Peri-Traumatic Near-Infrared Light Treatment Attenuates the Severity of Noise-Induced Hearing Loss by Rescuing (Type I) Spiral Ganglion Neurons in Mice
Abstract
1. Introduction
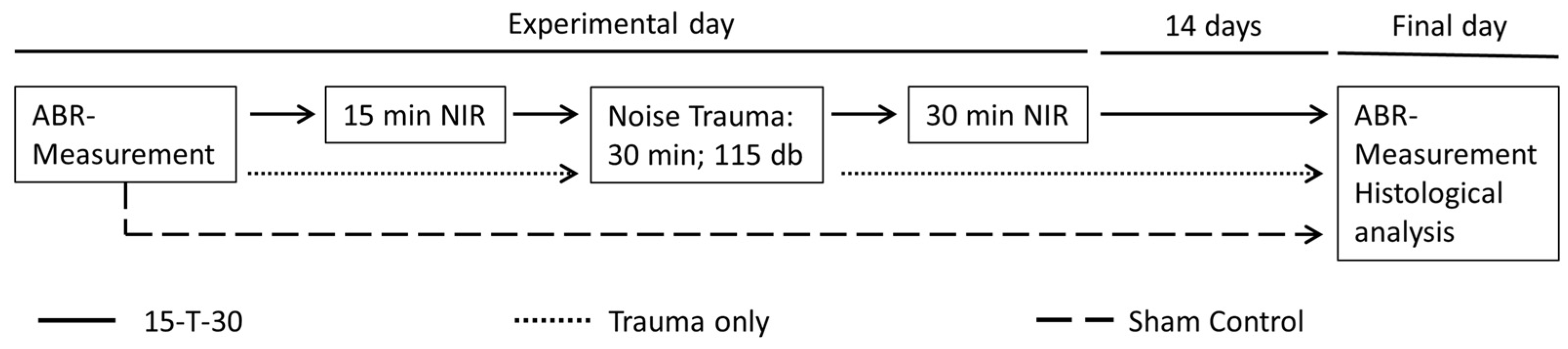
2. Materials and Methods
2.1. Animals
2.2. Frequency-Specific Auditory Brainstem Response Measurements
2.3. NIR Treatment
2.4. Noise Exposure
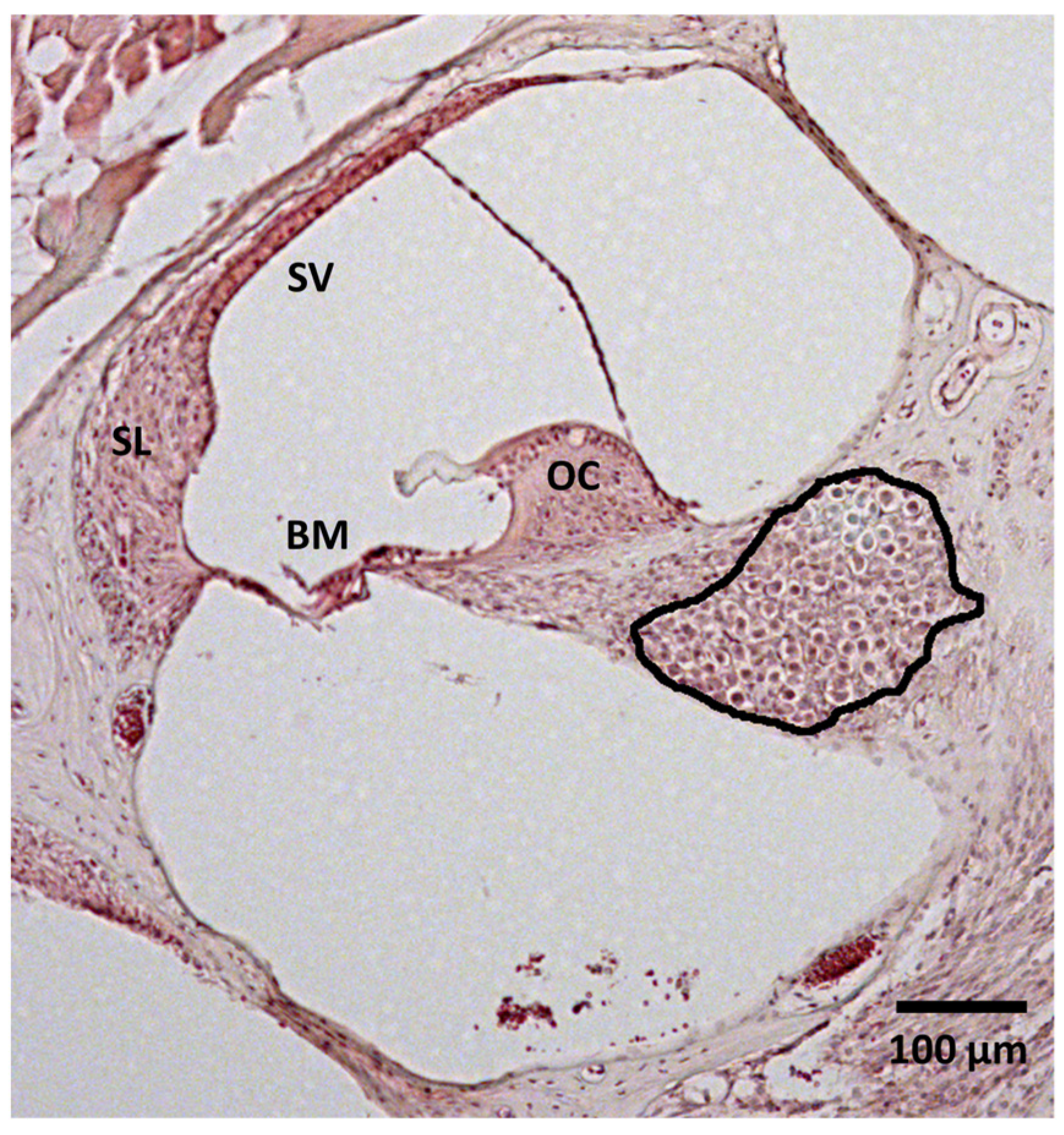
2.5. Histological Analysis
2.6. Image Analysis
2.7. Statistical Procedures
3. Results
3.1. ABR Threshold Shift
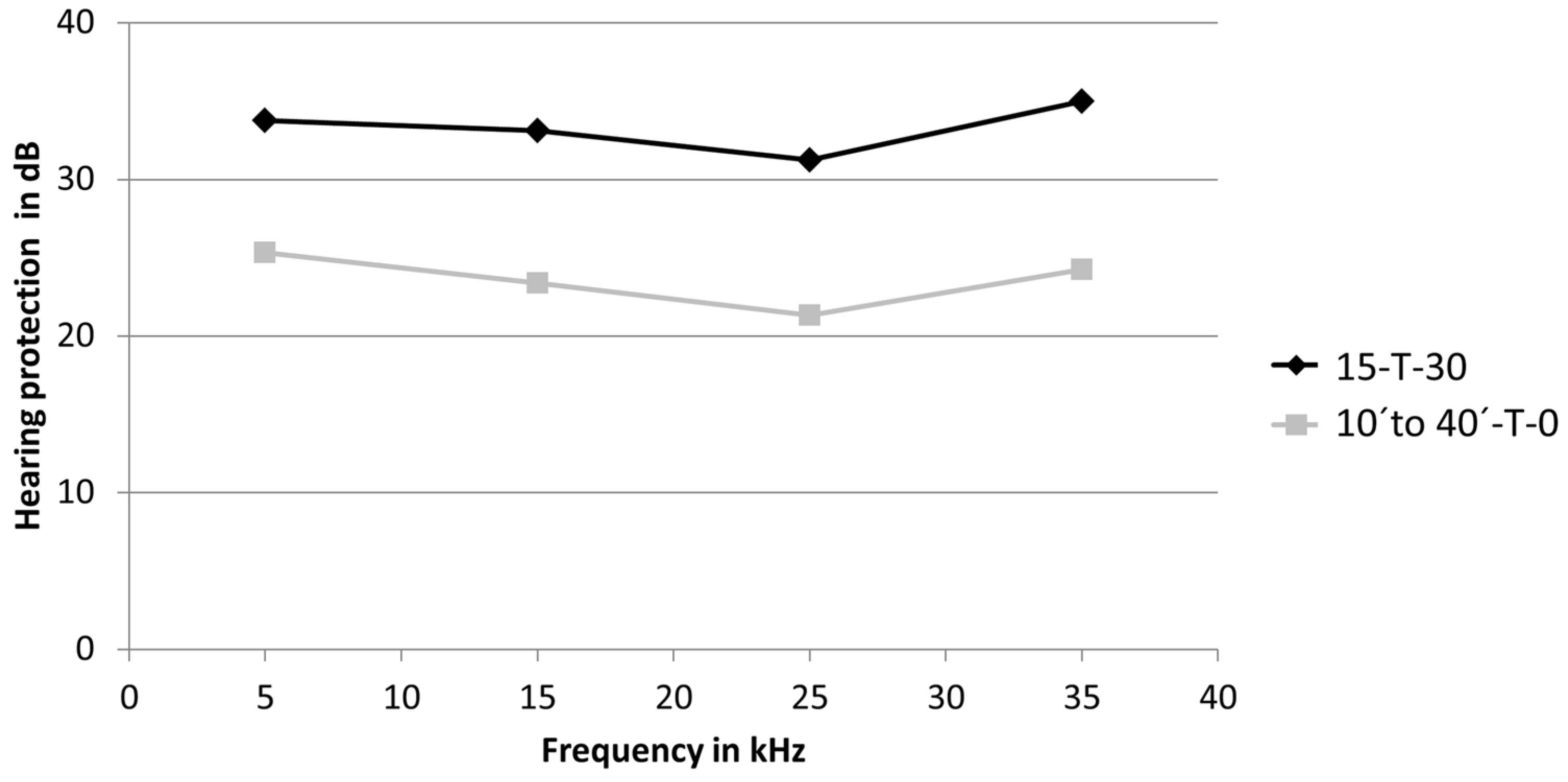
3.2. Hearing Protection
3.3. Spiral Ganglion Neuron Density
4. Discussion
4.1. Mechanism of NIR Action in Peri-Traumatic Treatment Regime
4.2. Spiral Ganglion Neuron Density
4.3. Potential for a Clinical Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabinowitz, P.M. Noise-induced hearing loss. Am. Fam. Physician 2000, 1, 2749–2756, 2759–2760. [Google Scholar]
- Goines, L.; Hagler, L. Noise Pollution: A Modern Plague. South Med. J. 2007, 100, 287–294. [Google Scholar] [CrossRef]
- Natarajan, N.; Batts, S.; Stankovic, K.M. Noise-Induced Hearing Loss. J. Clin. Med. 2023, 12, 2347. [Google Scholar] [CrossRef] [PubMed]
- Furness, D.N. Molecular basis of hair cell loss. Cell Tissue Res. 2015, 361, 387–399. [Google Scholar] [CrossRef]
- Salvi, R.; Sun, W.; Ding, D.; Chen, G.D.; Lobarinas, E.; Wang, J.; Radziwon, K.; Auerbach, B.D. Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain. Front. Neurosci. 2017, 10, 621. [Google Scholar] [CrossRef]
- Gröschel, M.; Manchev, T.; Fröhlich, F.; Voigt, S.; Ernst, A.; Basta, D. Early Loss of Spiral Ganglion Neurons in the Auditory System after Noise Trauma. Audiol. Neurootol. 2024, 29, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Gao, S.; Salam, I.; Ali, M.K.; Ma, J.; Wenyan, L. Inner Ear Hair Cell Protection in Mammals against the Noise-Induced Cochlear Damage. Neural Plast. 2018, 2018, 3170801. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, J.T.; Huang, Y.Y.; Sharma, S.K.; Kurup, D.B.; De Taboada, L.; Carroll, J.D.; Hamblin, M.R. Effect of pulsing in low-level light therapy. Lasers Surg. Med. 2010, 42, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Matsunobu, T.; Mizutari, K.; Niwa, K.; Kurioka, T.; Kawauchi, S.; Satoh, S.; Hiroi, S.; Satoh, Y.; Nibuya, M.; et al. Low-level laser therapy for prevention of noise-induced hearing loss in rats. Neurosci. Lett. 2015, 595, 81–86. [Google Scholar] [CrossRef]
