Withdrawal-Induced Delirium in Opioid Dependence: A Systematic Review
Abstract
1. Introduction
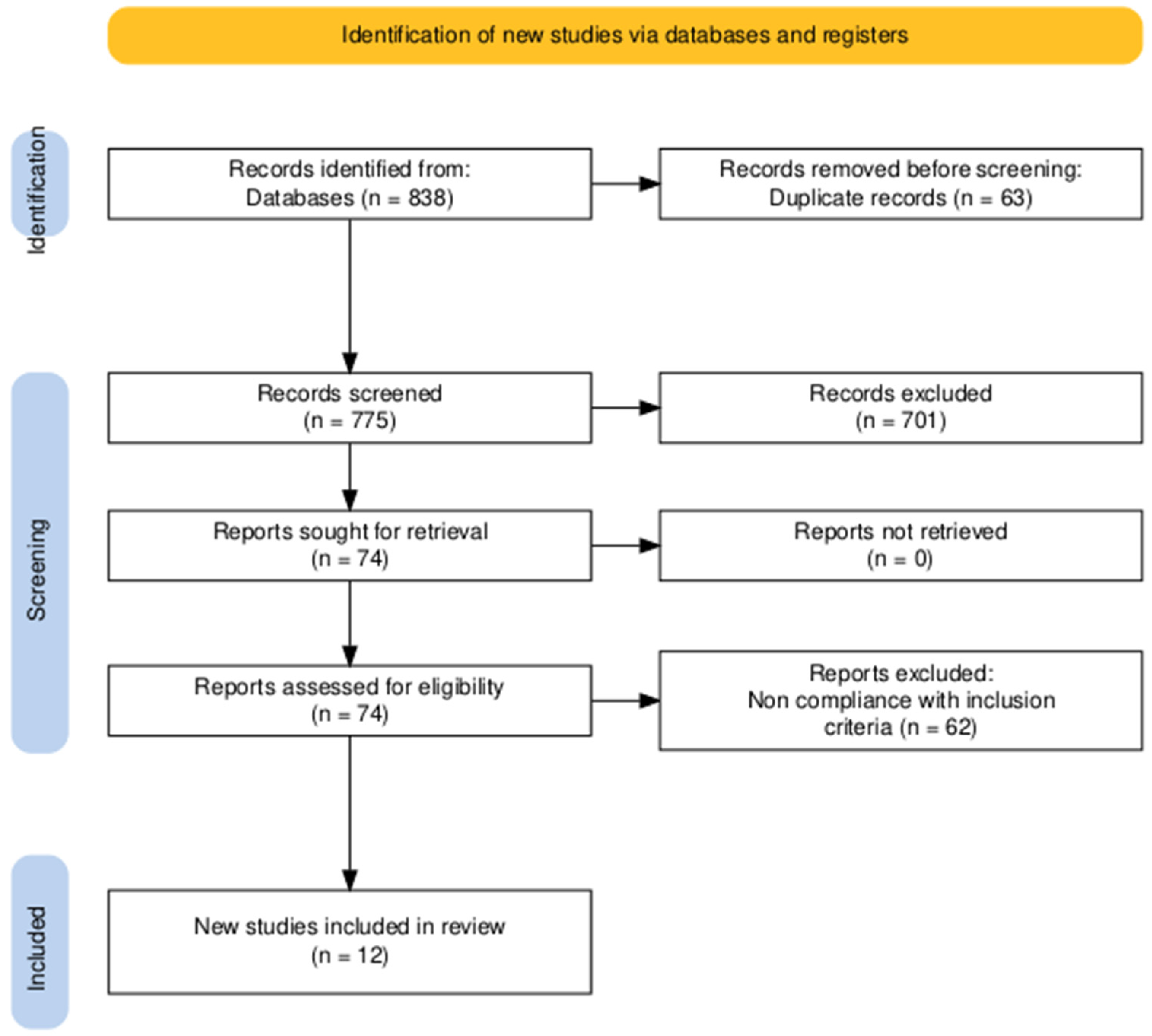
2. Materials and Methods
3. Results
4. Discussion
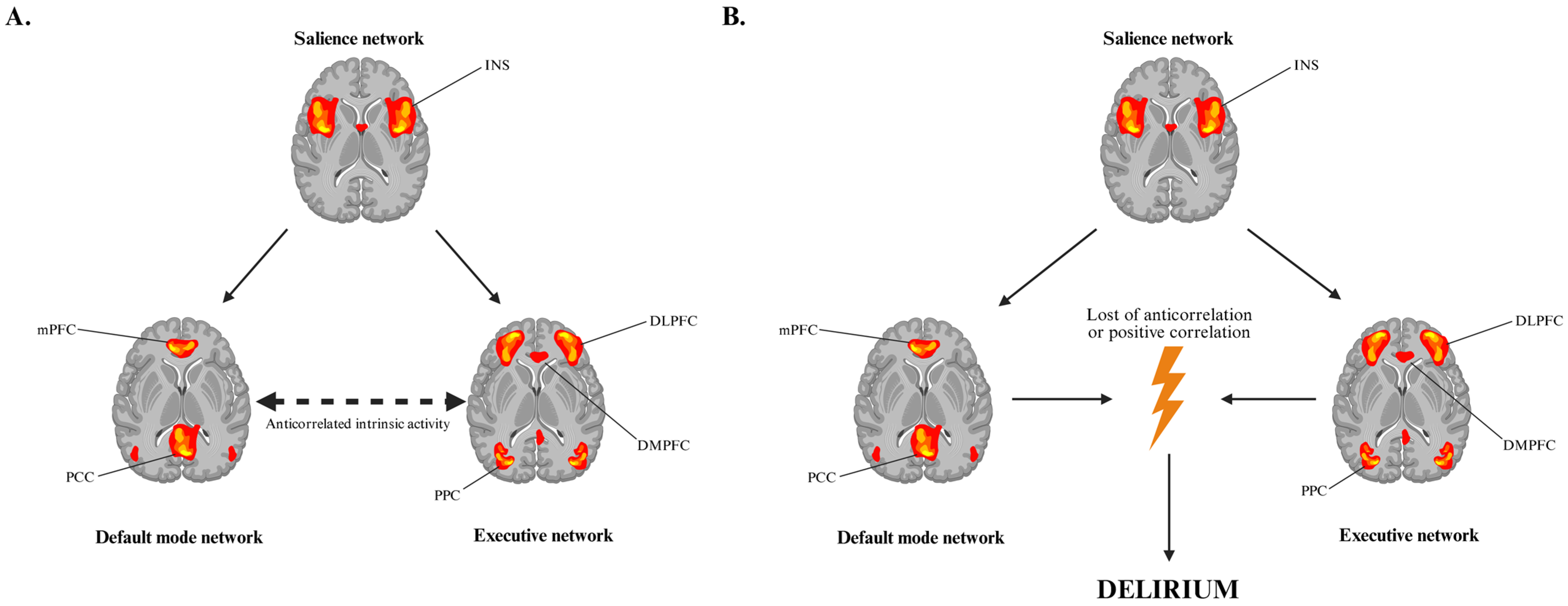
4.1. Neuroadaptive Changes and Network Dysfunction in Opioid Withdrawal-Associated Delirium
4.2. Antagonist-Precipitated Delirium and Rapid Opioid Detoxification (ROD)
4.3. Heterogeneity of Opioid Withdrawal Syndrome (OWS)
4.4. Future Directions: AI-Based Approaches in Opioid Withdrawal Delirium
4.5. Risk of Bias
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres-Lockhart, K.E.; Lu, T.Y.; Weimer, M.B.; Stein, M.R.; Cunningham, C.O. Clinical Management of Opioid Withdrawal. Addiction 2022, 117, 2540–2550. [Google Scholar] [CrossRef]
- Koob, G.F. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol. Psychiatry 2020, 87, 44–53. [Google Scholar] [CrossRef]
- Scavone, J.L.; Sterling, R.C.; Van Bockstaele, E.J. Cannabinoid and Opioid Interactions: Implications for Opiate Dependence and Withdrawal. Neuroscience 2013, 248, 637–654. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R.; Wu, M.-F.; Holmes, B.; Lam, H.A.; Maidment, N.T.; Gera, J.; Yamanaka, A.; Siegel, J.M. Hypocretin/Orexin Interactions with Norepinephrine Contribute to the Opiate Withdrawal Syndrome. J. Neurosci. 