Subjective Cognitive Impairment Can Be Detected from the Decline of Complex Cognition: Findings from the Examination of Remedes 4 Alzheimer’s (R4Alz) Structural Validity
Abstract
1. Introduction
The Purpose and the Hypotheses of the Study
2. Materials and Methods
2.1. Protocol Approval and Participant Consent
2.2. Participants
2.3. Exclusion Criteria
2.4. Inclusion Criteria
2.5. Tools
2.5.1. The R4Alz Battery
2.5.2. Working Memory Capacity Test
2.5.3. Attention Control Test (ACT)
2.5.4. Executive Functioning Test
- (a)
- The Inhibition part comprises four conditions. In the first, the examinee names animal sketches, whilst in the second, they recognize animal sounds. In the third condition, the examinee names the animal and ignores the heard sound, whilst in the last condition, the examinee names the sound of the animal and disregards the animal image. The total score ranges from a minimum of 0, indicating the best performance, to a maximum of 60, indicating the worst performance.
- (b)
- Task/rule-switching part: In the first condition, the examinee names the sound of animals he/she hears until a red pad activation appears. Then, the participant must change the rule and start naming the image of the animal. This procedure occurs several times. In the second condition, the examinee must repeatedly switch between naming the animal sounds and the animal sketches by keeping a specific rule in mind, which is “when the white pad is activated, name the sound you hear, and when the red pad is activated, name the sketch you see”. The total score ranges from a minimum of 0, indicating the best performance, to a maximum of 63, indicating the worst performance. Therefore, the total score, including both executive function subtests (1&2), has a minimum value of 0 and a maximum value of 123, with the score of 123 indicating the worst performance. At this point, it should be mentioned that regarding the first condition of the task/rule-switching subtest, two more variables are calculated: (a) switching errors (SEs), which measures how many times the subject failed in task/rule-switching between sets; and (b) failed sets (FSs), which measures the number of the sets of the test that contain at least one failure. There are five switches among sets; therefore, the best SE score is 0, whilst the worst is 5, indicating five switch errors. Also, there are six sets in total; therefore, the minimum FS score, indicating the best performance, is 0, whilst the maximum score is 6. These two scores are separate variables and do not add up to the subtest’s total score.
2.6. Procedure
2.7. Statistical Analysis
3. Results
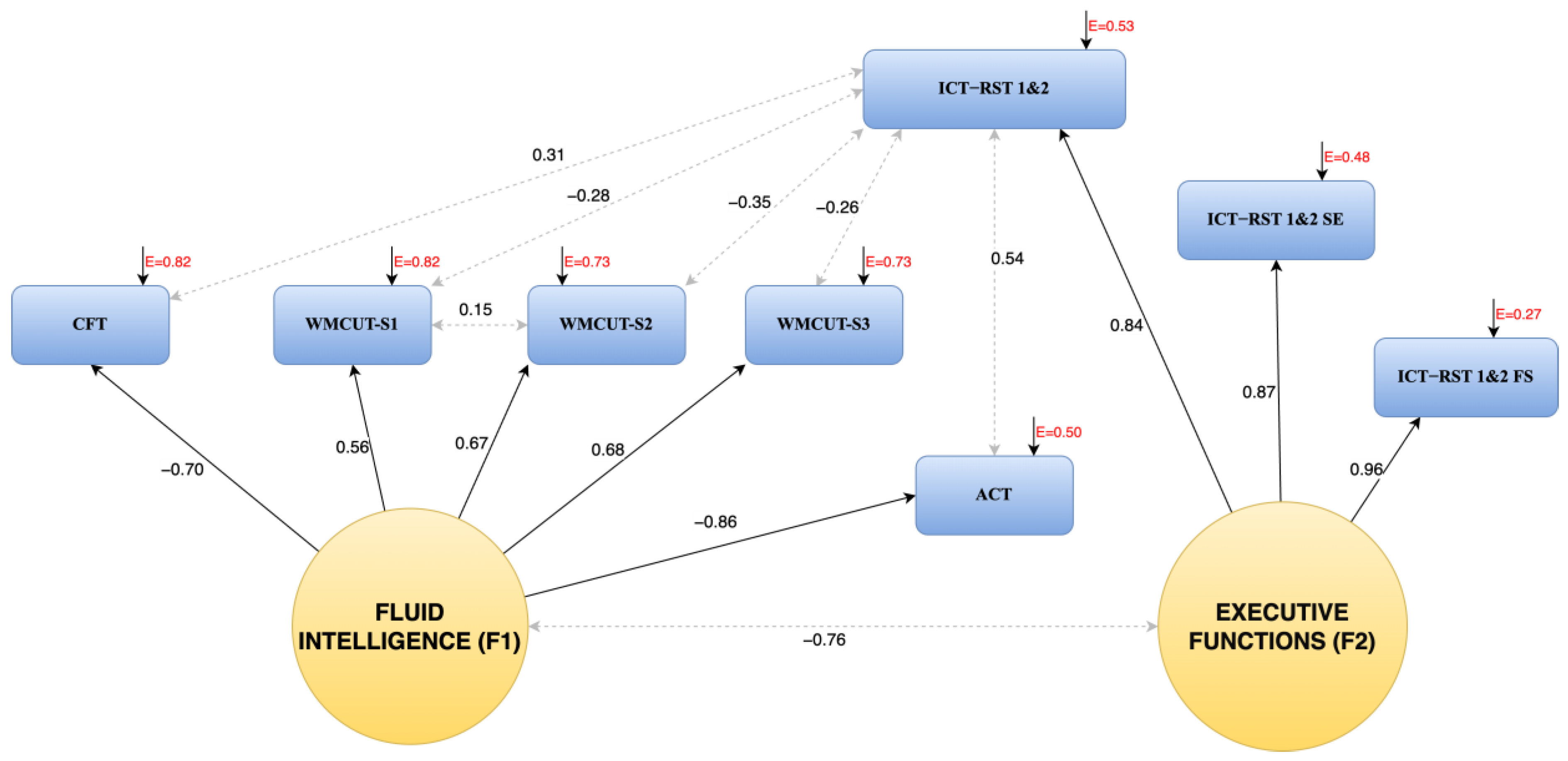
3.1. The Factor Structure of the R4Alz
3.2. Calculation of Total Scores for the Two Factors
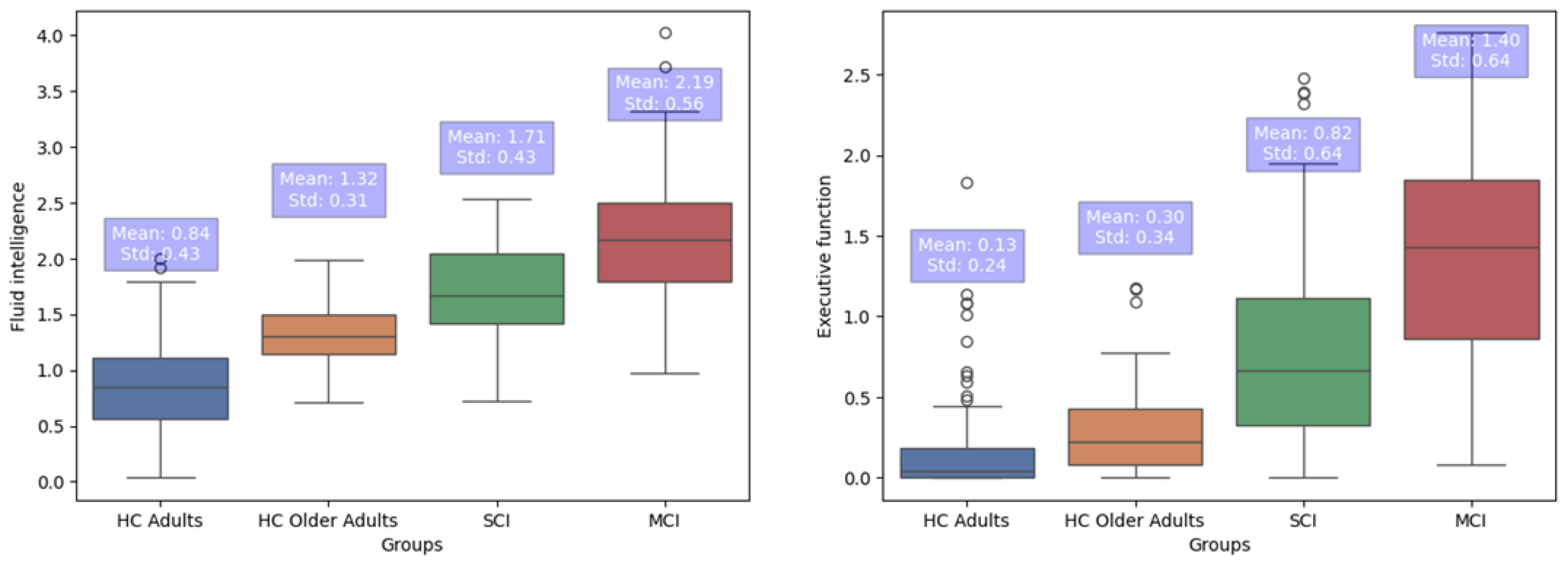
3.3. Group Differences in FI and EFs
4. Discussion
4.1. Construct Validity of the R4Alz
4.2. R4Alz’s Discriminant Potential
4.3. Limitations and Future Work