- Nikookam, Y.; Zia, N.; Lotfallah, A.; Muzaffar, J.; Davis-Manders, J.; Kullar, P.; Smith, M.; Bale, G.; Boyle, P.; Irving, R.; et al. The effect of photobiomodulation on hearing loss: A systematic review. Clin. Otolaryngol. 2024, 49, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chang, S.Y.; Moy, W.J.; Oh, C.; Kim, S.H.; Rhee, C.K.; Ahn, J.C.; Chung, P.S.; Jung, J.Y.; Lee, M.Y. Simultaneous bilateral laser therapy accelerates recovery after noise-induced hearing loss in a rat model. PeerJ 2016, 4, e2252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rhee, C.K.; Bahk, C.W.; Kim, S.H.; Ahn, J.C.; Jung, J.Y.; Chung, P.S.; Suh, M.W. Effect of low-level laser treatment on cochlea hair-cell recovery after acute acoustic trauma. J. Biomed. Opt. 2012, 17, 068002. [Google Scholar] [CrossRef]
- Basta, D.; Gröschel, M.; Strübing, I.; Boyle, P.; Fröhlich, F.; Ernst, A.; Seidl, R. Near-infrared-light pre-treatment attenuates noise-induced hearing loss in mice. PeerJ 2020, 8, e9384. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef]
- Mason, M.G.; Nicholls, P.; Cooper, C.E. Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: Implications for non invasive in vivo monitoring of tissues. BBA Bioenerg. 2014, 1837, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Barrientos, A.; Fontanesi, F.; Ott, M. The functional significance of mitochondrial respiratory chain supercomplexes. EMBO Rep. 2023, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Power Games. Nature 2006, 443, 26. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Lee, M.Y.; Chung, P.S.; Kim, S.; Choi, B.; Suh, M.W.; Rhee, C.K.; Jung, J.Y. Enhanced mitochondrial membrane potential and ATP synthesis by photobiomodulation increases viability of the auditory cell line after gentamicin-induced intrinsic apoptosis. Sci. Rep. 2019, 9, 19248. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T.; Bai, X.; Buchmann, E.; Whelan, H.T. Light-emitting diode treatment reverses the effect of TTX on cytochrome oxidase in neurons. Neuroreport 2001, 12, 3033–3037. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Wu, X.; Waynant, R.W.; Ilev, I.K.; Anders, J.J. Low power laser irradiation alters gene expression of olfactory ensheathing cells in vitro. Lasers Surg. Med. 2005, 37, 161–171. [Google Scholar] [CrossRef]
- Liang, H.L.; Whelan, H.T.; Eells, J.T.; Meng, H.; Buchmann, E.; Lerch-Gaggl, A.; Wong-Riley, M. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience 2006, 139, 639–649. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, H.S.; Song, J.J.; Chang, S.O.; Oh, S.H. Increased activity of mitochondrial respiratory chain complex in noise-damaged rat cochlea. Acta Otolaryngol. 2012, 132, 134–141. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Housley, G.D.; Muñoz, D.J.; Robson, S.C.; Sévigny, J.; Wang, C.J.; Thorne, P.R. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience 2004, 126, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Q.; Zheng, H.W.; Hill, K.; Sha, S.H. Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. J. Neurosci. 2012, 32, 12421–12430. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.; Yuan, H.; Wang, X.; Sha, S.H. Noise-Induced Loss of Hair Cells and Cochlear Synaptopathy are Mediated by the Activation of AMPK. J. Neurosci. 2016, 36, 7497–7510. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Chen, Y. Noise-Induced Hearing Loss: Updates on Molecular Targets and Potential Interventions. Neural Plast. 2021, 6, 4784385. [Google Scholar] [CrossRef]
- Zheng, H.W.; Chen, J.; Sha, S.H. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis. 2014, 5, e1262. [Google Scholar] [CrossRef]
- Ross, F.A.; MacKintosh, C.; Hardie, D.G. AMP-activated protein kinase: A cellular energy sensor that comes in 12 flavours. FEBS J. 2016, 283, 2987–3001. [Google Scholar] [CrossRef]
- Chen, A.C.-H.; Huan, J.J.; Arany, P.R.; Hamblin, M.R. Role of reactive oxygen species in low level light therapy. SPIE 2009, 7165, 9–19. [Google Scholar]