2022, 42, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.C.; Gipson, C.D.; Dunn, K.E. Dopamine Supersensitivity: A Novel Hypothesis of Opioid-Induced Neurobiological Mechanisms Underlying Opioid-Stimulant Co-Use and Opioid Relapse. Front. Psychiatry 2022, 13, 835816. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, K.; Ma, T.; Ji, Y.; Lou, Y.; Fu, X.; Lu, Y.; Liu, Y.; Dang, W.; Zhang, Q.; et al. Nucleus Accumbens D1/D2 Circuits Control Opioid Withdrawal Symptoms in Mice. J. Clin. Investig. 2023, 133, e163266. [Google Scholar] [CrossRef]
- Christie, M.J.; Williams, J.T.; Osborne, P.B.; Bellchambers, C.E. Where Is the Locus in Opioid Withdrawal? Trends Pharmacol. Sci. 1997, 18, 134–140. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Raffa, R.B.; Rosenblatt, M.H. Opioid Withdrawal Symptoms, a Consequence of Chronic Opioid Use and Opioid Use Disorder: Current Understanding and Approaches to Management. J. Clin. Pharm. Ther. 2020, 45, 892–903. [Google Scholar] [CrossRef]
- Lovrecic, B.; Lovrecic, M.; Gabrovec, B.; Carli, M.; Pacini, M.; Maremmani, A.G.I.; Maremmani, I. Non-Medical Use of Novel Synthetic Opioids: A New Challenge to Public Health. Int. J. Environ. Res. Public Health 2019, 16, 177. [Google Scholar] [CrossRef]
- World Drug Report. Available online: https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2023.html (accessed on 14 August 2025).
