Differences in the Efficiency of Cognitive Control across Young Adulthood: An ERP Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Psychological Tests
2.3. The Task
2.4. Procedure
2.5. Data Recording and Analysis
2.6. Statistical Analyses
3. Results
3.1. Psychological Assessment
3.2. Behavioral Performance
3.3. ERP Analysis
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knežević, M. When Do We Become Adults? Review of Theory, Research and Recent Advances from an Interdisciplinary Perspective. Psych. Topics 2018, 27, 267–289. [Google Scholar]
- Scales, P.C.; Benson, P.L.; Pesterče, S.; Hill, K.G.; Hawkins, J.D.; Pashak, T.J. The dimensions of successful young adult development: A conceptual and measurement framework. Appl. Dev. Sci. 2016, 20, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B. Cognitive efficiency: A conceptual and methodological comparison. Learn. Instr. 2012, 22, 133–144. [Google Scholar] [CrossRef]
- Luna, B. Developmental Changes in Cognitive Control through Adolescence. Adv. Child Dev. Behav. 2009, 37, 233–278. [Google Scholar] [PubMed]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F., 3rd; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef] [PubMed]
- Sowell, E.R.; Thompson, P.M.; Holmes, C.J.; Jernigan, T.L.; Toga, A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999, 2, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.; Luna, B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci. Biobehav. Rev. 2018, 94, 179–195. [Google Scholar] [CrossRef]
- Brown, T.T.; Kuperman, J.M.; Chung, Y.; Erhart, M.; McCabe, C.; Hagler, D.J., Jr.; Venkatraman, V.K.; Akshoomoff, N.; Amaral, D.G.; Bloss, C.S.; et al. Neuroanatomical Assessment of Biological Maturity. Curr. Biol. 2012, 22, 1693–1698. [Google Scholar] [CrossRef]
- Vara, A.S.; Pang, E.W.; Vidal, J.; Anagnostou, E.; Taylor, M.J. Neural mechanisms of inhibitory control continue to mature in adolescence. Dev. Cogn. Neurosci. 2014, 10, 129–139. [Google Scholar] [CrossRef]
- Knežević, M.; Marinković, K. Neurodynamic correlates of response inhibition from emerging to mid adulthood. Cogn. Dev. 2017, 43, 106–118. [Google Scholar] [CrossRef]
- Knežević, M.; Veroude, K.; Jolles, J.; Krabbendam, L. Neural Correlates of Performance Monitoring during the Transition to Young Adulthood. Mind Brain Educ. 2016, 10, 81–90. [Google Scholar] [CrossRef]
- Gavazzi, G.; Giovannelli, F.; Noferini, C.; Cincotta, M.; Cavaliere, C.; Salvatore, M.; Mascalchi, M.; Viggiano, M.P. Subregional prefrontal cortex recruitment as a function of inhibitory demand: An fMRI metanalysis. Neurosci. Biobehav. Rev. 2023, 152, 105285. [Google Scholar] [CrossRef] [PubMed]
