A Narrative Review of Current and Emerging Trends in the Treatment of Alcohol Use Disorder
Abstract
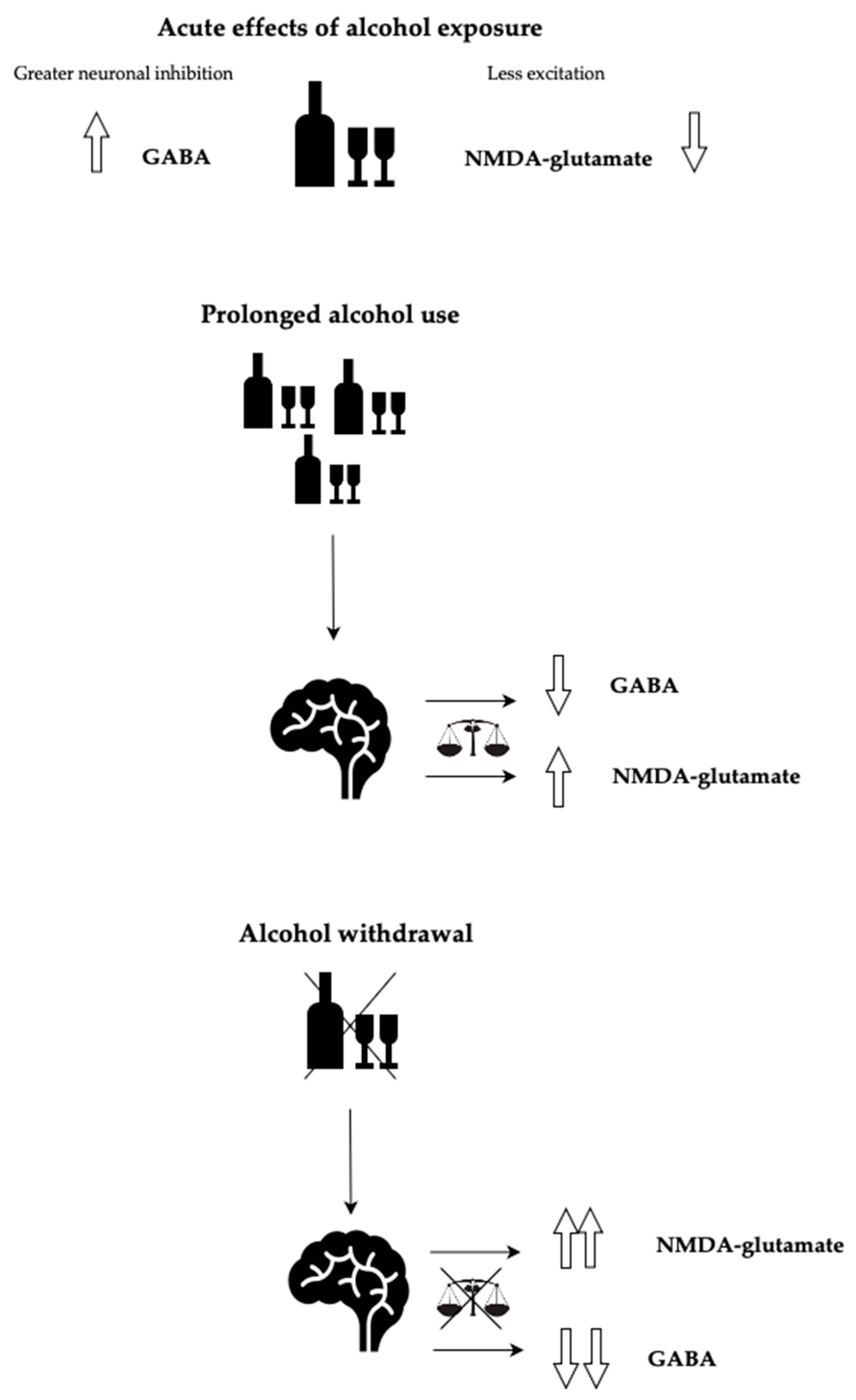
1. Introduction
2. Methods
3. Results
3.1. Benzodiazepines for AWS
3.2. Medications Other than Benzodiazepines for Alcohol Withdrawal Syndrome
3.2.1. GABA-B Receptor Agonists
Baclofen
3.2.2. Barbiturates
Phenobarbital
3.2.3. Anesthetics
Ketamine
Propofol
3.2.4. Anticonvulsants
Gabapentin
Valproate
3.2.5. Alpha-2-Agonists
Dexmedotimidine and Clonidine
3.2.6. Phosphodiesterase-4 Inhibitors
Ibudilast
3.2.7. Antipsychotics
3.2.8. Sodium Oxybate (SMO), Gamma-Hydroxybutyrate (GHB)
3.3. Treatment Modalities for Alcohol Use Disorder
3.3.1. FDA-Approved Medications
Naltrexone
Disulfiram
Acamprosate
3.3.2. Non-FDA Approved Treatment Modalities
Baclofen
ASP8062
Sodium Oxybate (SMO), Gamma-Hydroxybutyrate (GHB)
Topiramate
Gabapentin
Ondansetron
Psychedelics
LSD
Psilocybin
3.4. 4,5-Trimethoxyphenethylamine (Mescaline)
Ketamine
3.5. Phosphodiesterase-4 Inhibitors
3.5.1. Ibudilast, Apremilast
3.5.2. Ghrelin; PF-5190457
3.5.3. GLP-1 Receptor Agonists
3.5.4. Noninvasive Neural-Circuit-Based Interventions
Transcranial Magnetic Stimulation (TMS)
Deep Brain Stimulation (DBS)
Brief Interventions
Cognitive Behavioral Therapy (CBT)
Contingency Management (CM)
Alcoholics Anonymous/12-Step Facilitation (AA/TSF)
Third-Wave Therapies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- SAMHSA, Center for Behavioral Health Statistics and Quality, 2021. National Survey on Drug Use and Health. Table 2.25AdAlcohol Use in Lifetime: Among People Aged 12 or Older; by Age Group and Demographic Characteristics, Numbers in Thousands, 2021. Available online: https://www.samhsa.gov/data/sites/default/files/reports/rpt39441/NSDUHDetailedTabs2021/NSDUHDetailedTabs2021/NSDUHDetTabsSect2pe2021.htm#tab2.25a (accessed on 15 December 2023).
