Delayed Intracerebral Hemorrhage 15 Years After Indirect Revascularization in Moyamoya Disease: A Case Report and Review of the Literature
Abstract
1. Introduction
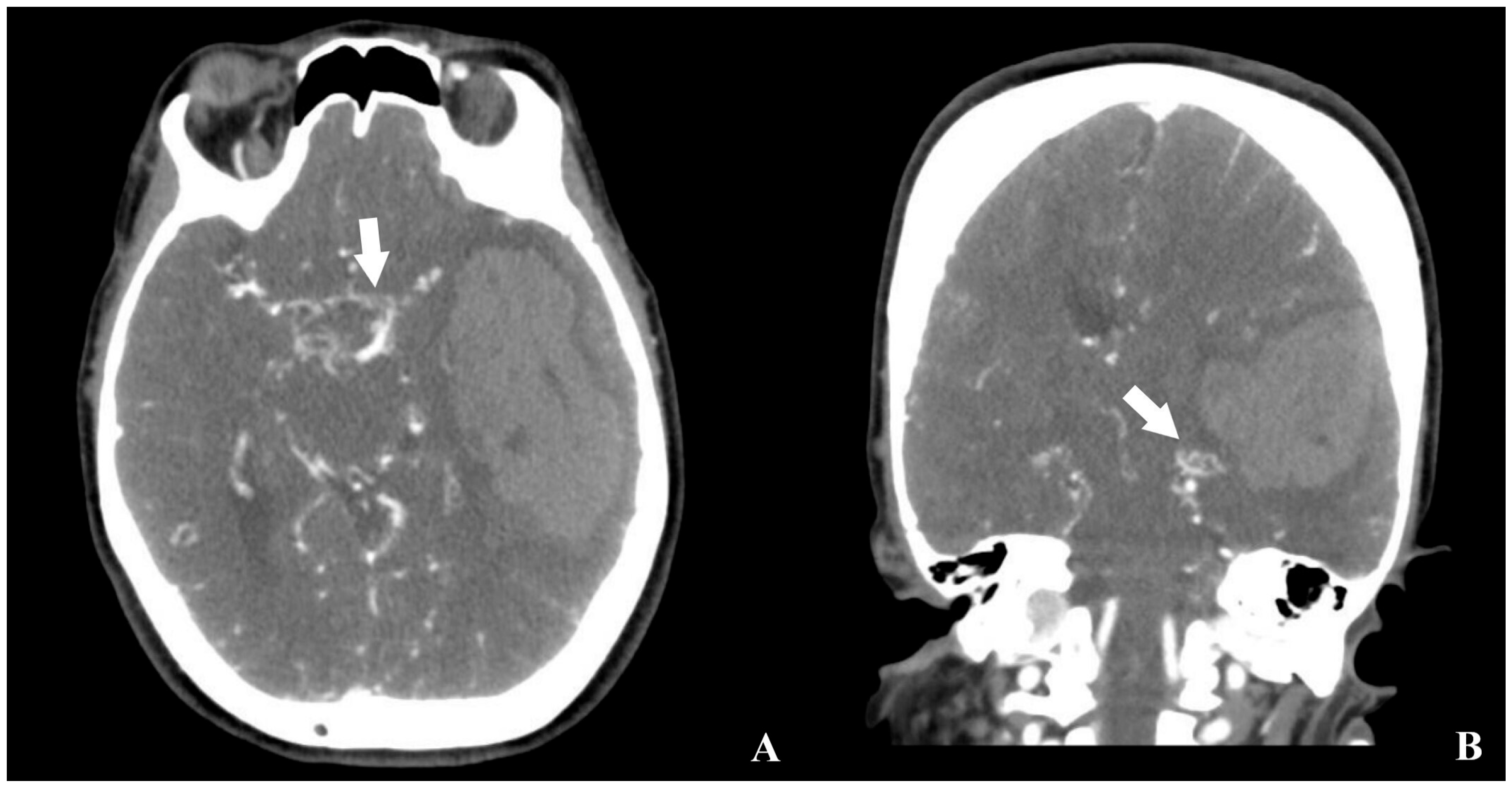
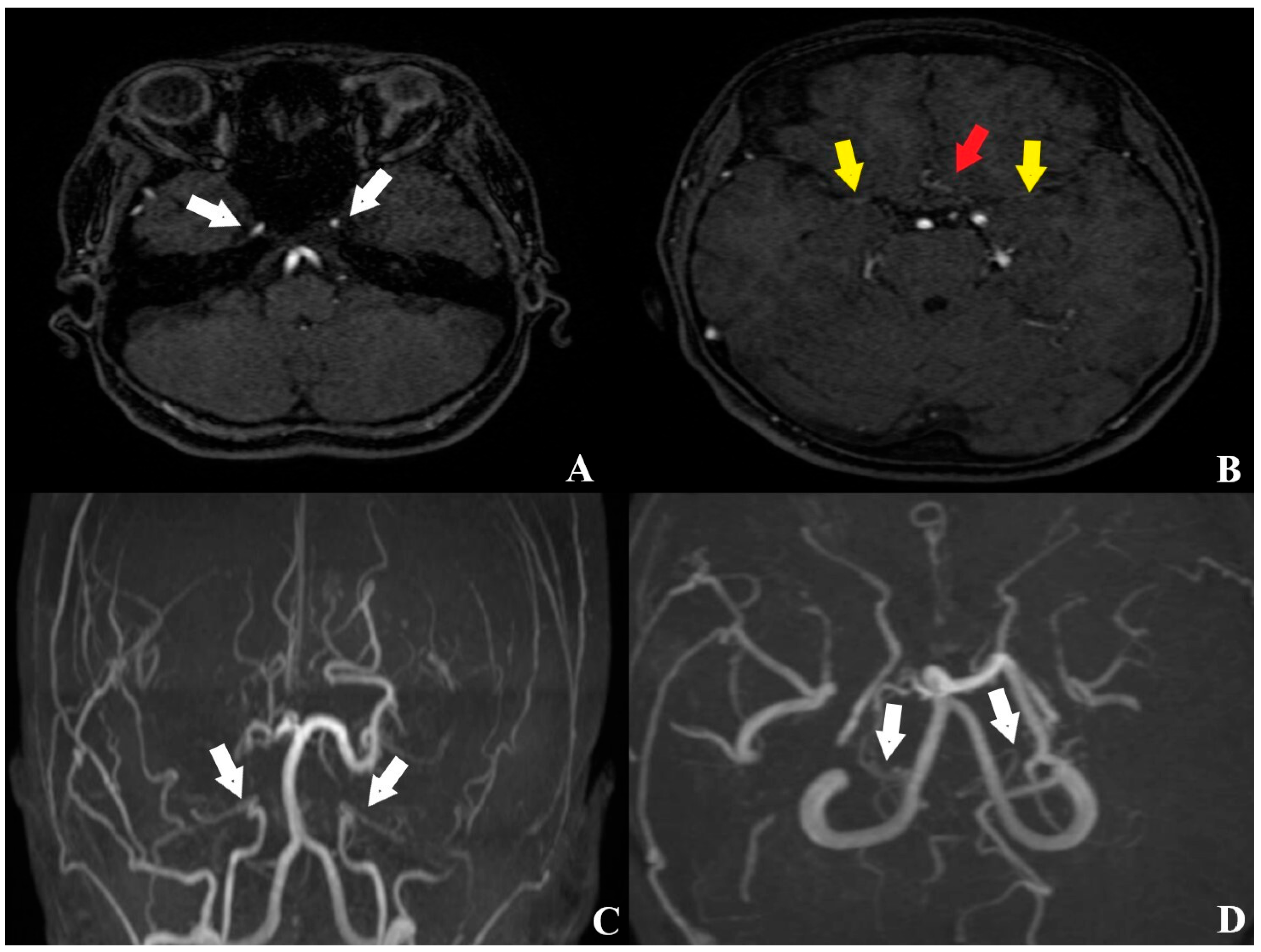
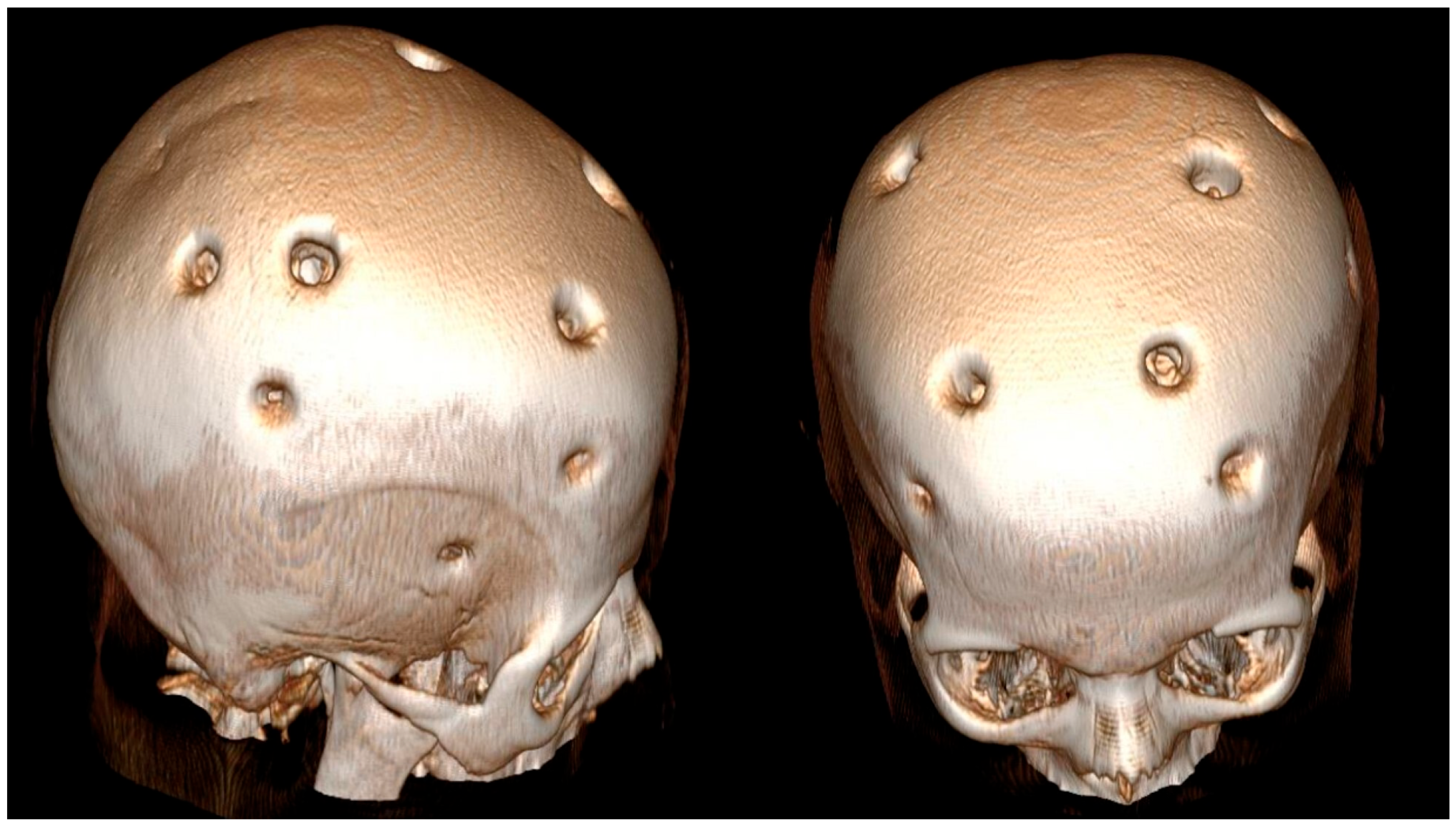
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MMD | Moyamoya disease |
| CTA | Computed tomography angiography |
| MRA | Magnetic resonance angiography |
| DSA | Digital subtraction angiography |
| GKS | Glasgow coma scale |
| mRS | Modified Rankin scale |
| TOF | Time of flight |
| FLAİR | Fluid-attenuated inversion recovery |
| IVWI | Intracranial vessel wall imaging |
| CTP | Computed tomography perfusion |
| BOLD-fMRI | Blood oxygen level–dependent functional MRI |
| SPECT | Single-photon emission computed tomography |
| PET | Positron emission tomography |
| MARS-MMA | MRI-Based Assessment of Risk for Stroke in Moyamoya Angiopathy |
| MBHs | Multiple burr holes |
References
- Scott, R.M.; Smith, E.R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 2009, 360, 1226–1237. [Google Scholar] [CrossRef]
- Kuroda, S.; Houkin, K. Moyamoya disease: Current concepts and future perspectives. Lancet Neurol. 2008, 7, 1056–1066. [Google Scholar] [CrossRef]
- Suzuki, J.; Kodama, N. Moyamoya disease–A review. Stroke 1983, 14, 104–109. [Google Scholar] [CrossRef]
- Baba, T.; Houkin, K.; Kuroda, S. Novel epidemiological features of moyamoya disease. J. Neurol. Neurosurg. Psychiatry 2008, 79, 900–904. [Google Scholar] [CrossRef]
- Wakai, K.; Tamakoshi, A.; Ikezaki, K.; Fukui, M.; Kawamura, T.; Aoki, R.; Kojima, M.; Lin, Y.; Ohno, Y. Epidemiological features of moyamoya disease in Japan: Findings from a nationwide survey. Clin. Neurol. Neurosurg. 1997, 99, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, Y.; Ogata, N.; Kaku, Y.; Taub, E.; Imhof, H.-G. Moyamoya disease in Europe, past and present status. Clin. Neurol. Neurosurg. 1997, 99, S58–S60. [Google Scholar] [CrossRef] [PubMed]
- Bagyinszky, E.; Yang, Y.; An, S.S.A. Multisystemic Impact of RNF213 Arg4810Lys: A Comprehensive Review of Moyamoya Disease and Associated Vasculopathies. Int. J. Mol. Sci. 2025, 26, 7864. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Liu, Y.H.; Hsu, C.Y.; Hogan, E.L.; Ryu, S.J. Moyamoya disease in Taiwan. Stroke 1988, 19, 53–59. [Google Scholar] [CrossRef]
