Sleep State Misperception in Insomnia: The Role of Sleep Instability and Emotional Dysregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Experimental Procedures
Missing Data
2.2. Sleep Recording
2.3. Sleep Diaries
2.4. Sleep State Misperception Index: TSTm
2.5. Statistical Analysis
3. Results
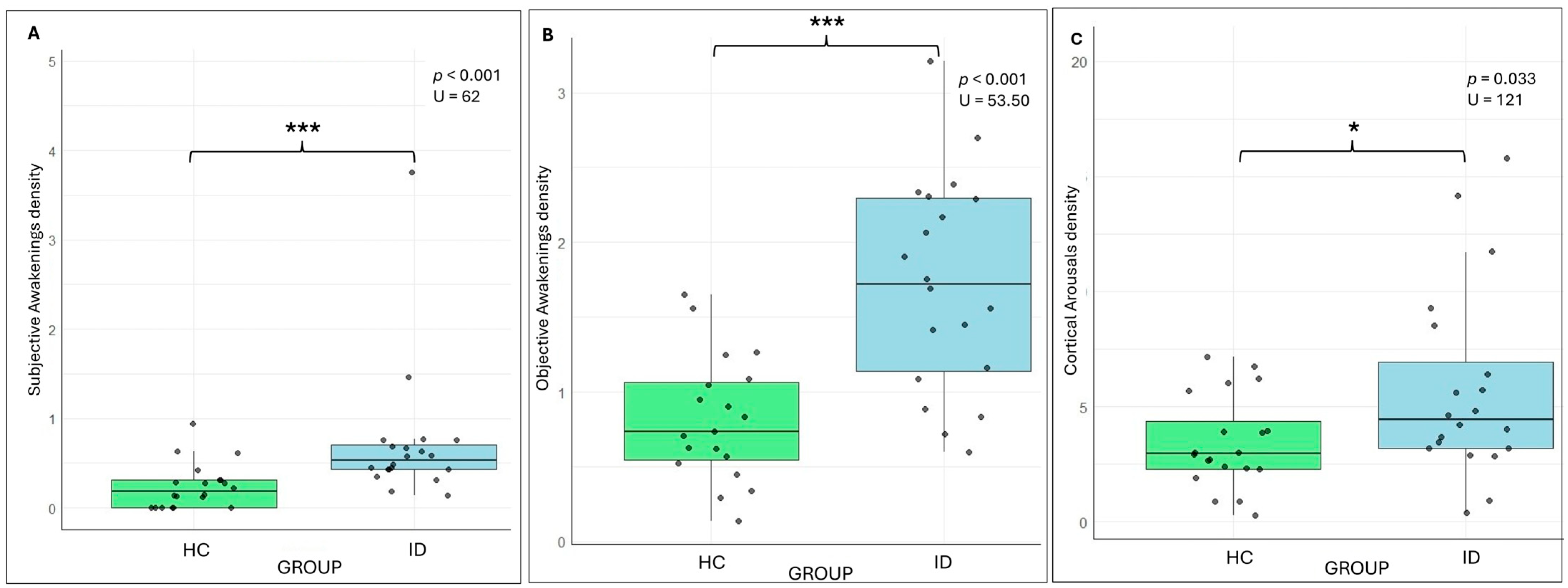
3.1. HCs vs. IDs
3.1.1. Clinical and Psychological Variables
3.1.2. Subjective and Objective Sleep Variables
3.1.3. Correlations Analysis
3.1.4. Regression Analyses
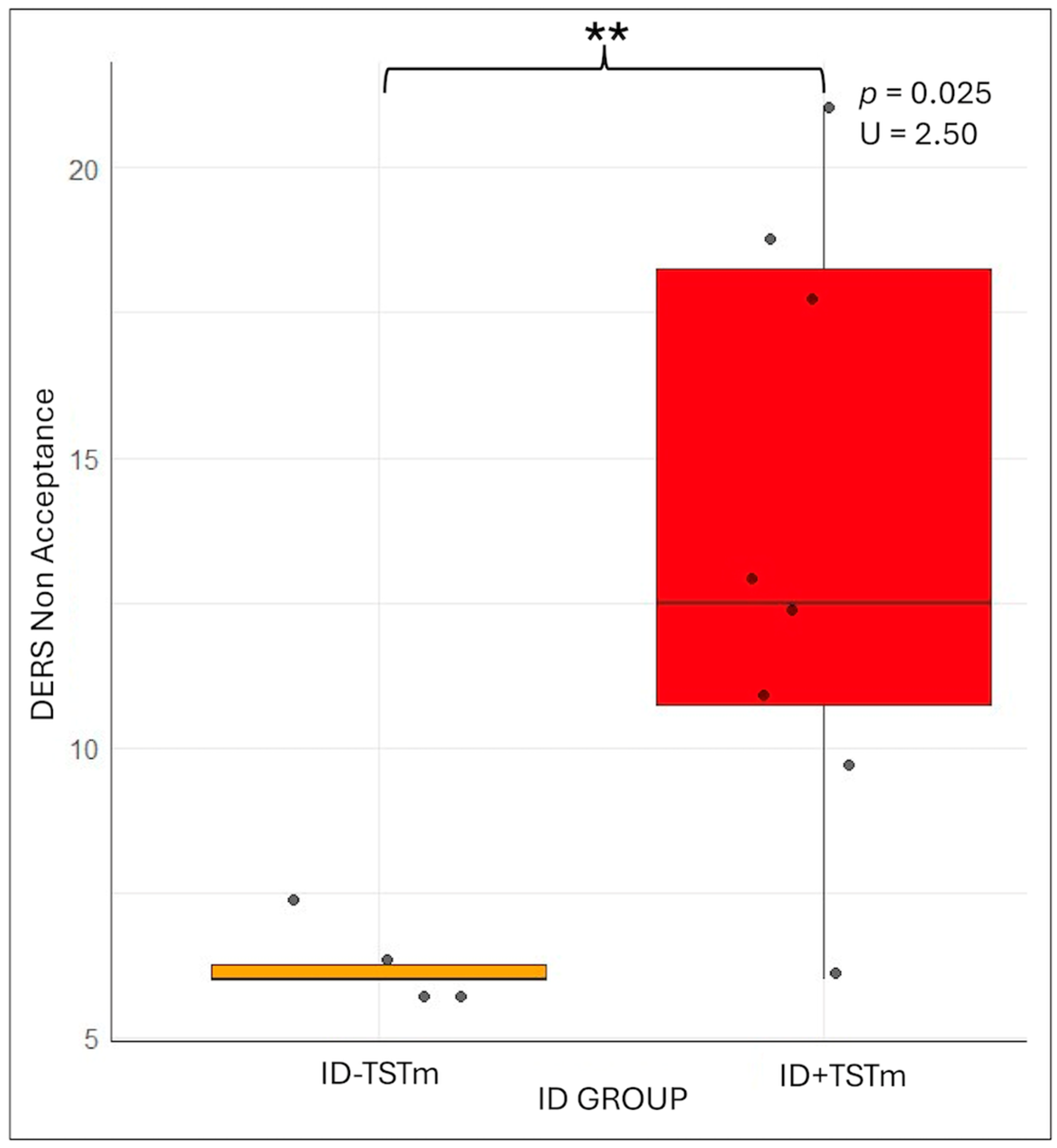
3.2. ID + TSTm vs. ID-TSTm
3.2.1. Clinical and Psychological Variables
3.2.2. Subjective and Objective Sleep Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wittchen, H.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Thorpy, M.J. Classification of Sleep disorders. J. Clin. Neurophysiol. 1990, 7, 67–82. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 2nd ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2005. [Google Scholar]
- Perlis, M.L.; Smith, M.T.; Andrews, P.J.; Orff, H.; Giles, D.E. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep 2001, 24, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.M.; Lecci, S.; Cataldi, J.; Siclari, F. Conscious experiences and high-density EEG patterns predicting subjective sleep depth. Curr. Biol. 2021, 31, 5487–5500.E3. [Google Scholar] [CrossRef]
- Fasiello, E.; Gorgoni, M.; Galbiati, A.; Sforza, M.; Berra, F.; Scarpelli, S.; Alfonsi, V.; Annarumma, L.; Casoni, F.; Zucconi, M.; et al. Decreased Delta/Beta ratio index as the sleep state-independent electrophysiological signature of sleep state misperception in Insomnia disorder: A focus on the sleep onset and the whole night. Neuroimage 2024, 298, 120782. [Google Scholar] [CrossRef]
- Andrillon, T.; Solelhac, G.; Bouchequet, P.; Romano, F.; Le Brun, M.-P.; Brigham, M.; Chennaoui, M.; Léger, D. Revisiting the value of polysomnographic data in insomnia: More than meets the eye. Sleep Med. 2020, 66, 184–200. [Google Scholar] [CrossRef]
- Lecci, S.; Cataldi, J.; Betta, M.; Bernardi, G.; Heinzer, R.; Siclari, F. Electroencephalographic changes associated with subjective under- and overestimation of sleep duration. Sleep 2020, 43, zsaa094. [Google Scholar] [CrossRef]
