Analysis of the Correlation Between Depression-like Behaviors and Lipid Peroxidation in the Prefrontal Cortex of Mice: The Impact of Early Life Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Maternal Separation
2.3. Behavioral Tests
2.3.1. Sucrose Preference Test (SPT)
2.3.2. Open Field Test (OFT)
2.3.3. Forced Swimming Test (FST)
2.3.4. Tail Suspension Test (TST)
2.4. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.5. Western Blot Analysis
2.6. Lipid Peroxidation Analysis
2.7. Statistical Analysis
3. Results
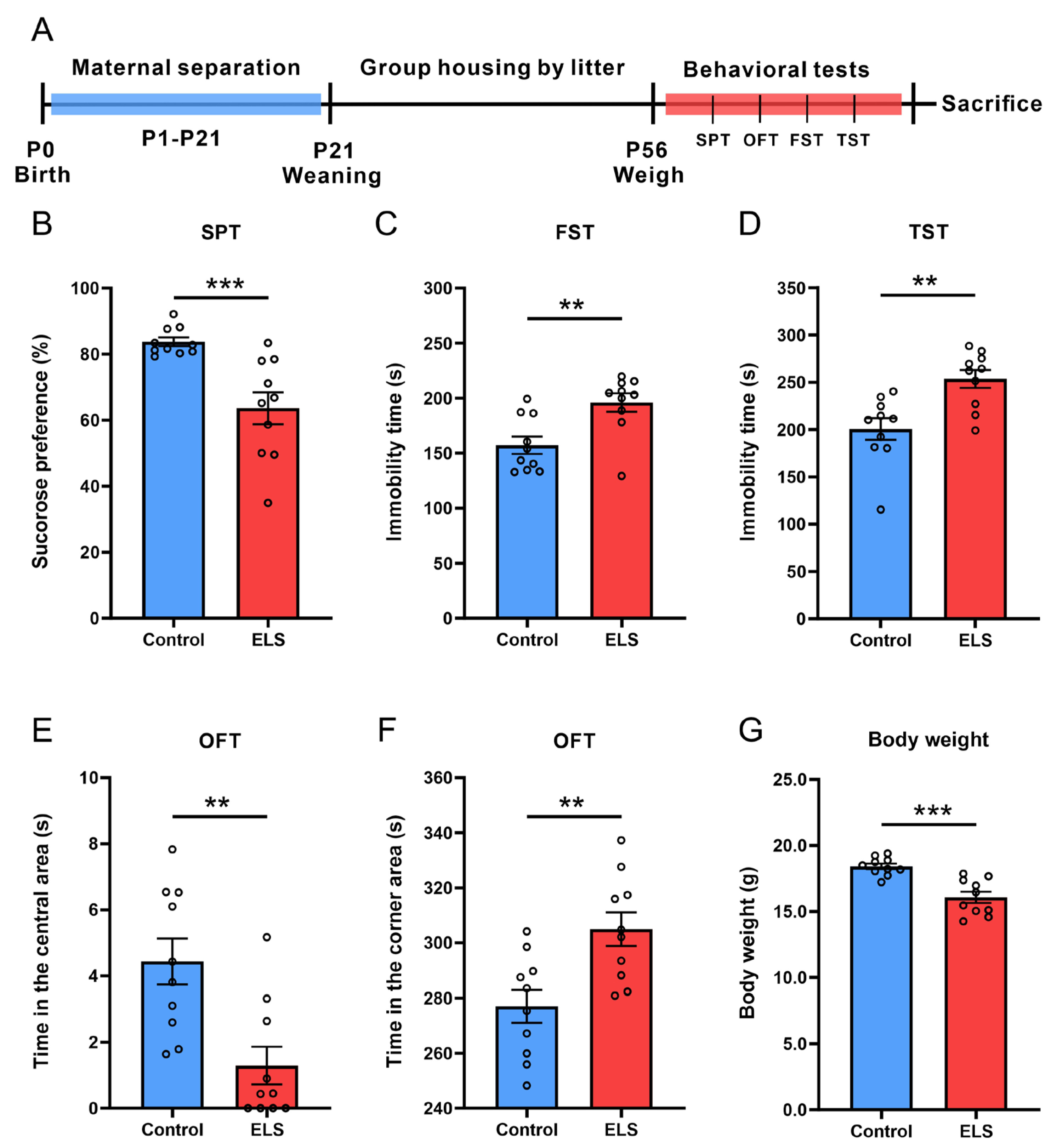
3.1. ELS-Induced Depression- and Anxiety-like Behaviors in Mice
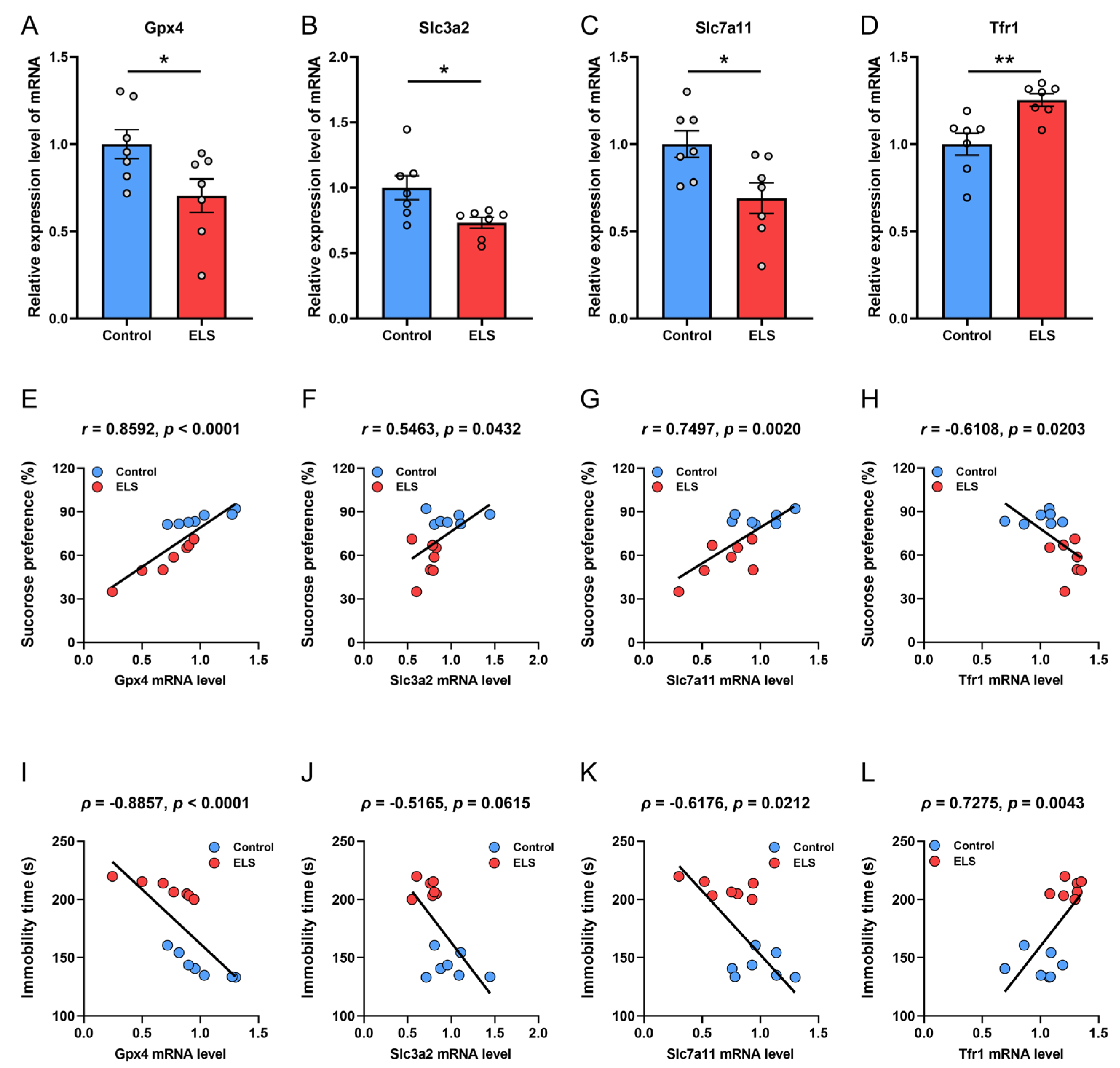
3.2. Correlation Analysis Between the mRNA Expression of Antioxidation Genes and Depression-like Behavior in Mice
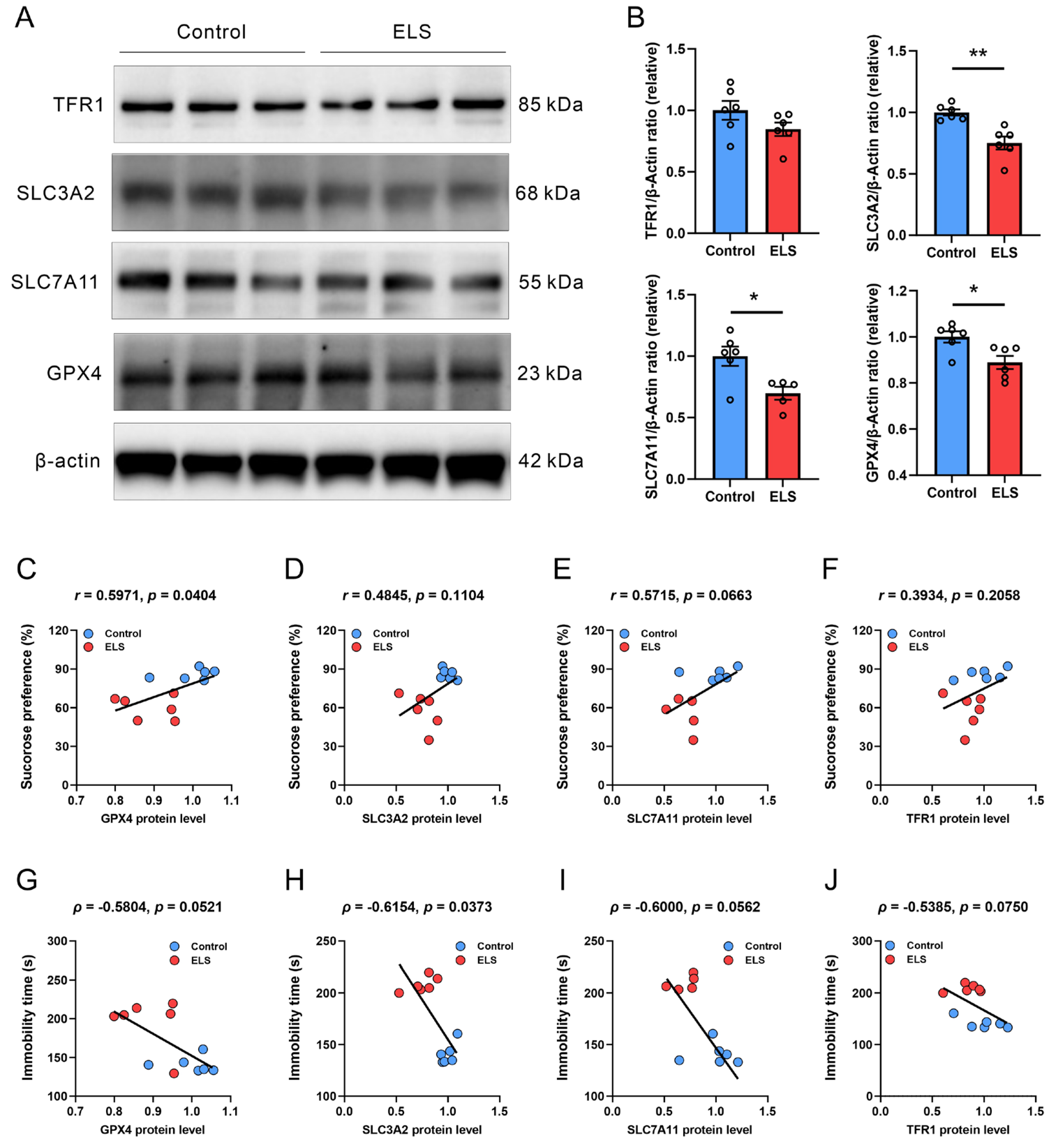
3.3. Correlation Analysis Between the Protein Expression of Antioxidation Genes and Depression-like Behavior in Mice
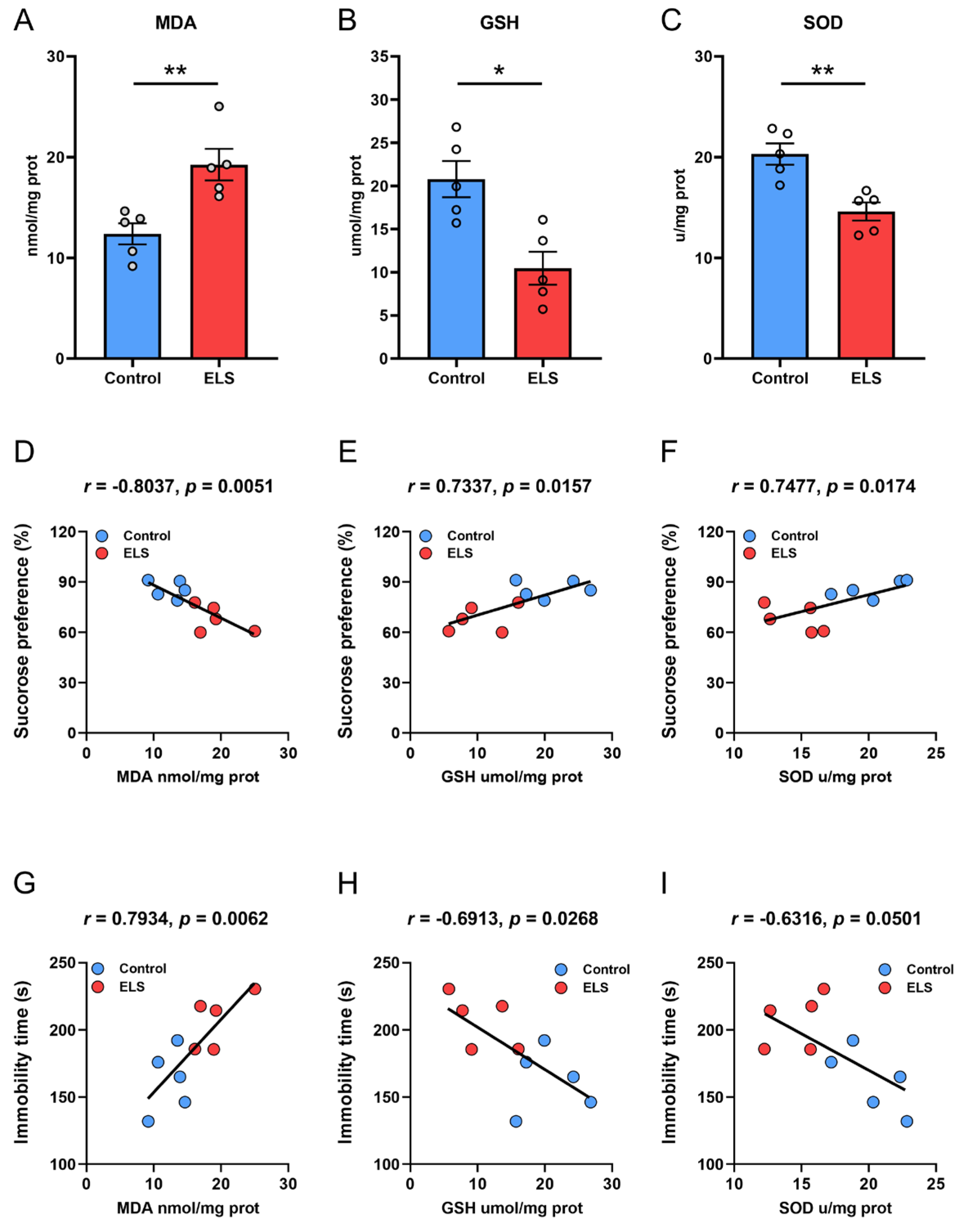
3.4. Correlation Analysis Between the Level of Lipid Peroxidation in the PFC and Behavior in ELS Mice
4. Discussion
5. Significance and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEs | Adverse childhood experiences |
| CSDS | Chronic social defeat stress |
| ELS | Early life stress |
| FST | Forced swim test |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| OFT | Open field test |
| PBS | Phosphate-buffered saline |
| PFC | Prefrontal cortex |
| ROS | Reactive oxygen species |
| RT-qPCR | Real-time quantitative PCR |
| SOD | Superoxide dismutase |
| SPT | Sucrose preference test |
| TST | Tail suspension test |
| YLDs | Years of life lost to disability |
References
- World Health Organization. World Mental Health Today: Latest Data; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- LeMoult, J.; Humphreys, K.L.; Tracy, A.; Hoffmeister, J.A.; Ip, E.; Gotlib, I.H. Meta-Analysis: Exposure to Early Life Stress and Risk for Depression in Childhood and Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; D’Arcy, C.; Meng, X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: Systematic review, meta-analysis, and proportional attributable fractions. Psychol. Med. 2016, 46, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Nanni, V.; Uher, R.; Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am. J. Psychiatry 2012, 169, 141–151. [Google Scholar] [CrossRef]
- Nielsen, J.D.; Mennies, R.J.; Olino, T.M. Application of a diathesis-stress model to the interplay of cortical structural development and emerging depression in youth. Clin. Psychol. Rev. 2020, 82, 101922. [Google Scholar] [CrossRef]
- Duman, C.H.; Duman, R.S. Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci. Lett. 2015, 601, 20–29. [Google Scholar] [CrossRef]
- Soztutar, E.; Colak, E.; Ulupinar, E. Gender- and anxiety level-dependent effects of perinatal stress exposure on medial prefrontal cortex. Exp. Neurol. 2016, 275 Pt 2, 274–284. [Google Scholar] [CrossRef]
- Rincel, M.; Lepinay, A.L.; Janthakhin, Y.; Soudain, G.; Yvon, S.; Da Silva, S.; Joffre, C.; Aubert, A.; Sere, A.; Laye, S.; et al. Maternal high-fat diet and early life stress differentially modulate spine density and dendritic morphology in the medial prefrontal cortex of juvenile and adult rats. Brain Struct. Funct. 2018, 223, 883–895. [Google Scholar] [CrossRef]
- Galts, C.P.C.; Bettio, L.E.B.; Jewett, D.C.; Yang, C.C.; Brocardo, P.S.; Rodrigues, A.L.S.; Thacker, J.S.; Gil-Mohapel, J. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019, 102, 56–84. [Google Scholar] [CrossRef]
- Sowa-Kucma, M.; Styczen, K.; Siwek, M.; Misztak, P.; Nowak, R.J.; Dudek, D.; Rybakowski, J.K.; Nowak, G.; Maes, M. Lipid Peroxidation and Immune Biomarkers Are Associated with Major Depression and Its Phenotypes, Including Treatment-Resistant Depression and Melancholia. Neurotox. Res. 2018, 33, 448–460. [Google Scholar] [CrossRef]