- Kraemer, M.; Trakolis, L.; Platzen, J.; Schwitalla, J.C.; Bersano, A.; Albrecht, P.; Schlamann, M.; Berlit, P. Movement symptoms in European Moyamoya angiopathy—First systematic questionnaire study. Clin. Neurol. Neurosurg. 2017, 152, 52–56. [Google Scholar] [CrossRef]
- Viteva, E.; Vasilev, P.; Vasilev, G.; Chompalov, K. Clinical Case of a 23-Year-Old Patient with Moyamoya Disease and Epilepsy in Bulgaria. Neurol. Int. 2024, 16, 869–879. [Google Scholar] [CrossRef]
- Morioka, M.; Hamada, J.-I.; Kawano, T.; Todaka, T.; Yano, S.; Kai, Y.; Ushio, Y. Angiographic dilatation and branch extension of the anterior choroidal and posterior communicating arteries are predictors of hemorrhage in adult moyamoya patients. Stroke 2003, 34, 90–95. [Google Scholar] [CrossRef]
- Liu, W.; Fu, H.; Fang, S.; Ni, J.; Peng, B. Construction and Analysis of Immune Infiltration and Competing Endogenous RNA Network in Moyamoya Disease. Int. J. Mol. Sci. 2025, 26, 7957. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Okune, S.; Tanaka, S.; Yamagami, H.; Matsumaru, Y. RNF213-Related Vasculopathy: An Entity with Diverse Phenotypic Expressions. Genes 2025, 16, 939. [Google Scholar] [CrossRef]
- Tan, C.; Wang, X.; Zhou, Z.; Liu, Y.; He, S.; Zhao, Y. A Machine Learning-Based Diagnostic Nomogram for Moyamoya Disease: The Validation of Hypoxia-Immune Gene Signatures. Bioengineering 2025, 12, 577. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, Q.; Liu, Y.; Zhang, X.; Huang, Y.; Zhang, H. The Role of Immune Cells in Moyamoya Disease. Brain Sci. 2025, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-J.; Wang, J.; Zhang, H.; Hao, F.-B.; Gao, G.; Liu, S.-M.; Wang, X.-P.; Li, J.-J.; Zou, Z.-X.; Guo, Q.-B. High level of serum complement C3 expression is associated with postoperative vasculopathy progression in moyamoya disease. J. Inflamm. Res. 2024, 2024, 1721–1733. [Google Scholar] [CrossRef]
- Si, X.; Li, L.; Fang, Y.; Yan, Y.; Pu, J. A patient with multiple sclerosis and coexisting Moyamoya disease: Why and how. Front. Neurol. 2020, 11, 516587. [Google Scholar] [CrossRef]