- Coch, D.; Meade, G. N1 and P2 to words and wordlike stimuli in late elementary school children and adults. J. Psychophysiol. 2016, 53, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Knežević, M. Error processing in young adulthood: Age-related differences in electrophysiology and behavioral performance. Neuropsychology 2024, 38, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Smit, F.; Aalten, P.; Batelaan, N.; Klein, A.; Salemink, E.; Spinhoven, P.; Struijs, S.; Vonk, P.; Wiers, R.W.; et al. The Associations of Common Psychological Problems with Mental Disorders Among College Students. Front. Psychiatry. 2021, 12, 573637. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Amminger, G.P.; Aguilar-Gaxiola, S.; Alonso, J.; Lee, S.; Ustün, T.B. Age of onset of mental disorders: A review of recent literature. Curr. Opin. Psychiatry 2007, 20, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Meijer, W.A.; Van Gerven, P.W.; de Groot, R.H.; Van Boxtel, M.P.; Jolles, J. The benefit of deep processing and high educational level for verbal learning in young and middle-aged adults. Aging Clin. Exp. Res. 2007, 19, 372–380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meijer, W.A.; de Groot, R.H.M.; van Gerven, P.W.M.; van Boxtel, M.P.J.; Jolles, J. Level of processing and reaction time in young and middle-aged adults and the effect of education. Eur. J. Cogn. Psychol. 2009, 21, 216–234. [Google Scholar] [CrossRef]
- Kutas, M.; Federmeier, K.D. Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP). Annu. Rev. Psychol. 2011, 62, 621–647. [Google Scholar] [CrossRef]
- Cnudde, K.; van Hees, S.; Brown, S.; van der Wijk, G.; Pexman, P.M.; Protzner, A.B. Increased Neural Efficiency in Visual Word Recognition: Evidence from Alterations in Event-Related Potentials and Multiscale Entropy. Entropy 2021, 23, 304. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y. Language Experience Modulates the Visual N200 Response for Disyllabic Chinese Words: An Event-Related Potential Study. Brain Sci. 2023, 13, 1321. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected studies of the Event-Related Potential. Int. J. Med. Sci. 2005, 2, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.J.; Carbine, K.A. Sample size calculations in human electrophysiology (EEG and ERP) studies: A systemati review and recommendations for increased rigor. Int. J. Psychophysiol. 2017, 111, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Picton, T.W.; Bentin, S.; Berg, P.; Donchin, E.; Hillyard, S.A.; Johnson, R., Jr.; Miller, G.A.; Ritter, W.; Ruchkin, D.S.; Rugg, M.D.; et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology 2000, 37, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Sučević, D.; Momirović, A.; Fruk, G.; Auguštin, B. Kognitivni Neverbalni Test—KNT; Naklada Slap: Jastrebarsko, Croatia, 2004. [Google Scholar]