- Sumimoto, H.; Minakami, R.; Miyano, K. Soluble Regulatory Proteins for Activation of NOX Family NADPH Oxidases. Methods Mol. Biol. 2019, 1982, 121–137. [Google Scholar] [PubMed]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Otorhinolaryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Wright, J.S.; Dugan, L.L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neurootol. 1999, 4, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Nuttall, A.L. Upregulated iNOS and oxidative damage to the cochlear stria vascularis due to noise stress. Brain Res. 2003, 967, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, D.; Jiang, H.Y.; Schacht, J.; Miller, J.M. Delayed production of free radicals following noise exposure. Brain Res. 2004, 1019, 201–209. [Google Scholar] [CrossRef]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Poirrier, A.L.; Pincemail, J.; Van Den Ackerveken, P.; Lefebvre, P.P.; Malgrange, B. Oxidative stress in the cochlea: An update. Curr. Med. Chem. 2010, 17, 3591–3604. [Google Scholar] [CrossRef]
- Wong, A.C.; Ryan, A.F. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci. 2015, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Yamashita, D.; Minami, S.B.; Kanzaki, S.; Ogawa, K.; Miller, J.M. Bcl-2 genes regulate noise-induced hearing loss. J. Neurosci. Res. 2008, 86, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Fujioka, M.; Kanzaki, S.; Okano, H.J.; Shibata, S.; Yamashita, D.; Masuda, M.; Mihara, M.; Ohsugi, Y.; Ogawa, K.; et al. Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci. Res. 2010, 66, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Keithley, E.M.; Wang, X.; Barkdull, G.C. Tumor necrosis factor alpha can induce recruitment of inflammatory cells to the cochlea. Otol. Neurotol. 2008, 29, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.H.; Lee, M.Y.; Jung, J.Y.; Ahn, J.C.; Chang, S.Y.; Chung, P.S.; Rhee, C.K.; Kim, Y.H.; Suh, M.W. Safety assessment of trans-tympanic photobiomodulation. Lasers Med. Sci. 2016, 31, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hast, A.; Hocke, T. Disproportional hoher Verlust an Sprachverstehen. HNO 2024, 72, 885–892. [Google Scholar] [CrossRef]