- Canavero, I.; Rifino, N.; Antozzi, C.; Caldiera, V.; Colombo, E.; Carrozzini, T.; Ganci, G.; Ferroli, P.; Acerbi, F.; Storti, B. Blurred by a “Puff of Smoke”—A Case-Based Review on the Challenging Recognition of Coexisting CNS Demyelinating Disease and Moyamoya Angiopathy. Int. J. Mol. Sci. 2025, 26, 5030. [Google Scholar] [CrossRef]
- Tominaga, T.; Suzuki, N.; Miyamoto, S.; Koizumi, A. Recommendations for the management of moyamoya disease: A statement from research committee on spontaneous occlusion of the circle of Willis (moyamoya disease). Surg. Cereb. Stroke 2018, 46, 1–24. [Google Scholar] [CrossRef]
- Karsonovich, T.; Lui, F. Moyamoya Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zedde, M.; Quaresima, M.; Capodanno, I.; Grisendi, I.; Assenza, F.; Napoli, M.; Moratti, C.; Pavone, C.; Bonacini, L.; Di Cecco, G. Neurovascular manifestations of sickle cell disease. Hemato 2024, 5, 277–320. [Google Scholar] [CrossRef]
- Lehman, L.L.; Ullrich, N.J. Cerebral vasculopathy in children with neurofibromatosis type 1. Cancers 2023, 15, 5111. [Google Scholar] [CrossRef]
- Matsuki, Y.; Kawakami, M.; Ishizuka, T.; Kawaguchi, Y.; Hidaka, T.; Suzuki, K.; Nakamura, H. SLE and Sjögren’s syndrome associated with unilateral moyamoya vessels in cerebral arteries. Scand. J. Rheumatol. 1997, 26, 392–394. [Google Scholar] [CrossRef]
- Asai, Y.; Nakayasu, H.; Fusayasu, E.; Nakashima, K. Moyamoya disease presenting as thalamic hemorrhage in a patient with neuromyelitis optica and Sjögren’s syndrome. J. Stroke Cerebrovasc. Dis. 2012, 21, 619.e7–619.e9. [Google Scholar] [CrossRef]
- Ge, P.; Zhang, Q.; Ye, X.; Liu, X.; Deng, X.; Wang, J.; Wang, R.; Zhang, Y.; Zhang, D.; Zhao, J.Z. Digital subtraction angiographic characteristics of progression of moyamoya disease 6 months prior to surgical revascularisation. Stroke Vasc. Neurol. 2020, 5, 97–102. [Google Scholar] [CrossRef]
- Ge, P.; Zhang, Q.; Ye, X.; Liu, X.; Deng, X.; Wang, J.; Wang, R.; Zhang, Y.; Zhang, D.; Zhao, J. Postoperative collateral formation after indirect bypass for hemorrhagic moyamoya disease. BMC Neurol. 2020, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin. Neurol. Neurosurg. 1997, 99, S238–S240. [Google Scholar] [CrossRef]
