Multimodal Neuroprotection in Ischemic Stroke: Emerging Non-Pharmacological Interventions from Bench to Bedside
Abstract
1. Introduction
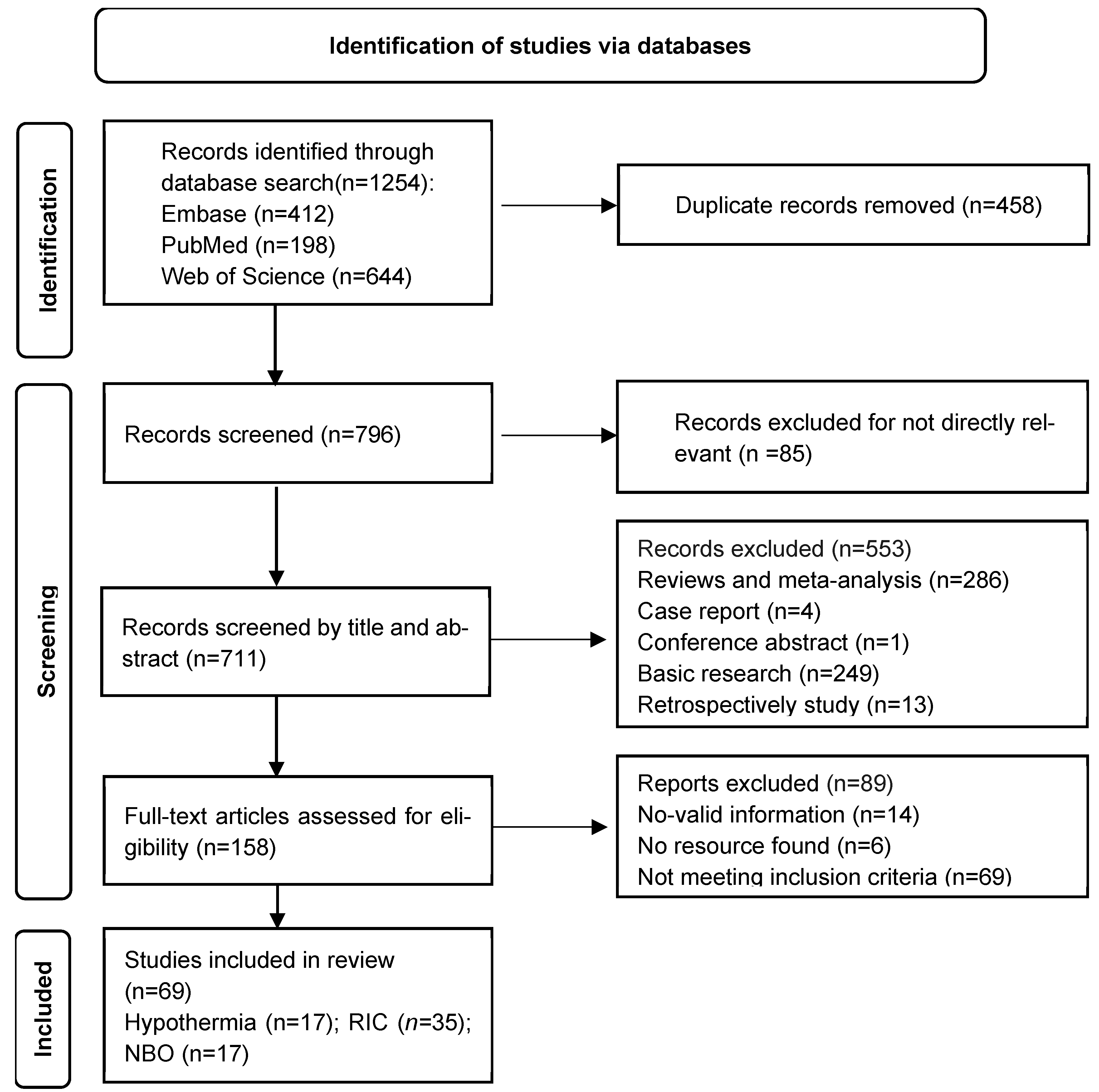
2. Methods
2.1. Search Strategy
2.2. Selection Process
2.3. Assessment of Bias
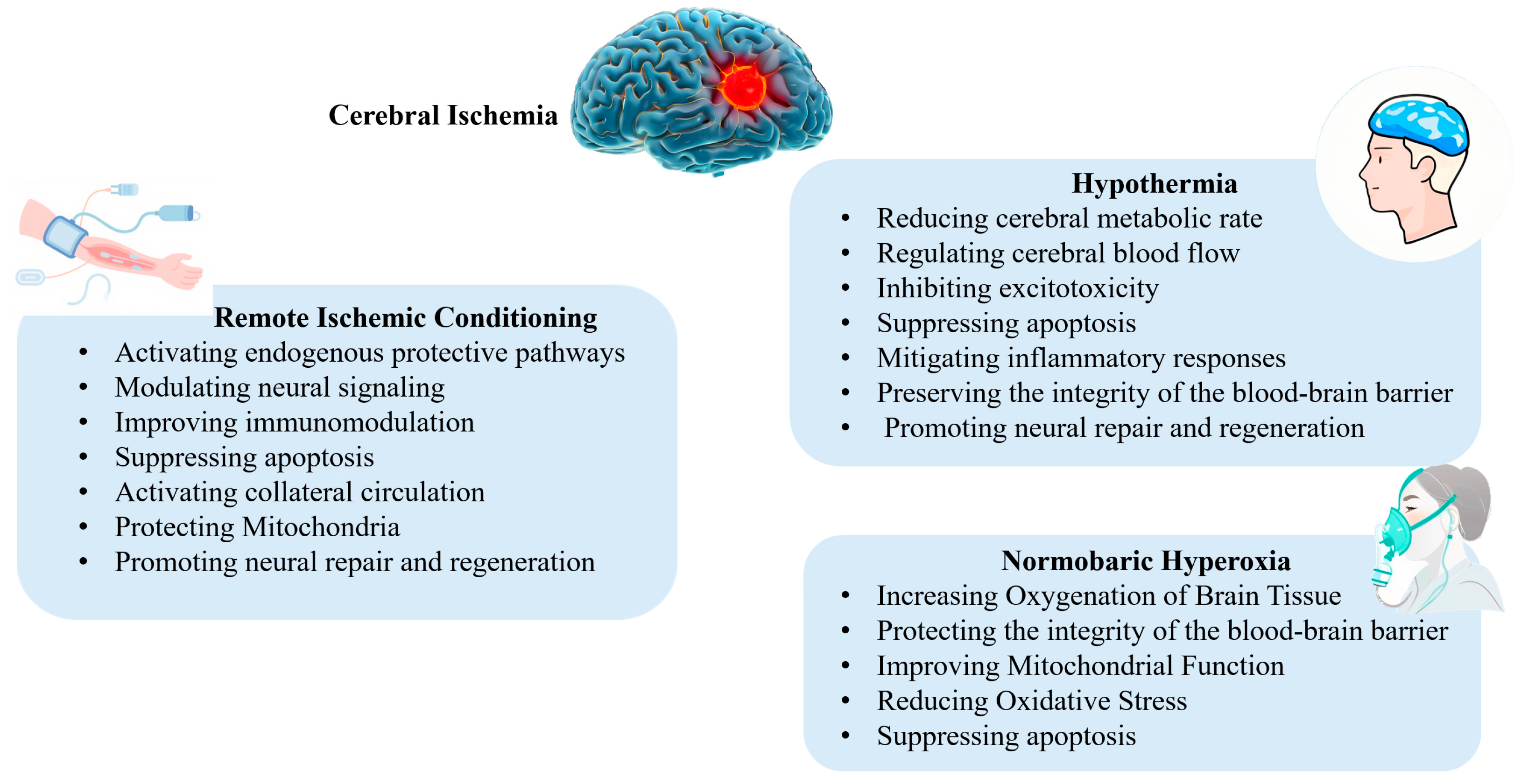
3. Hypothermia
3.1. Mechanisms of Hypothermia-Induced Neuroprotection
3.2. Methods of Hypothermia Induction
3.3. Induced Hypothermia and Synergistic Drug Strategies: Mechanistic Insights and Precision Modulation
3.4. Factors That Influence the Efficacy of Hypothermia
4. Remote Ischemic Conditioning
4.1. Neuroprotective Mechanisms of Remote Ischemic Conditioning
4.2. Research Progress on Remote Ischemic Conditioning in Neuroprotection Against Stroke
4.2.1. Preclinical Studies on RIC
4.2.2. Clinical Studies on RIC
| Study | Study Type | Sample Size | Type of Patients | Treatment | Primary Outcome | Main Results |
|---|---|---|---|---|---|---|
| RESCUE BRAIN (NCT02189928) | multi-center prospective RCT | 188 | Patients of AIS within 6 h of symptom onset | 4 cycles of 5 min of cuff inflation and deflation on the thigh of the unaffected side Cuff pressure: 110 mmHg above systolic pressure Times: Once prehospital | Brain MRI changes of DWI brain infarction volume between baseline and day 1 | RIC cannot limit brain infarction volume growth at 24 h after symptom onset. |
| RESIST (NCT03481777 ) | multicenter prospective RCT | 1500 | patients with prehospital stroke symptoms for less than 4 h | 5 cycles of 5 min of cuff inflation and deflation on 1 upper extremity Cuff pressure: 200 mm Hg or 35 mmHg higher than the systolic blood pressure Times: once prehospital and twice daily for 7 days in hospital | mRS at 3 months | RIC did not significantly improve functional outcome at 90 days among patients with acute stroke. |
| RICAMIS (NCT03740971) | multicenter prospective RCT | 1893 | patients with acute moderate ischemic stroke | 5 cycles of cuff inflation for 5 min and deflation for 5 min to the bilateral upper limbs Cuff pressure: 200 mm Hg Times: within 48 h after symptom onset; twice daily for 10 to 14 days | mRS at 3 months | RIC was safe and significantly increased the likelihood of excellent neurologic function at 90 days. |
| RICA (NCT02534545) | multicenter prospective RCT | 3033 | patients aged 40–80 years with ischemic stroke or transient ischemic attack attributable to angiographically verified 50–99% stenosis of a major intracranial artery | 5 cycles of cuff inflation for 5 min and deflation for 5 min to the bilateral upper limbs Cuff pressure: 200 mm Hg Times: once per day for the first 12 months after randomization | The time from randomization to the first occurrence of fatal or non-fatal ischemic stroke. | RIC did not reduce the risk of ischemic stroke in patients with symptomatic ICAS. |
| SERIC-IVT (NCT04980625) | multicenter prospective RCT | 558 | patients with acute ischemic stroke who underwent IVT | 5 cycles of cuff inflation for 5 min and deflation for 5 min to the unilateral upper limb of the unaffected side Cuff pressure: 200 mm Hg Times: twice daily for 7 days | mRS at 3 months | RIC was safe in patients with acute ischemic stroke who received IVT. However, it did not significantly improve excellent functional outcome. |
5. Normobaric Hyperoxia
5.1. Mechanisms of NBO
5.1.1. Increasing Oxygenation of Brain Tissue
5.1.2. Protecting the Integrity of the Blood–Brain Barrier (BBB)
5.1.3. Improving Mitochondrial Function
5.1.4. Reducing Oxidative Stress and Inhibiting Apoptosis
5.2. Advances in Clinical Research on NBO
5.2.1. NBO Monotherapy
5.2.2. Combination of NBO with Reperfusion Therapy
NBO Combined with Intravenous Thrombolysis
NBO Combined with Endovascular Therapy (EVT)
5.3. Factors Influencing NBO Efficacy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Acute Ischemic Stroke |
| RIC | Remote Ischemic Conditioning |
| NBO | Normobaric Hyperoxia |
| FDA | Food and Drug Administration |
| FAS ligand | FASL |
| iASPP | inhibitor of Apoptosis-Stimulating Protein of p53 |
| IACI | Intra-Arterial local Cooling |
| IA-CSI | Intra-Arterial Cold Saline Infusion |
| MT | Mechanical Thrombectomy |
| MgSO4 | Magnesium Sulfate |
| rtPA | Recombinant Tissue-type Plasminogen Activator |
| MCAO | Middle Cerebral Artery Occlusion |
| LRIC | Limb Remote Ischemic Conditioning |
| IVT | Intravenous Thrombolysis |
| ICAS | Intracranial Atherosclerotic Stenosis |
| BBB | Blood–Brain Barrier |
| EVT | Endovascular Treatment |
| MRI | Magnetic Resonance Imaging |
| ROS | Reactive Oxygen Species |
| mRS | modified Rankin Scale |
| RCTs | Randomized Controlled Trials |
| MMP-9 | Matrix Metalloproteinase-9 |
| CBF | Cerebral Blood Flow |
| NF-κB | Nuclear Factor κB |
References
- Global, regional, and national burden of stroke and its risk factors, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol 2024, 23, 973–1003. [CrossRef]
- Zaidat, O.O.; Lazzaro, M.A.; Liebeskind, D.S.; Janjua, N.; Wechsler, L.; Nogueira, R.G.; Edgell, R.C.; Kalia, J.S.; Badruddin, A.; English, J.; et al. Revascularization grading in endovascular acute ischemic stroke therapy. Neurology 2012, 79, S110–S116. [Google Scholar] [CrossRef]