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013.
- Pilar, M.R.; Eyler, A.A.; Moreland-Russell, S.; Brownson, R.C. Actual causes of death in relation to media, policy, and funding attention: Examining public health priorities. Front. Public Health 2020, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Hasin, D.S.; Stinson, F.S.; Ogburn, E.; Grant, B.F. Prevalence, correlates, disability, and comorbidity of dsm-iv alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 2007, 64, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.Z.; Chou, S.P.; Zhang, H.; Jung, J.; Grant, B.F. Prevalence and Correlates of Past-Year Recovery From DSM-5 Alcohol Use Disorder: Results from National Epidemiologic Survey on Alcohol and Related Conditions-III. Alcohol. Clin. Exp. Res. 2019, 43, 2406–2420. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C.; Lichstein, P.R.; Peden, J.G., Jr.; Busher, J.T.; Waivers, L.E. Alcohol withdrawal syndromes: A review of pathophysiology, clinical presentation, and treatment. J. Gen. Intern. Med. 1989, 4, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Kosten, T.R.; O’Connor, P.G. Management of Drug and Alcohol Withdrawal. N. Engl. J. Med. 2003, 348, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Reus, V.I.; Fochtmann, L.J.; Bukstein, O.; Eyler, A.E.; Hilty, D.M.; Horvitz-Lennon, M.; Mahoney, J.; Pasic, J.; Weaver, M.; Wills, C.D.; et al. The American psychiatric association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am. J. Psychiatry 2018, 175, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Amato, L.; Minozzi, S.; Vecchi, S.; Davoli, M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst. Rev. 2010, 3, CD005063. [Google Scholar] [CrossRef] [PubMed]
- Hecksel, K.A.; Bostwick, J.M.; Jaeger, T.M.; Cha, S.S. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin. Proc. 2008, 83, 274–279. [Google Scholar] [CrossRef]
- Daeppen, J.-B.; Gache, P.; Landry, U.; Sekera, E.; Schweizer, V.; Gloor, S.; Yersin, B. Symptom-Triggered vs Fixed-Schedule Doses of Benzodiazepine for Alcohol Withdrawal. Arch. Intern. Med. 2002, 162, 1117–1121. [Google Scholar] [CrossRef]
- Mayo-Smith, M.F. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA 1997, 278, 144–151. [Google Scholar] [CrossRef]
- Cooney, G.; Heydtmann, M.; Smith, I.D. Baclofen and the Alcohol Withdrawal Syndrome—A Short Review. Front. Psychiatry 2019, 9, 773. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.N. Baclofen for alcohol withdrawal. Cochrane Database Syst. Rev. 2015, 4, CD008502. [Google Scholar] [CrossRef]
- Crunelle, C.L.; Jegham, S.; Vanderbruggen, N.; Matthys, F. Baclofen during alcohol detoxification reduces the need for additional diazepam: A randomized placebo-controlled trial. Alcohol Alcohol. 2023, 58, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, K. Evaluation of Gabapentin and Baclofen Combination for Inpatient Management of Alcohol Withdrawal Syndrome. Fed. Pract. 2023, 40, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Suddock, J.T.; Cain, M.D. Barbiturate Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mo, Y.; Thomas, M.C.; Karras, G.E. Barbiturates for the treatment of alcohol withdrawal syndrome: A systematic review of clinical trials. J. Crit. Care 2016, 32, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, J.; Clements, C.; Simon, B.; Vieaux, J.; Graffman, S.; Vahidnia, F.; Cisse, B.; Lam, J.; Alter, H. Phenobarbital for acute alcohol withdrawal: A prospective randomized double-blind placebo-controlled study. J. Emerg. Med. 2013, 44, 592–598.e2. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, A.; AlRemeithi, R.; Kartiko, S.; Bronstein, D.; Tran, Q.K. Evaluation of phenobarbital based approach in treating patient with alcohol withdrawal syndrome: A systematic review and meta-analysis. Am. J. Emerg. Med. 2023, 69, 65–75. [Google Scholar] [CrossRef]
- Filewod, N.; Hwang, S.; Turner, C.J.; Rizvi, L.; Gray, S.; Klaiman, M.; Buell, D.; Ailon, J.; Caudarella, A.; Ginocchio, G.F.; et al. Phenobarbital for the management of severe acute alcohol withdrawal (the PHENOMANAL trial): A pilot randomized controlled trial. Pilot Feasibility Stud. 2022, 8, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Vlisides, P.E. Ketamine: 50 Years of Modulating the Mind. Front. Hum. Neurosci. 2016, 10, 612. [Google Scholar] [CrossRef]
- Pizon, A.F.; Lynch, M.J.; Benedict, N.J.; Yanta, J.H.; Frisch, A.; Menke, N.B.; Swartzentruber, G.S.; King, A.M.; Abesamis, M.G.; Kane-Gill, S.L. Adjunct Ketamine Use in the Management of Severe Ethanol Withdrawal. Crit. Care Med. 2018, 46, e768–e771. [Google Scholar] [CrossRef]
- Shah, P.; McDowell, M.; Ebisu, R.; Hanif, T.; Toerne, T. Adjunctive Use of Ketamine for Benzodiazepine-Resistant Severe Alcohol Withdrawal: A Retrospective Evaluation. J. Med. Toxicol. 2018, 14, 229–236. [Google Scholar] [CrossRef]
