Lumbosacral Endoscopic Ventral–Dorsal Rhizotomy: A Novel Approach for Tone Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Outcome Measures
2.2. Statistical Analysis
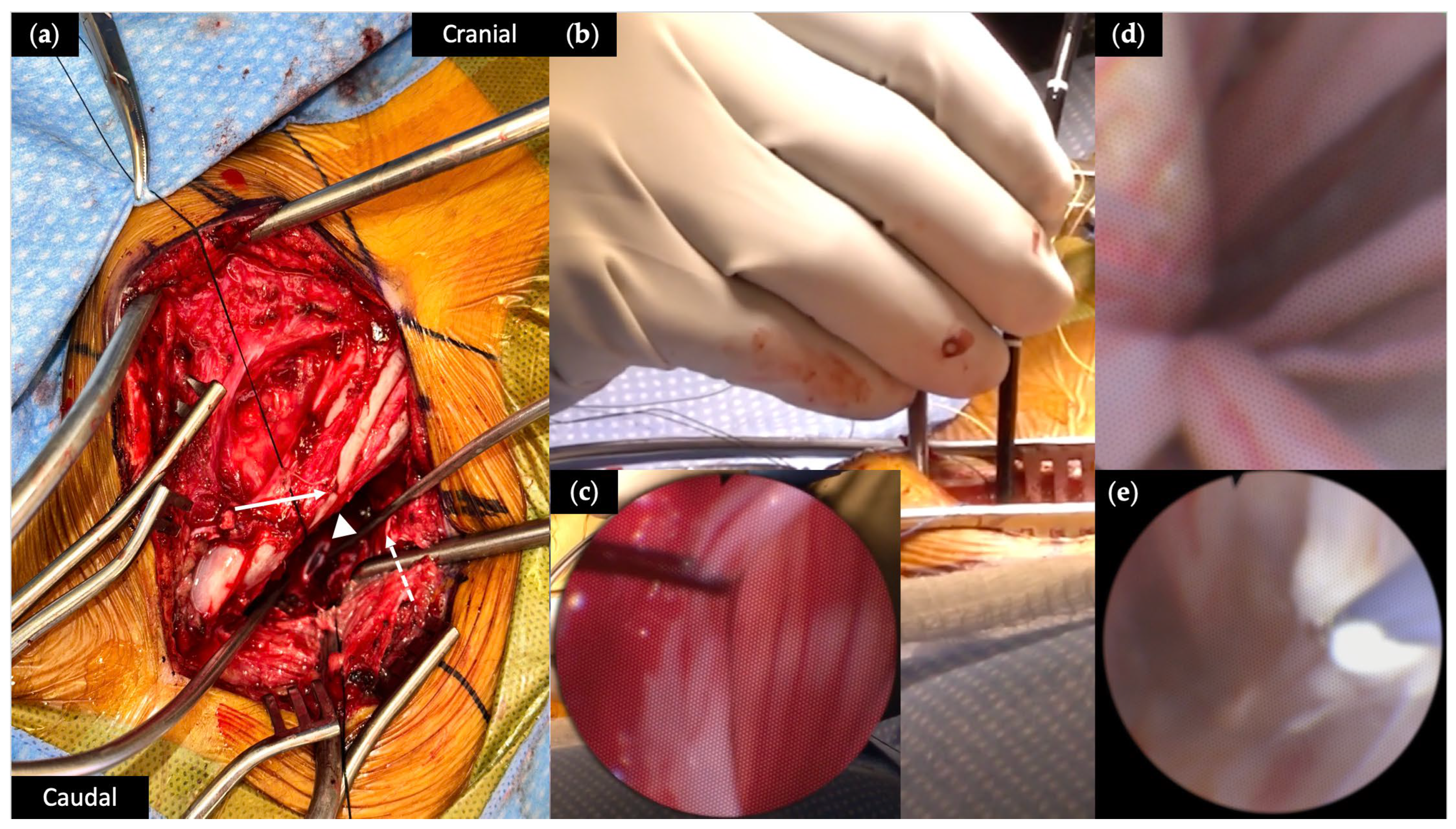
2.3. Surgical Procedure
3. Case Presentations
3.1. Patient Demographics
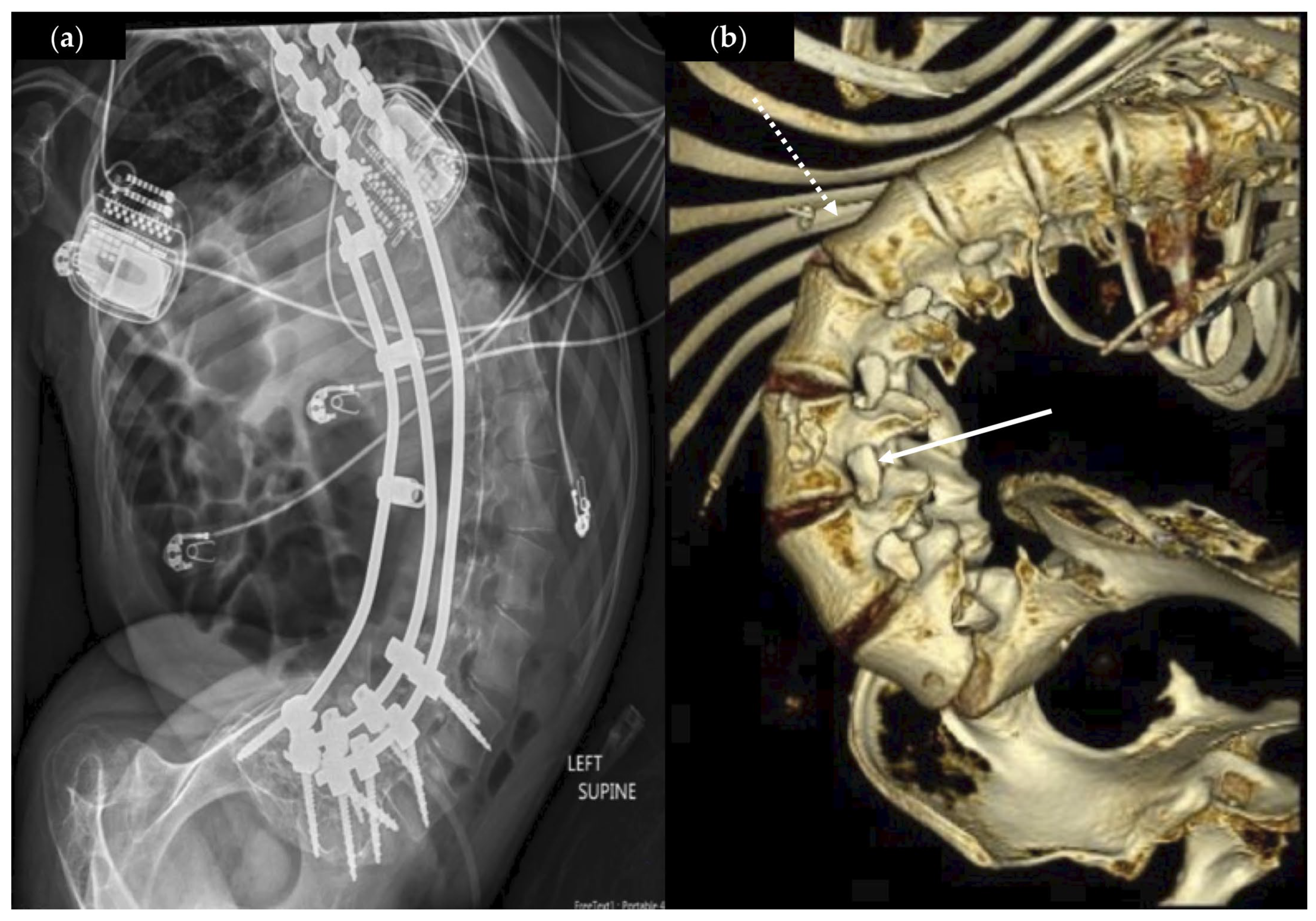
3.2. Operative Characteristics
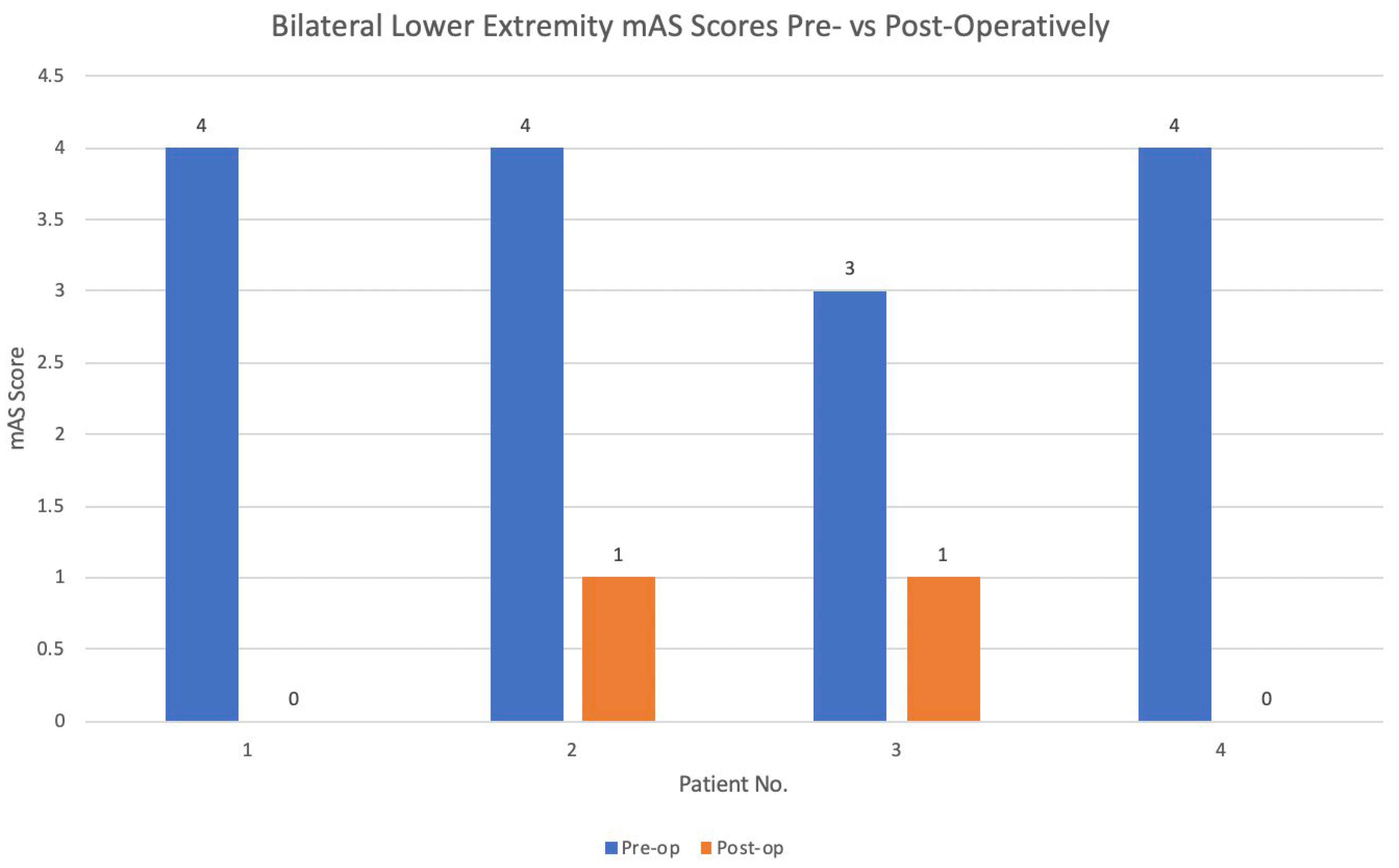
3.3. Surgical Outcomes
3.4. Perioperative Events
4. Discussion
4.1. Safety and Indications of eVDR
4.2. Efficacy of eVDR
4.3. Technical Challenges and Surgical Considerations
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | Cerebral palsy |
| SMA | Spinal muscular atrophy |
| eVDR | Endoscopic ventral dorsal rhizotomy |
| SDR | Selective dorsal rhizotomy |
| RFA | Radiofrequency ablation |
| mAS | Modified Ashworth Scale |
| BADS | Barry–Albright Dystonia Scale |
| GMFCS | Gross motor function classification score |
| ITB | Intrathecal baclofen |
| CSF | Cerebrospinal fluid |
| DBS | Deep brain stimulator |
| SSI | Surgical site infection |
References
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.; Goldsmith, S.; Webb, A.; Ehlinger, V.; Hollung, S.J.; McConnell, K.; Arnaud, C.; Smithers-Sheedy, H.; Oskoui, M.; Khandaker, G.; et al. Global prevalence of cerebral palsy: A systematic analysis. Dev. Med. Child Neurol. 2022, 64, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.; Benedict, R.; Christensen, D.; Dubois, L.A.; Fitzgerald, R.T.; Kirby, R.S.; Maenner, M.J.; Braun, K.V.N.; Wingate, M.S.; Yeargin-Allsopp, M. Prevalence of Cerebral Palsy among 8-Year-Old Children in 2010 and Preliminary Evidence of Trends in Its Relationship to Low Birthweight. Paediatr. Perinat. Epidemiol. 2016, 30, 496–510. [Google Scholar] [CrossRef]
- Bekteshi, S.; Monbaliu, E.; McIntyre, S.; Saloojee, G.; Hilberink, S.R.; Tatishvili, N.; Dan, B. Towards functional improvement of motor disorders associated with cerebral palsy. Lancet Neurol. 2023, 22, 229–243. [Google Scholar] [CrossRef]