- Castelnovo, A.; Ferri, R.; Galbiati, A.; Rossi, A.; Zucconi, M.; Castronovo, V.; Strambi, L.-F.; Manconi, M. Extreme sleep state misperception: From psychopathology to objective-subjective sleep measures. Int. J. Psychophysiol. 2021, 167, 77–85. [Google Scholar] [CrossRef]
- Stephan, A.M.; Siclari, F. Reconsidering sleep perception in insomnia: From misperception to mismeasurement. J. Sleep Res. 2023, 32, e14028. [Google Scholar] [CrossRef]
- Krystal, A.D.; Edinger, J.D.; Wohlgemuth, W.K.; Marsh, G.R. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep 2002, 25, 630–640. [Google Scholar] [CrossRef]
- Maes, J.; Verbraecken, J.; Willemen, M.; De Volder, I.; van Gastel, A.; Michiels, N.; Verbeek, I.; Vandekerckhove, M.; Wuyts, J.; Haex, B.; et al. Sleep misperception, EEG characteristics and autonomic nervous system activity in primary insomnia: A retrospective study on polysomnographic data. Int. J. Psychophysiol. 2014, 91, 163–171. [Google Scholar] [CrossRef]
- Berra, F.; Fasiello, E.; Zucconi, M.; Casoni, F.; De Gennaro, L.; Ferini-Strambi, L.; Galbiati, A. Neurophysiological Parameters Influencing Sleep-Wake Discrepancy in Insomnia Disorder: A Preliminary Analysis on Alpha Rhythm during Sleep Onset. Brain Sci. 2024, 14, 97. [Google Scholar] [CrossRef]
- Espie, C.A. Insomnia: Conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu. Rev. Psychol. 2002, 53, 215–243. [Google Scholar] [CrossRef]
- Galbiati, A.; Sforza, M.; Scarpellino, A.; Salibba, A.; Leitner, C.; D’Este, G.; Mombelli, S.; Ferini-Strambi, L.; Castronovo, V. “Thinking about thinking” in insomnia disorder: The effect of cognitive-behavioral therapy for insomnia on sleep-related metacognition. Front. Psychol. 2021, 12, 705112. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Berger, M.; Perlis, M.; Nissen, C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef]
- Moon, H.J.; Song, M.L.; Cho, Y.W. Clinical Characteristics of Primary Insomniacs with Sleep-State Misperception. J. Clin. Neurol. 2015, 11, 358–363. [Google Scholar] [CrossRef]