- Knežević, M.; Tarabić, B.N.; Tomac, P.; Vincek, A.; Ivanda, L. Role of age, gender and education in information processing speed. Psych. Topics 2015, 24, 173–185. [Google Scholar]
- van der Elst, W.; van Boxtel, M.P.; van Breukelen, G.J.; Jolles, J. The Letter Digit Substitution Test: Normative data for 1858 healthy participants aged 24–81 from the Maastricht Aging Study (MAAS): Influence of age, education, and sex. J. Clin. Exp. Neuropsychol. 2006, 28, 998–1009. [Google Scholar] [CrossRef]
- Spinella, M. Normative data and a short form of the Barratt Impulsiveness Scale. Neurosci. J. 2007, 117, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Eysenck, H.J.; Eysenck, S.B.G. Eysenckov Upitnik Licnosti—EPQ; Naklada Slap: Jastrebarsko, Croatia, 1994. [Google Scholar]
- Moguš, M.; Bratanić, M.; Tadić, M. Croatian Frequency Dictionary; Školska Knjiga: Zagreb, Croatia, 1999. [Google Scholar]
- Proverbio, A.M.; Vecchi, L.; Zani, A. From orthography to phonetics: ERP measures of grapheme-to-phoneme conversion mechanisms in reading. J. Cogn. Neurosci. 2004, 16, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Hauk, O.; Coutout, C.; Holden, A.; Chen, Y. The time-course of single-word reading: Evidence from fast behavioral and brain responses. Neuroimage 2012, 60, 1462–1477. [Google Scholar] [CrossRef]
- Barber, H.A.; Kutas, M. Interplay between computational models and cognitive electrophysiology in visual word recognition. Brain Res. Rev. 2007, 53, 98–123. [Google Scholar] [CrossRef]
- Bakker, A.; Cai, J.; English, L.; Kaiser, G.; Mesa, V.; Van Dooren, W. Beyond small, medium, or large: Points of consideration when interpreting effect sizes. Educ. Stud. Math. 2019, 102, 1–8. [Google Scholar] [CrossRef]
- Elsherif, M.M.; Preece, E.; Catling, J.C. Age-of-acquisition effects: A literature review. J. Exp. Psychol. Learn. Mem. Cogn. 2023, 49, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Brysbaert, M.; Buchmeier, M.; Conrad, M.; Jacobs, A.M.; Bölte, J.; Böhl, A. The word frequency effect: A review of recent developments and implications for the choice of frequency estimates in German. Exp. Psychol. 2011, 58, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Yeung, N.; van den Wildenberg, W.; Ridderinkhof, K.R. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cogn. Affect. Behav. Neurosci. 2003, 3, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Daffner, K.R.; Alperin, B.R.; Mott, K.K.; Holcomb, P.J. Age-related differences in the automatic processing of single letters: Implications for selective attention. Neuroreport 2014, 25, 77–82. [Google Scholar] [CrossRef]