- Ospel, J.M.; Singh, N.; Almekhlafi, M.A.; Menon, B.K.; Butt, A.; Poppe, A.Y.; Jadhav, A.; Silver, F.L.; Shah, R.; Dowlatshahi, D.; et al. Early Recanalization With Alteplase in Stroke Because of Large Vessel Occlusion in the ESCAPE Trial. Stroke 2021, 52, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, A.K.; Thrift, A.G.; Sturm, J.W.; Dewey, H.M.; Macdonell, R.A.; Donnan, G.A. Stroke units, tissue plasminogen activator, aspirin and neuroprotection: Which stroke intervention could provide the greatest community benefit? Cerebrovasc. Dis. 2005, 20, 239–244. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef]
- Minnerup, J.; Sutherland, B.A.; Buchan, A.M.; Kleinschnitz, C. Neuroprotection for stroke: Current status and future perspectives. Int. J. Mol. Sci. 2012, 13, 11753–11772. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Refocusing Neuroprotection in Cerebral Reperfusion Era: New Challenges and Strategies. Front. Neurol. 2018, 9, 249. [Google Scholar] [CrossRef]
- Hougaard, K.D.; Hjort, N.; Zeidler, D.; Sørensen, L.; Nørgaard, A.; Hansen, T.M.; von Weitzel-Mudersbach, P.; Simonsen, C.Z.; Damgaard, D.; Gottrup, H.; et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: A randomized trial. Stroke 2014, 45, 159–167. [Google Scholar] [CrossRef]
- Li, W.; Qi, Z.; Ma, Q.; Ding, J.; Wu, C.; Song, H.; Yang, Q.; Duan, J.; Liu, L.; Kang, H.; et al. Normobaric Hyperoxia Combined With Endovascular Treatment for Patients With Acute Ischemic Stroke: A Randomized Controlled Clinical Trial. Neurology 2022, 99, e824–e834. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, W.; An, H.; Wu, L.; Chen, J.; Hussain, M.; Ding, Y.; Li, C.; Wei, W.; Duan, J.; et al. Safety, feasibility, and potential efficacy of intraarterial selective cooling infusion for stroke patients treated with mechanical thrombectomy. J. Cereb. Blood Flow. Metab. 2018, 38, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, J.; Liu, G.; Li, S.; Ding, Y.; Ji, X.; Zhao, W. Multi-Target and Multi-Phase Adjunctive Cerebral Protection for Acute Ischemic Stroke in the Reperfusion Era. Biomolecules 2024, 14, 1181. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Choi, H.A.; Badjatia, N.; Mayer, S.A. Hypothermia for acute brain injury--mechanisms and practical aspects. Nat. Rev. Neurol. 2012, 8, 214–222. [Google Scholar] [CrossRef]
- Darwazeh, R.; Yan, Y. Mild hypothermia as a treatment for central nervous system injuries: Positive or negative effects. Neural Regen. Res. 2013, 8, 2677–2686. [Google Scholar] [CrossRef]
- Han, Z.; Liu, X.; Luo, Y.; Ji, X. Therapeutic hypothermia for stroke: Where to go? Exp. Neurol. 2015, 272, 67–77. [Google Scholar] [CrossRef]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, F.; Zhao, S.; Luo, Y.; Kang, J.; Zhao, H.; Yan, F.; Li, S.; Ji, X. MicroRNA-124-mediated regulation of inhibitory member of apoptosis-stimulating protein of p53 family in experimental stroke. Stroke 2013, 44, 1973–1980. [Google Scholar] [CrossRef]
- Liu, X.; Wen, S.; Zhao, S.; Yan, F.; Zhao, S.; Wu, D.; Ji, X. Mild Therapeutic Hypothermia Protects the Brain from Ischemia/Reperfusion Injury through Upregulation of iASPP. Aging Dis. 2018, 9, 401–411. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, X.N.; Yenari, M.A. The inflammatory response in stroke. J. Neuroimmunol. 2007, 184, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shimohata, T.; Wang, J.Q.; Sun, G.; Schaal, D.W.; Sapolsky, R.M.; Steinberg, G.K. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J. Neurosci. 2005, 25, 9794–9806. [Google Scholar] [CrossRef]
- Tang, X.N.; Liu, L.; Koike, M.A.; Yenari, M.A. Mild hypothermia reduces tissue plasminogen activator-related hemorrhage and blood brain barrier disruption after experimental stroke. Ther. Hypothermia Temp. Manag. 2013, 3, 74–83. [Google Scholar] [CrossRef]
- Nagel, S.; Su, Y.; Horstmann, S.; Heiland, S.; Gardner, H.; Koziol, J.; Martinez-Torres, F.J.; Wagner, S. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: Effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 2008, 1188, 198–206. [Google Scholar] [CrossRef]
- Nito, C.; Kamiya, T.; Amemiya, S.; Katoh, K.; Katayama, Y. The neuroprotective effect of a free radical scavenger and mild hypothermia following transient focal ischemia in rats. Acta Neurochir. Suppl. 2003, 86, 199–203. [Google Scholar] [CrossRef]
- Yenari, M.; Kitagawa, K.; Lyden, P.; Perez-Pinzon, M. Metabolic downregulation: A key to successful neuroprotection? Stroke 2008, 39, 2910–2917. [Google Scholar] [CrossRef]
- Kurisu, K.; Yenari, M.A. Therapeutic hypothermia for ischemic stroke; pathophysiology and future promise. Neuropharmacology 2018, 134, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, K.; Abumiya, T.; Nakamura, H.; Shimbo, D.; Shichinohe, H.; Nakayama, N.; Kazumata, K.; Shimizu, H.; Houkin, K. Transarterial Regional Brain Hypothermia Inhibits Acute Aquaporin-4 Surge and Sequential Microvascular Events in Ischemia/Reperfusion Injury. Neurosurgery 2016, 79, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Colbourne, F.; Grooms, S.Y.; Zukin, R.S.; Buchan, A.M.; Bennett, M.V. Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc. Natl. Acad. Sci. USA 2003, 100, 2906–2910. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yenari, M.A. Therapeutic hypothermia: Neuroprotective mechanisms. Front. Biosci. 2007, 12, 816–825. [Google Scholar] [CrossRef]
- Liu, L.; Kim, J.Y.; Koike, M.A.; Yoon, Y.J.; Tang, X.N.; Ma, H.; Lee, H.; Steinberg, G.K.; Lee, J.E.; Yenari, M.A. FasL shedding is reduced by hypothermia in experimental stroke. J. Neurochem. 2008, 106, 541–550. [Google Scholar] [CrossRef]