- Bess, F.H.; Lichtenstein, M.J.; Logan, S.A.; Burger, M.C.; Nelson, E. Hearing impairment as a determinant of function in the elderly. J. Am. Geriatr. Soc. 1989, 37, 123–128. [Google Scholar] [CrossRef]
- Lin, F.R.; Ferrucci, L. Hearing loss and falls among older adults in the United States. Arch. Intern. Med. 2012, 172, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Bartos, A.; Grondin, Y.; Bortoni, M.E.; Ghelfi, E.; Sepulveda, R.; Carroll, J.; Rogers, R.A. Pre-conditioning with near infrared photobiomodulation reduces inflammatory cytokines and markers of oxidative stress in cochlear hair cells. J. Biophotonics 2016, 9, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Avadhani, N.G. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Kharkwal, G.B.; Sajo, M.; Huang, Y.Y.; De Taboada, L.; McCarthy, T.; Hamblin, M.R. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg. Med. 2011, 43, 851–859. [Google Scholar] [CrossRef]
- Chen, A.C.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef]
- Tamura, A.; Matsunobu, T.; Tamura, R.; Kawauchi, S.; Sato, S.; Shiotani, A. Photobiomodulation rescues the cochlea from noise-induced hearing loss via upregulating nuclear factor κB expression in rats. Brain Res. 2016, 1646, 467–474. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Nagata, K.; Tedford, C.E.; McCarthy, T.; Hamblin, M.R. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J. Biophotonics 2013, 6, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.A.; Lyu, A.R.; Jeong, S.H.; Kim, T.H.; Park, M.J.; Park, Y.H. Acoustic Trauma Modulates Cochlear Blood Flow and Vasoactive Factors in a Rodent Model of Noise-Induced Hearing Loss. Int. J. Mol. Sci. 2019, 20, 5316. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K.; Girndt, M.; Meisner, C.; Fischer, I.; Böselt, I.; Löhler, J.; Ludwig-Kraus, B.; Richter, M.; Steighardt, J.; Reuter, B.; et al. HODOKORT Trial Investigators. High-Dose Glucocorticoids for the Treatment of Sudden Hearing Loss. NEJM Evid. 2024, 3, EVIDoa2300172. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K.; Meisner, C.; Agrawal, S.; Caye-Thomasen, P.; Galbraith, K.; Mikulec, A.A.; Parnes, L.; Premakumar, Y.; Reiber, J.; Schilder, A.G.; et al. Intratympanic corticosteroids for sudden sensorineural hearing loss. Cochrane Database Syst. Rev. 2022, 7, CD008080. [Google Scholar] [PubMed]
