Collateral Status Evaluation Using CT Angiography and Perfusion Source Images in Acute Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Image Acquisition and Processing
2.3. Imaging Analyses
2.4. Statistical Analyses
2.5. Generative AI Disclosure
3. Results
3.1. Patient Population
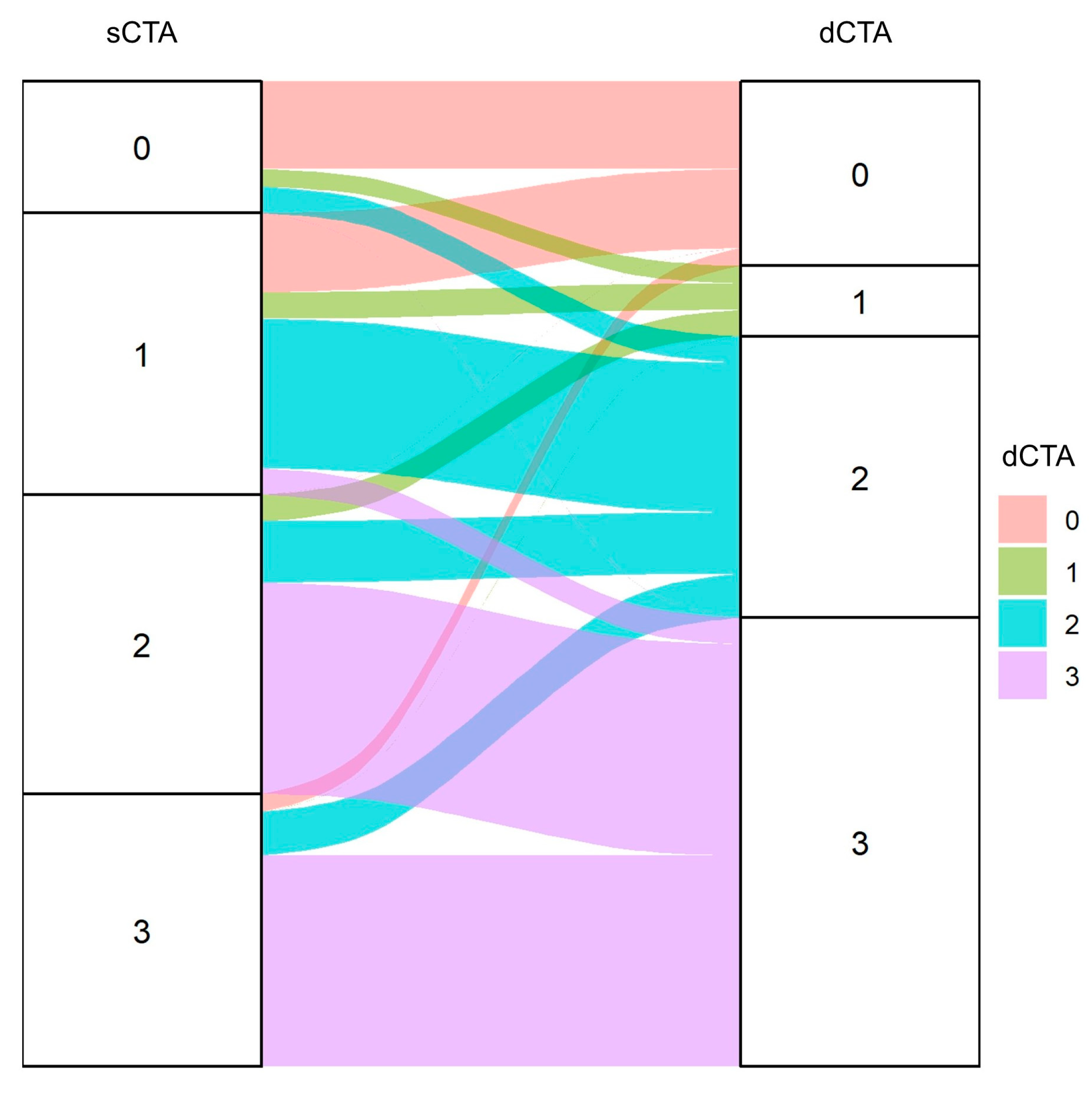
3.2. Comparison and Reliability of sCTA and dCTA Scores
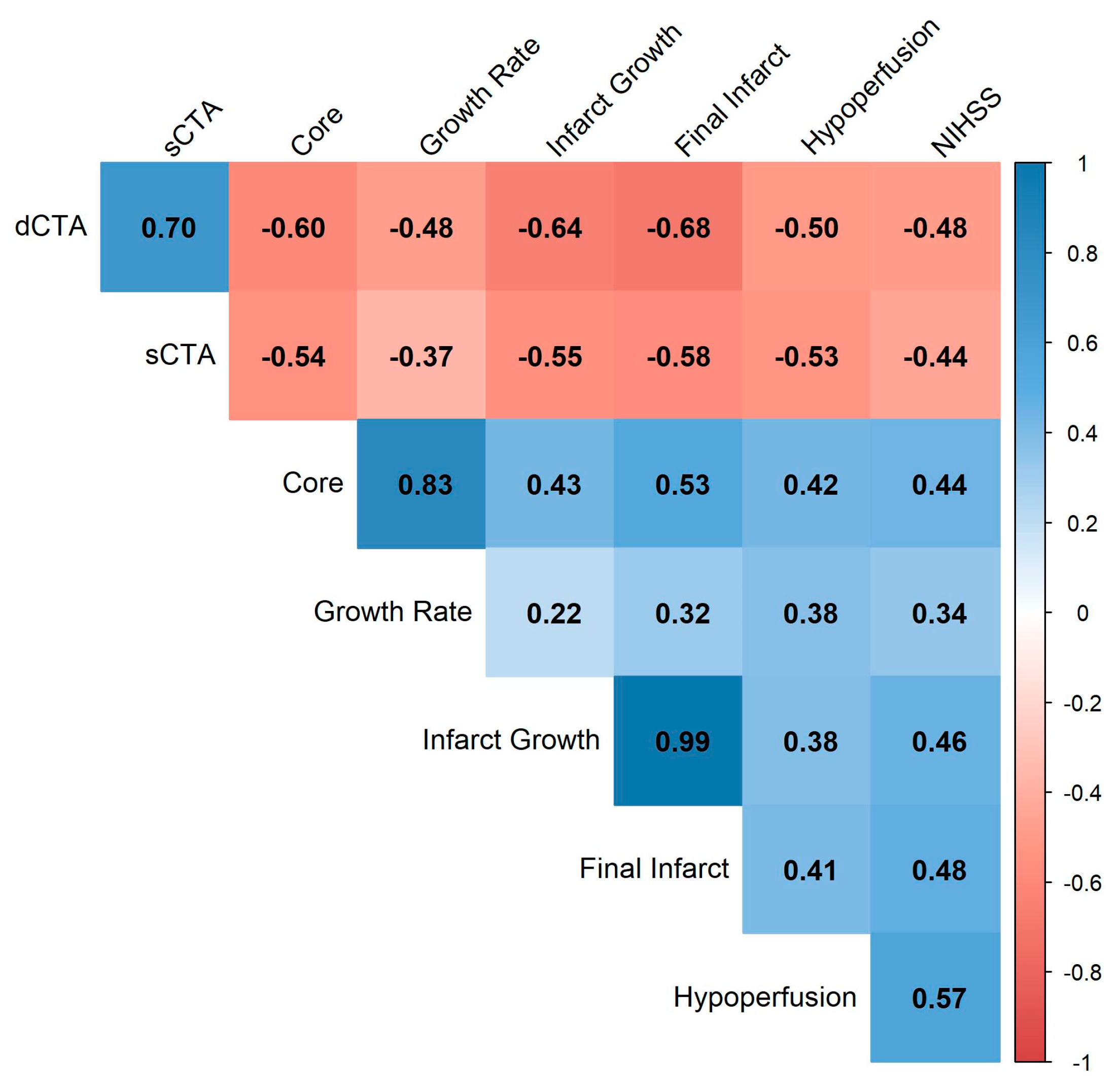
3.3. Association of Collateral Scores with Imaging Outcomes
3.4. Impact of Collaterals on Infarct Temporal Evolution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | acute ischemic stroke |
| CTP | computed tomography perfusion |
| dCTA | dynamic computed tomography angiography |
| sCTA | single-phase computed tomography angiography |
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, E344–E418. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Castonguay, A.C.; Siegler, J.E.; Nagel, S.; Lansberg, M.G.; de Havenon, A.; Sheth, S.A.; Abdalkader, M.; Tsai, J.P.; Albers, G.W.; et al. Mechanical Thrombectomy in the Late Presentation of Anterior Circulation Large Vessel Occlusion Stroke: A Guideline From the Society of Vascular and Interventional Neurology Guidelines and Practice Standards Committee. Stroke Vasc. Interv. Neurol. 2023, 3, 512. [Google Scholar] [CrossRef]
- Jovin, T.G.; Nogueira, R.G.; Lansberg, M.G.; Demchuk, A.M.; Martins, S.O.; Mocco, J.; Ribo, M.; Jadhav, A.P.; Ortega-Gutierrez, S.; Hill, M.D.; et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): A systematic review and individual patient data meta-analysis. Lancet 2022, 399, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; Van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van Der Lugt, A.; De Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Turc, G. Mechanical Thrombectomy in Late-Time Windows: Time to Treat More Patients. Stroke 2023, 54, 731–732. [Google Scholar] [CrossRef]
- Martins, S.O.; Mont’Alverne, F.; Rebello, L.C.; Abud, D.G.; Silva, G.S.; Lima, F.O.; Parente, B.S.; Nakiri, G.S.; Faria, M.B.; Frudit, M.E.; et al. Thrombectomy for Stroke in the Public Health Care System of Brazil. N. Engl. J. Med. 2020, 382, 2316–2326. [Google Scholar] [CrossRef]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Olthuis, S.G.; Pirson, F.A.V.; Pinckaers, F.M.; Hinsenveld, W.H.; Nieboer, D.; Ceulemans, A.; Knapen, R.R.; Robbe, M.Q.; Berkhemer, O.A.; van