; Draws, T.; van Kesteren, E.-J.; Derks, K.; Dablander, F.; Gronau, Q.F.; Kucharský, Š.; Raj, A. A tutorial on conducting and interpreting a Bayesian ANOVA in JASP. Année Psychol. 2020, 120, 73–91. [Google Scholar] [CrossRef]
- Robert, C.P.; Chopin, N.; Rousseau, J. Harold Jeffreys’s theory of probability revisited. Stat. Sci. 2009, 24, 141–172. [Google Scholar] [CrossRef]
- Kass, R.E.; Raftery, A.E. Bayes factors. J. Am. Stat. Assoc. 1995, 90, 773–795. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; (for Version 29.0.2.0, release year); IBM Corp: Armonk, NY, USA, 2022. [Google Scholar]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- The Kaiser Family Foundation. The US Government and the World Health Organization; The Kaiser Family Foundation: San Francisco, CA, USA, 2025. [Google Scholar]
- Australian Radiation Protection and Nuclear Safety Agency (ARPANSA). Fitzpatrick Skin Phototype. Australian Government. 2006. Available online: https://www.arpansa.gov.au/sites/default/files/legacy/pubs/RadiationProtection/FitzpatrickSkinType.pdf (accessed on 5 June 2019).
- Vogels, T.P.; Sprekeler, H.; Zenke, F.; Clopath, C.; Gerstner, W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 2011, 334, 1569–1573. [Google Scholar] [CrossRef]
- Wenner, P. Mechanisms of GABAergic homeostatic plasticity. Neural Plast. 2011, 2011, 489470. [Google Scholar] [CrossRef]
- Turrigiano, G. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 2012, 4, a005736. [Google Scholar] [CrossRef]
- Hattemer, K.; Knake, S.; Reis, J.; Rochon, J.; Oertel, W.H.; Rosenow, F.; Hamer, H.M. Excitability of the motor cortex during ovulatory and anovulatory cycles: A transcranial magnetic stimulation study. Clin. Endocrinol. 2007, 66, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Inghilleri, M.; Conte, A.; Currà, A.; Frasca, V.; Lorenzano, C.; Berardelli, A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin. Neurophysiol. 2004, 115, 1063–1068. [Google Scholar] [CrossRef]
- Smith, M.J.; Adams, L.F.; Schmidt, P.J.; Rubinow, D.R.; Wassermann, E.M. Effects of ovarian hormones on human cortical excitability. Ann. Neurol. 2002, 51, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, J.B.; Ogston, K.M.; Miles, T.S. Age and sex differences in human motor cortex input–output characteristics. J. Physiol. 2003, 546, 605–613. [Google Scholar] [CrossRef]
- Fujiyama, H.; Hinder, M.R.; Schmidt, M.W.; Garry, M.I.; Summers, J.J. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiol. Aging 2012, 33, 1484.e1–1484.e14. [Google Scholar] [CrossRef]
- Mishra, J.; Rolle, C.; Gazzaley, A. Neural plasticity underlying visual perceptual learning in aging. Brain Res. 2015, 1612, 140–151. [Google Scholar] [CrossRef]
- Ziemann, U.; Hallett, M.; Cohen, L.G. Mechanisms of deafferentation-induced plasticity in human motor cortex. J. Neurosci. 1998, 18, 7000–7007. [Google Scholar] [CrossRef] [PubMed]
- Hanajima, R.; Ugawa, Y.; Terao, Y.; Sakai, K.; Furubayashi, T.; Machii, K.; Kanazawa, I. Paired-pulse magnetic stimulation of the human motor cortex: Differences among I waves. J. Physiol. 1998, 509, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Kelsh, M.A.; Shum, M.; Sheppard, A.R.; Mcneely, M.; Kuster, N.; Lau, E.; Weidling, R.; Fordyce, T.; Kühn, S.; Sulser, C. Measured radiofrequency exposure during various mobile-phone use scenarios. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Jerram, M.; Poldrack, R.; Ahern, T.; Kennedy, D.N.; Seidman, L.J.; Makris, N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J. Neurosci. 2005, 25, 9309–9316. [Google Scholar] [CrossRef]
| (Study) | Participants’ Gender and Age Range (Mean) | Mobile Phone Specifications (Frequency, Power, SAR) | Exposure Time | Study Design (Crossover/Blinding) | TMS Device Specifications | Observed Effects | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Single Blind | Double Blind | CSE | ICF | SICI | LICI | |||||
| Inomata-Teradal., 2007 [49] | 10 (5 M, 5 F) 22–51 Y | 800 MHz/ 270 mW | 30 min | _ | √ | 200 (The Magstim Co., Ltd., Whitland, UK) | _ | ↑ | N/S | N/S |
| Ferreri, F., et al., 2006 [48] | 15 F 20–36 Y | 902.40 MHz/ 2 W | 45 min | _ | √ | 200 (The Magstim Co., Dyfed, UK) | _ | ↑ | ↓ | N/S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torkan, A.; Zoghi, M.; Foroughimehr, N.; Jaberzadeh, S. The Effect of 5G Mobile Phone Electromagnetic Exposure on Corticospinal and Intracortical Excitability in Healthy Adults: A Randomized Controlled Pilot Study. Brain Sci. 2025, 15, 1134. https://doi.org/10.3390/brainsci15111134
Torkan A, Zoghi M, Foroughimehr N, Jaberzadeh S. The Effect of 5G Mobile Phone Electromagnetic Exposure on Corticospinal and Intracortical Excitability in Healthy Adults: A Randomized Controlled Pilot Study. Brain Sciences. 2025; 15(11):1134. https://doi.org/10.3390/brainsci15111134
Chicago/Turabian StyleTorkan, Azadeh, Maryam Zoghi, Negin Foroughimehr, and Shapour Jaberzadeh. 2025. "The Effect of 5G Mobile Phone Electromagnetic Exposure on Corticospinal and Intracortical Excitability in Healthy Adults: A Randomized Controlled Pilot Study" Brain Sciences 15, no. 11: 1134. https://doi.org/10.3390/brainsci15111134
APA StyleTorkan, A., Zoghi, M., Foroughimehr, N., & Jaberzadeh, S. (2025). The Effect of 5G Mobile Phone Electromagnetic Exposure on Corticospinal and Intracortical Excitability in Healthy Adults: A Randomized Controlled Pilot Study. Brain Sciences, 15(11), 1134. https://doi.org/10.3390/brainsci15111134