- Fischer, B.; Keates, A.; Bühringer, G.; Reimer, J.; Rehm, J. Non-medical Use of Prescription Opioids and Prescription Opioid-related Harms: Why so Markedly Higher in North America Compared to the Rest of the World? Addiction 2014, 109, 177–181. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; pp. 591–593. [Google Scholar]
- Becker, G.L.; Gerak, L.R.; Koek, W.; France, C.P. Antagonist-Precipitated and Discontinuation-Induced Withdrawal in Morphine-Dependent Rhesus Monkeys. Psychopharmacology 2008, 201, 373–382. [Google Scholar] [CrossRef]
- Kunzler, N.M.; Wightman, R.S.; Nelson, L.S. Opioid Withdrawal Precipitated by Long-Acting Antagonists. J. Emerg. Med. 2020, 58, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.E.; Bird, H.E.; Bergeria, C.L.; Ware, O.D.; Strain, E.C.; Huhn, A.S. Operational Definition of Precipitated Opioid Withdrawal. Front. Psychiatry 2023, 14, 1141980. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.S.; Paul, S. Opioid Withdrawal Delirium without Convulsions: A Rare Case Report. Eur. Psychiatry 2022, 65, S828. [Google Scholar] [CrossRef]
- Pandey, K.; Chauhan, V.S.; Nath, S.; Singh, R. Opioid Withdrawal Manifesting with Delirium. Ind. Psychiatry J. 2024, 33, S303–S304. [Google Scholar] [CrossRef]
- Aggarwal, A.; Choudhary, S.; Jiloha, R.C. Opioid Withdrawal Delirium. J. Neuropsychiatry Clin. Neurosci. 2011, 23, E37. [Google Scholar] [CrossRef]
- Raj, B.N.; Manamohan, N.; Hegde, D.; Huded, C.B.; Pradeep, J. A Rare Case of Complicated Opioid Withdrawal in Delirium Without Convulsions. Indian J. Psychol. Med. 2017, 39, 191–193. [Google Scholar] [CrossRef]
- Sharma, R.C.; Kumar, R.; Sharma, D.D.; Kanwar, P. Opium Withdrawal Delirium: Two Case Reports. Psychopharmacol. Bull. 2017, 47, 48–51. [Google Scholar] [PubMed]
- Das, S.; Sah, D.; Nandi, S.; Das, P. Opioid Withdrawal Presenting as Delirium and Role of Buprenorphine: A Case Series. Indian J. Psychol. Med. 2017, 39, 665–667. [Google Scholar] [CrossRef]
- Aggarwal, K.; Ranjan, R.; Mallik, S.; Salian, H.H.; Shekhar, S. Delirium Secondary to Inadvertent Administration of Naltrexone in Patient with Opioid Dependence Syndrome: A Case Report. East Asian Arch. Psychiatry 2024, 34, 87–88. [Google Scholar] [CrossRef]
- Miles, I.; Kestler, A.; Scheuermeyer, F.X. Diarrhea and Delirium from Naltrexone-Precipitated Opioid Withdrawal. CJEM 2020, 22, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.; Seethalakshmi, R.; Adarkar, S.; Kharawala, S. Is This ‘complicated’ Opioid Withdrawal? Indian J. Psychiatry 2006, 48, 121. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Swetter, S. A Case of Delirium and Rhabdomyolysis in Severe Iatrogenic Opioid Withdrawal. Psychosomatics 2018, 59, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Van Der Mast, R.C. Pathophysiology of Delirium. J. Geriatr. Psychiatry Neurol. 1998, 11, 138–145. [Google Scholar] [CrossRef]
- Maldonado, J.R. Delirium Pathophysiology: An Updated Hypothesis of the Etiology of Acute Brain Failure. Int. J. Geriat. Psychiatry 2018, 33, 1428–1457. [Google Scholar] [CrossRef]
- Sibia, I.; Singh, A.H.; Joshi, R.; Khanduja, D.; Bathla, M. To Evaluate Serum Cortisol Levels in Patients with Alcohol Withdrawal Delirium v/s Patients with Delirium Due to Any Other Disorder. J. Fam. Med. Prim. Care 2023, 12, 986–989. [Google Scholar] [CrossRef]
- Mosharaf, M.P.; Alam, K.; Gow, J.; Mahumud, R.A. Cytokines and Inflammatory Biomarkers and Their Association with Post-Operative Delirium: A Meta-Analysis and Systematic Review. Sci. Rep. 2025, 15, 7830. [Google Scholar] [CrossRef]
- Navratilova, E.; Xie, J.Y.; Okun, A.; Qu, C.; Eyde, N.; Ci, S.; Ossipov, M.H.; King, T.; Fields, H.L.; Porreca, F. Pain Relief Produces Negative Reinforcement through Activation of Mesolimbic Reward–Valuation Circuitry. Proc. Natl. Acad. Sci. USA 2012, 109, 20709–20713. [Google Scholar] [CrossRef]