- Ruz, M.; Nobre, A.C. Attention modulates initial stages of visual word processing. J. Cogn. Neurosci. 2008, 20, 1727–1736. [Google Scholar] [CrossRef]
- Daffner, K.R.; Tarbi, E.; Haring, A.; Zhuravleva, T.; Sun, X.; Rentz, D.; Holcomb, P. The influence of executive capacity on selective attention and subsequent processing. Front. Hum. Neurosci. 2012, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.R.; Burman, D.D.; Meyer, J.R.; Lei, Z.; Trommer, B.L.; Davenport, N.D.; Li, W.; Parrish, T.B.; Gitelman, D.R.; Mesulam, M.M. Neural development of selective attention and response inhibition. Neuroimage 2003, 20, 737–751. [Google Scholar] [CrossRef]
- Ladouceur, C.D.; Dahl, R.E.; Carter, C.S. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev. Sci. 2007, 10, 874–891. [Google Scholar] [CrossRef]
- Pammer, K.; Hansen, P.C.; Kringelbach, M.L.; Holliday, I.; Barnes, G.; Hillebrand, A.; Singh, K.D.; Cornelissen, P.L. Visual word recognition: The first half second. Neuroimage 2004, 22, 1819–1825. [Google Scholar] [CrossRef]
- Wheat, K.L.; Cornelissen, P.L.; Frost, S.J.; Hansen, P.C. During Visual Word Recognition, Phonology Is Accessed within 100 ms and May Be Mediated by a Speech Production Code: Evidence from Magnetoencephalography. J. Neurosci. 2010, 30, 5229–5233. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, P.L.; Kringelbach, M.L.; Ellis, A.W.; Whitney, C.; Holliday, I.E.; Hansen, P.C. Activation of the left inferior frontal gyrus in the first 200 ms of reading: Evidence from magnetoencephalography (MEG). PLoS ONE 2009, 4, e5359. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.L.; Goddings, A.L.; Clasen, L.S.; Giedd, J.N.; Blakemore, S.J. The Developmental Mismatch in Structural Brain Maturation during Adolescence. Dev. Neurosci. 2014, 36, 147–160. [Google Scholar] [CrossRef]
- Eddy, M.D.; Grainger, J.; Holcomb, P.J.; Mitra, P.; Gabrieli, J.D. Masked priming and ERPs dissociate maturation of orthographic and semantic components of visual word recognition in children. Psychophysiology 2014, 51, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.M.; Maguire, M.J. Developmental differences in the neural correlates supporting semantics and syntax during sentence processing. Dev. Sci. 2019, 22, e12782. [Google Scholar] [CrossRef]