- Harvey, A.G.; Tang, N.K. (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychol. Bull. 2012, 138, 77–101. [Google Scholar] [CrossRef] [PubMed]
- Fasiello, E.; Ambrosio, A.; Berra, F.; Gorgoni, M.; Castronovo, V.; Ferini-Strambi, L.; De Gennaro, L.; Galbiati, A. Sleep stage transitions in patients with insomnia disorder: Implications of Markov chain analysis on sleep misperception. J. Sleep Res. 2024, 33, 14291. [Google Scholar]
- Fernandez-Mendoza, J.; Calhoun, S.L.; Bixler, E.O.; Karataraki, M.; Liao, D.; Vela-Bueno, A.; Jose Ramos-Platon, M.; Sauder, K.A.; Basta, M.; Vgontzas, A.N. Sleep misperception and chronic insomnia in the general population: Role of objective sleep duration and psychological profiles. Psychosom. Med. 2011, 73, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Giromini, L.; Velotti, P.; de Campora, G.; Bonalume, L.; Cesare Zavattini, G. Cultural adaptation of the difficulties in emotion regulation scale: Reliability and validity of an Italian version. J. Clin. Psychol. 2012, 68, 989–1007. [Google Scholar] [CrossRef]
- Sella, E.; De Min Tona, G.; De Beni, R. II metacognitions questionnaire-insomnia (MCQ-I) e il thought control questionnaire insomnia-revised (TCQI-R): Adattamento italiano di due questionari metacognitivi per la valutazione dei disturbi del sonno. Psicoter. Cogn. Comport. 2016, 22, 139–167. [Google Scholar]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Maltezos, A.; Perrault, A.A.; Walsh, N.A.; Phillips, E.-M.; Gong, K.; Tarelli, L.; Smith, D.; Cross, N.E.; Pomares, F.B.; Gouin, J.-P.; et al. Methodological approach to sleep state misperception in insomnia disorder: Comparison between multiple nights of actigraphy recordings and a single night of polysomnography recording. Sleep Med. 2024, 115, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Castronovo, V.; Galbiati, A.; Marelli, S.; Brombin, C.; Cugnata, F.; Giarolli, L.; Anelli, M.M.; Rinaldi, F.; Ferini-Strambi, L. Validation study of the Italian version of the Insomnia Severity Index (ISI). Neurol. Sci. 2016, 37, 1517–1524. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Vignatelli, L.; Plazzi, G.; Barbato, A.; Ferini-Strambi, L.; Manni, R.; Pompei, F.; D’ALessandro, R. Italian version of the Epworth sleepiness scale: External validity. Neurol. Sci. 2003, 23, 295–300. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Espie, C.A.; Inglis, S.J.; Harvey, L.; Tessier, S. Insomniacs’ attributions: Psychometric properties of the Dysfunctional Beliefs and Attitudes about Sleep Scale and the Sleep Disturbance Questionnaire. J. Psychosom. Res. 2000, 48, 141–148. [Google Scholar] [CrossRef]
- Coradeschi, D.; Novara, C.; Morin, C.M. Dysfunctional Beliefs and Attitudes About Sleep Questionnaire: Versione italiana ed analisi della fedeltà. Psicoter. Cogn. Comport. 2000, 6, 33–44. [Google Scholar]
- Mondo, M.; Sechi, C.; Cabras, C. Psychometric evaluation of three versions of the Italian Perceived Stress Scale. Curr. Psychol. 2021, 40, 1884–1892. [Google Scholar] [CrossRef]
- Sica, C.; Ghisi, M. The Italian versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric properties and discriminant power. In Leading-Edge Psychological Tests and Testing Research; Lange, M.A., Ed.; Nova Science Publishers: New York, NY, USA, 2007; pp. 27–50. [Google Scholar]
- Spielberger, C.D.; Pedrabissi, L.; Santinello, M. “Inventario per l’Ansia di “Stato” e di “tratto”: Nuova Versione Italiana dello STAI.” Forma Y: Manuale; Organizzazioni Speciali: Firenze, Italy, 1989. [Google Scholar]
- Gratz, K.L.; Roemer, L. Multidimensional Assesleep-wake state discrepancyent of Emotion Regulation and Dysregulation: Development, Factor Structure, and Initial Validation of the Difficulties in Emotion Regulation Scale. J. Psychopathol. Behav. Assess. 2004, 26, 41–54. [Google Scholar] [CrossRef]
- Jasper, H.H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0; American Academy of Sleep Medicine: Darien, IL, USA, 2012; Volume 176. [Google Scholar] [CrossRef]
- Hooper, R. To adjust, or not to adjust, for multiple comparisons. J. Clin. Epidemiol. 2025, 180, 111688. [Google Scholar] [CrossRef] [PubMed]
- Cabin, R.J.; Randall, J.M. To Bonferroni or Not to Bonferroni: When and How Are the Questions. In Bulletin of the Ecological Society of America; JSTOR: New York, NY, USA, 2000; Volume 81, pp. 246–248. Available online: https://www.jstor.org/stable/20168454 (accessed on 29 July 2025).