- Lee, S.M.; Zhao, H.; Maier, C.M.; Steinberg, G.K. The protective effect of early hypothermia on PTEN phosphorylation correlates with free radical inhibition in rat stroke. J. Cereb. Blood Flow. Metab. 2009, 29, 1589–1600. [Google Scholar] [CrossRef]
- Perrone, S.; Szabó, M.; Bellieni, C.V.; Longini, M.; Bangó, M.; Kelen, D.; Treszl, A.; Negro, S.; Tataranno, M.L.; Buonocore, G. Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury. Pediatr. Neurol. 2010, 43, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Qiao, Y.; Karabiyikoglu, M.; Giffard, R.G.; Yenari, M.A. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J. Neurosci. 2002, 22, 3921–3928. [Google Scholar] [CrossRef]
- Deng, H.; Han, H.S.; Cheng, D.; Sun, G.H.; Yenari, M.A. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke 2003, 34, 2495–2501. [Google Scholar] [CrossRef]
- Yenari, M.A.; Han, H.S. Influence of hypothermia on post-ischemic inflammation: Role of nuclear factor kappa B (NFkappaB). Neurochem. Int. 2006, 49, 164–169. [Google Scholar] [CrossRef]
- Baumann, E.; Preston, E.; Slinn, J.; Stanimirovic, D. Post-ischemic hypothermia attenuates loss of the vascular basement membrane proteins, agrin and SPARC, and the blood-brain barrier disruption after global cerebral ischemia. Brain Res. 2009, 1269, 185–197. [Google Scholar] [CrossRef]
- Xiong, M.; Cheng, G.Q.; Ma, S.M.; Yang, Y.; Shao, X.M.; Zhou, W.H. Post-ischemic hypothermia promotes generation of neural cells and reduces apoptosis by Bcl-2 in the striatum of neonatal rat brain. Neurochem. Int. 2011, 58, 625–633. [Google Scholar] [CrossRef]
- Kurisu, K.; Abumiya, T.; Ito, M.; Gekka, M.; Osanai, T.; Shichinohe, H.; Nakayama, N.; Kazumata, K.; Houkin, K. Transarterial regional hypothermia provides robust neuroprotection in a rat model of permanent middle cerebral artery occlusion with transient collateral hypoperfusion. Brain Res. 2016, 1651, 95–103. [Google Scholar] [CrossRef]
- Yenari, M.A.; Han, H.S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 2012, 13, 267–278. [Google Scholar] [CrossRef]
- Polderman, K.H.; Herold, I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: Practical considerations, side effects, and cooling methods. Crit. Care Med. 2009, 37, 1101–1120. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, D.; Yang, T.; Xu, J.; Chen, J.; Wang, L.; Xu, S.; Zhao, W.; Wu, C.; Ji, X. Hypothermic neuroprotection against acute ischemic stroke: The 2019 update. J. Cereb. Blood Flow Metab. 2020, 40, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, K.; Elmadhoun, O.; Ji, X.; Duan, Y.; Shi, J.; He, X.; Liu, X.; Wu, D.; Che, R.; et al. Synergistically Induced Hypothermia and Enhanced Neuroprotection by Pharmacological and Physical Approaches in Stroke. Aging Dis. 2018, 9, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Kammersgaard, L.P.; Rasmussen, B.H.; Jørgensen, H.S.; Reith, J.; Weber, U.; Olsen, T.S. Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: A case-control study: The Copenhagen Stroke Study. Stroke 2000, 31, 2251–2256. [Google Scholar] [CrossRef]
- Chen, X.; Xu, S.; Li, M.; Wu, D.; Ji, X. Transnasal cooling: New prospect of selective hypothermia in acute ischemic stroke. J. Cereb. Blood Flow. Metab. 2024, 44, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, J.; Luan, X.; Lai, Q.; McAllister, J.P., 2nd; Phillis, J.W.; Clark, J.C.; Guthikonda, M.; Diaz, F.G. Local saline infusion into ischemic territory induces regional brain cooling and neuroprotection in rats with transient middle cerebral artery occlusion. Neurosurgery 2004, 54, 956–964. [Google Scholar] [CrossRef]
- Li, J.; Luan, X.; Lai, Q.; Clark, J.C.; McAllister, J.P., 2nd; Fessler, R.; Diaz, F.G.; Ding, Y. Long-term neuroprotection induced by regional brain cooling with saline infusion into ischemic territory in rats: A behavioral analysis. Neurol. Res. 2004, 26, 677–683. [Google Scholar] [CrossRef]
- Furuse, M.; Preul, M.C.; Kinoshita, Y.; Nishihara, K.; Isono, N.; Kuroiwa, T. Rapid induction of brain hypothermia by endovascular intra-arterial perfusion. Neurol. Res. 2007, 29, 53–57. [Google Scholar] [CrossRef]
- Luan, X.; Li, J.; McAllister, J.P., 2nd; Diaz, F.G.; Clark, J.C.; Fessler, R.D.; Ding, Y. Regional brain cooling induced by vascular saline infusion into ischemic territory reduces brain inflammation in stroke. Acta Neuropathol. 2004, 107, 227–234. [Google Scholar] [CrossRef]
- Wang, B.; Wu, D.; Dornbos Iii, D.; Shi, J.; Ma, Y.; Zhang, M.; Liu, Y.; Chen, J.; Ding, Y.; Luo, Y.; et al. Local cerebral hypothermia induced by selective infusion of cold lactated ringer’s: A feasibility study in rhesus monkeys. Neurol. Res. 2016, 38, 545–552. [Google Scholar] [CrossRef]