- Brotherton, A.L.; Hamilton, E.P.; Kloss, H.G.; Hammond, D.A. Propofol for Treatment of Refractory Alcohol Withdrawal Syndrome: A Review of the Literature. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Love, K.; Zimmermann, A.E. Use of Propofol Plus Dexmedetomidine in Patients Experiencing Severe Alcohol Withdrawal in the Intensive Care Unit. J. Clin. Pharmacol. 2020, 60, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.J.; Quello, S.; Shadan, F. Gabapentin for the treatment of alcohol use disorder. Expert Opin. Investig. Drugs 2018, 27, 113–124. [Google Scholar] [CrossRef] [PubMed]
- DeFoster, R.E.; Morgan, R.J.; Leung, J.G.; Schenzel, H.; Vijapura, P.; Kashiwagi, D.T.; Fischer, K.M.; Philbrick, K.L.; Kung, S. Use of Gabapentin for Alcohol Withdrawal Syndrome in the Hospital Setting: A Randomized Open-Label Controlled Trial. Subst. Use Misuse 2023, 58, 1643–1650. [Google Scholar] [CrossRef]
- Mattle, A.G.; McGrath, P.; Sanu, A.; Kunadharaju, R.; Kersten, B.; Zammit, K.; Mammen, M.J. Gabapentin to treat acute alcohol withdrawal in hospitalized patients: A systematic review and meta-analysis. Drug Alcohol Depend. 2022, 241, 109671. [Google Scholar] [CrossRef]
- Myrick, H.; Malcolm, R.; Randall, P.K.; Boyle, E.; Anton, R.F.; Becker, H.C.; Randall, C.L. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol. Clin. Exp. Res. 2009, 33, 1582–1588. [Google Scholar] [CrossRef]
- Lambie, D.G.; Johnson, R.H.; Vijayasenan, M.E.; Whiteside, E.A. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust. N. Z. J. Psychiatry 1980, 14, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Hillbom, M.; Tokola, R.; Kuusela, V.; Kärkkäinen, P.; Källi-Lemma, L.; Pilke, A.; Kaste, M. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol 1989, 6, 223–226. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/study/NCT03235531?id=NCT03235531&rank=1 (accessed on 15 December 2023).
- Muzyk, A.J.; A Fowler, J.; Norwood, D.K.; Chilipko, A. Role of α2-agonists in the treatment of acute alcohol withdrawal. Ann. Pharmacother. 2011, 45, 649–657. [Google Scholar] [CrossRef]
- Woods, A.D.; Giometti, R.; Weeks, S.M. The use of dexmedetomidine as an adjuvant to benzodiazepine-based therapy to decrease the severity of delirium in alcohol withdrawal in adult intensive care unit patients: A systematic review. JBI Database Syst. Rev. Implement Rep. 2015, 13, 224–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tolonen, J.; Rossinen, J.; Alho, H.; Harjola, V.-P. Dexmedetomidine in addition to benzodiazepine-based sedation in patients with alcohol withdrawal delirium. Eur. J. Emerg. Med. 2013, 20, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Enătescuv, V.R.; Kalinovic, R.; Dehelean, C.A.; Giurgi-Oncu, C.; Hogea, L.M.; Ifteni, P.; Vlad, G.; Neda-Stepan, O.; Simu, M.; Bedreag, O.H.; et al. The efficacy of clonidine in the pharmacological management of alcohol withdrawal syndrome: Preliminary results. Farmácia 2020, 68, 1069–1074. [Google Scholar]
- Mayfield, J.; Harris, R.A. The Neuroimmune Basis of Excessive Alcohol Consumption. Neuropsychopharmacology 2017, 42, 376. [Google Scholar] [CrossRef]
- Johnson, K.W.; Matsuda, K.; Iwaki, Y. Ibudilast for the treatment of drug addiction and other neurological conditions. Clin. Investig. 2014, 4, 269–279. [Google Scholar] [CrossRef]
- Meredith, L.R.; Grodin, E.N.; Montoya, A.K.; Miranda, R.; Squeglia, L.M.; Towns, B.; Evans, C.; Ray, L.A. The effect of neuroimmune modulation on subjective response to alcohol in the natural environment. Alcohol. Clin. Exp. Res. 2022, 46, 876–890. [Google Scholar] [CrossRef]
- Faustmann, T.J.; Paschali, M.; Kojda, G.; Schilbach, L.; Kamp, D. Systematische Übersichtsarbeit Antipsychotische Behandlung des Alkoholentzugssyndroms: Fokus Delirium Tremens [Antipsychotic Treatment of Alcohol Withdrawal Syndrome with Focus on Delirium Tremens: A Systematic Review]. Fortschr. Neurol. Psychiatr. 2023. [Google Scholar] [CrossRef]
- Blum, K.; Eubanks, J.D.; Wallace, J.E.; Hamilton, H. Enhancement of alcohol withdrawal convulsions in mice by haloperidol. Clin. Toxicol. 1976, 9, 427–434. [Google Scholar] [CrossRef]
- Caputo, F.; Vignoli, T.; Maremmani, I.; Bernardi, M.; Zoli, G. Gamma hydroxybutyric acid (ghb) for the treatment of alcohol dependence: A review. Int. J. Environ. Res. Public Health 2009, 6, 1917–1929. [Google Scholar] [CrossRef]
- Leone, M.A.; Vigna-Taglianti, F.; Avanzi, G.; Brambilla, R.; Faggiano, F. Gamma-hydroxybutyrate (GHB) for treatment of alcohol withdrawal and prevention of relapses. Cochrane Database Syst. Rev. 2010, 2, CD006266. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Skala, K.; Mirijello, A.; Ferrulli, A.; Walter, H.; Lesch, O.; Addolorato, G. Sodium oxybate in the treatment of alcohol withdrawal syndrome: A randomized double-blind comparative study versus oxazepam. The GATE 1 Trial. CNS Drugs 2014, 28, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Department of Veterans Affairs and Department of Defense: VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders 2021. 2023. Available online: https://www.healthquality.va.gov/guidelines/mh/sud (accessed on 25 December 2023).