- Gorgoni, M.; Fasiello, E.; Leonori, V.; Galbiati, A.; Scarpelli, S.; Alfonsi, V.; Annarumma, L.; Casoni, F.; Castronovo, V.; Ferini-Strambi, L.; et al. K-Complex morphological alterations in insomnia disorder and their relationship with sleep state misperception. Sleep 2025, 48, zsaf040. [Google Scholar] [CrossRef]
- Herzog, R.; Crosbie, F.; Aloulou, A.; Hanif, U.; Chennaoui, M.; Léger, D.; Andrillon, T. A continuous approach to explain insomnia and subjective-objective sleep discrepancy. Commun. Biol. 2025, 8, 423. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Suh, S.; Nowakowski, S.; Bernert, R.A.; Ong, J.C.; Siebern, A.T.; Dowdle, C.L.; Manber, R. Clinical significance of night-to-night sleep variability in insomnia. Sleep Med. 2012, 13, 469–475. [Google Scholar] [CrossRef]
- Parrino, L.; Milioli, G.; De Paolis, F.; Grassi, A.; Terzano, M.G. Paradoxical insomnia: The role of CAP and arousals in sleep misperception. Sleep Med. 2009, 10, 1139–1145. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Nissen, C.; Hirscher, V.; Baglioni, C.; Feige, B. REM sleep instability–a new pathway for insomnia? Pharmacopsychiatry 2012, 45, 167–176. [Google Scholar] [CrossRef]
- Schiel, J.E.; Holub, F.; Petri, R.; Leerssen, J.; Tamm, S.; Tahmasian, M.; Riemann, D.; Spiegelhalder, K. Affect and Arousal in Insomnia: Through a Lens of Neuroimaging Studies. Curr. Psychiatry Rep. 2020, 22, 44. [Google Scholar] [CrossRef]
- Wassing, R.; Benjamins, J.S.; Dekker, K.; Moens, S.; Spiegelhalder, K.; Feige, B.; Riemann, D.; van der Sluis, S.; Van Der Werf, Y.D.; Talamini, L.M.; et al. Slow dissolving of emotional distress contributes to hyperarousal. Proc. Natl. Acad. Sci. USA 2016, 113, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, A.; Sforza, M.; Fasiello, E.; Casoni, F.; Marrella, N.; Leitner, C.; Zucconi, M.; Ferini-Strambi, L. The association between emotional dysregulation and REM sleep features in insomnia disorder. Brain Cogn. 2020, 146, 105642. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Z.; Li, Y.; Luo, X.; Ru, T.; Chen, Q.; Zhou, G. Insomnia and emotional dysfunction: Altered brain network connectivity across sleep and wakefulness states. Sleep Med. 2025, 133, 106582. [Google Scholar] [CrossRef] [PubMed]
- Samea, F.; Mortazavi, N.; Reimann, G.M.; Ebneabbasi, A.; Zarei, M.; Khazaie, H.; Goldstein-Piekarski, A.N.; Spiegelhalder, K.; Baglioni, C.; Sepehry, A.A.; et al. Insomnia and emotion dysregulation: A meta-analytical perspective integrating regulatory strategies and dispositional difficulties. Sleep Med. Rev. 2025, 82, 102111. [Google Scholar] [CrossRef]
- Riemann, D.; Dressle, R.J.; Benz, F.; Spiegelhalder, K.; Johann, A.F.; Nissen, C.; Hertenstein, E.; Baglioni, C.; Palagini, L.; Krone, L.; et al. Chronic insomnia, REM sleep instability and emotional dysregulation: A pathway to anxiety and depression? J. Sleep Res. 2025, 34, e14252. [Google Scholar] [CrossRef]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
- Bender, R.; Lange, S. Adjusting for multiple testing–when and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Kawai, K.; Iwamoto, K.; Miyata, S.; Okada, I.; Ando, M.; Fujishiro, H.; Noda, A.; Ozaki, N. A Study of Factors Causing Sleep State Misperception in Patients with Depression. Nat. Sci. Sleep 2022, 14, 1273–1283. [Google Scholar] [CrossRef]