4.4. Clinical Implication of the R4Alz Battery and Innovative Contributions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Rabin, L.A.; Smart, C.M.; Amariglio, R.E. Subjective Cognitive Decline in Preclinical Alzheimer’s Disease. Annu. Rev. Clin. Psychol. 2017, 13, 369–396. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A Conceptual Framework for Research on Subjective Cognitive Decline in Preclinical Alzheimer’s Disease. Alzheimers Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Stephan, B.C.M.; Hunter, S.; Harris, D.; Llewellyn, D.J.; Siervo, M.; Matthews, F.E.; Brayne, C. The Neuropathological Profile of Mild Cognitive Impairment (MCI): A Systematic Review. Mol. Psychiatry 2012, 17, 1056–1076. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, I.; Moraitou, D.; Papatheodorou, M.; Vavouras, I.; Lokantidou, C.; Agogiatou, C.; Gialaoutzis, M.; Nikolopoulos, S.; Stavropoulos, T.G.; Kompatsiaris, I.; et al. Adaptation and Validation of the Memory Alteration Test (M@T) in Greek Middle-Aged, Older, and Older-Old Population with Subjective Cognitive Decline and Mild Cognitive Impairment. J. Alzheimers Dis. 2021, 84, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, E.; Moraitou, D.; Eleftheriou, M.; Kounti-Zafeiropoulou, F.; Papasozomenou, C.; Agogiatou, C.; Bakoglidou, E.; Batsila, G.; Liapi, D.; Markou, N.; et al. Normative Data for the Montreal Cognitive Assessment in Greek Older Adults with Subjective Cognitive Decline, Mild Cognitive Impairment and Dementia. J. Geriatr. Psychiatry Neurol. 2019, 32, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, E.; Tsardoulias, E.; Moraitou, D.; Symeonidis, A.L.; Tsolaki, M. REMEDES for Alzheimer-R4Alz Battery: Design and Development of a New Tool of Cognitive Control Assessment for the Diagnosis of Minor and Major Neurocognitive Disorders. J. Alzheimers Dis. 2019, 72, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Rami, L.; Mollica, M.A.; García-Sanchez, C.; Saldaña, J.; Sanchez, B.; Sala, I.; Valls-Pedret, C.; Castellví, M.; Olives, J.; Molinuevo, J.L. The Subjective Cognitive Decline Questionnaire (SCD-Q): A Validation Study. J. Alzheimers Dis. 2014, 41, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Guerreiro, M.; Faria, C.; Maroco, J.; Schmand, B.A.; de Mendonça, A. Significance of Subjective Memory Complaints in the Clinical Setting. J. Geriatr. Psychiatry Neurol. 2014, 27, 259–265. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Vogt, B.; Frisch, S.; Becker, G.; Barthel, H.; Mueller, K.; Villringer, A.; Sabri, O. Executive Deficits Are Related to the Inferior Frontal Junction in Early Dementia. Brain 2011, 135, 201–215. [Google Scholar] [CrossRef]
- Poptsi, E.; Moraitou, D.; Tsardoulias, E.; Symeonidis, A.L.; Papaliagkas, V.; Tsolaki, M. R4Alz-Revised: A Tool Able to Strongly Discriminate “Subjective Cognitive Decline” from Healthy Cognition and “Minor Neurocognitive Disorder”. Diagnostics 2023, 13, 338. [Google Scholar] [CrossRef]