- Srivastava, A.B.; Gold, M.S. Naltrexone: A History and Future Directions. Cerebrum 2018, 2018, cer-13-18. [Google Scholar] [PubMed]
- Leighty, A.E.; Ansara, E.D. Treatment outcomes of long-acting injectable naltrexone versus oral naltrexone in alcohol use disorder in veterans. Ment. Health Clin. 2019, 9, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; O’neil, J.P.; Janabi, M.; Marks, S.M.; Jagust, W.J.; Fields, H.L. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci. Transl. Med. 2012, 4, 116ra6. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Amick, H.R.; Feltner, C.; Bobashev, G.; Thomas, K.; Wines, R.; Kim, M.M.; Shanahan, E.; Gass, C.E.; Rowe, C.J.; et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: A systematic review and meta-analysis. JAMA 2014, 311, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.D.; Lahmek, P.; Pham, H.; Aubin, H.-J. Disulfiram efficacy in the treatment of alcohol dependence: A meta-analysis. PLoS ONE 2014, 9, e87366. [Google Scholar] [CrossRef]
- Kranzler, H.R.; Soyka, M. Diagnosis and Pharmacotherapy of Alcohol Use Disorder. JAMA 2018, 320, 815–824. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Harris, R.A. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: Relationship to acamprosate actions. Int. J. Neuropsychopharmacol. 2008, 11, 775–793. [Google Scholar] [CrossRef]
- Rösner, S.; Hackl-Herrwerth, A.; Leucht, S.; Lehert, P.; Vecchi, S.; Soyka, M. Acamprosate for alcohol dependence. Cochrane Database Syst. Rev. 2010, 9, CD004332. [Google Scholar] [CrossRef]
- Garbutt, J.C.; Kampov-Polevoy, A.B.; Pedersen, C.; Stansbury, M.; Jordan, R.; Willing, L.; Gallop, R.J. Efficacy and tolerability of baclofen in a U.S. community population with alcohol use disorder: A dose-response, randomized, controlled trial. Neuropsychopharmacology 2021, 46, 2250–2256. [Google Scholar] [CrossRef]
- Minozzi, S.; Saulle, R.; Rösner, S. Baclofen for alcohol use disorder. Emergencias 2018, 2018, CD012557. [Google Scholar] [CrossRef]
- Maccioni, P.; Colombo, G. Potential of GABAB Receptor Positive Allosteric Modulators in the Treatment of Alcohol Use Disorder. CNS Drugs 2019, 33, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Murai, N.; Kondo, Y.; Akuzawa, S.; Mihara, T.; Shiraishi, N.; Kakimoto, S.; Matsumoto, M. A novel GABAB receptor positive allosteric modulator, ASP8062, exerts analgesic effects in a rat model of fibromyalgia. Eur. J. Pharmacol. 2019, 865, 172750. [Google Scholar] [CrossRef]
- Walzer, M.; Marek, G.J.; Wu, R.; Nagata, M.; Han, D. Single- and Multiple-Dose Safety, Tolerability, and Pharmacokinetic Profiles of ASP8062: Results From 2 Phase 1 Studies. Clin. Pharmacol. Drug Dev. 2020, 9, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, J.; Addolorato, G.; Aubin, H.-J.; Batel, P.; de Bejczy, A.; Caputo, F.; Goudriaan, A.E.; Gual, A.; Lesch, O.; Maremmani, I.; et al. Treating alcohol dependence with an abuse and misuse deterrent formulation of sodium oxybate: Results of a randomised, double-blind, placebo-controlled study. Eur. Neuropsychopharmacol. 2021, 52, 18–30. [Google Scholar] [CrossRef] [PubMed]
- A Johnson, B.; Ait-Daoud, N.; Bowden, C.L.; DiClemente, C.C.; Roache, J.D.; Lawson, K.; A Javors, M.; Ma, J.Z. Oral topiramate for treatment of alcohol dependence: A randomised controlled trial. Lancet 2003, 361, 1677–1685. [Google Scholar] [CrossRef]
- Johnson, B.A.; Rosenthal, N.; Capece, J.A.; Wiegand, F.; Mao, L.; Beyers, K.; McKay, A.; Ait-Daoud, N.; Anton, R.F.; Ciraulo, D.A.; et al. Topiramate for treating alcohol dependencea randomized controlled trial. JAMA 2007, 298, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Jose, N.; Yadav, P.; Mahla, V.P. Comparison between baclofen and topiramate in alcohol dependence: A prospective study. Ind. Psychiatry J. 2019, 28, 44–50. [Google Scholar] [CrossRef]
- Fluyau, D.; Kailasam, V.K.; Pierre, C.G. A Bayesian meta-analysis of topiramate’s effectiveness for individuals with alcohol use disorder. J. Psychopharmacol. 2023, 37, 155–163. [Google Scholar] [CrossRef]
- Anton, R.F.; Latham, P.; Voronin, K.; Book, S.; Hoffman, M.; Prisciandaro, J.; Bristol, E. Efficacy of Gabapentin for the Treatment of Alcohol Use Disorder in Patients with Alcohol Withdrawal Symptoms: A Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 728–736. [Google Scholar] [CrossRef]
- Falk, D.E.; Ryan, M.L.; Fertig, J.B.; Devine, E.G.; Cruz, R.; Brown, E.S.; Burns, H.; Salloum, I.M.; Newport, D.J.; Mendelson, J.; et al. Gabapentin Enacarbil Extended-Release for Alcohol Use Disorder: A Randomized, Double-Blind, Placebo-Controlled, Multisite Trial Assessing Efficacy and Safety. Alcohol. Clin. Exp. Res. 2019, 43, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Kranzler, H.R.; Feinn, R.; Morris, P.; Hartwell, E.E. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction 2019, 114, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.V.; Havens, J.R.; Walsh, S.L. Gabapentin misuse, abuse and diversion: A systematic review. Addiction 2016, 111, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Kenna, G.A.; Zywiak, W.H.; Swift, R.M.; McGeary, J.E.; Clifford, J.S.; Shoaff, J.R.; Fricchione, S.; Brickley, M.; Beaucage, K.; Haass-Koffler, C.L.; et al. Ondansetron and sertraline may interact with 5-HTTLPR and DRD4 polymorphisms to reduce drinking in non-treatment seeking alcohol-dependent women: Exploratory findings. Alcohol 2014, 48, 515–522. [Google Scholar] [CrossRef]