Walderveen, M.A.; et al. Endovascular treatment versus no endovascular treatment after 6–24 h in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: A multicentre, open-label, blinded-endpoint, randomised, controlled, phase 3 trial. Lancet 2023, 401, 1371–1380. [Google Scholar] [CrossRef]
- Lyndon, D.; van den Broek, M.; Niu, B.; Yip, S.; Rohr, A.; Settecase, F. Hypoperfusion Intensity Ratio Correlates with CTA Collateral Status in Large-Vessel Occlusion Acute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2021, 42, 1380–1386. [Google Scholar] [CrossRef]
- Christoforidis, G.A.; Vakil, P.; Ansari, S.A.; Dehkordi, F.H.; Carroll, T.J. Impact of pial collaterals on infarct growth rate in experimental acute ischemic stroke. AJNR Am. J. Neuroradiol. 2017, 38, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Liebeskind, D.S. Collateral circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef]
- Bang, O.Y.; Goyal, M.; Liebeskind, D.S. Collateral Circulation in Ischemic Stroke: Assessment Tools and Therapeutic Strategies. Stroke 2015, 46, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.C.B.; Pacheco, F.T.; Rocha, A.J. Collateral blood vessels in acute ischemic stroke: A physiological window to predict future outcomes. Arq. Neuropsiquiatr. 2016, 74, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Boers, A.M.; Jansen, I.G.; Berkhemer, O.A.; Yoo, A.J.; Lingsma, H.F.; Slump, C.H.; Roos, Y.B.; van Oostenbrugge, R.J.; Dippel, D.W.; van der Lugt, A.; et al. Collateral status and tissue outcome after intra-arterial therapy for patients with acute ischemic stroke. J. Cereb. Blood Flow. Metab. 2017, 37, 3589–3598. [Google Scholar] [CrossRef]
- Heit, J.J.; Zaharchuk, G.; Wintermark, M. Advanced Neuroimaging of Acute Ischemic Stroke. Neuroimaging Clin. N. Am. 2018, 28, 585–597. [Google Scholar] [CrossRef]
- Seker, F.; Potreck, A.; Möhlenbruch, M.; Bendszus, M.; Pham, M. Comparison of four different collateral scores in acute ischemic stroke by CT angiography. J. Neurointerv. Surg. 2016, 8, 1116–1118. [Google Scholar] [CrossRef]
- Tan, I.Y.L.; Demchuk, A.M.; Hopyan, J.; Zhang, L.; Gladstone, D.; Wong, K.; Martin, M.; Symons, S.P.; Fox, A.J.; Aviv, R.I. CT angiography clot burden score and collateral score: Correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am. J. Neuroradiol. 2009, 30, 525–531. [Google Scholar] [CrossRef]