- Wolfsgruber, S.; Kleineidam, L.; Guski, J.; Polcher, A.; Frommann, I.; Roeske, S.; Spruth, E.J.; Franke, C.; Priller, J.; Kilimann, I.; et al. Minor Neuropsychological Deficits in Patients with Subjective Cognitive Decline. Neurology 2020, 95, e1134–e1143. [Google Scholar] [CrossRef] [PubMed]
- Macoir, J.; Tremblay, P.; Hudon, C. The Use of Executive Fluency Tasks to Detect Cognitive Impairment in Individuals with Subjective Cognitive Decline. Behav. Sci. 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Oliver, M.D. Subjective Cognitive Decline Is Associated with Lower Baseline Cognition and Increased Rate of Cognitive Decline. J. Gerontol. Ser. B 2023, 78, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Huff, M.J.; Balota, D.A.; Aschenbrenner, A.J.; Duchek, J.M.; Fagan, A.M.; Holtzman, D.M.; Benzinger, T.L.S.; Morris, J.C. P2-085: Task-Switching Errors Show Sensitivity to Preclinical Alzheimer’s Disease Biomarkers. Alzheimers Dement. 2015, 11, P516. [Google Scholar] [CrossRef]
- Valech, N.; Tort-Merino, A.; Coll-Padrós, N.; Olives, J.; León, M.; Rami, L.; Molinuevo, J.L. Executive and Language Subjective Cognitive Decline Complaints Discriminate Preclinical Alzheimer’s Disease from Normal Aging. J. Alzheimers Dis. 2017, 61, 689–703. [Google Scholar] [CrossRef]
- Sternberg, R.J.; Sternberg, K. The Psychologist’s Companion; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Baddeley, A.D. Working Memory; Oxford University Press: Oxford, UK, 1986. [Google Scholar]
- Botvinick, M.; Braver, T. Motivation and Cognitive Control: From Behavior to Neural Mechanism. Annu. Rev. Psychol. 2015, 66, 83–113. [Google Scholar] [CrossRef]
- Checa, P.; Rodríguez-Bailón, R.; Rueda, M.R. Neurocognitive and Temperamental Systems of Self-Regulation and Early Adolescents’ Social and Academic Outcomes. Mind Brain Educ. 2008, 2, 177–187. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Lavie, N.; Hirst, A.; de Fockert, J.W.; Viding, E. Load Theory of Selective Attention and Cognitive Control. J. Exp. Psychol. Gen. 2004, 133, 339–354. [Google Scholar] [CrossRef]
- Engle, R.W.; Kane, M.J. Executive Attention, Working Memory Capacity, and a Two-Factor Theory of Cognitive Control. Psychol. Learn. Motiv. 2003, 44, 145–199. [Google Scholar] [CrossRef]
- Friedman, N.P.; Miyake, A. Unity and Diversity of Executive Functions: Individual Differences as a Window on Cognitive Structure. Cortex 2017, 86, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.G.; Chiang, J.; Pogoda, J.M.; Gomez, M.; Thomas, K.; Marion, S.D.; Miller, K.J.; Siddarth, P.; Yi, X.; Zhou, F.; et al. Executive Function Changes before Memory in Preclinical Alzheimer’s Pathology: A Prospective, Cross-Sectional, Case Control Study. PLoS ONE 2013, 8, e79378. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.M.; Krawitz, A. The Impact of Subjective Cognitive Decline on Iowa Gambling Task Performance. Neuropsychology 2015, 29, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, B.P.; Smart, C.M.; Ali, J.I. Relationship of Subjective and Objective Performance Indicators in Subjective Cognitive Decline. Psychol. Neurosci. 2016, 9, 362–378. [Google Scholar] [CrossRef]