- Myrick, H.; Anton, R.F.; Li, X.; Henderson, S.; Randall, P.K.; Voronin, K. Effect of naltrexone and ondansetron on alcohol cue–induced activation of the ventral striatum in alcohol-dependent people. Arch. Gen. Psychiatry 2008, 65, 466–475. [Google Scholar] [CrossRef]
- Filho, J.M.C.; Baltieri, D.A. A pilot study of full-dose ondansetron to treat heavy-drinking men withdrawing from alcohol in Brazil. Addict. Behav. 2013, 38, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.N.; Meshkat, S.; Benitah, K.; Lipsitz, O.; Gill, H.; Lui, L.M.; Teopiz, K.M.; McIntyre, R.S.; Rosenblat, J.D. Registered clinical studies investigating psychedelic drugs for psychiatric disorders. J. Psychiatr. Res. 2021, 139, 71–81. [Google Scholar] [CrossRef]
- Bogenschutz, M.P. Studying the Effects of Classic Hallucinogens in the treatment of alcoholism: Rationale, methodology, and current research with psilocybin. Curr. Drug Abus. Rev. 2013, 6, 17–29. [Google Scholar] [CrossRef]
- Krebs, T.S.; Johansen, P. Lysergic acid diethylamide (LSD) for alcoholism: Meta-analysis of randomized controlled trials. J. Psychopharmacol. 2012, 26, 994–1002. [Google Scholar] [CrossRef]
- Alper, K.; Dong, B.; Shah, R.; Sershen, H.; Vinod, K.Y. LSD Administered as a Single Dose Reduces Alcohol Consumption in C57BL/6J Mice. Front. Pharmacol. 2018, 9, 994. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, M.W.; Güngör, C.; Skorodumov, I.; Mertens, L.J.; Spanagel, R. Psilocybin and LSD have no long-lasting effects in an animal model of alcohol relapse. Neuropsychopharmacology 2020, 45, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Ross, S.; Bhatt, S.; Baron, T.; Forcehimes, A.A.; Laska, E.; Mennenga, S.E.; O’donnell, K.; Owens, L.T.; Podrebarac, S.; et al. Percentage of Heavy Drinking Days Following Psilocybin-Assisted Psychotherapy vs Placebo in the Treatment of Adult Patients with Alcohol Use Disorder: A Randomized Clinical Trial [published correction appears in JAMA Psychiatry. JAMA Psychiatry 2022, 79, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, J.G.; De Smet, P.A.; El-Seedi, H.R.; Beck, O. Mescaline use for 5700 years. Lancet 2002, 359, 1866. [Google Scholar] [CrossRef]
- Albaugh, B.J.; Anderson, P.O. Peyote in the Treatment of Alcoholism Among American Indians. Am. J. Psychiatry 1974, 131, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Agin-Liebes, G.; Haas, T.F.; Lancelotta, R.; Uthaug, M.V.; Ramaekers, J.G.; Davis, A.K. Naturalistic Use of Mescaline Is Associated with Self-Reported Psychiatric Improvements and Enduring Positive Life Changes. ACS Pharmacol. Transl. Sci. 2021, 4, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Krupitsky, E.M.; Grineko, A.Y.; Berkaliev, T.N.; Paley, A.I.; Tetrov, U.N.; Mushkov, K.A.; Borodikin, Y.S. The combination of psychedelic and aversive approaches in alcoholism treatment: The affective contra-attribution method. Alcohol. Treat. Q. 1992, 9, 99–105. [Google Scholar] [CrossRef]
- Kolp, E.; Friedman, H.L.; Young, M.S.; Krupitsky, E. Ketamine Enhanced Psychotherapy: Preliminary Clinical Observations on Its Effectiveness in Treating Alcoholism. Humanist. Psychol. 2006, 34, 399–422. [Google Scholar] [CrossRef]
- Dakwar, E.; Levin, F.; Hart, C.L.; Basaraba, C.; Choi, J.; Pavlicova, M.; Nunes, E.V. A Single Ketamine Infusion Combined with Motivational Enhancement Therapy for Alcohol Use Disorder: A Randomized Midazolam-Controlled Pilot Trial. Am. J. Psychiatry 2020, 177, 125–133. [Google Scholar] [CrossRef]
- Grabski, M.; McAndrew, A.; Lawn, W.; Marsh, B.; Raymen, L.; Stevens, T.; Hardy, L.; Warren, F.; Bloomfield, M.; Borissova, A.; et al. Adjunctive Ketamine with Relapse Prevention–Based Psychological Therapy in the Treatment of Alcohol Use Disorder. Am. J. Psychiatry 2022, 179, 152–162. [Google Scholar] [CrossRef]
- A Ray, L.; Bujarski, S.; Shoptaw, S.; Roche, D.J.; Heinzerling, K.; Miotto, K. Development of the Neuroimmune Modulator Ibudilast for the Treatment of Alcoholism: A Randomized, Placebo-Controlled, Human Laboratory Trial. Neuropsychopharmacology 2017, 42, 1776–1788. [Google Scholar] [CrossRef]
- Grodin, E.N.; Nieto, S.J.; Meredith, L.R.; Burnette, E.; O’Neill, J.; Alger, J.; London, E.D.; Miotto, K.; Evans, C.J.; Irwin, M.R.; et al. Effects of ibudilast on central and peripheral markers of inflammation in alcohol use disorder: A randomized clinical trial. Addict. Biol. 2022, 27, e13182. [Google Scholar] [CrossRef]
- Grigsby, K.B.; Mangieri, R.A.; Roberts, A.J.; Lopez, M.F.; Firsick, E.J.; Townsley, K.G.; Beneze, A.; Bess, J.; Eisenstein, T.K.; Meissler, J.J.; et al. Preclinical and clinical evidence for suppression of alcohol intake by apremilast. J. Clin. Investig. 2023, 133, e159103. [Google Scholar] [CrossRef]