- Menon, B.K.; d’Esterre, C.D.; Qazi, E.M.; Almekhlafi, M.; Hahn, L.; Demchuk, A.M.; Goyal, M. Multiphase CT Angiography: A New Tool for the Imaging Triage of Patients with Acute Ischemic Stroke. Radiology 2015, 275, 510–520. [Google Scholar] [CrossRef]
- Van Den Wijngaard, I.R.; Boiten, J.; Holswilder, G.; Algra, A.; Dippel, D.W.; Velthuis, B.K.; Wermer, M.J.; Van Walderveen, M.A. Impact of collateral status evaluated by dynamic computed tomographic angiography on clinical outcome in patients with ischemic stroke. Stroke 2015, 46, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Van Den Wijngaard, I.R.; Holswilder, G.; Wermer, M.J.H.; Boiten, J.; Algra, A.; Dippel, D.W.J.; Dankbaar, J.W.; Velthuis, B.K.; Boers, A.M.M.; Majoie, C.B.L.M.; et al. Assessment of collateral status by dynamic ct angiography in acute mca stroke: Timing of acquisition and relationship with final infarct volume. AJNR Am. J. Neuroradiol. 2016, 37, 1231–1236. [Google Scholar] [CrossRef]
- Potreck, A.; Scheidecker, E.; Weyland, C.S.; Neuberger, U.; Herweh, C.; Möhlenbruch, M.A.; Chen, M.; Nagel, S.; Bendszus, M.; Seker, F. RAPID CT Perfusion–Based Relative CBF Identifies Good Collateral Status Better Than Hypoperfusion Intensity Ratio, CBV-Index, and Time-to-Maximum in Anterior Circulation Stroke. AJNR Am. J. Neuroradiol. 2022, 43, 960–965. [Google Scholar] [CrossRef] [PubMed]
- McDougall, C.C.; Chan, L.; Sachan, S.; Guo, J.; Sah, R.G.; Menon, B.K.; Demchuk, A.M.; Hill, M.D.; Forkert, N.D.; d’Esterre, C.D.; et al. Dynamic CTA-Derived Perfusion Maps Predict Final Infarct Volume: The Simple Perfusion Reconstruction Algorithm. AJNR Am. J. Neuroradiol. 2020, 41, 2034–2040. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Klein, P.; Berberich, A.; Nagel, S.; Abdalkader, M.; Herning, A.; Chen, Y.; Huo, X.; Miao, Z.; Sheth, S.A.; et al. Late Window Imaging Selection for Endovascular Therapy of Large Vessel Occlusion Stroke: An International Survey. Stroke Vasc. Interv. Neurol. 2023, 3, e000595. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Xu, J.; Dai, F.; Wang, B.; Wang, Y.; Li, J.; Pan, L.; Liu, J.; Liu, H.; He, S. Predictive Value of CT Perfusion in Hemorrhagic Transformation after Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Brain Sci. 2023, 13, 156. [Google Scholar] [CrossRef]