- Mattsson, N.; Insel, P.S.; Nosheny, R.; Tosun, D.; Trojanowski, J.Q.; Shaw, L.M.; Jack, C.R.; Donohue, M.C.; Weiner, M.W. Emerging β-Amyloid Pathology and Accelerated Cortical Atrophy. JAMA Neurol. 2014, 71, 725. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Mormino, E.C.; Madison, C.; Hayenga, A.; Smiljic, A.; Jagust, W.J. β-Amyloid Affects Frontal and Posterior Brain Networks in Normal Aging. NeuroImage 2011, 54, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Vlassenko, A.G.; Mintun, M.A.; Xiong, C.; Sheline, Y.I.; Goate, A.M.; Benzinger, T.L.S.; Morris, J.C. Amyloid-Beta Plaque Growth in Cognitively Normal Adults: Longitudinal [11C] Pittsburgh Compound B Data. Ann. Neurol. 2011, 70, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Rabi, R.; Vasquez, B.P.; Alain, C.; Hasher, L.; Belleville, S.; Anderson, N.D. Inhibitory Control Deficits in Individuals with Amnestic Mild Cognitive Impairment: A Meta-Analysis. Neuropsychol. Rev. 2020, 30, 97–125. [Google Scholar] [CrossRef]
- Braver, T.S.; Barch, D.M. A Theory of Cognitive Control, Aging Cognition, and Neuromodulation. Neurosci. Biobehav. Rev. 2002, 26, 809–817. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, M.; Poptsi, E.; Aggogiatou, C.; Kounti, F.; Zafeiropoulos, S.; Markou, N. Computer-Based Cognitive Training versus Paper and Pencil Training: Which Is More Effective? A Randomized Controlled Trial in People with Mild Cognitive Impairment. JSM Alzheimers Dis. Related Dement. 2017, 4, 1032. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Tsolaki, M.; Chantzi, H.; Kazis, A. Mini Mental State Examination (MMSE): A Validation Study in Greece. Am. J. Alzheimers Dis. Other Dement. 2000, 15, 342–345. [Google Scholar] [CrossRef]
- Kounti, F.; Tsolaki, M.; Kiosseoglou, G. Functional Cognitive Assessment Scale (FUCAS): A New Scale to Assess Executive Cognitive Function in Daily Life Activities in Patients with Dementia and Mild Cognitive Impairment. Hum. Psychopharmacol. Clin. Exp. 2006, 21, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hutton, J.T. Alzheimer’s Disease. In Conn’s Current Therapy; W B Saunders Co.: Philadelphia, PA, USA, 1990; pp. 778–781. [Google Scholar]
- Rubé, P. L’examen Clinique En Psychologie. Am. J. Psychother. 1959, 13, 989–990. [Google Scholar] [CrossRef]
- MESSINIS, L.; TSAKONA, I.; MALEFAKI, S.; PAPATHANASOPOULOS, P. Normative Data and Discriminant Validity of Rey’s Verbal Learning Test for the Greek Adult Population. Arch. Clin. Neuropsychol. 2007, 22, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Rey, A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch. Psychol. 1941, 28, 286–340. [Google Scholar]
- Kosmidis, M.H.; Vlahou, C.H.; Panagiotaki, P.; Kiosseoglou, G. The Verbal Fluency Task in the Greek Population: Normative Data, and Clustering and Switching Strategies. J. Int. Neuropsychol. Soc. 2004, 10, 164–172. [Google Scholar] [CrossRef]