- Davis, J.F.; Schurdak, J.D.; Magrisso, I.J.; Mul, J.D.; Grayson, B.E.; Pfluger, P.T.; Tschöp, M.H.; Seeley, R.J.; Benoit, S.C. Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biol. Psychiatry 2012, 72, 354–360. [Google Scholar] [CrossRef]
- Stievenard, A.; Méquinion, M.; Andrews, Z.B.; Destée, A.; Chartier-Harlin, M.-C.; Viltart, O.; Vanbesien-Mailliot, C.C. Is there a role for ghrelin in central dopaminergic systems? Focus on nigrostriatal and mesocorticolimbic pathways. Neurosci. Biobehav. Rev. 2017, 73, 255–275. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Andrews, K.; Beveridge, R.; Cameron, K.O.; Chen, C.; Dunn, M.; Fernando, D.; Gao, H.; Hepworth, D.; Jackson, V.M.; et al. Discovery of PF-5190457, a Potent, Selective, and Orally Bioavailable Ghrelin Receptor Inverse Agonist Clinical Candidate. ACS Med. Chem. Lett. 2014, 5, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Du, X.; Li, Y.; Gong, B.; Shi, L.; Tang, T.; Jiang, H. The neurological effects of ghrelin in brain diseases: Beyond metabolic functions. Neurosci. Biobehav. Rev. 2017, 73, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Tapocik, J.D.; Ghareeb, M.; Schwandt, M.L.; Dias, A.A.; Le, A.N.; Cobbina, E.; Farinelli, L.A.; Bouhlal, S.; Farokhnia, M.; et al. The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: Preclinical safety experiments and a phase 1b human laboratory study. Mol. Psychiatry 2020, 25, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef]
- Cork, S.C.; Richards, J.E.; Holt, M.K.; Gribble, F.M.; Reimann, F.; Trapp, S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab. 2015, 4, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Suchankova, P.; Yan, J.; Schwandt, M.L.; Stangl, B.L.; Caparelli, E.C.; Momenan, R.; Jerlhag, E.; A Engel, J.; A Hodgkinson, C.; Egli, M.; et al. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: Evidence from human genetic association studies and a mouse model of alcohol dependence. Transl. Psychiatry 2015, 5, e583. [Google Scholar] [CrossRef]
- Farokhnia, M.; Browning, B.D.; Crozier, M.E.; Sun, H.; Akhlaghi, F.; Leggio, L. The glucagon-like peptide-1 system is modulated by acute and chronic alcohol exposure: Findings from human laboratory experiments and a post-mortem brain study. Addict. Biol. 2022, 27, e13211. [Google Scholar] [CrossRef]
- Vallöf, D.; Kalafateli, A.L.; Jerlhag, E. Long-term treatment with a glucagon-like peptide-1 receptor agonist reduces ethanol intake in male and female rats. Transl. Psychiatry 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Egecioglu, E.; Steensland, P.; Fredriksson, I.; Feltmann, K.; Engel, J.A.; Jerlhag, E. The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 2013, 38, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Chuong, V.; Farokhnia, M.; Khom, S.; Pince, C.L.; Elvig, S.K.; Vlkolinsky, R.; Marchette, R.C.; Koob, G.F.; Roberto, M.; Vendruscolo, L.F.; et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. J. Clin. Investig. 2023, 8, e170671. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.R.; Dorand, M.F.; Royal, K.; Mnajjed, L.; Paszkowiak, M.; Simmons, W.K. Significant Decrease in Alcohol Use Disorder Symptoms Secondary to Semaglutide Therapy for Weight Loss. J. Clin. Psychiatry 2023, 85, 50515. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, I.K.; Wium-Andersen, M.K.; Fink-Jensen, A.; Rungby, J.; Jørgensen, M.B.; Osler, M. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 2022, 131, 372–379. [Google Scholar] [CrossRef]
- Klausen, M.K.; Jensen, M.E.; Møller, M.; Le Dous, N.; Jensen, A.-M.; Zeeman, V.A.; Johannsen, C.-F.; Lee, A.M.; Thomsen, G.K.; Macoveanu, J.; et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. J. Clin. Investig. 2022, 7, e159863. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef]
- Strafella, A.P.; Paus, T.; Barrett, J.; Dagher, A. Repetitive Transcranial Magnetic Stimulation of the Human Prefrontal Cortex Induces Dopamine Release in the Caudate Nucleus. J. Neurosci. 2001, 21, RC157. [Google Scholar] [CrossRef]
- Zangen, A.; Hyodo, K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. NeuroReport 2002, 13, 2401–2405. [Google Scholar] [CrossRef]
- Kearney-Ramos, T.E.; Dowdle, L.T.; Lench, D.H.; Mithoefer, O.J.; Devries, W.H.; George, M.S.; Anton, R.F.; Hanlon, C.A. Transdiagnostic Effects of Ventromedial Prefrontal Cortex Transcranial Magnetic Stimulation on Cue Reactivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 599–609. [Google Scholar] [CrossRef]
- Harel, M.; Perini, I.; Kämpe, R.; Alyagon, U.; Shalev, H.; Besser, I.; Sommer, W.H.; Heilig, M.; Zangen, A. Repetitive Transcranial Magnetic Stimulation in Alcohol Dependence: A Randomized, Double-Blind, Sham-Controlled Proof-of-Concept Trial Targeting the Medial Prefrontal and Anterior Cingulate Cortices. Biol. Psychiatry 2022, 91, 1061–1069. [Google Scholar] [CrossRef]