- Bivard, A.; Levi, C.; Spratt, N.; Parsons, M. Perfusion CT in acute stroke: A comprehensive analysis of infarct and penumbra. Radiology 2013, 267, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Puetz, V.; Dzialowski, I.; Hill, M.D.; Subramaniam, S.; Sylaja, P.N.; Krol, A.; O'Reilly, C.; Hudon, M.E.; Hu, W.Y.; Coutts, S.B.; et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: The clot burden score. Int. J. Stroke 2008, 3, 230–236. [Google Scholar] [CrossRef]
- Nael, K.; Sakai, Y.; Larson, J.; Goldstein, J.; Deutsch, J.; Awad, A.J.; Pawha, P.; Aggarwal, A.; Fifi, J.; Deleacy, R.; et al. CT Perfusion collateral index in assessment of collaterals in acute ischemic stroke with delayed presentation: Comparison to single phase CTA. J. Neuroradiol. 2022, 49, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Vagal, A.; Menon, B.K.; Foster, L.D.; Livorine, A.; Yeatts, S.D.; Qazi, E.; d’Esterre, C.; Shi, J.; Demchuk, A.M.; Hill, M.D.; et al. Association between CT Angiogram Collaterals and CT Perfusion in the Interventional Management of Stroke III Trial. Stroke 2016, 47, 535–538. [Google Scholar] [CrossRef]
- Nannoni, S.; Cereda, C.W.; Sirimarco, G.; Lambrou, D.; Strambo, D.; Eskandari, A.; Dunet, V.; Wintermark, M.; Michel, P. Collaterals are a major determinant of the core but not the penumbra volume in acute ischemic stroke. Neuroradiology 2019, 61, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.S.; Eaton, R.G.; Cua, S.; Dornbos, I.I.I.D.; Hoang, N.; Schunemann, V.; Nimjee, S.; Youssef, P.; Powers, C.J. Scoring of Middle Cerebral Artery Collaterals Predicts RAPID CT-Perfusion Analysis and Short-Term Outcomes in Acute Ischemic Stroke Patients Undergoing Thrombectomy. World Neurosurg. 2020, 135, e494–e499. [Google Scholar] [CrossRef] [PubMed]
- Voleti, S.; Aziz, Y.N.; Vidovich, J.; Corcoran, B.; Zhang, B.; Mistry, E.; Khandwala, V.; Khatri, P.; Tomsick, T.; Wang, L.; et al. Association Between CT Angiogram Collaterals and CT Perfusion in Delayed Time Windows for Large Vessel Occlusion Ischemic Strokes. J. Stroke Cerebrovasc. Dis. 2022, 31, 106263. [Google Scholar] [CrossRef]