- Abelaira, H.M.; Veron, D.C.; de Moura, A.B.; Carlessi, A.S.; Borba, L.A.; Botelho, M.E.M.; Andrade, N.M.; Martinello, N.S.; Zabot, G.C.; Joaquim, L.; et al. Sex differences on the behavior and oxidative stress after ketamine treatment in adult rats subjected to early life stress. Brain Res. Bull. 2021, 172, 129–138. [Google Scholar] [CrossRef]
- Visentin, A.P.V.; Colombo, R.; Scotton, E.; Fracasso, D.S.; da Rosa, A.R.; Branco, C.S.; Salvador, M. Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxidative Med. Cell. Longev. 2020, 2020, 2972968. [Google Scholar] [CrossRef]
- Ruigrok, S.R.; Yim, K.; Emmerzaal, T.L.; Geenen, B.; Stoberl, N.; den Blaauwen, J.L.; Abbink, M.R.; Kiliaan, A.J.; van Schothorst, E.M.; Kozicz, T.; et al. Effects of early-life stress on peripheral and central mitochondria in male mice across ages. Psychoneuroendocrinology 2021, 132, 105346. [Google Scholar] [CrossRef]
- Gawryluk, J.W.; Wang, J.F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mon, M.A.; Ortega, M.A.; Garcia-Montero, C.; Fraile-Martinez, O.; Lahera, G.; Monserrat, J.; Gomez-Lahoz, A.M.; Molero, P.; Gutierrez-Rojas, L.; Rodriguez-Jimenez, R.; et al. Differential malondialdehyde (MDA) detection in plasma samples of patients with major depressive disorder (MDD): A potential biomarker. J. Int. Med. Res. 2022, 50, 3000605221094995. [Google Scholar] [CrossRef] [PubMed]
- Khanzode, S.D.; Dakhale, G.N.; Khanzode, S.S.; Saoji, A.; Palasodkar, R. Oxidative damage and major depression: The potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003, 8, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Costa, I.; Barbosa, D.J.; Benfeito, S.; Silva, V.; Chavarria, D.; Borges, F.; Remiao, F.; Silva, R. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol. Ther. 2023, 244, 108373. [Google Scholar] [CrossRef]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xin, Y.; Zhang, J.; Wang, S.; Yang, Z.; Liu, C. Hydrogen sulfide alleviates the anxiety-like and depressive-like behaviors of type 1 diabetic mice via inhibiting inflammation and ferroptosis. Life Sci. 2021, 278, 119551. [Google Scholar] [CrossRef]