- Scannell, B.; Yaszay, B. Scoliosis, Spinal Fusion, and Intrathecal Baclofen Pump Implantation. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Koop, S. Scoliosis in cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 92–98. [Google Scholar] [CrossRef]
- Persson-Bunke, M.; Hägglund, G.; Lauge-Pedersen, H.; Ma, P.; Westbom, L. Scoliosis in a Total Population of Children with Cerebral Palsy. Spine 2012, 37, E708. [Google Scholar] [CrossRef]
- Willoughby, K.; Ang, S.; Thomason, P.; Rutz, E.; Shore, B.; Buckland, A.J.; Johnson, M.B.; Graham, H.K. Epidemiology of scoliosis in cerebral palsy: A population-based study at skeletal maturity. J. Paediatr. Child Health 2022, 58, 295–301. [Google Scholar] [CrossRef]
- Abdelmageed, S.; Dalmage, M.; Mossner, J.; Trierweiler, R.; Krater, T.; Raskin, J. Nonselective lumbosacral ventral-dorsal rhizotomy for the management of lower-limb hypertonia in nonambulatory children with cerebral palsy. Neurosurg. Focus 2024, 56, E9. [Google Scholar] [CrossRef]
- Albright, A.; Tyler-Kabara, E. Combined ventral and dorsal rhizotomies for dystonic and spastic extremities. J. Neurosurg. Pediatr. 2007, 107, 324–327. [Google Scholar] [CrossRef]
- Miller, S.; Lewis, E.; Lau, J.; Juricic, M.B.; Nguyen, V.; Steinbok, P.M.; Miyanji, F.; Mulpuri, K.M. The Effect of Selective Dorsal Rhizotomy on Scoliosis in Children with Cerebral Palsy: A Long-term Follow-up Study. J. Pediatr. Orthop. 2025, 45, 158. [Google Scholar] [CrossRef]
- Wheelwright, M.; Selvey, P.; Steinbok, P.; Singhal, A.; Ibrahim, G.; Fallah, A.; Weil, A.G.; Halvorson, K.; Tu, A. Systematic review of spinal deformities following multi-level selective dorsal rhizotomy. Childs Nerv. Syst. 2020, 36, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, R.; Bass, P.; Flynn, L.; Martin, E.; Riordan, H.; Lawrence, A.; Naftel, R.P. Conus-level combined dorsal and ventral lumbar rhizotomy for treatment of mixed hypertonia: Technical note and complications. J. Neurosurg. Pediatr. 2020, 27, 102–107. [Google Scholar] [CrossRef]