- Wu, L.; Huber, M.; Wu, D.; Chen, J.; Li, M.; Ding, Y.; Ji, X. Intra-arterial Cold Saline Infusion in Stroke: Historical Evolution and Future Prospects. Aging Dis. 2020, 11, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, L.; Zhang, H.; Geng, X.; Jiao, L.; Li, G.; Coutinho, J.M.; Ding, Y.; Liebeskind, D.S.; Ji, X. Endovascular Hypothermia in Acute Ischemic Stroke: Pilot Study of Selective Intra-Arterial Cold Saline Infusion. Stroke 2016, 47, 1933–1935. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, D.; Huber, M.; Shi, J.; An, H.; Wei, W.; He, X.; Ding, Y.; Ji, X. New Endovascular Approach for Hypothermia With Intrajugular Cooling and Neuroprotective Effect in Ischemic Stroke. Stroke 2020, 51, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Covaciu, L.; Weis, J.; Bengtsson, C.; Allers, M.; Lunderquist, A.; Ahlström, H.; Rubertsson, S. Brain temperature in volunteers subjected to intranasal cooling. Intensive Care Med. 2011, 37, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, M.; Wei, M.; Wu, C.; Huang, Y.; Song, B.; Xu, Y.; Zhang, H.; Shen, Y.; Wu, D.; et al. MgSO4 as a novel hypothermia infusion solution promotes ischemic stroke recovery through Ca2+ regulation of neurovascular units. Theranostics 2025, 15, 1896–1913. [Google Scholar] [CrossRef]
- An, H.; Duan, Y.; Wu, D.; Yip, J.; Elmadhoun, O.; Wright, J.C.; Shi, W.; Liu, K.; He, X.; Shi, J.; et al. Phenothiazines Enhance Mild Hypothermia-induced Neuroprotection via PI3K/Akt Regulation in Experimental Stroke. Sci. Rep. 2017, 7, 7469. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Schabitz, W.R.; Wolf, M.; Mueller, H.; Sommer, C.; Schwab, S. Hypothermia and brain-derived neurotrophic factor reduce glutamate synergistically in acute stroke. Exp. Neurol. 2004, 185, 305–312. [Google Scholar] [CrossRef]
- Doerfler, A.; Schwab, S.; Hoffmann, T.T.; Engelhorn, T.; Forsting, M. Combination of decompressive craniectomy and mild hypothermia ameliorates infarction volume after permanent focal ischemia in rats. Stroke 2001, 32, 2675–2681. [Google Scholar] [CrossRef]
- Hemmen, T.M.; Raman, R.; Guluma, K.Z.; Meyer, B.C.; Gomes, J.A.; Cruz-Flores, S.; Wijman, C.A.; Rapp, K.S.; Grotta, J.C.; Lyden, P.D. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): Final results. Stroke 2010, 41, 2265–2270. [Google Scholar] [CrossRef]
- Bi, M.; Ma, Q.; Zhang, S.; Li, J.; Zhang, Y.; Lin, L.; Tong, S.; Wang, D. Local mild hypothermia with thrombolysis for acute ischemic stroke within a 6-h window. Clin. Neurol. Neurosurg. 2011, 113, 768–773. [Google Scholar] [CrossRef]
- Kurasako, T.; Zhao, L.; Pulsinelli, W.A.; Nowak, T.S., Jr. Transient cooling during early reperfusion attenuates delayed edema and infarct progression in the Spontaneously Hypertensive Rat. Distribution and time course of regional brain temperature change in a model of postischemic hypothermic protection. J. Cereb. Blood Flow. Metab. 2007, 27, 1919–1930. [Google Scholar] [CrossRef]
- van der Worp, H.B.; Sena, E.S.; Donnan, G.A.; Howells, D.W.; Macleod, M.R. Hypothermia in animal models of acute ischaemic stroke: A systematic review and meta-analysis. Brain 2007, 130, 3063–3074. [Google Scholar] [CrossRef]
- Clark, D.L.; Penner, M.; Orellana-Jordan, I.M.; Colbourne, F. Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Exp. Neurol. 2008, 212, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Dae, M.W.; Gao, D.W.; Ursell, P.C.; Stillson, C.A.; Sessler, D.I. Safety and efficacy of endovascular cooling and rewarming for induction and reversal of hypothermia in human-sized pigs. Stroke 2003, 34, 734–738. [Google Scholar] [CrossRef]
- Schwab, S.; Georgiadis, D.; Berrouschot, J.; Schellinger, P.D.; Graffagnino, C.; Mayer, S.A. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke 2001, 32, 2033–2035. [Google Scholar] [CrossRef]
- Krieger, D.W.; Yenari, M.A. Therapeutic hypothermia for acute ischemic stroke: What do laboratory studies teach us? Stroke 2004, 35, 1482–1489. [Google Scholar] [CrossRef]
- Meng, R.; Ding, Y.; Asmaro, K.; Brogan, D.; Meng, L.; Sui, M.; Shi, J.; Duan, Y.; Sun, Z.; Yu, Y.; et al. Ischemic Conditioning Is Safe and Effective for Octo- and Nonagenarians in Stroke Prevention and Treatment. Neurotherapeutics 2015, 12, 667–677. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Sadowsky, M.G.; Meng, R.; Ding, Y.; Ji, X. Remote ischaemic conditioning for preventing and treating ischaemic stroke. Cochrane Database Syst. Rev. 2018, 7, Cd012503. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, F.; Li, S.; Liu, G.; Wu, C.; Wang, Y.; Ren, C.; Zhang, J.; Gu, F.; Zhang, Q.; et al. Safety and efficacy of remote ischemic conditioning for the treatment of intracerebral hemorrhage: A proof-of-concept randomized controlled trial. Int. J. Stroke 2022, 17, 425–433. [Google Scholar] [CrossRef]
- Li, S.; Han, C.; Asmaro, K.; Quan, S.; Li, M.; Ren, C.; Zhang, J.; Zhao, W.; Xu, J.; Liu, Z.; et al. Remote Ischemic Conditioning Improves Attention Network Function and Blood Oxygen Levels in Unacclimatized Adults Exposed to High Altitude. Aging Dis. 2020, 11, 820–827. [Google Scholar] [CrossRef]
- Guo, W.; Ren, C.; Zhang, B.; Zhao, W.; Gao, Y.; Yu, W.; Ji, X. Chronic Limb Remote Ischemic Conditioning may have an Antihypertensive Effect in Patients with Hypertension. Aging Dis. 2021, 12, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, X.; Ma, J.; Fayyaz, A.; Wang, L.; Qin, P.; Ding, Y.; Ji, X.; Li, S. Remote ischemic conditioning after stroke: Research progress in clinical study. CNS Neurosci. Ther. 2024, 30, e14507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Chen, C.; Li, X.R.; Ran, Y.Y.; Xu, T.; Zhang, Y.; Geng, X.K.; Zhang, Y.; Du, H.S.; Leak, R.K.; et al. Remote Ischemic Preconditioning-Mediated Neuroprotection against Stroke is Associated with Significant Alterations in Peripheral Immune Responses. CNS Neurosci. Ther. 2016, 22, 43–52. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Du, X.; Liu, M.; Ji, X.; Du, H.; Zhao, H. Hypoxia Inducible Factor 1α Plays a Key Role in Remote Ischemic Preconditioning Against Stroke by Modulating Inflammatory Responses in Rats. J. Am. Heart Assoc. 2018, 7, e007589. [Google Scholar] [CrossRef]