- Ryška, P.; Lojík, M.; Habalová, J.; Kajzrová, C.; Česák, T.; Vítková, E.; Bartoš, M.; Bělobrádek, Z.; Krajina, A. Endovascular Therapy of Ruptured Aneurysms on Moyamoya Collateral Vessels: Two Cases. Medicina 2024, 60, 1499. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jiang, H.; Li, J.; Duan, T.; Zhou, C.; Yan, F. Imaging methods for surgical revascularization in patients with moyamoya disease: An updated review. Neurosurg. Rev. 2022, 45, 343–356. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Guo, X.; Shi, Z.; Sun, Z.; Gao, L.; Jin, F.; Wang, J.; Chen, W.; Yang, Y. CT perfusion assessment of Moyamoya syndrome before and after direct revascularization (superficial temporal artery to middle cerebral artery bypass). Eur. Radiol. 2016, 26, 254–261. [Google Scholar] [CrossRef]
- Uchino, H.; Yamamoto, S.; Kashiwazaki, D.; Akioka, N.; Kuwayama, N.; Noguchi, K.; Kuroda, S. Using postoperative remodeling of donor arteries on MR angiography to predict the development of surgical collaterals in moyamoya disease. J. Neurosurg. 2019, 134, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qi, R.; Cheng, X.; Zhou, C.; Luo, S.; Ni, L.; Huang, W. Assessment of extracranial-intracranial bypass in Moyamoya disease using 3T time-of-flight MR angiography: Comparison with CT angiography. Vasa 2014, 43, 278–283. [Google Scholar] [CrossRef]
- Ryoo, S.; Cha, J.; Kim, S.J.; Choi, J.W.; Ki, C.-S.; Kim, K.H.; Jeon, P.; Kim, J.-S.; Hong, S.-C.; Bang, O.Y. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke 2014, 45, 2457–2460. [Google Scholar] [CrossRef]
- Ya, J.; Zhou, D.; Ding, J.; Ding, Y.; Ji, X.; Yang, Q.; Meng, R. High-resolution combined arterial spin labeling MR for identifying cerebral arterial stenosis induced by moyamoya disease or atherosclerosis. Ann. Transl. Med. 2020, 8, 87. [Google Scholar] [CrossRef]
- Haas, P.; Hauser, T.-K.; Wiggenhauser, L.M.; Zerweck, L.; Tatagiba, M.; Khan, N.; Roder, C. Coincidence of Concentric Vessel-Wall Contrast Enhancement in Moyamoya Disease and Acute Postoperative Ischemic Stroke During Revascularization Procedures. Brain Sci. 2024, 14, 1190. [Google Scholar] [CrossRef]
- Kaku, Y.; Iihara, K.; Nakajima, N.; Kataoka, H.; Fukushima, K.; Iida, H.; Hashimoto, N. The leptomeningeal ivy sign on fluid-attenuated inversion recovery images in moyamoya disease: Positron emission tomography study. Cerebrovasc. Dis. 2013, 36, 19–25. [Google Scholar] [CrossRef]