- Shapkin, A.; Iakimov, I.; Sufianov, R.; Sufianova, G.; Sufianov, A. Percutaneous thermal radiofrequency rhizotomy of L2–S1 spinal nerve roots in children with cerebral palsy. Neurosurg. Focus 2024, 56, E7. [Google Scholar] [CrossRef] [PubMed]
- LoPresti, M.; Horak, V.; Trierweiler, R.; Stone, L.; Krater, T.; Raskin, J. Navigated Radiofrequency Ablation Peripheral Rhizotomy for Lumbosacral Hypertonia in a Nonambulatory Patient with Spinal Fusion: Indications, Surgical Techniques, and Lessons Learned. Oper. Neurosurg. 2023, 25, 461–468. [Google Scholar] [CrossRef]
- Richards, D.; Prasad, D.; Trierweiler, R.; Rourke, K.; Berton, E.; Katholi, B.; Raskin, J.S. Novel Intradural Endoscopic Lumbosacral Ventral-Dorsal Rhizotomy: A Technical Note with Operative Video. Oper. Neurosurg. 2022, 00, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.; Burton, D. Adolescent idiopathic scoliosis: Natural history and long term treatment effects. Scoliosis 2006, 1, 2. [Google Scholar] [CrossRef]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Screening for Adolescent Idiopathic Scoliosis: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 165–172. [Google Scholar] [CrossRef]
- Lam, G.; Hill, D.; Le, L.; Raso, J.; Lou, E. Vertebral rotation measurement: A summary and comparison of common radiographic and CT methods. Scoliosis 2008, 3, 16. [Google Scholar] [CrossRef]
- Nash, C.; Moe, J. A Study of Vertebral Rotation. J. Bone Jt. Surg. 1969, 51, 223. [Google Scholar] [CrossRef]
- Ng, S.; Bettany-Saltikov, J. Imaging in the Diagnosis and Monitoring of Children with Idiopathic Scoliosis. Open Orthop. J. 2017, 11, 150–160. [Google Scholar] [CrossRef]
- Abdelmageed, S.; Villalba, N.; Mossner, J.; Krater, T.; Raskin, J. Heterotopic Osteotomy for Intrathecal Baclofen Test Dose Administration in a Pediatric Patient with Spinal Fusion: A Technical Note. Oper. Neurosurg. 2024, 29, 131–134. [Google Scholar] [CrossRef]