- Olivot, J.M.; Mlynash, M.; Inoue, M.; Marks, M.P.; Wheeler, H.M.; Kemp, S.; Straka, M.; Zaharchuk, G.; Bammer, R.; Lansberg, M.G.; et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 cohort. Stroke 2014, 45, 1018–1023. [Google Scholar] [CrossRef]
- Lu, S.S.; Zhang, X.; Xu, X.Q.; Cao, Y.Z.; Zhao, L.B.; Liu, Q.H.; Wu, F.Y.; Liu, S.; Shi, H.B. Comparison of CT angiography collaterals for predicting target perfusion profile and clinical outcome in patients with acute ischemic stroke. Eur. Radiol. 2019, 29, 4922–4929. [Google Scholar] [CrossRef]
- Tian, H.; Chen, C.; Garcia-Esperon, C.; Parsons, M.W.; Lin, L.; Levi, C.R.; Bivard, A. Dynamic CT but Not Optimized Multiphase CT Angiography Accurately Identifies CT Perfusion Target Mismatch Ischemic Stroke Patients. Front Neurol. 2019, 10, 1130. [Google Scholar] [CrossRef] [PubMed]


| Single-Phase CTA Collaterals | CTP Source Images Collaterals | |||||
|---|---|---|---|---|---|---|
| Poor | Good | p Value | Poor | Good | p Value | |
| Number (n) | 47 | 65 | 29 | 83 | ||
| Age, median [IQR] | 64 [56, 77] | 62 [51, 73] | 0.26 | 58 [51, 71] | 64 [56, 76] | 0.12 |
| Sex, male (%) | 27 (57.4) | 32 (49.2) | 0.50 | 19 (65.5) | 40 (48.2) | 0.16 |
| Intravenous alteplase, n (%) | 13 (27.7) | 17 (26.2) | 1.00 | 7 (24.1) | 23 (27.7) | 0.90 |
| Endovascular treatment, n (%) | 1 (2.1) | 1 (1.5) | 1.00 | 0 (0.0) | 2 (2.4) | 0.98 |
| Ictus in minutes, median [IQR] | 120 [67, 180] | 120 [60, 210] | 0.64 | 120 [60, 180] | 135 [72, 210] | 0.57 |
| NIHSS, median [IQR] | 15 [13, 17] | 9 [6, 14] | <0.001 | 15 [13, 16] | 12 [6, 15] | 0.01 |
| 24 h NIHSS, median [IQR] | 12 [7, 14] | 5 [3, 9] | <0.001 | 13 [10, 14] | 5 [2, 10] | <0.001 |
| SBP—mean mm Hg (SD) | 150 [134, 180] | 150 [130, 160] | 0.11 | 148 [120, 200] | 150 [135, 166] | 0.93 |
| DBP—mean mm Hg (SD) | 90 [80, 100] | 90 [80, 100] | 0.19 | 90 [77, 100] | 90 [80, 100] | 0.97 |
| Baseline glucose, median [IQR] | 143 [123, 202] | 120 [99, 147] | <0.001 | 143 [121, 213] | 126 [104, 162] | 0.074 |
| Medical history | ||||||
| Atrial fibrillation—n. (%) | 14 (30.4) | 10 (16.4) | 0.17 | 6 (21.4) | 18 (22.8) | 1.00 |
| Diabetes mellitus—n. (%) | 13 (28.3) | 12 (19.7) | 0.42 | 9 (32.1) | 16 (20.3) | 0.31 |
| Hypertension—n. (%) | 35 (76.1) | 35 (57.4) | 0.07 | 18 (64.3) | 52 (65.8) | 1.00 |
| Dyslipidemia, n. (%) | 3 (6.5) | 4 (6.6) | 1.00 | 0 (0.0) | 7 (8.9) | 0.236 |
| Ischemic stroke—n. (%) | 4 (8.7) | 7 (11.5) | 0.88 | 2 (7.1) | 9 (11.4) | 0.78 |