- Alexander, T.H.; Weisman, M.H.; Derebery, J.M.; Espeland, M.A.; Gantz, B.J.; Gulya, A.J.; Hammerschlag, P.E.; Hannley, M.; Hughes, G.B.; Moscicki, R.; et al. Safety of high-dose corticosteroids for the treatment of autoimmune inner ear disease. Otol. Neurotol. 2009, 30, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Minesinger, K.; Gallagher, H.; Stefanson, J.R.; Bridges, N.; Jackson, N.; Stark, V.; Coto, J.; Rajguru, S.; Yankaskas, K.; et al. Examining the utility of near infrared light as pre-exposure therapy to mitigate temporary noise-induced hearing loss in humans. Front. Neurol. 2024, 22, 1366239. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meuser, M.; Schwitzer, S.; Faraji, P.; Ernst, A.; Basta, D. Peri-Traumatic Near-Infrared Light Treatment Attenuates the Severity of Noise-Induced Hearing Loss by Rescuing (Type I) Spiral Ganglion Neurons in Mice. Brain Sci. 2025, 15, 62. https://doi.org/10.3390/brainsci15010062
Meuser M, Schwitzer S, Faraji P, Ernst A, Basta D. Peri-Traumatic Near-Infrared Light Treatment Attenuates the Severity of Noise-Induced Hearing Loss by Rescuing (Type I) Spiral Ganglion Neurons in Mice. Brain Sciences. 2025; 15(1):62. https://doi.org/10.3390/brainsci15010062
Chicago/Turabian StyleMeuser, Max, Susanne Schwitzer, Parisa Faraji, Arne Ernst, and Dietmar Basta. 2025. "Peri-Traumatic Near-Infrared Light Treatment Attenuates the Severity of Noise-Induced Hearing Loss by Rescuing (Type I) Spiral Ganglion Neurons in Mice" Brain Sciences 15, no. 1: 62. https://doi.org/10.3390/brainsci15010062
APA StyleMeuser, M., Schwitzer, S., Faraji, P., Ernst, A., & Basta, D. (2025). Peri-Traumatic Near-Infrared Light Treatment Attenuates the Severity of Noise-Induced Hearing Loss by Rescuing (Type I) Spiral Ganglion Neurons in Mice. Brain Sciences, 15(1), 62. https://doi.org/10.3390/brainsci15010062