- Cao, H.; Zuo, C.; Huang, Y.; Zhu, L.; Zhao, J.; Yang, Y.; Jiang, Y.; Wang, F. Hippocampal proteomic analysis reveals activation of necroptosis and ferroptosis in a mouse model of chronic unpredictable mild stress-induced depression. Behav. Brain Res. 2021, 407, 113261. [Google Scholar] [CrossRef]
- Jiao, H.; Yang, H.; Yan, Z.; Chen, J.; Xu, M.; Jiang, Y.; Liu, Y.; Xue, Z.; Ma, Q.; Li, X.; et al. Traditional Chinese Formula Xiaoyaosan Alleviates Depressive-Like Behavior in CUMS Mice by Regulating PEBP1-GPX4-Mediated Ferroptosis in the Hippocampus. Neuropsychiatr. Dis. Treat. 2021, 17, 1001–1019. [Google Scholar] [CrossRef]
- Pena, C.J.; Smith, M.; Ramakrishnan, A.; Cates, H.M.; Bagot, R.C.; Kronman, H.G.; Patel, B.; Chang, A.B.; Purushothaman, I.; Dudley, J.; et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat. Commun. 2019, 10, 5098. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Yin, C.Y.; Zhu, L.J.; Zhu, X.H.; Xu, C.; Luo, C.X.; Chen, H.; Zhu, D.Y.; Zhou, Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Zeng, G.R.; Liu, J.Q.; Luo, Z.S.; Zhang, L.; Dai, X.M.; Fang, W.T.; Zhang, J.; Chen, X.C. Ganoderma Lucidum Triterpenoids Improve Maternal Separation-Induced Anxiety- and Depression-like Behaviors in Mice by Mitigating Inflammation in the Periphery and Brain. Nutrients 2022, 14, 2268. [Google Scholar] [CrossRef] [PubMed]
- Pena, C.J.; Kronman, H.G.; Walker, D.M.; Cates, H.M.; Bagot, R.C.; Purushothaman, I.; Issler, O.; Loh, Y.E.; Leong, T.; Kiraly, D.D.; et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 2017, 356, 1185–1188. [Google Scholar] [CrossRef]
- Mi, X.; Ruan, X.; Lin, R.; Huang, S.; Cai, P.; Chen, X.; Liao, J.; Dai, X. Intranasal administration of Ganoderma lucidum-derived exosome-like nanovesicles ameliorates cognitive impairment by reducing inflammation in a mouse model of Alzheimer’s disease. Front. Pharmacol. 2025, 16, 1572771. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Wang, R.; Shi, M.; Zhang, Q.; Zhang, J.; Sun, L.; Jia, Y.; Zhu, Z.; Xu, T.; Zhang, Y. The association of early life factors with depression and anxiety in adults aged 40–69 years: A population-based cohort study. Transl. Psychiatry 2024, 14, 299. [Google Scholar] [CrossRef]