| Myocardial infarction—n. (%) | 5 (10.9) | 5 (8.2) | 0.89 | 3 (10.7) | 7 (8.9) | 1.00 |
| Peripheral artery disease—n. (%) | 2 (4.3) | 2 (3.3) | 1.00 | 3 (10.7) | 1 (1.3) | 0.09 |
| Imaging | ||||||
| Occlusion level on CTA, n (%) | <0.001 | <0.001 | ||||
| ICA-I | 3 (6.4) | 4 (6.2) | 2 (6.9) | 5 (6.0) | ||
| ICA-T | 17 (36.2) | 5 (7.7) | 12 (41.4) | 10 (12.0) | ||
| M1 proximal | 13 (27.7) | 7 (10.8) | 7 (24.1) | 13 (15.7) | ||
| M1 distal | 9 (19.1) | 12 (18.5) | 3 (10.3) | 18 (21.7) | ||
| M2 | 5 (10.6) | 18 (27.7) | 5 (17.2) | 18 (21.7) | ||
| M3 | 0 (0.0) | 16 (24.6) | 0 (0.0) | 16 (19.3) | ||
| M4 | 0 (0.0) | 3 (4.6) | 0 (0.0) | 3 (3.6) | ||
| Clot Burden Score, median [IQR] | 4 [2, 6] | 9 [6, 10] | <0.001 | 4 [2, 6] | 7 [5, 9] | <0.001 |
| Hyperdense artery sign, n. (%) | 38 (80.9) | 25 (39.1) | <0.001 | 22 (75.9) | 41 (50.0) | 0.03 |
| Thrombus length in mm, median [IQR] | 18.25 [13.32, 24.63] | 9.09 [4.36, 12.90] | <0.001 | 15.40 [10.84, 22.50] | 11.73 [5.52, 17.93] | 0.02 |
| Tandem occlusion, n. (%) | 5 (11.6) | 2 (3.3) | 0.20 | 5 (18.5) | 2 (2.6) | 0.01 |
| sCTA | dCTA | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| 0 | 10 | 2 | 3 | 0 |
| 1 | 9 | 3 | 17 | 3 |
| 2 | 0 | 3 | 7 | 24 |
| 3 | 2 | 0 | 5 | 24 |
| Single-Phase CTA Collaterals | CTP Source Images Collaterals | |||||
|---|---|---|---|---|---|---|
| Poor | Good | p Value | Poor | Good | p Value | |
| Number (n) | 47 | 65 | 29 | 83 | ||
| Core volume (mL), median [IQR] | 21.5 [8.8, 36.0] | 3.9 [1.1, 7.6] | <0.001 | 27.1 [11.0, 66.8] | 4.63 [1.3, 11.8] | <0.001 |
| Hypoperfusion volume (mL), median [IQR] | 113.3 [82.3, 135.7] | 33.9 [13.3, 81.2] | <0.001 | 123.3 [83.6, 151.8] | 51.4 [22.1, 99.1] | <0.001 |
| Infarct growth volume (mL), median [IQR] | 118.6 [48.4, 290.5] | 24.9 [7.6, 42.9] | <0.001 | 182.6 [107.6, 345.4] | 27.5 [11.8, 78.3] | <0.001 |
| Final infarct volume (mL), median [IQR] | 120.6 [53.1, 303.4] | 25.4 [12.2, 44.9] | <0.001 | 176.0 [101.3, 350.1] | 27.3 [13.1, 92.0] | <0.001 |
| Acute growth rate (mL/min), median [IQR] | 1.41 [0.1, 6.0] | 0.0 [0.0, 0.16] | <0.001 | 2.3 [0.4, 13.1] | 0.0 [0.0, 1.1] | <0.001 |
| sCTA | dCTA | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes | Model | β (95% CI) | R-Squared | p-Value | β (95% CI) | R-Squared | p-Value |