- Hess, D.C.; Blauenfeldt, R.A.; Andersen, G.; Hougaard, K.D.; Hoda, M.N.; Ding, Y.; Ji, X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015, 11, 698–710. [Google Scholar] [CrossRef]
- Hess, D.C.; Hoda, M.N.; Khan, M.B. Humoral Mediators of Remote Ischemic Conditioning: Important Role of eNOS/NO/Nitrite. Acta Neurochir. Suppl. 2016, 121, 45–48. [Google Scholar] [CrossRef]
- Davidson, S.M.; Selvaraj, P.; He, D.; Boi-Doku, C.; Yellon, R.L.; Vicencio, J.M.; Yellon, D.M. Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic. Res. Cardiol. 2013, 108, 377. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.P.; Parajuli, N.; Zheng, X.; Becker, L. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic. Res. Cardiol. 2012, 107, 277. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rohailla, S.; Gelber, N.; Rutka, J.; Sabah, N.; Gladstone, R.A.; Wei, C.; Hu, P.; Kharbanda, R.K.; Redington, A.N. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic. Res. Cardiol. 2014, 109, 423. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Naggar, I.; Stewart, M.; Rosenbaum, D.M. Neurogenic pathway mediated remote preconditioning protects the brain from transient focal ischemic injury. Brain Res. 2011, 1386, 184–190. [Google Scholar] [CrossRef]
- Wei, D.; Ren, C.; Chen, X.; Zhao, H. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS ONE 2012, 7, e30892. [Google Scholar] [CrossRef]
- Mastitskaya, S.; Marina, N.; Gourine, A.; Gilbey, M.P.; Spyer, K.M.; Teschemacher, A.G.; Kasparov, S.; Trapp, S.; Ackland, G.L.; Gourine, A.V. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc. Res. 2012, 95, 487–494. [Google Scholar] [CrossRef]
- Zhao, L.; Nowak, T.S., Jr. CBF changes associated with focal ischemic preconditioning in the spontaneously hypertensive rat. J. Cereb. Blood Flow. Metab. 2006, 26, 1128–1140. [Google Scholar] [CrossRef]
- Hoyte, L.C.; Papadakis, M.; Barber, P.A.; Buchan, A.M. Improved regional cerebral blood flow is important for the protection seen in a mouse model of late phase ischemic preconditioning. Brain Res. 2006, 1121, 231–237. [Google Scholar] [CrossRef]
- Hoda, M.N.; Bhatia, K.; Hafez, S.S.; Johnson, M.H.; Siddiqui, S.; Ergul, A.; Zaidi, S.K.; Fagan, S.C.; Hess, D.C. Remote ischemic perconditioning is effective after embolic stroke in ovariectomized female mice. Transl. Stroke Res. 2014, 5, 484–490. [Google Scholar] [CrossRef]
- Hoda, M.N.; Fagan, S.C.; Khan, M.B.; Vaibhav, K.; Chaudhary, A.; Wang, P.; Dhandapani, K.M.; Waller, J.L.; Hess, D.C. A 2 × 2 factorial design for the combination therapy of minocycline and remote ischemic perconditioning: Efficacy in a preclinical trial in murine thromboembolic stroke model. Exp. Transl. Stroke Med. 2014, 6, 10. [Google Scholar] [CrossRef]
- Ong, S.B.; Dongworth, R.K.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Role of the MPTP in conditioning the heart—Translatability and mechanism. Br. J. Pharmacol. 2015, 172, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tong, L.; Luan, Q.; Deng, J.; Li, Y.; Li, Z.; Dong, H.; Xiong, L. Protective effect of delayed remote limb ischemic postconditioning: Role of mitochondrial K(ATP) channels in a rat model of focal cerebral ischemic reperfusion injury. J. Cereb. Blood Flow. Metab. 2012, 32, 851–859. [Google Scholar] [CrossRef]
- Shiva, S. Nitrite: A Physiological Store of Nitric Oxide and Modulator of Mitochondrial Function. Redox Biol. 2013, 1, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Sack, M.N.; Greer, J.J.; Duranski, M.; Ringwood, L.A.; Burwell, L.; Wang, X.; MacArthur, P.H.; Shoja, A.; Raghavachari, N.; et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 2007, 204, 2089–2102. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, M.; Wu, J.; Jiang, Q.; Zheng, X. Exosomes isolated from the plasma of remote ischemic conditioning rats improved cardiac function and angiogenesis after myocardial infarction through targeting Hsp70. Aging 2020, 12, 3682–3693. [Google Scholar] [CrossRef]
- Esposito, E.; Hayakawa, K.; Ahn, B.J.; Chan, S.J.; Xing, C.; Liang, A.C.; Kim, K.W.; Arai, K.; Lo, E.H. Effects of ischemic post-conditioning on neuronal VEGF regulation and microglial polarization in a rat model of focal cerebral ischemia. J. Neurochem. 2018, 146, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. Brain-Derived Neurotrophic Factor, Depression, and Physical Activity: Making the Neuroplastic Connection. Neural Plast. 2017, 2017, 7260130. [Google Scholar] [CrossRef]
- Ramagiri, S.; Taliyan, R. Protective effect of remote limb post conditioning via upregulation of heme oxygenase-1/BDNF pathway in rat model of cerebral ischemic reperfusion injury. Brain Res. 2017, 1669, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.W.; Yang, Y.R.; Sun, S.H.; Liang, K.C.; Wang, R.Y. Post ischemia intermittent hypoxia induces hippocampal neurogenesis and synaptic alterations and alleviates long-term memory impairment. J. Cereb. Blood Flow. Metab. 2013, 33, 764–773. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ma, Y.; Fang, C.; Deng, Z.; Wang, F.; Qu, Y.; Yin, M.; Zhao, R.; Zhang, D.; Guo, F.; et al. Remote ischemic conditioning attenuates blood-brain barrier disruption after recombinant tissue plasminogen activator treatment via reducing PDGF-CC. Pharmacol. Res. 2023, 187, 106641. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Zhu, H.J.; Zhao, R.Y.; Zhou, S.Y.; Wang, M.Q.; Yang, Y.; Guo, Z.N. Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice. Redox Biol. 2023, 66, 102852. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Li, N.; Li, H.; Xu, J.; Zhao, W.; Wang, X.; Ma, L.; Gao, C.; Ding, Y.; et al. Limb Remote Ischemic Conditioning Promotes Neurogenesis after Cerebral Ischemia by Modulating miR-449b/Notch1 Pathway in Mice. Biomolecules 2022, 12, 1137. [Google Scholar] [CrossRef]