- McCalley, D.M.; Kaur, N.; Wolf, J.P.; Contreras, I.E.; Book, S.W.; Smith, J.P.; Hanlon, C.A. Medial Prefrontal Cortex Theta Burst Stimulation Improves Treatment Outcomes in Alcohol Use Disorder: A Double-Blind, Sham-Controlled Neuroimaging Study. Biol. Psychiatry Glob. Open Sci. 2022, 3, 301–310. [Google Scholar] [CrossRef]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Swanson, J.M.; Telang, F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch. Neurol. 2007, 64, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Schacht, J.P.; Anton, R.F.; Myrick, H. Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addict. Biol. 2013, 18, 121–133. [Google Scholar] [CrossRef]
- Davidson, B.; Giacobbe, P.; George, T.P.; Nestor, S.M.; Rabin, J.S.; Goubran, M.; Nyman, A.J.; Baskaran, A.; Meng, Y.; Pople, C.B.; et al. Deep brain stimulation of the nucleus accumbens in the treatment of severe alcohol use disorder: A phase I pilot trial. Mol. Psychiatry 2022, 27, 3992–4000. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.; Luderer, M.; Müller, U.J.; Jakobs, M.; Baldermann, J.C.; Voges, J.; Kiening, K.; Lux, A.; Visser-Vandewalle, V.; Klosterkötter, J.; et al. Deep brain stimulation of the nucleus accumbens in treatment-resistant alcohol use disorder: A double-blind randomized controlled multi-center trial. Transl. Psychiatry 2023, 13, 1–11. [Google Scholar] [CrossRef]
- O’Donnell, A.; Anderson, P.; Newbury-Birch, D.; Schulte, B.; Schmidt, C.; Reimer, J.; Kaner, E. The impact of brief alcohol interventions in primary healthcare: A systematic review of reviews. Alcohol Alcohol. 2014, 49, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Garbutt, J.C.; Amick, H.R.; Brown, J.M.; Brownley, K.A.; Council, C.L.; Viera, A.J.; Wilkins, T.M.; Schwartz, C.J.; Richmond, E.M.; et al. Behavioral counseling after screening for alcohol misuse in primary care: A systematic review and meta-analysis for the u.s. preventive services task force. Ann. Intern. Med. 2012, 157, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Schwenker, R.; Dietrich, C.E.; Hirpa, S.; Nothacker, M.; Smedslund, G.; Frese, T.; Unverzagt, S. Motivational interviewing for substance use reduction. Cochrane Database Syst. Rev. 2023, 12, CD008063. [Google Scholar] [CrossRef] [PubMed]
- Harder, V.S.; Musau, A.M.; Musyimi, C.W.; Ndetei, D.M.; Mutiso, V.N. A randomized clinical trial of mobile phone motivational interviewing for alcohol use problems in Kenya. Addiction 2020, 115, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Magill, M.; Ray, L.; Kiluk, B.; Hoadley, A.; Bernstein, M.; Tonigan, J.S.; Carroll, K. A meta-analysis of cognitive-behavioral therapy for alcohol or other drug use disorders: Treatment efficacy by contrast condition. J. Consult. Clin. Psychol. 2019, 87, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.M.; Lake, S.L.; Hill-Kapturczak, N.; Liang, Y.; Karns, T.E.; Mullen, J.; Roache, J.D. Using contingency management procedures to reduce at-risk drinking in heavy drinkers. Alcohol. Clin. Exp. Res. 2015, 39, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Barnett, N.P.; Celio, M.A.; Tidey, J.W.; Murphy, J.G.; Colby, S.M.; Swift, R.M. A preliminary randomized controlled trial of contingency management for alcohol use reduction using a transdermal alcohol sensor. Addiction 2017, 112, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Yalom, I.D.; Leszcz, M. The Theory and Practice of Group Psychotherapy, 5th ed.; Basic Books: New York, NY, USA, 2008. [Google Scholar]
- Kelly, J.F.; Humphreys, K.; Ferri, M. Alcoholics Anonymous and other 12-step programs for alcohol use disorder. Cochrane Database Syst. Rev. 2020, 3, CD012880. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Full Catastrophe Living, Revised Edition: How to Cope with Stress, Pain and Illness Using Mindfulness Meditation; Hachette: London, UK, 2013. [Google Scholar]
- Harvey, S.T.; Henricksen, A.; Bimler, D.; Dickson, D. Addressing anger, stress and alcohol-related difficulties in the military: An ACT intervention. Mil. Psychol. 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Kamboj, S.K.; Irez, D.; Serfaty, S.; Thomas, E.; Das, R.K.; Freeman, T.P. Ultra-Brief Mindfulness Training Reduces Alcohol Consumption in At-Risk Drinkers: A Randomized Double-Blind Active-Controlled Experiment. Int. J. Neuropsychopharmacol. 2017, 20, 936–947. [Google Scholar] [CrossRef]
- McLellan, A.T.; Koob, G.F.; Volkow, N.D. Preaddiction—A Missing Concept for Treating Substance Use Disorders. JAMA Psychiatry 2022, 79, 749–751. [Google Scholar] [CrossRef] [PubMed]
| Treatment Modality | Mechanism of Action | Results |
|---|---|---|
| GABA-A receptor agonists (Benzodiazepines) | Stimulation of GABA-A receptors | Gold standard for AWS; ↓ withdrawal severity; ↓ DTs |
| GABA-B receptor agonists (Baclofen) | ↓ Excitatory neurotransmitter release; stimulation of GABA-B receptors | Effective as a benzodiazepine sparing agent; ↓ length of hospital stay |
| Barbiturates (Phenobarbital) | ↑ duration chloride ion channel opening; ↓ glutamate signaling | No sufficient evidence for monotherapy; ↓ ICU admission and intubation rates when used with benzodiazepines |
| Anesthetics (Ketamine, propofol) | NMDA antagonism; GABA-A receptor agonism | No sufficient evidence for monotherapy; ↓ Intubation rates when used with benzodiazepines; useful in refractory DTs |