- Reitan, R.M.; Wolfson, D. Instructor’s Manual for the Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation; Neuropsychological Press: Tucson, AZ, USA, 1985. [Google Scholar]
- Zalonis, I.; Kararizou, E.; Triantafyllou, N.I.; Kapaki, E.; Papageorgiou, S.; Sgouropoulos, P.; Vassilopoulos, D. A Normative Study of the Trail Making Test a and B in Greek Adults. Clin. Neuropsychol. 2008, 22, 842–850. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol. Gen. 1935, 121, 15–23. [Google Scholar] [CrossRef]
- Wechsler, D.; Psychological Corporation. WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale; Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Yesavage, J.A.; Sheikh, J.I. Geriatric Depression Scale (GDS). Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Tsolaki, M.; Iacovides, A.; Yesavage, J.; O’Hara, R.; Kazis, A.; Ierodiakonou, C. The Validation of the Short Form of the Geriatric Depression Scale (GDS) in Greece. Aging Clin. Exp. Res. 1999, 11, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive Assessment of Psychopathology in Dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef] [PubMed]
- Politis, A.; Mayer, L.D.; Passa, M.; Maillis, A.; Lyketsos, C.G. Validity and Reliability of the Newly Translated Hellenic Neuropsychiatric Inventory(H-NPI) Applied to Greek Outpatients with Alzheimer’s Disease: A Study of Disturbing Behaviors among Referrals to a Memory Clinic. Int. J. Geriatr. Psychiatry 2004, 19, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Ferris, S.; de Leon, M.; Crook, T. The Global Deterioration Scale for Assessment of Primary Degenerative Dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Bentler, P.M. EQS 6 Structural Equations Program Manual; Multivariate Software: Encino, CA, USA, 2005. [Google Scholar]
- Brown, T.A. Confirmatory Factor Analysis for Applied Research; Guilford: New York, NY, USA, 2015. [Google Scholar]
- Anthony, H.; Richard, A.A. Statnote 6: Post-hoc ANOVA tests. Microbiologist 2006, 9, 34–36. [Google Scholar]
- Staudinger, U.M.; Cornelius, S.W.; Baltes, P.B. The Aging of Intelligence: Potential and Limits. ANNALS Am. Acad. Political Soc. Sci. 1989, 503, 43–59. [Google Scholar] [CrossRef]
- Baltes, P.B. The Aging Mind: Potential and Limits. Gerontology 1993, 33, 580–594. [Google Scholar] [CrossRef]
- Flanagan, D.P.; McGrew, K.S.; Ortiz, S.O. The Wechsler Intelligence Scales and Gf-Gc Theory; Allyn & Bacon: Boston, MA, USA, 2000. [Google Scholar]
- Wilhelm, O.; Hildebrandt, A.; Oberauer, K. What Is Working Memory Capacity, and How Can We Measure It? Front. Psychol. 2013, 4, 433. [Google Scholar] [CrossRef]
- Schneider, J.; McGrew, K.S. The Cattell-Horn-Carroll (CHC) Model of Intelligence, Contemporary Intellectual Assessment: Theories, Tests, and Issues, 3rd ed.; Guilford: New York, NY, USA, 2012. [Google Scholar]