- Heim, C.; Nemeroff, C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, A.; Liu, W.; Tao, F.; Sun, Y. Childhood separation from parents with cognitive and psychopathological outcomes in adolescence. Dev. Sci. 2023, 26, e13324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Liu, L.; Mo, X.; He, D.; Chen, X.; Xiao, R.; Cheng, Q.; Fatima, M.; Du, Y.; et al. Maternal separation regulates sensitivity of stress-induced depression in mice by affecting hippocampal metabolism. Physiol. Behav. 2024, 279, 114530. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, K.; Lin, H.; Cui, S.; Shen, C.; Wen, W.; Mo, H.; Dong, Z.; Bai, S.; Yang, L.; et al. Early-Life Stress Induces Depression-Like Behavior and Synaptic-Plasticity Changes in a Maternal Separation Rat Model: Gender Difference and Metabolomics Study. Front. Pharmacol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Galler, J.R.; Bryce, C.P.; Waber, D.; Hock, R.S.; Exner, N.; Eaglesfield, D.; Fitzmaurice, G.; Harrison, R. Early childhood malnutrition predicts depressive symptoms at ages 11–17. J. Child Psychol. Psychiatry 2010, 51, 789–798. [Google Scholar] [CrossRef]
- Danese, A.; Moffitt, T.E.; Harrington, H.; Milne, B.J.; Polanczyk, G.; Pariante, C.M.; Poulton, R.; Caspi, A. Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009, 163, 1135–1143. [Google Scholar] [CrossRef]
- Hughes, K.; Bellis, M.A.; Hardcastle, K.A.; Sethi, D.; Butchart, A.; Mikton, C.; Jones, L.; Dunne, M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e356–e366. [Google Scholar] [CrossRef]
- Goodwin, R.D.; Stein, M.B. Association between childhood trauma and physical disorders among adults in the United States. Psychol. Med. 2004, 34, 509–520. [Google Scholar] [CrossRef]