- Horak, V.; Jimenez, M.; LoPresti, M.; Raskin, J. Pediatric intraspinal arachnoid cyst: Successful endoscopic fenestration. Illustrative case. J. Neurosurg. Case Lessons 2023, 6, 1–4. [Google Scholar] [CrossRef]
- Lee, S.; Hyun, C.; KIM, K.; Kwon, H.; Woo, M.; Koh, S. Effect of Intrathecal Baclofen Pump on Scoliosis in Children with Cerebral Palsy: A Meta-Analysis. Ann. Rehabil. Med. 2023, 47, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Albright, L.; Barry, M.; Shafron, D.; Ferson, S. Intrathecal baclofen for generalized dystonia. Dev. Med. Child Neurol. 2001, 43, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Di, N.R.; Padalia, D. Implantable Intrathecal Drug Delivery System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wach, J.; Yildiz, Ö.; Sarikaya-Seiwert, S.; Vatter, H.; Haberl, H. Predictors of postoperative complications after selective dorsal rhizotomy. Acta Neurochir. 2021, 163, 463–474. [Google Scholar] [CrossRef]
- Fjelstad, A.; Hommelstad, J.; Sorteberg, A. Infections related to intrathecal baclofen therapy in children and adults: Frequency and risk factors. J. Neurosurg. Pediatr. 2009, 4, 487–493. [Google Scholar] [CrossRef]
- Costici, P.; Russo, R.; Brigato, P.; De Salvatore, S.; Vescio, A.; Oggiano, L.; Donati, F. Safety and efficacy of the novel subfascial with umbilicus detachment technique for intrathecal baclofen therapy in pediatric patients with cerebral palsy and low body mass index. Childs Nerv. Syst. 2025, 41, 118. [Google Scholar] [CrossRef]
- Spader, H.; Bollo, R.; Bowers, C.; Riva-Cambrin, J. Risk factors for baclofen pump infection in children: A multivariate analysis. J. Neurosurg. Pediatr. 2016, 17, 756–762. [Google Scholar] [CrossRef]