- Cochrane, A.; Simmering, V.; Green, C.S. Fluid Intelligence Is Related to Capacity in Memory as Well as Attention: Evidence from Middle Childhood and Adulthood. PLoS ONE 2019, 14, e0221353. [Google Scholar] [CrossRef] [PubMed]
- Birney, D.P.; Beckmann, J.F. Intelligence IS Cognitive Flexibility: Why Multilevel Models of Within-Individual Processes Are Needed to Realise This. J. Intell. 2022, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Viviano, R.P.; Hayes, J.M.; Pruitt, P.J.; Fernandez, Z.J.; van Rooden, S.; van der Grond, J.; Rombouts, S.A.R.B.; Damoiseaux, J.S. Aberrant Memory System Connectivity and Working Memory Performance in Subjective Cognitive Decline. NeuroImage 2019, 185, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhao, X.; Cui, X.; Liu, X.; Zhu, X.; Jiang, Y.; Li, J. Subtle Pathophysiological Changes in Working Memory–Related Potentials and Intrinsic Theta Power in Community-Dwelling Older Adults with Subjective Cognitive Decline. Innov. Aging 2023, 7, igad004. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.; Nejati, V.; Shati, M.; Vatan, R.F.; Chehrehnegar, N.; Foroughan, M. Attentional Network Changes in Subjective Cognitive Decline. Aging Clin. Exp. Res. 2022, 34, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.-C.; Lo, C.-P.; Huang, C.-F.; Huang, W.-H.; Deng, J.F.; Hsu, Y.-H. Visual Attention Performances and Related Cerebral Microstructural Integrity among Subjects with Subjective Cognitive Decline and Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.L.J.; Summers, M.J. Attention and Working Memory Deficits in Mild Cognitive Impairment. J. Clin. Exp. Neuropsychol. 2009, 32, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.K.; Sze, S.L.; Woo, J.; Kwok, T.; Shum, D.H.K.; Yu, R.; Chan, A.S. Reduced Frontal Activations at High Working Memory Load in Mild Cognitive Impairment: Near-Infrared Spectroscopy. Dement. Geriatr. Cogn. Disord. 2016, 42, 278–296. [Google Scholar] [CrossRef]
- Rao, A.; Chatterjee, P.; Rao, A.R.; Dey, A.B. A Cross-Sectional Study of Various Memory Domains in Normal Ageing Population and Subjective Cognitive Decline Using PGIMS and Stroop Color-Word Test. J. Indian Acad. Geriatr. 2023, 19, 180. [Google Scholar]
- Fonseca, J.A.S.; Ducksbury, R.; Rodda, J.; Whitfield, T.; Nagaraj, C.; Suresh, K.; Stevens, T.; Walker, Z. Factors That Predict Cognitive Decline in Patients with Subjective Cognitive Impairment. Int. Psychogeriatr. 2015, 27, 1671–1677. [Google Scholar] [CrossRef]
- Borella, E.; Carretti, B.; Mitolo, M.; Zavagnin, M.; Caffarra, P.; Mammarella, N.; Fairfield, B.; Gamboz, N.; Piras, F. Characterizing Cognitive Inhibitory Deficits in Mild Cognitive Impairment. Psychiatry Res. 2017, 251, 342–348. [Google Scholar] [CrossRef]
- Hafiz, N.J.; Lohse, A.; Haas, R.; Reiche, S.; Sedlaczek, L.; Brandl, E.J.; Riemer, T.G. Trail Making Test Error Analysis in Subjective Cognitive Decline, Mild Cognitive Impairment, and Alzheimer’s Dementia with and without Depression. Arch. Clin. Neuropsychol. 2023, 38, 25–36. [Google Scholar] [CrossRef]