- Ren, C.; Li, S.; Wang, B.; Han, R.; Li, N.; Gao, J.; Li, X.; Jin, K.; Ji, X. Limb remote ischemic conditioning increases Notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav. Brain Res. 2018, 340, 87–93. [Google Scholar] [CrossRef]
- Pico, F.; Lapergue, B.; Ferrigno, M.; Rosso, C.; Meseguer, E.; Chadenat, M.L.; Bourdain, F.; Obadia, M.; Hirel, C.; Duong, D.L.; et al. Effect of In-Hospital Remote Ischemic Perconditioning on Brain Infarction Growth and Clinical Outcomes in Patients With Acute Ischemic Stroke: The RESCUE BRAIN Randomized Clinical Trial. JAMA Neurol. 2020, 77, 725–734. [Google Scholar] [CrossRef]
- Blauenfeldt, R.A.; Hjort, N.; Valentin, J.B.; Homburg, A.M.; Modrau, B.; Sandal, B.F.; Gude, M.F.; Hougaard, K.D.; Damgaard, D.; Poulsen, M.; et al. Remote Ischemic Conditioning for Acute Stroke: The RESIST Randomized Clinical Trial. JAMA 2023, 330, 1236–1246. [Google Scholar] [CrossRef]
- Blauenfeldt, R.A.; Mortensen, J.K.; Hjort, N.; Valentin, J.B.; Homburg, A.M.; Modrau, B.; Sandal, B.F.; Gude, M.F.; Berhndtz, A.B.; Johnsen, S.P.; et al. Effect of Remote Ischemic Conditioning in Ischemic Stroke Subtypes: A Post Hoc Subgroup Analysis From the RESIST Trial. Stroke 2024, 55, 874–879. [Google Scholar] [CrossRef]
- Chen, H.S.; Cui, Y.; Li, X.Q.; Wang, X.H.; Ma, Y.T.; Zhao, Y.; Han, J.; Deng, C.Q.; Hong, M.; Bao, Y.; et al. Effect of Remote Ischemic Conditioning vs Usual Care on Neurologic Function in Patients With Acute Moderate Ischemic Stroke: The RICAMIS Randomized Clinical Trial. JAMA 2022, 328, 627–636. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.H.; Shang, Z.Y.; Wang, L.; Chen, H.S. Baseline neurologic deficit and efficacy of remote ischemic conditioning after acute ischemic stroke: A post hoc analysis of RICAMIS. Neurotherapeutics 2024, 21, e00294. [Google Scholar] [CrossRef]
- Abuduxukuer, R.; Guo, Z.N.; Zhang, P.; Qu, Y.; Yang, Y. Safety and efficacy of remote ischemic conditioning combined with intravenous thrombolysis for acute ischemic stroke: A multicenter, randomized, parallel-controlled clinical trial (SERIC-IVT) Study design and protocol. Int. J. Stroke 2023, 18, 370–374. [Google Scholar] [CrossRef]
- Hou, C.; Lan, J.; Lin, Y.; Song, H.; Wang, Y.; Zhao, W.; Li, S.; Meng, R.; Hao, J.; Ding, Y.; et al. Chronic remote ischaemic conditioning in patients with symptomatic intracranial atherosclerotic stenosis (the RICA trial): A multicentre, randomised, double-blind sham-controlled trial in China. Lancet Neurol. 2022, 21, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Chazalviel, L.; David, H.N.; Haelewyn, B.; Blatteau, J.E.; Vallée, N.; Risso, J.J.; Besnard, S.; Abraini, J.H. The underestimated effect of normobaric hyperoxia on cerebral blood flow and its relationship to neuroprotection. Brain 2016, 139, e62. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Liang, Z.; Wang, T.; Li, W.; Ren, L.; Huang, S.; Liu, W. Normobaric hyperoxia retards the evolution of ischemic brain tissue toward infarction in a rat model of transient focal cerebral ischemia. Neurol. Res. 2016, 38, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, W.; Mangal, R.; Jia, M.; Ji, X.; Ding, Y. Normobaric Hyperoxia (NBHO): An Adjunctive Therapy to Cerebrovascular Recanalization in Ischemic Stroke. Aging Dis. 2023, 14, 1483–1487. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Suo, Y.; Chen, Z.; Wu, M.; Wen, X.; Lai, Q.; Yin, X.; Bao, B. Normobaric hyperoxia therapy in acute ischemic stroke: A literature review. Heliyon 2024, 10, e23744. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Ding, W.; Miyake, M.; Rosenberg, G.A.; Liu, K.J. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 2006, 26, 1274–1284. [Google Scholar] [CrossRef]
- Shin, H.K.; Dunn, A.K.; Jones, P.B.; Boas, D.A.; Lo, E.H.; Moskowitz, M.A.; Ayata, C. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain 2007, 130, 1631–1642. [Google Scholar] [CrossRef]
- Jin, X.; Liu, J.; Yang, Y.; Liu, K.J.; Yang, Y.; Liu, W. Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3h after ischemia onset. Neurobiol. Dis. 2012, 48, 309–316. [Google Scholar] [CrossRef]
- Liang, J.; Qi, Z.; Liu, W.; Wang, P.; Shi, W.; Dong, W.; Ji, X.; Luo, Y.; Liu, K.J. Normobaric hyperoxia slows blood-brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia. Stroke 2015, 46, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, W.; Yuan, S.; Chen, J.; Wang, Q.; Ding, J.; Chen, Z.; Qi, Z.; Han, J. Enhancing Blood-Brain Barrier Integrity in Patients With Acute Ischemic Stroke Via Normobaric Hyperoxia. J. Am. Heart Assoc. 2024, 13, e036474. [Google Scholar] [CrossRef]
- Hiebert, J.B.; Shen, Q.; Thimmesch, A.R.; Pierce, J.D. Traumatic brain injury and mitochondrial dysfunction. Am. J. Med. Sci. 2015, 350, 132–138. [Google Scholar] [CrossRef]