- Flohe, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free. Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Gregolin, C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta 1985, 839, 62–70. [Google Scholar] [CrossRef]
- Yant, L.J.; Ran, Q.; Rao, L.; Van Remmen, H.; Shibatani, T.; Belter, J.G.; Motta, L.; Richardson, A.; Prolla, T.A. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free. Radic. Biol. Med. 2003, 34, 496–502. [Google Scholar] [CrossRef]
- Scheerer, P.; Borchert, A.; Krauss, N.; Wessner, H.; Gerth, C.; Hohne, W.; Kuhn, H. Structural basis for catalytic activity and enzyme polymerization of phospholipid hydroperoxide glutathione peroxidase-4 (GPx4). Biochemistry 2007, 46, 9041–9049. [Google Scholar] [CrossRef]
- da Silva, T.N.X.; Angeli, J.P.F.; Ingold, I. GPX4: Old lessons, new features. Biochem. Soc. Trans. 2022, 50, 1205–1213. [Google Scholar] [CrossRef]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef]
- Portugal-Nunes, C.; Castanho, T.C.; Amorim, L.; Moreira, P.S.; Mariz, J.; Marques, F.; Sousa, N.; Santos, N.C.; Palha, J.A. Iron Status is Associated with Mood, Cognition, and Functional Ability in Older Adults: A Cross-Sectional Study. Nutrients 2020, 12, 3594. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free. Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Sears, S.M.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Bilici, M.; Efe, H.; Koroglu, M.A.; Uydu, H.A.; Bekaroglu, M.; Deger, O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J. Affect. Disord. 2001, 64, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kucma, M.; Styczen, K.; Siwek, M.; Misztak, P.; Nowak, R.J.; Dudek, D.; Rybakowski, J.K.; Nowak, G.; Maes, M. Are there differences in lipid peroxidation and immune biomarkers between major depression and bipolar disorder: Effects of melancholia, atypical depression, severity of illness, episode number, suicidal ideation and prior suicide attempts. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bonifacio, K.L.; Morelli, N.R.; Vargas, H.O.; Moreira, E.G.; Stoyanov, D.S.; Barbosa, D.S.; Carvalho, A.F.; Nunes, S.O.V. Generalized Anxiety Disorder (GAD) and Comorbid Major Depression with GAD Are Characterized by Enhanced Nitro-oxidative Stress, Increased Lipid Peroxidation, and Lowered Lipid-Associated Antioxidant Defenses. Neurotox. Res. 2018, 34, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Ballinger, S.W.; Darley-Usmar, V.M.; Landar, A. Free radicals, mitochondria, and oxidized lipids: The emerging role in signal transduction in vascular cells. Circ. Res. 2006, 99, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Walker, T.; Ayton, S. Neuroferroptosis in health and diseases. Nat. Rev. Neurosci. 2025, 26, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Almulla, A.F.; Thipakorn, Y.; Algon, A.A.A.; Tunvirachaisakul, C.; Al-Hakeim, H.K.; Maes, M. Reverse cholesterol transport and lipid peroxidation biomarkers in major depression and bipolar disorder: A systematic review and meta-analysis. Brain Behav. Immun. 2023, 113, 374–388. [Google Scholar] [CrossRef]
- Thorwald, M.A.; Godoy-Lugo, J.A.; Garcia, G.; Silva, J.; Kim, M.; Christensen, A.; Mack, W.J.; Head, E.; O’Day, P.A.; Benayoun, B.A.; et al. Iron-associated lipid peroxidation in Alzheimer’s disease is increased in lipid rafts with decreased ferroptosis suppressors, tested by chelation in mice. Alzheimers Dement. 2025, 21, e14541. [Google Scholar] [CrossRef]
- Yan, H.F.; Zou, T.; Tuo, Q.Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free. Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Mohamed, H.M.; Mahmoud, A.M. Chronic exposure to the opioid tramadol induces oxidative damage, inflammation and apoptosis, and alters cerebral monoamine neurotransmitters in rats. Biomed. Pharmacother. 2019, 110, 239–247. [Google Scholar] [CrossRef]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Sun, Z.W.; Zhang, L.; Zhu, S.J.; Chen, W.C.; Mei, B. Excitotoxicity effects of glutamate on human neuroblastoma SH-SY5Y cells via oxidative damage. Neurosci. Bull. 2010, 26, 8–16. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Li, H.; Song, L.; Cen, M.; Fu, X.; Gao, X.; Zuo, Q.; Wu, J. Oxidative balance scores and depressive symptoms: Mediating effects of oxidative stress and inflammatory factors. J. Affect. Disord. 2023, 334, 205–212. [Google Scholar] [CrossRef]
| Gene | Primer Sequences | |
|---|---|---|
| Gpx4 | F | GATGGAGCCCATTCCTGAACC |
| R | CCCTGTACTTATCCAGGCAGA | |
| Slc3a2 | F | GGTGCTCAACTTCCGAGATTC |
| R | TCATAAGGATTCAGGCTCAGGTT | |
| Slc7a11 | F | AGGGCATACTCCAGAACACG |
| R | GGACCAAAGACCTCCAGAATG | |
| Tfr1 | F | CAGCAAAGTCTGGCGAGATG |
| R | GAATGCCACATAACCCTCGG | |
| β-actin | F | TGTCCACCTTCCAGCAGATGT |
| R | AGCTCAGTAACAGTCCGCCTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, X.; Ye, Z.-L.; Zhang, X.-J.; Dai, X.-M.; Luo, Z.-S. Analysis of the Correlation Between Depression-like Behaviors and Lipid Peroxidation in the Prefrontal Cortex of Mice: The Impact of Early Life Stress. Brain Sci. 2025, 15, 1112. https://doi.org/10.3390/brainsci15101112
Mi X, Ye Z-L, Zhang X-J, Dai X-M, Luo Z-S. Analysis of the Correlation Between Depression-like Behaviors and Lipid Peroxidation in the Prefrontal Cortex of Mice: The Impact of Early Life Stress. Brain Sciences. 2025; 15(10):1112. https://doi.org/10.3390/brainsci15101112
Chicago/Turabian StyleMi, Xue, Zi-Ling Ye, Xu-Jun Zhang, Xiao-Man Dai, and Zhou-Song Luo. 2025. "Analysis of the Correlation Between Depression-like Behaviors and Lipid Peroxidation in the Prefrontal Cortex of Mice: The Impact of Early Life Stress" Brain Sciences 15, no. 10: 1112. https://doi.org/10.3390/brainsci15101112
APA StyleMi, X., Ye, Z.-L., Zhang, X.-J., Dai, X.-M., & Luo, Z.-S. (2025). Analysis of the Correlation Between Depression-like Behaviors and Lipid Peroxidation in the Prefrontal Cortex of Mice: The Impact of Early Life Stress. Brain Sciences, 15(10), 1112. https://doi.org/10.3390/brainsci15101112