- Desai, V.; Raskin, J.; Mohan, A.; Montojo, J.; Briceño, V.; Curry, D.J.; Lam, S. A standardized protocol to reduce pediatric baclofen pump infections: A quality improvement initiative. J. Neurosurg. Pediatr. 2018, 21, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Bollo, R.; Gross, P.; Rocque, B.; Browd, S.R.; Raskin, J.S.; Leonard, J.R.; Albarqawi, L.; Bailes, A.F. A multicenter initiative to reduce intrathecal baclofen pump surgical site infection: A Cerebral Palsy Research Network quality improvement project. J. Neurosurg. Pediatr. 2023, 31, 444–452. [Google Scholar] [CrossRef]
- Westbom, L.; Lundkvist, J.A.; Wagner, P.; Nordmark, E. Growth in Children with Cerebral Palsy during five years after Selective Dorsal Rhizotomy: A practice-based study. BMC Neurol. 2010, 10, 57. [Google Scholar] [CrossRef]
- Borowski, A.; Shah, S.; Littleton, A.; Dabney, K.; Miller, F. Baclofen pump implantation and spinal fusion in children: Techniques and complications. Spine 2008, 33, 1995–2000. [Google Scholar] [CrossRef]
- Chen, K.; Kim, J.; Huang, A.; Lin, M.; Chen, C. Current Indications for Spinal Endoscopic Surgery and Potential for Future Expansion. Neurospine 2023, 20, 33–42. [Google Scholar] [CrossRef]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: A prospective, randomized, controlled study. J. Neurosurg. Spine 2009, 10, 476–485. [Google Scholar] [CrossRef]
- Birkenmaier, C.; Komp, M.; Leu, H.; Wegener, B.; Ruetten, S. The current state of endoscopic disc surgery: Review of controlled studies comparing full-endoscopic procedures for disc herniations to standard procedures. Pain Physician 2013, 16, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ordaz, J.; Huh, A.; Desai, V.; Raskin, J.S. Iatrogenic Spinal Deformity Following Spinal Intradural Arachnoid Cyst Fenestration Despite Minimal Access with Laminoplasty and Endoscopy in a Pediatric Patient. Cureus. 2022, 14, e22053. [Google Scholar] [CrossRef]
- Chern, J.; Gordon, A.; Naftel, R.; Tubbs, R.; Oakes, W.; Wellons, J. Intradural spinal endoscopy in children. J. Neurosurg. Pediatr. 2011, 8, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Adams, I.; Jayaweera, R.; Lewis, J.; Badawi, N.; Abdel-Latif, M.; Paget, S. Postoperative pain and pain management following selective dorsal rhizotomy. BMJ Paediatr. Open 2024, 8, e002381. [Google Scholar] [CrossRef]