- Dong, W.; Qi, Z.; Liang, J.; Shi, W.; Zhao, Y.; Luo, Y.; Ji, X.; Liu, K.J. Reduction of zinc accumulation in mitochondria contributes to decreased cerebral ischemic injury by normobaric hyperoxia treatment in an experimental stroke model. Exp. Neurol. 2015, 272, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, K.J.; Ramu, J.; Chen, Q.; Li, T.; Liu, W. Inhibition of gp91(phox) contributes towards normobaric hyperoxia afforded neuroprotection in focal cerebral ischemia. Brain Res. 2010, 1348, 174–180. [Google Scholar] [CrossRef]
- Jin, X.; Liu, J.; Liu, K.J.; Rosenberg, G.A.; Yang, Y.; Liu, W. Normobaric hyperoxia combined with minocycline provides greater neuroprotection than either alone in transient focal cerebral ischemia. Exp. Neurol. 2013, 240, 9–16. [Google Scholar] [CrossRef]
- Wu, O.; Benner, T.; Roccatagliata, L.; Zhu, M.; Schaefer, P.W.; Sorensen, A.G.; Singhal, A.B. Evaluating effects of normobaric oxygen therapy in acute stroke with MRI-based predictive models. Med. Gas. Res. 2012, 2, 5. [Google Scholar] [CrossRef]
- Shi, S.; Qi, Z.; Ma, Q.; Pan, R.; Timmins, G.S.; Zhao, Y.; Shi, W.; Zhang, Y.; Ji, X.; Liu, K.J. Normobaric Hyperoxia Reduces Blood Occludin Fragments in Rats and Patients With Acute Ischemic Stroke. Stroke 2017, 48, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Geng, X.; Tong, Y.; Dornbos, D., 3rd; Hussain, M.; Rajah, G.B.; Gao, J.; Ma, L.; Li, F.; Du, H.; et al. Adjuvant High-Flow Normobaric Oxygen After Mechanical Thrombectomy for Anterior Circulation Stroke: A Randomized Clinical Trial. Neurotherapeutics 2021, 18, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Poli, S.; Mbroh, J.; Baron, J.C.; Singhal, A.B.; Strbian, D.; Molina, C.; Lemmens, R.; Turc, G.; Mikulik, R.; Michel, P.; et al. Penumbral Rescue by normobaric O = O administration in patients with ischemic stroke and target mismatch proFile (PROOF): Study protocol of a phase IIb trial. Int. J. Stroke 2024, 19, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, L.; Zhao, W.; Dornbos, D., 3rd; Wu, C.; Li, W.; Wu, D.; Ding, J.; Ding, Y.; Xie, Y.; et al. Efficacy and safety of normobaric hyperoxia combined with intravenous thrombolysis on acute ischemic stroke patients. Neurol. Res. 2021, 43, 809–814. [Google Scholar] [CrossRef]
- Li, W.; Lan, J.; Wei, M.; Liu, L.; Hou, C.; Qi, Z.; Li, C.; Jiao, L.; Yang, Q.; Chen, W.; et al. Normobaric hyperoxia combined with endovascular treatment for acute ischaemic stroke in China (OPENS-2 trial): A multicentre, randomised, single-blind, sham-controlled trial. Lancet 2025, 405, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, X.; Wang, S.; Liu, S.; Ji, X.; Li, W. Protocol for a prospective 1-year follow-up investigation on normobaric hyperoxia in conjunction with endovascular treatment for acute ischemic stroke (OPENS-2L) trial. Brain Circ. 2025, 11, 127–134. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Liu, L.; Chen, J.; Lan, J.; Ding, J.; Chen, Z.; Yuan, S.; Qi, Z.; Wei, M.; et al. Normobaric Hyperoxia Combined With Endovascular Treatment Based on Temporal Gradient: A Dose-Escalation Study. Stroke 2024, 55, 1468–1476. [Google Scholar] [CrossRef]
- Henninger, N.; Bouley, J.; Nelligan, J.M.; Sicard, K.M.; Fisher, M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J. Cereb. Blood Flow. Metab. 2007, 27, 1632–1642. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, Y.; Ji, X.; Meng, R. Advances in Normobaric Hyperoxia Brain Protection in Experimental Stroke. Front. Neurol. 2020, 11, 50. [Google Scholar] [CrossRef]
| Study | Study Type | Sample Size | Type of Patients | Treatment | Main Results |
|---|---|---|---|---|---|
| L Covaciu et al. (2011) [52] | prospective, nonrandomized and single arm study | 10 | awake volunteers | Intranasal balloons catheters circulated with saline at 20 °C were applied for 60 min | Brain temperature decreased in volunteers subjected to intranasal cooling. |
| Chen et al. (2016) [50] | nonrandomized and single arm observational study | 26 | patients with LVO within 8 h after symptom onset | 50 mL cold 0.9% sodium chloride (4 °C) into the ischemic territory at 10 mL/min before recanalization, 30 mL/minute for 10 min as soon as blood flow was restored | Intra-arterial hypothermia with endovascular recanalization therapy in AIS appears feasible and safe. |
| Wu et al. (2018) [10] | prospective non-RCT | 113 | patients with LVO-induced AIS receiving MT | IA-SCI with 350 mL 0.9% saline at 4 °C for 15 min pre- and post-reperfusion | Combining short-duration IA-SCI with MT was safe. IA-CSI was effective in reducing infarct volume but cannot improve the functional outcomes at 90 days. |
| Study Sponsor | Year | Study Type | Sample Size | Type of Patients | NBO Treatment and Duration | Primary Outcome | Results |
|---|---|---|---|---|---|---|---|
| NBO Monotherapy | |||||||
| Ona Wu et al. [118] | 2012 | Prospective RCT | 16 | AIS within 12 h of onset or <15 h after last seen neurologically intact with PWI/DWI mismatch >20% NBO group: n = 10, Control group: n = 6 | High-flow oxygen via facemask for 8 h | Lesion volume change (4 h, 24 h and discharge) | NBO significantly attenuated ischemic lesion growth during therapy (4 h). No significant differences in lesion volumes at later time points (24 h and discharge). |
| Combination of NBO with Reperfusion Therapy | |||||||