| Core volume | unadjusted | −13.22 (−16.97 to −9.46) | 0.32 | <0.01 | −12.68 (−15.92 to −9.44) | 0.36 | <0.01 |
| Adjusted * | −10.17 (−16.48 to −3.86) | 0.32 | <0.01 | −9.08 (−14.61 to −3.55) | 0.33 | <0.01 | |
| Hypoperfusion volume | Unadjusted | −29.64 (−39.06 to −20.21) | 0.27 | <0.01 | −30.96 (−38.82 to −23.09) | 0.37 | <0.01 |
| Adjusted * | −8.25 (−23.9 to 7.39) | 0.42 | 0.29 | −11.56 (−23.93 to 0.81) | 0.44 | 0.07 | |
| Infarct growth volume | Unadjusted | −62.25 (−87.56 to −36.93) | 0.24 | <0.01 | −65.88 (−87.22 to −44.54) | 0.33 | <0.01 |
| Adjusted * | −34.89 (−71.7 to 1.92) | 0.36 | 0.06 | −48.88 (−79.2 to −18.56) | 0.43 | <0.01 | |
| Final infarct volume | Unadjusted | −68.07 (−93.35 to −42.80) | 0.26 | <0.01 | −68.38 (−89.98 to −46.78) | 0.33 | <0.01 |
| Adjusted * | −45.69 (−78.6 to −12.78) | 0.40 | <0.01 | −49.29 (−80.25 to −18.33) | 0.43 | <0.01 | |
| Acute growth rate | Unadjusted | −3.73 (−6.39 to −1.06) | 0.07 | <0.01 | −4.76 (−7.02 to −2.49) | 0.15 | <0.01 |
| Adjusted * | −5.28 (−11.46 to 0.9) | 0.12 | 0.09 | −6.59 (−11.39 to −1.79) | 0.19 | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, H.C.B.R.; Dutra, B.G.; Gagliardi, V.; Gagliardi, R.J.; Pacheco, F.T.; Maia, A.C.M., Jr.; da Rocha, A.J. Collateral Status Evaluation Using CT Angiography and Perfusion Source Images in Acute Stroke Patients. Brain Sci. 2025, 15, 1092. https://doi.org/10.3390/brainsci15101092
Alves HCBR, Dutra BG, Gagliardi V, Gagliardi RJ, Pacheco FT, Maia ACM Jr., da Rocha AJ. Collateral Status Evaluation Using CT Angiography and Perfusion Source Images in Acute Stroke Patients. Brain Sciences. 2025; 15(10):1092. https://doi.org/10.3390/brainsci15101092
Chicago/Turabian StyleAlves, Heitor C. B. R., Bruna G. Dutra, Vivian Gagliardi, Rubens J. Gagliardi, Felipe T. Pacheco, Antonio C. M. Maia, Jr., and Antônio J. da Rocha. 2025. "Collateral Status Evaluation Using CT Angiography and Perfusion Source Images in Acute Stroke Patients" Brain Sciences 15, no. 10: 1092. https://doi.org/10.3390/brainsci15101092
APA StyleAlves, H. C. B. R., Dutra, B. G., Gagliardi, V., Gagliardi, R. J., Pacheco, F. T., Maia, A. C. M., Jr., & da Rocha, A. J. (2025). Collateral Status Evaluation Using CT Angiography and Perfusion Source Images in Acute Stroke Patients. Brain Sciences, 15(10), 1092. https://doi.org/10.3390/brainsci15101092