- Hesselgard, K.; Reinstrup, P.; Stromblad, L.; Undén, J.; Romner, B. Selective Dorsal Rhizotomy and Postoperative Pain Management: A Worldwide Survey. Pediatr. Neurosurg. 2007, 43, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, S.; Markia, B.; Pople, I.; Aquilina, K.; Smith, J.; Mohamed, A.Z.; Burchell, A.; Jenkins, L.; Walsh, P.; Clark, N.; et al. Surgical Outcomes of Single-Level Bilateral Selective Dorsal Rhizotomy for Spastic Diplegia in 150 Consecutive Patients. World Neurosurg. 2019, 125, e60–e66. [Google Scholar] [CrossRef] [PubMed]
- Steinbok, P.; Schrag, C. Complications after Selective Posterior Rhizotomy for Spasticity in Children with Cerebral Palsy. Pediatr. Neurosurg. 1998, 28, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Barik, S.; Raj, V.; Kandwal, P. A systematic review of complications following selective dorsal rhizotomy in cerebral palsy. Neurochirurgie 2023, 69, 101425. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, C.; Chen, H.; Wu, C.; Lin, K.; Hsieh, Y.-W.; Shen, I. Responsiveness and minimal clinically important difference of Modified Ashworth Scale in patients with stroke. Eur. J. Phys. Rehabil. Med. 2019, 55, 754–760. [Google Scholar] [CrossRef]
- Merckx, L.; Poncelet, F.; De, H.H.; Laumen, A.; Peeters, L.; Nuttin, B.; Pauwels, P.; Van Campenhout, A.; Ortibus, E.; De Vloo, P. Upper-extremity spasticity and functionality after selective dorsal rhizotomy for cerebral palsy: A systematic review. J. Neurosurg. Pediatr. 2023, 32, 673–685. [Google Scholar] [CrossRef]

| Case No. | Age (Years), Sex | Race/ Ethnicity | Dominant Movement Disorder | Diagnosis Etiology | GMFCS (I–V) | Trach? | G-Tube? | BMI | Scoliosis, Cobb (°), Curve | Scoliosis, Nash-Moe | Prior Spinal Fusion | Prior Functional Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22, M | Asian | Quadriplegia, mixed | CP (HIE) | V | Y | Y | 19.9 | T10-L4, 51.5 L | +2, L3 | Y | Y, bolus ITB test dose |