| Shi et al. [119] (NCT02974283) | 2017 | Cohort Study | 18 | AIS patients receiving IVT NBO group: n = 9, Normoxia group: n = 9 | Oxygen, a flow rate of 10 L/min, 4 h, by facemask | Blood Occludin and Claudin-5 Levels (at admission, 24 h, 72 h) NIHSS (at admission, 24 h, 72 h and 1 week) | NBO reduced blood occludin and improved neurological functions in AIS patients. |
| Cheng et al. [120] (ChiCTR-INR-17013685) | 2017 | Prospective RCT | 175 | AIS patients with anterior circulation LVO receiving MT NBO group: n = 88, Control group: n = 87 | FiO2 50%, flow 15 L/min by Venturi mask for 6 h after MT | mRS at 90 days | High-flow NBO therapy after MT is safe and effective in improving functional outcomes. |
| Li et al. [9] (OPENS-1) (NCT03620370) | 2018 | Single-center, assessor-blinded RCT | 86 | AIS patients who received EVT NBO + EVT group: n = 43, EVT group: n = 43 | Oxygen, 10 L/min for 4 h via facemask initiated before vascular recanalization | Cerebral infarct volume within 24–48 h after randomization | NBO in combination with EVT could significantly reduce infarct volume and enhance clinical outcomes at 90 days. |
| Poli et al. (PROOF trial) [121] (NCT03500939) | 2019 | Multi-center RCT | 456 | AIS patients due to anterior-circulation LVO likely to receive EVT Treatment group: Control group (1:1) | ≥40 L/min oxygen via non-rebreather mask (FiO2 ≈100%) or ventilator (FiO2 1.0) continued until MT completion or for 4 h if MT was not attempted/stopped | Ischemic core growth from baseline to 24 h | Terminated |
| Li na et al. [122] | 2021 | Single-center observational cohort study | 227 | AIS patients with anterior circulation LVO receiving IVT NBO group: n = 125, Control group: n = 102 | Oxygen, 10 L/min for 4 h via facemask at the beginning of IVT | mRS at 90 days | Better prognosis in the NBO group. |
| Li et al. [123] (OPENS-2) (NCT04681651) | 2021 | Multi-center, single-blind, RCT | 282 | AIS patients with anterior circulation LVO within 6 h, who were candidates for endovascular treatment. NBO group: n = 140, Sham normobaric hyperoxia group: n = 142 | 100% oxygen at a flow rate of 10 L/min through a non-rebreather mask for 4 h | mRS at 90 days | Normobaric hyperoxia combined with endovascular treatment significantly improved 90-day functional outcome in AIS patients. |
| Wei et al. (OPENS-2L) [124] (NCT05039697) | 2021 | Multi-center, double-blind, RCT | 282 | AIS patients with anterior circulation LVO within 6 h, who were candidates for endovascular treatment. NBO group: n = 140, Sham normobaric hyperoxia group: n = 142 | 100% oxygen at a flow rate of 10 L/min through a non-rebreather mask for 4 h | mRS at 1 year | Completed |
| Li et al. (TD-NBO) [125] (NCT05404373) | 2022 | Single-center, single-blind, RCT | 100 | AIS patients who had an indication for endovascular treatment NBO group (2 h, n = 25; 4 h, n = 25; 6 h, n = 25); Low flow oxygen group (n = 25) | 100% oxygen at a ventilation rate of 10 L/min using an oxygen storage mask for 2 h, 4 h and 6 h | The infarct volume within 72 h after randomization | NBO therapy for 4 and 6 h was found to be more effective. |
| Ji et al. (OPENS-3) (NCT05965687) | 2023 | Multi-center, single-blind, RCT | 1230 | AIS patients who undergo IVT within 4.5 h from onset NBO group: NBO+ rt-PA; Control group: Nasal oxygen+ rt-PA | 100% oxygen at a ventilation rate of 10 L/min using a sealed non-ventilating oxygen storage mask for 4 h | Utility-weighted mRS at 90 days | On going |
| Ji et al. (OPENS-3L) (NCT05965193) | 2023 | Multi-center, single-blind, RCT | 1230 | AIS patients who undergo IVT within 4.5 h from onset NBO group: NBO+ rt-PA; Control group: Nasal oxygen+ rt-PA | 100% oxygen at a ventilation rate of 10 L/min using a sealed non-ventilating oxygen storage mask for 4 h | Utility-weighted mRS at 12 months | On going |
| Ji et al. (AN-O2-Trans) (NCT06666764) | 2024 | Multi-center, single-blind, RCT | 1500 | AIS patients due to anterior-circulation LVO likely to receive EVT NBO group: NBO+ best medical practice, Control group: best medical practice | 100% oxygen | mRS at 90 days | On going |
| Ji et al. (AN-O2-EMS) (NCT06801457) | 2025 | Multi-center, single-blind, RCT | 1230 | Patients with suspected AIS due to LVO presenting within 6 h of symptom onset NBO group: NBO+ best medical practice, Control group: best medical practice | 100% oxygen | Utility-weighted mRS at 90 days | On going |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Yang, J.; Liu, L.; Liu, X.; Ji, X. Multimodal Neuroprotection in Ischemic Stroke: Emerging Non-Pharmacological Interventions from Bench to Bedside. Brain Sci. 2025, 15, 1111. https://doi.org/10.3390/brainsci15101111
Cui J, Yang J, Liu L, Liu X, Ji X. Multimodal Neuroprotection in Ischemic Stroke: Emerging Non-Pharmacological Interventions from Bench to Bedside. Brain Sciences. 2025; 15(10):1111. https://doi.org/10.3390/brainsci15101111
Chicago/Turabian StyleCui, Junzhao, Jingyi Yang, Luji Liu, Xiaoyun Liu, and Xunming Ji. 2025. "Multimodal Neuroprotection in Ischemic Stroke: Emerging Non-Pharmacological Interventions from Bench to Bedside" Brain Sciences 15, no. 10: 1111. https://doi.org/10.3390/brainsci15101111
APA StyleCui, J., Yang, J., Liu, L., Liu, X., & Ji, X. (2025). Multimodal Neuroprotection in Ischemic Stroke: Emerging Non-Pharmacological Interventions from Bench to Bedside. Brain Sciences, 15(10), 1111. https://doi.org/10.3390/brainsci15101111