- Thompson, B.L.; Oscar-Berman, M.; Kaplan, G.B. Opioid-Induced Structural and Functional Plasticity of Medium-Spiny Neurons in the Nucleus Accumbens. Neurosci. Biobehav. Rev. 2021, 120, 417–430. [Google Scholar] [CrossRef]
- Chan, P.; Lutfy, K. Molecular Changes in Opioid Addiction: The Role of Adenylyl Cyclase and cAMP/PKA System. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 203–227. ISBN 978-0-12-803786-7. [Google Scholar]
- Jensen, K.P.; DeVito, E.E.; Yip, S.; Carroll, K.M.; Sofuoglu, M. The Cholinergic System as a Treatment Target for Opioid Use Disorder. CNS Drugs 2018, 32, 981–996. [Google Scholar] [CrossRef]
- Li, C.; McElroy, B.D.; Phillips, J.; McCloskey, N.S.; Shi, X.; Unterwald, E.M.; Kirby, L.G. Role of A1-GABAA Receptors in the Serotonergic Dorsal Raphe Nucleus in Models of Opioid Reward, Anxiety, and Depression. J. Psychopharmacol. 2024, 38, 188–199. [Google Scholar] [CrossRef]
- Monroe, S.C.; Radke, A.K. Opioid Withdrawal: Role in Addiction and Neural Mechanisms. Psychopharmacology 2023, 240, 1417–1433. [Google Scholar] [CrossRef]
- Świderski, N.; Rodek, P.; Kucia, K. Methylphenidate as a Novel Adjunct in Opioid-Taking Patients: Insights into Dopaminergic Neuroadaptation and Hypoactive Delirium. Brain Sci. 2025, 15, 850. [Google Scholar] [CrossRef]
- Ciucă Anghel, D.-M.; Nițescu, G.V.; Tiron, A.-T.; Guțu, C.M.; Baconi, D.L. Understanding the Mechanisms of Action and Effects of Drugs of Abuse. Molecules 2023, 28, 4969. [Google Scholar] [CrossRef]
- Matinfar, M.; Esfahani, M.; Aslany, N.; Davoodi, S.R.; Parsaei, P.; Zarei, G.; Reisi, P. Effect of Repeated Morphine Withdrawal on Spatial Learning, Memory and Serum Cortisol Level in Mice. Adv. Biomed. Res. 2013, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Thomas, P.; White, J.; Mcgregor, C.; Danz, C.; Gowing, L.; Stegink, A.; Athanasos, P. Antagonist-precipitated Heroin Withdrawal under Anaesthetic Prior to Maintenance Naltrexone Treatment: Determinants of Withdrawal Severity. Drug Alcohol Rev. 2003, 22, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.L.; Gerak, L.R.; Li, J.-X.; Koek, W.; France, C.P. Precipitated and Conditioned Withdrawal in Morphine-Treated Rats. Psychopharmacology 2010, 209, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The Human Brain Is Intrinsically Organized into Dynamic, Anticorrelated Functional Networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Curtis, C.E.; D’Esposito, M. Persistent Activity in the Prefrontal Cortex during Working Memory. Trends Cogn. Sci. 2003, 7, 415–423. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Ullsperger, M.; Crone, E.A.; Nieuwenhuis, S. The Role of the Medial Frontal Cortex in Cognitive Control. Science 2004, 306, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Rogers, R.D.; Passingham, R.E. Dissociating Response Selection and Conflict in the Medial Frontal Surface. NeuroImage 2006, 29, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Lee, H.; Chung, T.-S.; Park, K.-M.; Jung, Y.-C.; Kim, S.I.; Kim, J.-J. Neural Network Functional Connectivity During and After an Episode of Delirium. Am. J. Psychiatry 2012, 169, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.M. The Ascending Reticular Activating System. Neurocrit. Care 2019, 31, 419–422. [Google Scholar] [CrossRef]
- Figiel, G.S.; Krishnan, K.R.; Breitner, J.C.; Nemeroff, C.B. Radiologic Correlates of Antidepressant-Induced Delirium: The Possible Significance of Basal-Ganglia Lesions. J. Neuropsychiatry Clin. Neurosci. 1989, 1, 188–190. [Google Scholar] [CrossRef]
- Davis, J.P.; Eddie, D.; Prindle, J.; Dworkin, E.R.; Christie, N.C.; Saba, S.; DiGuiseppi, G.T.; Clapp, J.D.; Kelly, J.F. Sex Differences in Factors Predicting Post-treatment Opioid Use. Addiction 2021, 116, 2116–2126. [Google Scholar] [CrossRef]