- Horie, N.; Morikawa, M.; Morofuji, Y.; Hiu, T.; Izumo, T.; Hayashi, K.; Nagata, I. De novo ivy sign indicates postoperative hyperperfusion in moyamoya disease. Stroke 2014, 45, 1488–1491. [Google Scholar] [CrossRef]
- Sam, K.; Poublanc, J.; Sobczyk, O.; Han, J.S.; Battisti-Charbonney, A.; Mandell, D.M.; Tymianski, M.; Crawley, A.P.; Fisher, J.A.; Mikulis, D.J. Assessing the effect of unilateral cerebral revascularisation on the vascular reactivity of the non-intervened hemisphere: A retrospective observational study. BMJ Open 2015, 5, e006014. [Google Scholar] [CrossRef]
- Garbani Nerini, L.; Bellomo, J.; Höbner, L.M.; Stumpo, V.; Colombo, E.; van Niftrik, C.H.B.; Schubert, T.; Kulcsár, Z.; Wegener, S.; Luft, A. BOLD cerebrovascular reactivity and NOVA quantitative MR angiography in adult patients with moyamoya vasculopathy undergoing cerebral bypass surgery. Brain Sci. 2024, 14, 762. [Google Scholar] [CrossRef] [PubMed]
- Sebök, M. BOLD Cerebrovascular Reactivity as a Novel Marker for Hemodynamic Impairment in Symptomatic Cerebrovascular Steno-Occlusive Disease. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 2022. [Google Scholar]
- Setta, K.; Kojima, D.; Shimada, Y.; Yoshida, J.; Oshida, S.; Fujimoto, K.; Tsutsui, S.; Chiba, T.; Fujiwara, S.; Terasaki, K. Accuracy of brain perfusion single-photon emission computed tomography for detecting misery perfusion in adult patients with symptomatic ischemic moyamoya disease. Ann. Nucl. Med. 2018, 32, 611–619. [Google Scholar] [CrossRef] [PubMed]
- So, Y.; Lee, H.-Y.; Kim, S.-K.; Lee, J.S.; Wang, K.-C.; Cho, B.-K.; Kang, E.; Lee, D.S. Prediction of the clinical outcome of pediatric moyamoya disease with postoperative basal/acetazolamide stress brain perfusion SPECT after revascularization surgery. Stroke 2005, 36, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Kazumata, K.; Tokairin, K.; Sugiyama, T.; Ito, M.; Uchino, H.; Osanai, T.; Kawabori, M.; Nakayama, N.; Houkin, K. Association of cognitive function with cerebral blood flow in children with moyamoya disease. J. Neurosurg. Pediatr. 2019, 25, 62–68. [Google Scholar] [CrossRef]