- Feige, B.; Al-Shajlawi, A.; Nissen, C.; Voderholzer, U.; Hornyak, M.; Spiegelhalder, K.; Kloepfer, C.; Perlis, M.; Riemann, D. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J. Sleep Res. 2008, 17, 180–190, Correction in J. Sleep Res. 2012, 21, 484. [Google Scholar] [CrossRef]
- Bensen-Boakes, D.B.; Lovato, N.; Meaklim, H.; Bei, B.; Scott, H. “Sleep-wake state discrepancy”: Toward a common understanding and standardized nomenclature. Sleep 2022, 45, zsac187. [Google Scholar] [CrossRef]


| Psychological Test | Abbreviation | Definition |
|---|---|---|
| Insomnia Severity Index [24] | ISI | Three core items assessing specific aspects of insomnia symptoms were analyzed: difficulty initiating sleep (item 1-a), difficulty maintaining sleep (item 1-b), and early morning awakenings (item 1-c). Together, these items constitute the Symptom Severity subscale. Two additional dimensions were also considered: Satisfaction, which evaluates the individual’s dissatisfaction with their current sleep pattern, and Diurnal Impact, which measures the perceived consequences of sleep difficulties on daytime functioning and their visibility to others. |
| Dysfunctional Beliefs and Attitudes about Sleep [31] | DBAS | It assesses maladaptive beliefs and attitudes related to sleep, reflecting (a) misconceptions or amplifications about the causes and the consequences of insomnia (Consequences); (b) unrealistic sleep expectations (Expectations); (c) diminished perceptions of control and predictability of sleep (Worry/Helplessness); and (d) faulty beliefs about sleep-promoting practices (Medication) [32]. |
| Perceived Stress Scale [33] | PSS | It measures the extent to which individuals perceive their lives as stressful. |
| Beck Depression Inventory-II [34] | BDI-II | It assesses the severity of depressive symptoms. |
| State-Trait Anxiety Inventory [35] | STAY-I | It differentiates between temporary (state) and enduring (trait) anxiety. |
| the Difficulties in Emotion Regulation Scale—36 items [22] | DERS-36 | It evaluates various aspects of emotional dysregulation across six subcomponents: (a) awareness and (b) understanding of emotions (Awareness; Clarity); (b) non acceptance of emotions (Non acceptance); (c) the ability to engage in goal-directed behavior (Goals), and refrain from impulsive behavior, when experiencing negative emotions (Impulse); and (d) access to emotion regulation strategies perceived as effective (Strategies) [36]. |
| Metacognitions Questionnaire Insomnia [23] | MCQI | It assesses the metacognitive beliefs and processes associated with insomnia. |
| Parameter | Abbreviation | Unit |
|---|---|---|
| Sleep Latency to N2, N3, REM | oSL | Minutes |
| Total Sleep Time | oTST | Minutes |
| Sleep Stage Percentages | N1%, N2%, N3%, REM%, NREM% | Percentage (%) |
| Wake After Sleep Onset | WASO | Minutes |
| Nocturnal Awakenings | oAWK | Count |
| Cortical Arousals | CA | Count |
| Time In Bed | TIB | Minutes |
| Sleep Efficiency (oTST/TIB) × 100 | SE | Percentage (%) |
| Awakening Density oAWK is divided by total hours of sleep. | oAWK_d | Events/hour |
| Arousal Density CA is divided by total hours of sleep. | CA_d | Events/hour |
| Parameter | Abbreviation | Unit |
|---|---|---|
| Subjective Sleep Latency | sSL | Minutes |
| Subjective Total Sleep Time | sTST | Minutes |
| Subjective Nocturnal Awakenings | sAWK | Count |
| Awakening Density (Subjective) sAWK is divided by total hours of self-reported sleep. | sAWK_d | Events/hour |