- Chiu, A.W.; Contreras, S.; Mehta, S.; Korman, J.; Perreault, M.M.; Williamson, D.R.; Burry, L.D. Iatrogenic Opioid Withdrawal in Critically Ill Patients: A Review of Assessment Tools and Management. Ann. Pharmacother. 2017, 51, 1099–1111. [Google Scholar] [CrossRef]
- Fishbain, D.; Rosomoff, H.; Cutler, R.; Rosomoff, R.S. Opiate Detoxification Protocols: A Clinical Manual. Ann. Clin. Psychiatry 1993, 5, 53–65. [Google Scholar] [CrossRef]
- Van Dorp, E.L.; Yassen, A.; Dahan, A. Naloxone Treatment in Opioid Addiction: The Risks and Benefits. Expert Opin. Drug Saf. 2007, 6, 125–132. [Google Scholar] [CrossRef]
- Fishbain, D.A. Opioid Tapering/Detoxification Protocols, A Compendium: Narrative Review. Pain Med. 2021, 22, 1676–1697. [Google Scholar] [CrossRef]
- Golden, S.A.; Sakhrani, D.L. Unexpected Delirium During Rapid Opioid Detoxification (ROD). J. Addict. Dis. 2004, 23, 65–75. [Google Scholar] [CrossRef]
- Bell, J.R.; Young, M.R.; Masterman, S.C.; Mattick, R.P.; Bammer, G. A Pilot Study of Naltrexone-accelerated Detoxification in Opioid Dependence. Med. J. Aust. 1999, 171, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Safari, F.; Mohajerani, S.A.; Hashemian, M.; Kolahi, A.-A.; Mottaghi, K. Long-Term Relapse of Ultra-Rapid Opioid Detoxification. J. Addict. Dis. 2014, 33, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Streel, E.; Verbanck, P. Ultra-rapid Opiate Detoxification: From Clinical Applications to Basic Science. Addict. Biol. 2003, 8, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Saari, T.I.; Strang, J.; Dale, O. Clinical Pharmacokinetics and Pharmacodynamics of Naloxone. Clin. Pharmacokinet. 2024, 63, 397–422. [Google Scholar] [CrossRef]
- Bodnar, R.J. Supraspinal Circuitry Mediating Opioid Antinociception: Antagonist and Synergy Studies in Multiple Sites. J. Biomed. Sci. 2000, 7, 181–194. [Google Scholar] [CrossRef]
- Schulteis, G.; Chiang, D.; Archer, C. Relative Potency of the Opioid Antagonists Naloxone and 6-Alpha-Naloxol to Precipitate Withdrawal from Acute Morphine Dependence Varies with Time Post-Antagonist. Pharmacol. Biochem. Behav. 2009, 92, 157–163. [Google Scholar] [CrossRef]
- Margolis, E.B.; Lock, H.; Chefer, V.I.; Shippenberg, T.S.; Hjelmstad, G.O.; Fields, H.L. κ Opioids Selectively Control Dopaminergic Neurons Projecting to the Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 2938–2942. [Google Scholar] [CrossRef]
- Estave, P.M.; Spodnick, M.B.; Karkhanis, A.N. KOR Control over Addiction Processing: An Exploration of the Mesolimbic Dopamine Pathway. In The Kappa Opioid Receptor; Liu-Chen, L.-Y., Inan, S., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2020; Volume 271, pp. 351–377. ISBN 978-3-030-89073-5. [Google Scholar]
- Escobar, A.D.P.; Casanova, J.P.; Andrés, M.E.; Fuentealba, J.A. Crosstalk Between Kappa Opioid and Dopamine Systems in Compulsive Behaviors. Front. Pharmacol. 2020, 11, 57. [Google Scholar] [CrossRef]
- Buchta, W.C.; Riegel, A.C. Chronic Cocaine Disrupts Mesocortical Learning Mechanisms. Brain Res. 2015, 1628, 88–103. [Google Scholar] [CrossRef]
- Lapish, C.C.; Kroener, S.; Durstewitz, D.; Lavin, A.; Seamans, J.K. The Ability of the Mesocortical Dopamine System to Operate in Distinct Temporal Modes. Psychopharmacology 2007, 191, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, G. A Mesocortical Glutamatergic Pathway Modulates Neuropathic Pain Independent of Dopamine Co-Release. Nat. Commun. 2024, 15, 643. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, P.; Gottheil, E.; Van Bockstaele, E.J. Antagonist Treatment of Opioid Withdrawal: Translational Low Dose Approach. J. Addict. Dis. 2006, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hyun, D.; Huh, J.W.; Hong, S.-B.; Koh, Y.; Lim, C.-M. Iatrogenic Opioid Withdrawal Syndrome in Critically Ill Patients: A Retrospective Cohort Study. J. Korean Med. Sci. 2020, 35, e106. [Google Scholar] [CrossRef]