- Poptsi, E.; Moraitou, D.; Tsardoulias, E.; Symeonidisd, A.L.; Tsolaki, M. Is the Discrimination of Subjective Cognitive Decline from Cognitively Healthy Adulthood and Mild Cognitive Impairment Possible? A Pilot Study Utilizing the R4Alz Battery. J. Alzheimers Dis. 2020, 77, 715–732. [Google Scholar] [CrossRef]
- Petrazzuoli, F.; Vestberg, S.; Midlöv, P.; Thulesius, H.; Stomrud, E.; Palmqvist, S. Brief Cognitive Tests Used in Primary Care Cannot Accurately Differentiate Mild Cognitive Impairment from Subjective Cognitive Decline. J. Alzheimers Dis. 2020, 75, 1191–1201. [Google Scholar] [CrossRef]
| The “unity and diversity” model [22] | Three cognitive-control abilities engaged in complex executive tasks:
|
| The “load theory” [23] | Cognitive control & perceptual load are associated with selective attention. Two mechanisms are activated against distractor intrusions:
|
| The “two-factor” theory of cognitive control [24] | Tests of working memory (WM) and fluid intelligence are related.
|
| The “unity and diversity” model of executive functions in a behavioral and a genetic level [25] | At a behavioral level
|
| Diagnostic Groups | ||||
|---|---|---|---|---|
| Characteristics | HC Adults (n = 192) | HC Older Adults (n = 29) | SCI (n = 74) | MCI (n = 109) |
| Age M (SD) | 36.95 (12.88) | 66.65 (4.76) | 65.90 (7.75) | 69.46 (7.87) |
| Gender (Male/Female) | 70 M/122 F | 11 M/18 F | 16 M/58 F | 28 M/81 F |
| Education M (SD) | 16.54 (2.70) | 15.41 (2.84) | 13.56 (4.17) | 12.59 (4.13) |
| MoCA M (SD) | 28.20 (1.68) | 28.24 (1.20) | 26.96 (2.03) | 24.62 (3.12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poptsi, E.; Moraitou, D.; Tsardoulias, E.; Symeonidis, A.L.; Tsolaki, M. Subjective Cognitive Impairment Can Be Detected from the Decline of Complex Cognition: Findings from the Examination of Remedes 4 Alzheimer’s (R4Alz) Structural Validity. Brain Sci. 2024, 14, 548. https://doi.org/10.3390/brainsci14060548
Poptsi E, Moraitou D, Tsardoulias E, Symeonidis AL, Tsolaki M. Subjective Cognitive Impairment Can Be Detected from the Decline of Complex Cognition: Findings from the Examination of Remedes 4 Alzheimer’s (R4Alz) Structural Validity. Brain Sciences. 2024; 14(6):548. https://doi.org/10.3390/brainsci14060548
Chicago/Turabian StylePoptsi, Eleni, Despina Moraitou, Emmanouil Tsardoulias, Andreas L. Symeonidis, and Magda Tsolaki. 2024. "Subjective Cognitive Impairment Can Be Detected from the Decline of Complex Cognition: Findings from the Examination of Remedes 4 Alzheimer’s (R4Alz) Structural Validity" Brain Sciences 14, no. 6: 548. https://doi.org/10.3390/brainsci14060548
APA StylePoptsi, E., Moraitou, D., Tsardoulias, E., Symeonidis, A. L., & Tsolaki, M. (2024). Subjective Cognitive Impairment Can Be Detected from the Decline of Complex Cognition: Findings from the Examination of Remedes 4 Alzheimer’s (R4Alz) Structural Validity. Brain Sciences, 14(6), 548. https://doi.org/10.3390/brainsci14060548