| Gabapentin | ↓ GABA receptor mediated inhibitory post synaptic currents (IPSCS); voltage gated calcium channel blockage | No sufficient evidence for monotherapy; As an adjunctive in inpatient settings and outpatient management of AWS |
| GHB | GABA-B partial agonist | No recent studies; Some effectiveness in uncomplicated AWS |
| Alpha-2-agonists | ↑ central presynaptic a2-autoreceptor stimulation; ↓ autonomic hyperactivity | Could be useful as adjunctive medications; ↓ delirium severity and intubation in ICU settings |
| Phosphodiesterase-4 inhibitors | ↓ proinflammatory cytokines; Selective phosphodiesterase inhibition | No sufficient evidence; ↓ alcohol craving; positive mood effects |
| Antipsychotics | Dopamine antagonism | No recent studies; Useful in uncontrolled agitation, hallucinations |
| Treatment Modality | Mechanism of Action | Results |
|---|---|---|
| Naltrexone | Mu-opioid receptor antagonism | ↓ cravings, reinforcing effects of alcohol, binge drinking; ↓ relapse |
| Disulfiram | ALDH inhibition resulting in acetaldehyde accumulation | ↑ abstinence; questionable efficacy as monotherapy |
| Acamprosate | NMDA receptor antagonist; mGluR5 receptor modulation | ↑ abstinence; not effective in heavy drinking |
| Treatment Modality | Mechanism of Action | Results |
|---|---|---|
| Baclofen | ↓ Excitatory neurotransmitter release; stimulation of GABA-B receptors | Conflicting clinical results ↓ heavy drinking days ↑ abstinent days |
| Asp8062 | Allosteric modulator of GABA-B | ↓ alcohol consumption in animal studies Good safety and tolerability in human studies |
| GHB | Partial agonist for GABA-B receptors; ↑ production of GABA from GHB | ↑ abstinent days ↓ daily alcohol consumption ↓ withdrawal symptoms |
| Topiramate | Inhibits voltage-dependent sodium channels; ↑ inhibitory activity of GAB | ↓ percentage of heavy drinking days ↑ increased percent days abstinent ↓ alcohol craving |
| Gabapentin | ↓ GABA receptor mediated inhibitory post synaptic currents (IPSCS); blocks voltage gated calcium channels | Conflicting results ↑ non-heavy drinking days ↑ abstinent days Failed effectiveness in extended-release gabapentin (GE-XR) |
| Ondansetron | 5-HT3 receptor blockage | ↓ alcohol craving (in combination with naltrexone) ↓ percentage of heavy drinking days |
| LSD | Interaction with the 5-HT2A receptors | Old clinical trials from 70s Conflicting animal studies; ↓ alcohol intake and preference in rats |
| Psilocybin | Interaction with the 5-HT2A receptors | Psychotherapy, psilocybin combination: ↓ percentage of heavy drinking days Failed recent psilocybin/LSD microdosing in rats |
| Mescaline | Interaction with the 5-HT2A receptors | No RCTs Self-reported cathartic experiences leading to AUD alleviation ↓ self-reported daily alcohol consumption |
| Ketamine | NMDA antagonism | Psychotherapy, ketamine combination; ↓ percentage of heavy drinking days ↑ increased percent days abstinent No difference in relapse rates |
| Ibudilast; apremilast | ↓ proinflammatory cytokines; Selective phosphodiesterase inhibition | ↓ alcohol craving ↓ neural cue-reactivity ↓ daily alcohol consumption |
| Pf-5190457 | Ghrelin receptor inverse agonism | ↓ alcohol craving ↓ neural cue-reactivity |
| Dulaglutide, Exendin-4 (Ex4), exenatide, Semaglutide | GLP-1 receptor agonism | ↓ alcohol intake and preference, locomotor stimulation and dopamine release in rats ↓ alcohol cue reactivity, heavy drinking and total alcohol intake in obese patients |
| TMS, DBS | Electrical current induction to depolarize neurons | ↓ alcohol craving ↓ daily alcohol consumption ↓ neural cue-reactivity No difference in continuous abstinence |
| Brief interventions | Behavioral modification | ↓ self-reported daily alcohol consumption ↓ self-reported heavy drinking episodes |
| CBT | Behavioral modification | ↓ consumption frequency and quantity Not superior compared to MI and CM |
| AA | Behavioral modification | ↑ abstinent days Cost effective Superior compared to CBT |
| Contingency management | Behavioral modification | ↓ self-reported daily alcohol consumption ↓ self-reported heavy drinking days ↑abstinent days |
| Third-wave therapies | Behavioral modification | ↓ self-reported daily alcohol consumption ↓ self-reported past week alcohol consumption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celik, M.; Gold, M.S.; Fuehrlein, B. A Narrative Review of Current and Emerging Trends in the Treatment of Alcohol Use Disorder. Brain Sci. 2024, 14, 294. https://doi.org/10.3390/brainsci14030294
Celik M, Gold MS, Fuehrlein B. A Narrative Review of Current and Emerging Trends in the Treatment of Alcohol Use Disorder. Brain Sciences. 2024; 14(3):294. https://doi.org/10.3390/brainsci14030294
Chicago/Turabian StyleCelik, Muhammet, Mark S. Gold, and Brian Fuehrlein. 2024. "A Narrative Review of Current and Emerging Trends in the Treatment of Alcohol Use Disorder" Brain Sciences 14, no. 3: 294. https://doi.org/10.3390/brainsci14030294
APA StyleCelik, M., Gold, M. S., & Fuehrlein, B. (2024). A Narrative Review of Current and Emerging Trends in the Treatment of Alcohol Use Disorder. Brain Sciences, 14(3), 294. https://doi.org/10.3390/brainsci14030294