- Bluthenthal, R.N.; Simpson, K.; Ceasar, R.C.; Zhao, J.; Wenger, L.; Kral, A.H. Opioid Withdrawal Symptoms, Frequency, and Pain Characteristics as Correlates of Health Risk among People Who Inject Drugs. Drug Alcohol Depend. 2020, 211, 107932. [Google Scholar] [CrossRef]
- Kaplan, G.B.; Thompson, B.L. Neuroplasticity of the Extended Amygdala in Opioid Withdrawal and Prolonged Opioid Abstinence. Front. Pharmacol. 2023, 14, 1253736. [Google Scholar] [CrossRef]
- Lozano-López, M.T.; Gamonal-Limcaoco, S.; Casado-Espada, N.; Aguilar, L.; Vicente-Hernández, B.; Grau-López, L.; Álvarez-Navares, A.; Roncero, C. Psychosis after Buprenorphine, Heroin, Methadone, Morphine, Oxycodone, and Tramadol Withdrawal: A Systematic Review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4554–4562. [Google Scholar] [CrossRef]
- Lay, S.; Nguyen, L.L.; Sangani, A. Catatonia and Opioid Withdrawal: A Case Report. Cureus 2024, 16, e56396. [Google Scholar] [CrossRef]
- White, W.; White, I.M. Amphetamine and Morphine May Produce Acute-Withdrawal Related Hypoactivity by Initially Activating a Common Dopamine Pathway. Physiol. Behav. 2016, 165, 187–194. [Google Scholar] [CrossRef]
- Sayles, B.D.; Royer, E.G.; Karre, V.M.M.; Balasanova, A.A. Malignant Catatonia Induced by Concurrent Opioid and Benzodiazepine Withdrawal. Prim. Care Companion CNS Disord. 2023, 25, 22cr03346. [Google Scholar] [CrossRef]
- Abbasi, B.; Elyasi, F.; Heydari, F.; Ghasemian, R. Benzodiazepine and Opioid Withdrawal Induced Neuroleptic Malignant Like Syndrome: A Clinical Case Report With Management Complexities. Neuropsychopharm. Rep. 2025, 45, e70020. [Google Scholar] [CrossRef]
- Shah, D.B.; Fichadia, P.A.; Shah, F.H.; Patel, S.S.; Jain, I.N. Acute Psychotic Episode Precipitated by Opioid Withdrawal in a Case of Bipolar I Disorder. Cureus 2023, 5, e45538. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.R.; Ling, W. The Clinical Opiate Withdrawal Scale (COWS). J. Psychoact. Drugs 2003, 35, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.D.; Wilson, B.L.; Jurkovitz, C.T.; Melson, J.A.; Reitz, J.A.; Pal, C.K.; Hausman, S.P.; Booker, E.; Lang, L.J.; Horton, T.L. Implementation of a Clinical Pathway to Screen and Treat Medical Inpatients for Opioid Withdrawal. Implement. Res. Pract. 2022, 3, 26334895221096290. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Van Dyck, C.H.; Alessi, C.A.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying Confusion: The Confusion Assessment Method: A New Method for Detection of Delirium. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef]
- Gusmao-Flores, D.; Salluh, J.I.F.; Chalhub, R.Á.; Quarantini, L.C. The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and Intensive Care Delirium Screening Checklist (ICDSC) for the Diagnosis of Delirium: A Systematic Review and Meta-Analysis of Clinical Studies. Crit. Care 2012, 16, R115. [Google Scholar] [CrossRef]
- Avelino-Silva, T.J.; Bittencourt, J.A.S.; Miguel, C.G.; Rozzino, T.P.D.C.; Vaccari, A.M.H.; Barbosa, M.S.; Szlejf, C. The Confusion Assessment Method in Action: Implementation of a Protocol to Increase Delirium Screening and Diagnosis. Geriatr. Nurs. 2023, 54, 32–36. [Google Scholar] [CrossRef]
- Chen, T.-J.; Chung, Y.-W.; Chang, H.-C.; Chen, P.-Y.; Wu, C.-R.; Hsieh, S.-H.; Chiu, H.-Y. Diagnostic Accuracy of the CAM-ICU and ICDSC in Detecting Intensive Care Unit Delirium: A Bivariate Meta-Analysis. Int. J. Nurs. Studies 2021, 113, 103782. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in Elderly People. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Zhao, H.; Ying, H.-L.; Zhang, C.; Zhang, S. Relative Hypoglycemia Is Associated with Delirium in Critically Ill Patients with Diabetes: A Cohort Study. Diabetes Metab. Syndr. Obes. 2022, 15, 3339–3346. [Google Scholar] [CrossRef]