- Ikezaki, K.; Matsushima, T.; Kuwabara, Y.; Suzuki, S.O.; Nomura, T.; Fukui, M. Cerebral circulation and oxygen metabolism in childhood moyamoya disease: A perioperative positron emission tomography study. J. Neurosurg. 1994, 81, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Kudo, T.; Hayashi, S.; Inaji, M.; Tanaka, Y.; Maehara, T.; Ishii, K.; Nariai, T. Improvement in cognitive decline after indirect bypass surgery in adult moyamoya disease: Implication of 15 O-gas positron emission tomography. Ann. Nucl. Med. 2020, 34, 467–475. [Google Scholar] [CrossRef]
- Roder, C.; Haas, P.; Fudali, M.; Milian, M.; Ernemann, U.; Meyer, P.T.; Tatagiba, M.; Khan, N. Neuropsychological impairment in adults with moyamoya angiopathy: Preoperative assessment and correlation to MRI and H215O PET. Neurosurg. Rev. 2020, 43, 1615–1622. [Google Scholar] [CrossRef]
- Zerweck, L.; Roder, C.; Blazhenets, G.; Martus, P.; Thurow, J.; Haas, P.; Estler, A.; Gohla, G.; Ruff, C.; Selo, N. MRI-Based Assessment of Risk for Stroke in Moyamoya Angiopathy (MARS-MMA): An MRI-Based Scoring System for the Severity of Moyamoya Angiopathy. Diagnostics 2024, 14, 1437. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Takaku, A. Cerebrovascular moyamoya disease: Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969, 20, 288–299. [Google Scholar] [CrossRef]
- Liu, J.J.; Steinberg, G.K. Direct versus indirect bypass for moyamoya disease. Neurosurg. Clin. 2017, 28, 361–374. [Google Scholar] [CrossRef]
- Kazumata, K.; Ito, M.; Tokairin, K.; Ito, Y.; Houkin, K.; Nakayama, N.; Kuroda, S.; Ishikawa, T.; Kamiyama, H. The frequency of postoperative stroke in moyamoya disease following combined revascularization: A single-university series and systematic review. J. Neurosurg. 2014, 121, 432–440. [Google Scholar] [CrossRef]
- Liu, W.; Huang, K.; Zhang, J.; Zhou, D.; Chen, J. Clinical features and risk factors of postoperative stroke in adult moyamoya disease. Brain Sci. 2023, 13, 1696. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, L.; Lu, J.; Chen, X.; Ye, X.; Zhang, D.; Zhang, Y.; Wang, R.; Zhao, Y. Postoperative hemorrhage during the acute phase after direct or combined revascularization for moyamoya disease: Risk factors, prognosis, and literature review. J. Neurosurg. 2019, 133, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Tokairin, K.; Kazumata, K.; Uchino, H.; Ito, M.; Ono, K.; Tatezawa, R.; Shindo, T.; Kawabori, M.; Nakayama, N.; Houkin, K. Postoperative intracerebral hemorrhage after combined revascularization surgery in moyamoya disease: Profiles and clinical associations. World Neurosurg. 2018, 120, e593–e600. [Google Scholar] [CrossRef]