- Schel, M.A.; Ridderinkhof, K.R.; Crone, E.A. Choosing not to act: Neural bases of the development of intentional inhibition. Dev. Cogn. Neurosci. 2014, 10, 93–103. [Google Scholar] [CrossRef]
- Crone, E.A.; Ridderinkhof, K.R. The developing brain: From theory to neuroimaging and back. Dev. Cogn. Neurosci. 2011, 1, 101–109. [Google Scholar] [CrossRef]
| Early 20s M (±SD) | Mid 20s M (±SD) | Early 30s M (±SD) | F(2,104) | p | ηp2 | |
|---|---|---|---|---|---|---|
| Non-verbal IQ | 33.8 (±3.96) | 31.7 (±5.19) | 31.7 (±5.47) | 2.1 | 0.13 | 0.04 |
| Speed of info. processing | 45.1 (±4.86) | 46.0 (±5.55) | 44.6 (±4.99) | 0.7 | 0.49 | 0.01 |
| Impulsivity | 29.2 (±4.74) | 28.9 (±5.60) | 29.5 (±5.05) | 0.2 | 0.86 | 0.003 |
| Extraversion | 14.4 (±4.94) | 15.3 (±4.17) | 14.4 (±4.36) | 0.4 | 0.64 | 0.01 |
| Psychoticism | 3.5 (±2.45) | 3.7 (±2.14) | 4.4 (±2.02) | 0.7 | 0.48 | 0.01 |
| Neuroticism | 8.9 (±4.58) | 7.9 (±5.35) | 6.8 (±4.35) | 1.9 | 0.16 | 0.03 |
| Variable | F | p | ηp2 |
|---|---|---|---|
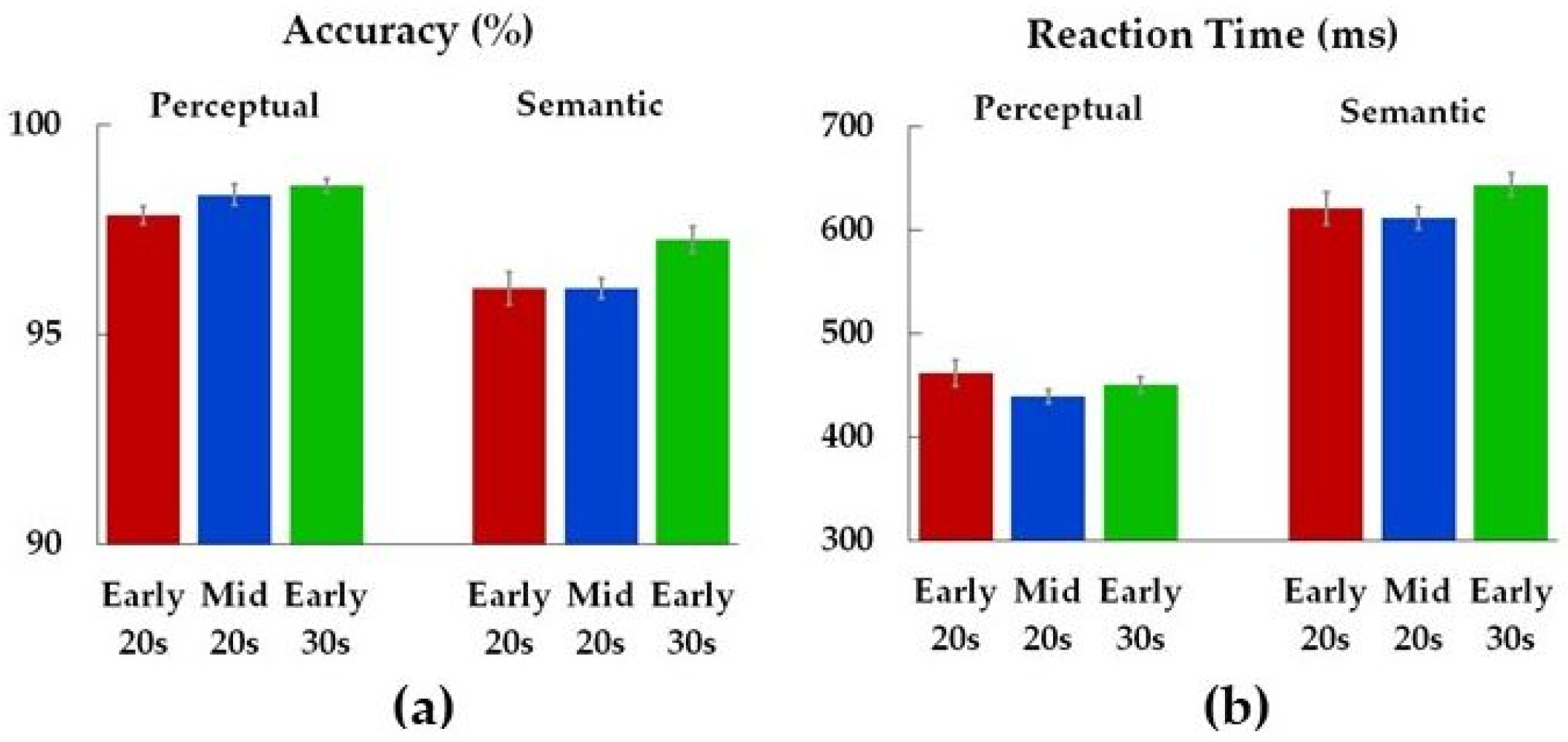
| Accuracy | |||
| Perceptual | 2.7 | 0.07 | 0.05 |
| Semantic | 3.5 | 0.04 * | 0.06 |
| Reaction Time | |||
| Perceptual | 1.4 | 0.24 | 0.03 |
| Semantic | 1.7 | 0.18 | 0.03 |
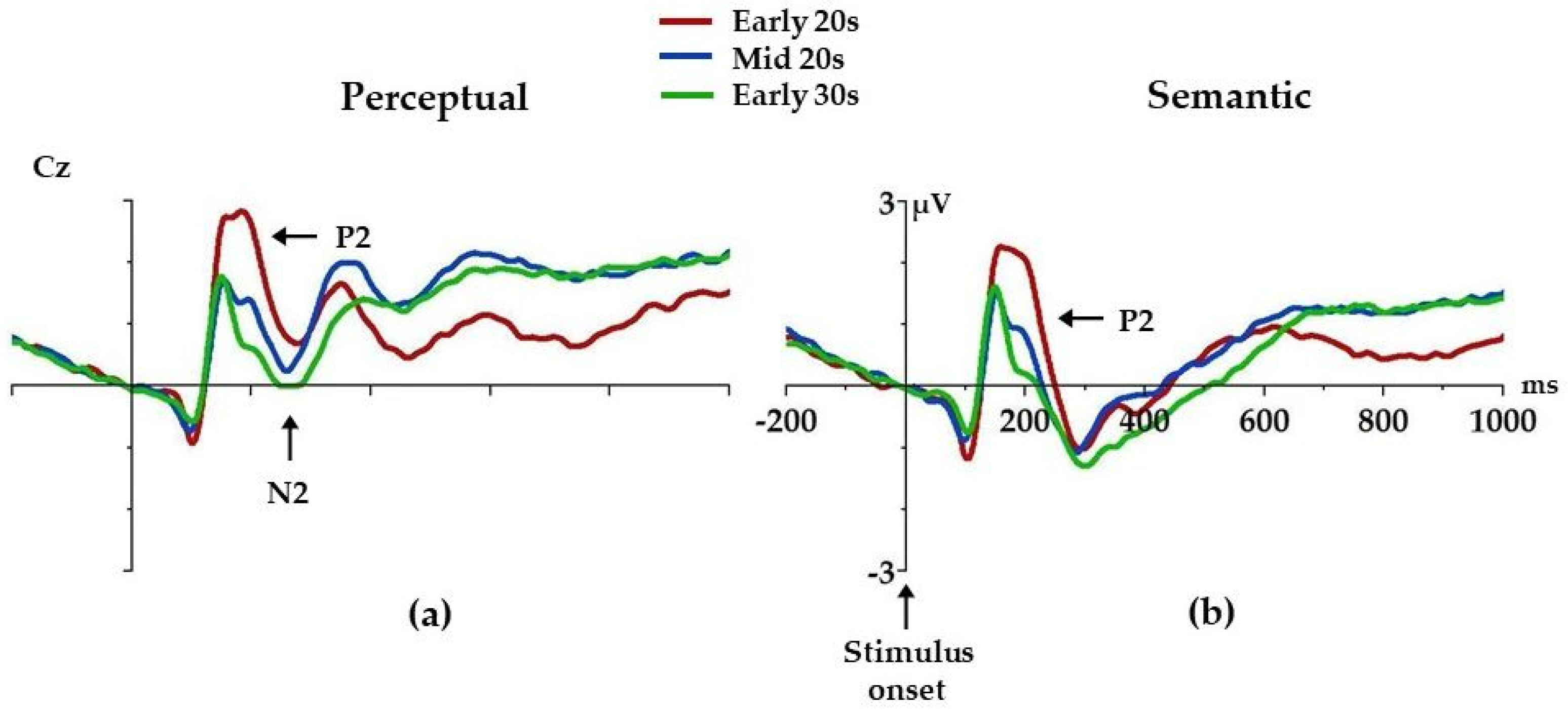
| Variable | F(2,104) | p | ηp2 |
|---|---|---|---|
| P2 Peak Latency | |||
| Perceptual | 3.1 | 0.05 | 0.06 |
| Semantic | 2.2 | 0.11 | 0.04 |
| P2 Peak Amplitude | |||
| Perceptual | 9.4 | <0.001 *** | 0.15 |
| Semantic | 7.8 | 0.001 ** | 0.13 |
| N2 Mean Amplitude | |||
| Perceptual | 3.8 | 0.03 * | 0.07 |
| Semantic | 2.0 | 0.14 | 0.04 |
| N400 Mean Amplitude | |||
| Perceptual | 3.2 | 0.05 | 0.06 |
| Semantic | 1.1 | 0.35 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knežević, M. Differences in the Efficiency of Cognitive Control across Young Adulthood: An ERP Perspective. Brain Sci. 2024, 14, 347. https://doi.org/10.3390/brainsci14040347
Knežević M. Differences in the Efficiency of Cognitive Control across Young Adulthood: An ERP Perspective. Brain Sciences. 2024; 14(4):347. https://doi.org/10.3390/brainsci14040347
Chicago/Turabian StyleKnežević, Martina. 2024. "Differences in the Efficiency of Cognitive Control across Young Adulthood: An ERP Perspective" Brain Sciences 14, no. 4: 347. https://doi.org/10.3390/brainsci14040347
APA StyleKnežević, M. (2024). Differences in the Efficiency of Cognitive Control across Young Adulthood: An ERP Perspective. Brain Sciences, 14(4), 347. https://doi.org/10.3390/brainsci14040347