| 2 | 21, M | Hispanic | Quadriplegia, spastic | CP (unknown) | V | N | Y | 19.3 | T7-L4, 64 D | +4, L3 | Y | N |
| 3 | 21, F | Hispanic | Quadriplegia, spastic | SMA2 | V | Y * | Y | 14.8 | T12-S1, 143 D ^ | +4, T12 | N | N |
| 4 | 18, M | Hispanic | Quadriplegia, mixed | CP (pre-term) | V | N | Y | 19.1 | T9-L5, 82 L | +4, T12 | Y | Y, DBS |
| Case No. | eVDR Procedure Levels | Access Laminotomy Level | Operative Time (Minutes) | EBL (mLs) | LOS (Days) | Follow-Up (Months) | Perioperative Events? | Caregiving Improvement |
|---|---|---|---|---|---|---|---|---|
| 1 | Left T12, bilateral L1-S1 | L3 | 233 | 50 | 2 | 8 | No | Transfers |
| 2 | Bilateral L1-S1 | L3 | 230 | <5 | 5 | 9 | No | Positioning |
| 3 | Bilateral L1-S1 | T12 | 209 | 50 | 3 | 3 | Mild neuropathic pain | Improved ROM |
| 4 | Bilateral L1-S1 | T12-L1 | 229 | 10 | 1 | 3 | Superficial wound dehiscence | Positioning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, L.T.; Weiss, B.E.; Pertsch, N.; Rogers, O.; Katholi, B.; Raskin, J.S. Lumbosacral Endoscopic Ventral–Dorsal Rhizotomy: A Novel Approach for Tone Reduction. Brain Sci. 2025, 15, 1030. https://doi.org/10.3390/brainsci15101030
Chiu LT, Weiss BE, Pertsch N, Rogers O, Katholi B, Raskin JS. Lumbosacral Endoscopic Ventral–Dorsal Rhizotomy: A Novel Approach for Tone Reduction. Brain Sciences. 2025; 15(10):1030. https://doi.org/10.3390/brainsci15101030
Chicago/Turabian StyleChiu, Lucinda T., Benjamin E. Weiss, Nathan Pertsch, Olivia Rogers, Benjamin Katholi, and Jeffrey S. Raskin. 2025. "Lumbosacral Endoscopic Ventral–Dorsal Rhizotomy: A Novel Approach for Tone Reduction" Brain Sciences 15, no. 10: 1030. https://doi.org/10.3390/brainsci15101030
APA StyleChiu, L. T., Weiss, B. E., Pertsch, N., Rogers, O., Katholi, B., & Raskin, J. S. (2025). Lumbosacral Endoscopic Ventral–Dorsal Rhizotomy: A Novel Approach for Tone Reduction. Brain Sciences, 15(10), 1030. https://doi.org/10.3390/brainsci15101030