- Feinkohl, I.; Janke, J.; Slooter, A.J.C.; Winterer, G.; Spies, C.; Pischon, T. Metabolic Syndrome and the Risk of Postoperative Delirium and Postoperative Cognitive Dysfunction: A Multi-Centre Cohort Study. Br. J. Anaesth. 2023, 131, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Krinitski, D.; Kasina, R.; Klöppel, S.; Lenouvel, E. Associations of Delirium with Urinary Tract Infections and Asymptomatic Bacteriuria in Adults Aged 65 and Older: A Systematic Review and Meta-analysis. J. Am. Geriatr. Soc. 2021, 69, 3312–3323. [Google Scholar] [CrossRef] [PubMed]
- Rebora, P.; Rozzini, R.; Bianchetti, A.; Blangiardo, P.; Marchegiani, A.; Piazzoli, A.; Mazzeo, F.; Cesaroni, G.; Chizzoli, A.; Guerini, F.; et al. Delirium in Patients with SARS-CoV-2 Infection: A Multicenter Study. J. Am. Geriatr. Soc. 2021, 69, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Mossello, E.; Rivasi, G.; Tortù, V.; Giordano, A.; Iacomelli, I.; Cavallini, M.C.; Rafanelli, M.; Ceccofiglio, A.; Cartei, A.; Rostagno, C.; et al. Renal Function and Delirium in Older Fracture Patients: Different Information from Different Formulas? Eur. J. Intern. Med. 2020, 71, 70–75. [Google Scholar] [CrossRef]
- Prendergast, N.T.; Tiberio, P.J.; Girard, T.D. Treatment of Delirium During Critical Illness. Annu. Rev. Med. 2022, 73, 407–421. [Google Scholar] [CrossRef]
- Devlin, J.W.; Peelen, L.M.; Slooter, A. Benzodiazepine-Associated Delirium: Further Considerations. Intens. Care Med. 2016, 42, 1517–1518. [Google Scholar] [CrossRef]
- Aono-Setoguchi, H.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Factors Associated with Intensive Care Unit Delirium in Patients with Acute Myocardial Infarction. Heart Vessel. 2023, 38, 478–487. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Y.; Ding, Y.; Ni, J.; Zhu, B.; Shen, J.; Miao, L. The Role of Hormones in the Pathogenesis and Treatment Mechanisms of Delirium in ICU: The Past, the Present, and the Future. J. Steroid Biochem. Mol. Biol. 2023, 233, 106356. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, X.; Pei, J.; Wu, Y.; Guo, Q.; Su, Y.; Yan, H.; Nan, R.; Chen, H.; Dou, X. Machine Learning–Based Prediction Models for Delirium: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2022, 23, 1655–1668.e6. [Google Scholar] [CrossRef]
- Contreras, M.; Kapoor, S.; Zhang, J.; Davidson, A.; Ren, Y.; Guan, Z.; Ozrazgat-Baslanti, T.; Sena, J.; Nerella, S.; Bihorac, A.; et al. DeLLiriuM: A Large Language Model for Delirium Prediction in the ICU Using Structured HER. arXiv 2024, arXiv:2410.17363. [Google Scholar]
- Sun, H.; Kimchi, E.; Akeju, O.; Nagaraj, S.B.; McClain, L.M.; Zhou, D.W.; Boyle, E.; Zheng, W.-L.; Ge, W.; Westover, M.B. Automated Tracking of Level of Consciousness and Delirium in Critical Illness Using Deep Learning. Npj Digit. Med. 2019, 2, 89. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Cecil, A.; Sena, J.; Davidson, A.; Guan, Z.; Nerella, S.; Zhang, J.; Khezeli, K.; Armfield, B.; Bihorac, A.; et al. Predicting Risk of Delirium from Ambient Noise and Light Information in the ICU. arXiv 2023, arXiv:2303.06253. [Google Scholar] [CrossRef]


| Author(s) (Year) | Age/Sex | Opioid | Cessation Sequence | Onset Time of Delirium | Clinical Presentation | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|
| Singh & Parker (2022) [16] | 20/M | Non specified chronic opioid use | Opioid withdrawal (in general) | 2 days | Psychotic manifestations (delusions, auditory pseudohallucinations), agitation, prosopagnosia, sleep disturbances | 10 mg/day lorazepam, 10 mg/day haloperidol, discharged on 50 mg naltrexone per day | Complete resolution |