- Funaki, T.; Kataoka, H.; Yoshida, K.; Kikuchi, T.; Mineharu, Y.; Okawa, M.; Yamao, Y.; Miyamoto, S. The targeted bypass strategy for preventing hemorrhage in moyamoya disease. Neurol. Med.-Chir. 2019, 59, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Kawashima, A.; Ishiguro, T.; Hashimoto, K.; Hodotsuka, K.; Nakamura, A.; Kuwano, A.; Tanaka, Y.; Murakami, M.; Shiono, T. Five-day bed rest reduces postoperative intracerebral hemorrhage after direct bypass for moyamoya disease. World Neurosurg. 2022, 159, e267–e272. [Google Scholar] [CrossRef]
- Houkin, K.; Kamiyama, H.; Abe, H.; Takahashi, A.; Kuroda, S. Surgical therapy for adult moyamoya disease: Can surgical revascularization prevent the recurrence of intracerebral hemorrhage? Stroke 1996, 27, 1342–1346. [Google Scholar] [CrossRef]
- Ryan, R.W.; Chowdhary, A.; Britz, G.W. Hemorrhage and risk of further hemorrhagic strokes following cerebral revascularization in Moyamoya disease: A review of the literature. Surg. Neurol. Int. 2012, 3, 72. [Google Scholar] [CrossRef]
- Guo, Q.; Xie, M.; Ji, H.; Wang, Q.N.; Bao, X.; Duan, L. Long-Term Outcomes in Patients with Hemorrhagic Moyamoya Disease Combined with Hypertension After Encephaloduroarteriosynangiosis. J. Am. Heart Assoc. 2025, 14, e039054. [Google Scholar] [CrossRef]
- Lucia, K.; Acker, G.; Rubarth, K.; Beyaztas, D.; Vajkoczy, P. The development and effect of systemic hypertension on clinical and radiological outcome in adult Moyamoya angiopathy following revascularization surgery: Experience of a single European institution. J. Clin. Med. 2023, 12, 4219. [Google Scholar] [CrossRef]
- Kim, J.W.; Phi, J.H.; Lee, J.Y.; Koh, E.J.; Kim, K.H.; Kim, H.-S.; Kim, S.-K. Comparison of bifrontal craniotomy and multiple burr hole encephalogaleoperiosteal-synangiosis for pediatric moyamoya disease: An experience of 346 patients. Neurosurgery 2023, 93, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Soler-Rico, M.; Di Santo, M.; Vaz, G.; Joris, V.; Fomekong, E.; Guillaume, S.; Van Boven, M.; Raftopoulos, C. How to reduce the complication rate of multiple burr holes surgery in moyamoya angiopathy. Acta Neurochir. 