| Subjective Wake After Sleep Onset | sWASO | Minutes |
| Subjective Time in Bed | sTIB | Hours |
| Subjective Sleep Efficiency (sTST/sTIB) × 100 | sSE | Percentage (%) |
| Sleep Disturbance | — | Likert (0–10) |
| Sleep Quality | — | Likert (0–10) |
| HC Means ± SD (N = 20) | ID Means ± SD | U-Test | p-Value | |
|---|---|---|---|---|
| Gender | 10 M/10 F | 7 M/13 F | 170 | 0.352 |
| Age | 41.05 ± 11.55 (26–63) | 43.50 ± 12.75 (18–65) | 174 | 0.490 |
| ESS | 5.55 ± 2.95 | 4.44 ± 4.96 | 241.000 | 0.076 |
| ISI | 4.25 ± 2.38 | 16.94 ± 4.51 | 0.000 | <0.001 |
| ISI-1a | 0.35 ± 0.59 | 1.53 ± 1.23 | 75.500 | 0.002 |
| ISI-1b | 0.3 ± 0.47 | 2.88 ± 0.93 | 3.000 | <0.001 |
| ISI-1c | 0.5 ± 0.61 | 2.77 ± 1.09 | 16.500 | <0.001 |
| ISI_Symptoms Severity | 1.15 ± 1.14 | 7.18 ± 2.07 | 1.500 | <0.001 |
| ISI_Diurnal Impact | 1.35 ± 1.42 | 6.47 ± 3 | 21.500 | <0.001 |
| ISI_Satisfaction | 2.1 ± 1.33 | 7.28 ± 2.27 | 3.000 | <0.001 |
| DBAS | 51.3 ± 16.66 | 80.11 ± 23.82 | 56.500 | <0.001 |
| DBAS_Consequences | 16.75 ± 7.43 | 24.13 ± 12.88 | 93.000 | 0.034 |
| DBAS_Worry/Helplessness | 14.3 ± 9.63 | 33-38 ± 9.23 | 26.500 | <0.001 |
| DBAS_Expectations | 13.75 ± 3.8 | 9.1 ± 5.7 | 238.000 | 0.013 |
| DBAS_Medication | 6.5 ± 3.44 | 11.38 ± 5.6 | 70.000 | 0.004 |
| BDI-II | 6.75 ± 5.6 | 12.47 ± 8.57 | 98.500 | 0.030 |
| PSS | 14.35 ± 4.84 | 19.13 ± 5.73 | 81.000 | 0.012 |
| DERS | 72.85 ± 20.85 | 72.42 ± 20.9 | 121.000 | 0.984 |
| DERS_Non-acceptance | 11.2 ± 4.87 | 11.25 ± 5.51 | 127.500 | 0.784 |
| DERS_Goals | 11.05 ± 3.46 | 11.25 ± 3.57 | 124.000 | 0.891 |
| DERS_Strategies | 14.1 ± 5.1 | 13.83 ± 4.84 | 124.000 | 0.891 |
| DERS_Impulse | 10.3 ± 3.8 | 10.83 ± 3.76 | 105.000 | 0.570 |
| DERS_Clarity | 9.85 ± 3.42 | 10.33 ± 3.7 | 108.500 | 0.666 |
| DERS_Awarness | 8.25 ± 4.71 | 6.67 ± 3.11 | 144.000 | 0.357 |
| TOT STAY-1s | 36.05 ± 8.4 | 42.47 ±10.71 | 108.500 | 0.063 |
| TOT STAY-2t | 42.8 ± 4.99 | 46.24 ± 9.1 | 121.500 | 0.142 |
| MCQ-I | 96.79 ± 22.73 | 123.27 ± 28.94 | 79.000 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cini, E.; Bolengo, F.; Fasiello, E.; Berra, F.; Gorgoni, M.; Sforza, M.; Casoni, F.; Proserpio, P.; Castronovo, V.; De Gennaro, L.; et al. Sleep State Misperception in Insomnia: The Role of Sleep Instability and Emotional Dysregulation. Brain Sci. 2025, 15, 1078. https://doi.org/10.3390/brainsci15101078
Cini E, Bolengo F, Fasiello E, Berra F, Gorgoni M, Sforza M, Casoni F, Proserpio P, Castronovo V, De Gennaro L, et al. Sleep State Misperception in Insomnia: The Role of Sleep Instability and Emotional Dysregulation. Brain Sciences. 2025; 15(10):1078. https://doi.org/10.3390/brainsci15101078
Chicago/Turabian StyleCini, Elettra, Francesca Bolengo, Elisabetta Fasiello, Francesca Berra, Maurizio Gorgoni, Marco Sforza, Francesca Casoni, Paola Proserpio, Vincenza Castronovo, Luigi De Gennaro, and et al. 2025. "Sleep State Misperception in Insomnia: The Role of Sleep Instability and Emotional Dysregulation" Brain Sciences 15, no. 10: 1078. https://doi.org/10.3390/brainsci15101078
APA StyleCini, E., Bolengo, F., Fasiello, E., Berra, F., Gorgoni, M., Sforza, M., Casoni, F., Proserpio, P., Castronovo, V., De Gennaro, L., Ferini-Strambi, L., & Galbiati, A. (2025). Sleep State Misperception in Insomnia: The Role of Sleep Instability and Emotional Dysregulation. Brain Sciences, 15(10), 1078. https://doi.org/10.3390/brainsci15101078