| Pandey et al. (2024) [17] | 23/M | Heroin | Opioid withdrawal (in general) | 5 days | Severe agitation, sleep disturbances, disturbance of consciousness | 2 mg/h midazolam, 32 μg/h dexmedetomidine, discharged on 50 mg naltrexone per day | Complete resolution in 24 h |
| Aggarwal et al. (2011) [18] | 23/M | Heroin | Opioid withdrawal (in general) | 4 days | Fully disoriented, agitation, prosopagnosia, irrelevant speech, sleep disturbances | 200 mg/day tramadol, 3 mg/day clonazepam, discharged on 50 mg naltrexone per day | Complete resolution in 2 weeks |
| Raj et al. (2017) [19] | 25/M | Codeine and heroine | Opioid withdrawal (in general) | 7 days | Psychotic manifestations (delusions, multimodal hallucinations), agitation, prosopagnosia, irrelevant speech, sleep disturbances | Initiatively 5 mg haloperidol, 4 mg lorazepam, 0.1 mg clonidine, 15 mg diazepam, then 1.5 mg/day clonidine and 8 mg/day lorazepam, discharged on 50 mg naltrexone per day | Complete resolution in 7 days, lapsed 3 times and was readmitted with similar presentation |
| Sharma et al. (2017) [20] | 56/M | Opium | Detoxification protocol | 2 days | Severe agitation, irrelevant speech, sleep disturbances | Initiatively 8 mg lorazepam, 5 mg haloperidol, 50 mg promethazine, then 300 mg/day tramadol and 1 mg/day clonazepam, discharged on 50 mg/day naltrexone and 50 mg/day quetiapine | Complete resolution in 58 h |
| Sharma et al. (2017) [20] | 38/M | Opium | Opioid withdrawal (in general) | 2 days | Confusion, agitation, stereotypical movements, sleep disturbances | 200 mg/day tramadol, 1.5 mg/day risperidone, 3 mg/day clonazepam, discharged on 50 mg/day quetiapine | Complete resolution in 48 h |
| Das et al. (2017) [21] | 26/M | Heroin | Substitution therapy | 5 days | Confusion, agitation, disorientation, sleep disturbances | 8 mg/day buprenorphine, 2 mg/day naloxone | Complete resolution in 48–72 h |
| Das et al. (2017) [21] | 29/M | Heroin | Substitution therapy | 4 days | Confusion, agitation, disorientation, sleep disturbances | 8 mg/day buprenorphine, 2 mg/day naloxone | Complete resolution in 48–72 h |
| Aggarwal et al. (2024) [22] | 24/M | Non specified chronic opioid use | Precipitated opioid withdrawal | Same day | Psychotic manifestations (delusion, visual hallucinations), irrelevant speech, agitation, disorientation | Initiatively 70 mg diazepam, 17.5 mg haloperidol, then 15 mg/day diazepam and 1.5 mg/day clonidine | Complete resolution in 7 days |
| Miles et al. (2020) [23] | 45/M | Non specified chronic opioid use | Precipitated opioid withdrawal | Same day | Severe agitation | 2 mg lorazepam | Complete resolution in 2 days |
| Parkar et al. (2006) [24] | 4 patients aged 20–38 years | Opium | Opioid withdrawal (in general) | 3–7 days | Not specified, the diagnosis of delirium made by the attending physician | Not specified | Complete resolution |
| Hanna & Swetter (2018) [25] | 70/M | Chronic alcohol and opioid use | Precipitated opioid withdrawal | 1 day | Severe agitation, disorientation | 1 mg clonidine, 2 mg lorazepam, methadone | Complete resolution in 7 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świderski, N.; Rodek, P.; Kucia, K. Withdrawal-Induced Delirium in Opioid Dependence: A Systematic Review. Brain Sci. 2025, 15, 1118. https://doi.org/10.3390/brainsci15101118
Świderski N, Rodek P, Kucia K. Withdrawal-Induced Delirium in Opioid Dependence: A Systematic Review. Brain Sciences. 2025; 15(10):1118. https://doi.org/10.3390/brainsci15101118
Chicago/Turabian StyleŚwiderski, Nikodem, Patryk Rodek, and Krzysztof Kucia. 2025. "Withdrawal-Induced Delirium in Opioid Dependence: A Systematic Review" Brain Sciences 15, no. 10: 1118. https://doi.org/10.3390/brainsci15101118
APA StyleŚwiderski, N., Rodek, P., & Kucia, K. (2025). Withdrawal-Induced Delirium in Opioid Dependence: A Systematic Review. Brain Sciences, 15(10), 1118. https://doi.org/10.3390/brainsci15101118