2023, 165, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.S.; Lee, S.-J.; Hong, J.M.; Lim, Y.C. Multiple burr hole and erythropoietin combination therapy: Optimal early surgical intervention for patients with acute stroke episode of moyamoya disease or moyamoya syndrome. Front. Neurol. 2024, 15, 1479379. [Google Scholar] [CrossRef]
- Aoki, N. Cerebrovascular bypass surgery for the treatment of Moyamoya disease: Unsatisfactory outcome in the patients presenting with intracranial hemorrhage. Surg. Neurol. 1993, 40, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Otawara, Y.; Ogasawara, K.; Seki, K.; Kibe, M.; Kubo, Y.; Ogawa, A. Intracerebral hemorrhage after prophylactic revascularization in a patient with adult moyamoya disease. Surg. Neurol. 2007, 68, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, T.; Yuki, K.; Sasaki, T.; Murakami, T.; Kodama, Y.; Kurisu, K. A ruptured middle cerebral artery aneurysm originating from the site of anastomosis 20 years after extracranial-intracranial bypass for moyamoya disease: Case report. Surg. Neurol. 2005, 64, 261–265. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, M.C.; Aydin, K. Delayed Intracerebral Hemorrhage 15 Years After Indirect Revascularization in Moyamoya Disease: A Case Report and Review of the Literature. Brain Sci. 2025, 15, 1077. https://doi.org/10.3390/brainsci15101077
Yilmaz MC, Aydin K. Delayed Intracerebral Hemorrhage 15 Years After Indirect Revascularization in Moyamoya Disease: A Case Report and Review of the Literature. Brain Sciences. 2025; 15(10):1077. https://doi.org/10.3390/brainsci15101077
Chicago/Turabian StyleYilmaz, Merih C., and Keramettin Aydin. 2025. "Delayed Intracerebral Hemorrhage 15 Years After Indirect Revascularization in Moyamoya Disease: A Case Report and Review of the Literature" Brain Sciences 15, no. 10: 1077. https://doi.org/10.3390/brainsci15101077
APA StyleYilmaz, M. C., & Aydin, K. (2025). Delayed Intracerebral Hemorrhage 15 Years After Indirect Revascularization in Moyamoya Disease: A Case Report and Review of the Literature. Brain Sciences, 15(10), 1077. https://doi.org/10.3390/brainsci15101077






